Movement of microbial pathogens plays an important role in pathogen biology, host-pathogen interaction, and disease pathogenesis. Microbial pathogens employ a variety of mechanisms for cell locomotion, including passive movement within their host's circulation, cooptation of host cytoskeletal and membrane transport pathways, and active self-propulsion through the action of flagellar, amoeboid, or gliding motility. The contribution of pathogen locomotion to virulence is well documented for bacterial and viral pathogens; it functions in chemotaxis, survival in the environment, host cell attachment and invasion, intracellular locomotion, colonization of host tissues, and dissemination within the host (46, 49, 53). In the case of protozoan pathogens, e.g., Plasmodium spp., Toxoplasma gondii, trypanosomes, and Leishmania spp., the contribution of cell motility to host-pathogen interactions has been largely unexplored. The complex life cycles of these organisms, generally requiring passage through multiple hosts, as well as the variety of hosts and host tissues that they colonize, provide numerous barriers to cell movement that must be overcome. Investigation of cell motility in these organisms therefore presents an opportunity not only for advancing our understanding of microbial pathogenesis but also for illuminating novel aspects of cellular locomotion.
Recent studies on Plasmodium and Toxoplasma have demonstrated a role for parasite motility in the mammalian host and/or insect vector (19-21, 52, 58, 70). For most protozoa, however, a specific requirement for active parasite movement remains strongly implied but not tested. Likewise, we have only just begun to understand the molecular mechanisms behind the diverse forms of motility employed by parasites to navigate within their environment. Some of these mechanisms resemble those employed for motility in other organisms, while others have features that represent unique adaptations to the demands imposed on a particular parasite. A more complete understanding of these mechanisms is therefore likely to facilitate identification of novel targets for therapeutic intervention in parasitic disease. Finally, protozoa also provide important model systems for investigating the fundamental mechanisms of cell locomotion. Examples include structural and functional studies of cilia and flagella in paramecia and trypanosomes (22, 68, 74) and of gliding motility in apicomplexan parasites (52).
This review will discuss biological and mechanistic aspects of cell motility in African trypanosomes, protozoan parasites that are the causative agent of African sleeping sickness. We will first discuss the importance of trypanosome cell motility for the interaction of the parasite with its mammalian host and insect vector. Next we will summarize what is known about the unusual and distinctive swimming behavior of trypanosomes. Finally, we will discuss the main structural features of the trypanosome motility apparatus and evidence for the requirement of these structures for normal cell motility. Emphasis will be placed on features that are unique to trypanosomes, and for the most part, we will restrict our discussion to Trypanosoma brucei, drawing comparisons to other kinetoplastid parasites where appropriate.
FUNCTIONS FOR MOTILITY IN TRYPANOSOMES
African trypanosomes, e.g., T. brucei and related subspecies, are uniflagellated parasites that cause African trypanosomiasis in humans and in wild and domestic animals. T. brucei is the causative agent of human African trypanosomiasis, a fatal disease that is commonly referred to as “African sleeping sickness.” These parasites are digenetic organisms, completing part of their life cycle in a mammalian host and part in an insect vector, the tsetse fly. T. brucei is transmitted to the bloodstream of a mammalian host through the bite of an infected tsetse fly. Once in the bloodstream, the parasites multiply extracellularly for a period of weeks to months. They eventually penetrate the blood vessel endothelium, spread within the connective tissues, and infiltrate the host's central nervous system (CNS), where they initiate a cascade of events that result in fatal sleeping sickness. Clinical manifestations of sleeping sickness are divided into an early stage, in which parasites are found in the blood and lymph, and a late stage, when parasites have invaded the CNS. The early and late stages of the disease are characterized by distinct clinical symptoms and respond very differently to antiparasitic drugs (57). If untreated, sleeping sickness is always fatal, and the fatal course of the disease is directly linked to the presence of parasites in the CNS (57). Hence, the pathogenic features of sleeping sickness are directly related to migration of the parasite to specific host tissues. Since T. brucei is extracellular at all stages of its life cycle, it is dependent upon its own vigorous cell motility for extravasation and dissemination within the host.
The requirement for trypanosome cell motility is especially acute during transmission through the tsetse fly, where the parasite must undergo an ordered series of developmental transformations and directed migrations in order to achieve its goal of being delivered to a new, mammalian host (77, 79, 81, 85). Development within the tsetse fly has been extensively characterized by Vickerman (79, 81), Van Den Abbeele (77), and others (85) and is briefly summarized here. Following a blood meal, ingested quiescent bloodstream-form trypomastigotes first differentiate into actively dividing procyclic trypomastigotes and establish an infection in the tsetse fly midgut. The parasites then migrate from the midgut into the ectoperitrophic space and then through the proventriculus into the foregut, where they differentiate into elongated and asymmetrically dividing “postmesocyclic” epimastigotes (77). These elongated epimastigotes complete the journey through the proboscis and hypopharynx to reach the lumen of the salivary gland, where the final stage of development occurs. Parasites advancing to the foregut and proboscis exhibit dramatically increased motility compared to those found in the midgut (77). Once parasites are in the salivary gland, cell division is completed, generating short epimastigotes, which attach themselves to the gland epithelium through intricate membrane and cytoskeletal connections that are established between the parasite flagellum and the epithelial cell membrane (75, 79, 81). These attached epimastigotes differentiate into variant surface glycoprotein (VSG)-coated metacyclic trypomastigotes that detach from the epithelium and are now uniquely suited for survival in the mammalian bloodstream. Thus, migration of the parasite from the midgut to the salivary gland and the concomitant developmental changes that occur along the way are required for transmission to the mammalian host.
The importance of trypanosome motility for completion of the journey from the midgut to the salivary gland is obvious but remains to be tested experimentally. In addition, other important questions arise concerning development in the tsetse. Do changes in cell morphology and motility within specific compartments of the fly occur in response to environmental cues? Does the parasite arrive in the salivary gland by chance, or is this movement directed in response to chemotactic signals from the host? What is the nature of the highly structured attachment sites that form between the parasite flagellum and the salivary gland epithelium? Are these structures related to the desmosome-like adhesion junctions (see below) between the flagellum and the trypanosome cell body? The answers to these important questions await further investigation.
PHYSIOLOGICAL ASPECTS OF TRYPANOSOME CELL MOTILITY
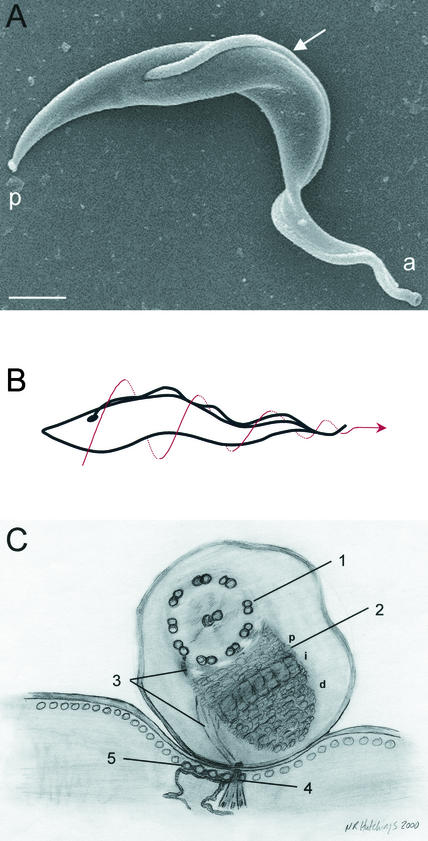
The trypanosome cell body is roughly cylindrical in shape, approximately 10 to 20 μm long, with tapered anterior and posterior ends (Fig. 1A), though some developmental stages within the tsetse fly may be much longer (77). Cell motility is accomplished through the action of a single flagellum that emerges from the basal body apparatus near the posterior end of the cell. The flagellum is surrounded by its own membrane that is distinct from, but contiguous with, the plasma membrane (1). A specialized compartment called the “flagellar pocket” forms from an invagination of the plasma membrane at the position where the flagellum emerges from the cell (84). Unlike the situation in most flagellated cells, the trypanosome flagellum is attached to the cell not only through the basal body but also along the length of the flagellum. This attachment is mediated by a highly ordered array of transmembrane cross-links that form a unique cytoskeleton-membrane domain called the flagellum attachment zone (FAZ) (see below) (26, 80). Because of this unusual arrangement, movement of the cell body is tightly coupled to flagellar wave propagation, giving the appearance of an “undulating membrane” on one side of the cell when live parasites are examined by light microscopy. The possibility that undulations produced by cytoskeletal elements within the cell body, rather than the flagellum, also contribute to cell motility (34) is intriguing but difficult to test experimentally.
FIG. 1.
T. brucei cell structure. (A) Scanning electron micrograph of T. brucei. The flagellum (arrow) is attached to the cell body along its length. The flagellum emerges from the posterior (p) end of the cell and charts a left-handed helical path as it extends toward the anterior end of the cell (a). Bar, 1 μm. (B) Cartoon depicting the auger-like movement of the trypanosome cell. The dotted arrow indicates cell motion. (C) Schematic illustration of a transverse section through the FAZ, as viewed from the posterior end of the cell. The flagellum (top) contains a canonical 9 + 2 axoneme (1) and the PFR (2). A network of thin filaments (3) connects the axoneme and PFR to the cytoplasmic FAZ filament (4). Together with a specialized quartet of reticulum-associated microtubules (5), these filaments comprise the FAZ, which extends from the flagellar pocket to the anterior end of the cell. See the text for further details. The proximal (p), intermediate (i), and distal (d) regions of the PFR are indicated. (Reprinted from references 28 and 33 with permission of the publishers.)
High-speed cinematography and stroboscopic illumination studies have demonstrated that the flagellar waves in T. brucei and other trypanosomatids initiate at the distal tip of the flagellum and move toward the basal body [30, 31, 82, 83; M. E. J. Holwill, abstract from the 17th Meet. Soc. Protozool., J. Protozool. 11(Suppl.):40, abstr. 122, 1964]. As a result, the direction of cell movement is toward the flagellar tip. Many trypanosomatid species are capable of reversing the direction of flagellar wave propagation and consequently the direction of cell movement [31, 74; Holwill, J. Protozool. 11(Suppl.):40, 1964], although this has not been demonstrated for T. brucei. Flagellar wave reversal has been examined in detail for the trypanosomatid Crithidia oncopelti, where it is used as an avoidance response and is influenced by the Ca2+ ion concentration (74). Tip-to-base wave propagation is unusual, since most flagellated cells propagate flagellar waves from base to tip (29). It remains to be determined whether the unusual directionality of wave propagation in trypanosomes results from specialized structural features of the trypanosome flagellum or the presence of specific regulatory factors. Tip-to-base wave propagation does not require an attached flagellum, since it is also observed in kinetoplastid parasites with a free flagellum, e.g., Leishmania spp. and C. oncopelti (31, 67).
The T. brucei flagellum wraps around the cell body in a left-handed helix as it extends from the posterior to the anterior end of the cell (Fig. 1A) (26, 80). As a result, beating of the flagellum produces a spiral waveform and causes the entire cell to rotate, driving it forward toward the flagellum tip in an “auger-like” motion (Fig. 1B) (82, 83). This spiral movement is a distinguishing feature of trypanosome cells. Indeed, the genus name Trypanosoma stems from the Greek word for auger, “trypanon,” and translates as “auger cell.” Thus, the movement of the trypanosome cell through its environment resembles a corkscrew threading into a cork rather than a boat being driven forward by a twirling propeller or rowing oars. The unusual spiral motility of trypanosomes, also observed in treponemes and other spirochetes with attached flagella (7), is an extremely efficient means of cell locomotion and is thought to facilitate movement through very viscous environments (34), such as the bloodstream and connective tissues of the mammalian host. It remains to be determined experimentally whether the motility of T. brucei actively facilitates extravasation or influences disease pathogenesis.
Trypanosomes are vigorous swimmers, moving with a forward velocity as high as 20 μm/s, and are capable of highly directional cell motility, i.e., moving for extended periods in one direction. Careful observation of wild-type trypanosomes revealed an interesting aspect of this organism's swimming behavior: the parasites occasionally stop their forward motion and tumble or spin in one location, then move forward again, often in a new direction (33). During this tumbling period, the trypanosome flagellum assumes a bent hook shape that is similar to the large curvature seen in sperm flagella during the transition from linear swimming to nonprogressive tumbling (42). The tumbling, or “hyperactivated” motility, of sperm cells occurs in response to Ca2+ in vitro and as yet unidentified physiological cues in vivo (42). As discussed above, Ca2+ also affects flagellar beat and cell movement in the trypanosomatid C. oncopelti (74). At present, it is not known whether environmental factors, such as Ca2+, influence flagellar beat in T. brucei. The presence of calmodulin and other Ca-binding proteins (61, 86) in the flagellum of this parasite, together with the established role of Ca2+ in regulating flagellar beat in other organisms (42, 74), makes this a likely possibility. The “run-and-tumble” motility of trypanosomes is also reminiscent of the chemotactic behavior of bacterial cells (73), and these observations together raise the intriguing possibility that trypanosomes may be capable of chemotaxis. Although chemotaxis has not yet been demonstrated in trypanosomes, such an idea is not inconsistent with the physiology of trypanosome infection in the mammalian host or with requirements for parasite development within the tsetse fly vector (see above).
STRUCTURAL FEATURES OF THE TRYPANSOME FLAGELLAR APPARATUS
Flagellar axoneme
The axoneme is the basic unit of wave generation in the eukaryotic flagellum and consists of an estimated 250 proteins (23, 43) arranged in a core structure of 9 peripheral microtubule doublets surrounding 2 singlet microtubules (68). This canonical “9 + 2” arrangement is highly conserved throughout evolution, and electron microscopy studies indicate that it is the same in trypanosomes as it is in other eukaryotic organisms (Fig. 1C) (14, 68, 71, 80). Although biochemical analysis of the axoneme has not been conducted for trypanosomes, the same general mechanism for wave propagation is presumed to operate in all eukaryotic flagella. A simplified description of this general mechanism follows. For detailed reviews of axoneme structure and models for wave generation, see references 14, 68, and 71 and references therein.
Within the axoneme, ATP-dependent binding and release of dyneins between adjacent peripheral microtubule doublets causes these microtubules to slide past one another. This sliding produces a localized bend that is propagated along the length of the flagellum through precisely coordinated activation and deactivation of dynein subsets, together with coordinated resistance forces on the side opposite the active dyneins (15, 68). This generalized model of flagellar wave generation and propagation is widely accepted and is presumed to operate in all flagellated eukaryotic cells. Four decades of careful biochemical and genetic studies with a variety of organisms have identified key structural and motor proteins and have provided a detailed picture of the arrangement of protein subunits within the axoneme (25, 68, 71). Many current efforts are therefore focused on the identification and characterization of regulatory proteins that are responsible for the precisely coordinated action of sliding and resisting forces necessary for wave propagation (60). The recent development of powerful tools for the molecular genetic analysis of trypanosomes (11, 41, 48, 76) makes them an excellent model system for investigating the molecular mechanisms of axoneme function. In particular, analysis of gene function through targeted gene disruption, RNAi knockdown of gene expression, and inducible ectopic gene expression in T. brucei will nicely complement the elegant genetic approaches that have been used with Chlamydomonas reinhardtii (23, 71).
Paraflagellar rod
In addition to the axoneme, the other major structural feature of the trypanosome flagellum is the paraflagellar rod (PFR), a large lattice-like filament that begins just anterior to the flagellar pocket and runs parallel to the axoneme within the flagellar membrane (3, 10, 44). Unlike the axoneme, which is a universal feature of all eukaryotic flagella, the PFR is observed only in kinetoplastids, euglenoids, and dinoflagellates (10). It is composed of two major protein subunits, designated PFRA and PFRC, in T. brucei (3, 44). The corresponding proteins are designated PFR2-PFR1 and PAR2-PAR3 in Leishmania spp. and T. cruzi, respectively (3, 44). A few less-abundant components have recently been described (4), but most of the biochemical data on the PFR come from studies of its two main subunits. The T. brucei PFR has a diameter of approximately 150 nm and, when viewed in cross section, consists of three structurally distinct domains designated proximal, intermediate, and distal, based on their positions relative to the axoneme (Fig. 1C) (10). The PFR is connected to the axoneme by filaments between the PFR proximal domain and axonemal doublets 4 to 7 (10).
Until recently, a function for the PFR in cell motility was entirely speculative, since this enigmatic structure is not a universal feature of motile flagella or even of those of kinetoplastids (10). Nevertheless, independent studies of T. brucei and Leishmania mexicana have now unequivocally demonstrated that the PFR is required for normal cell motility (6, 32, 67). In L. mexicana, the PFR2 gene was deleted by conventional gene disruption (67). The resultant PFR2 knockout parasites were viable in culture but possessed an incompletely formed PFR and exhibited pronounced changes in flagellar waveform and a fourfold reduction in forward swimming velocity. Stroboscopic photomicroscopy revealed that the frequency and amplitude of flagellar beat were reduced, and previous studies indicated that such an effect could result from reduced flagellum rigidity (9, 31, 67). Similar effects on motility and flagellar beat were observed in PFR1 knockout and PFR1 PFR2 double-knockout L. mexicana mutants (45). Interestingly, the PFR2 homologue in T. brucei, PFRA, appears to be essential, since efforts to delete both PFRA alleles by conventional gene disruption were unsuccessful (32). Gull and colleagues circumvented this problem by using RNAi to knock down PFRA levels without completely blocking gene expression (6). The resultant PFRA-deficient mutants are viable but have only a rudimentary PFR and are paralyzed (5, 6). These experiments with L. mexicana and T. brucei not only provide the first demonstration of a motility function for the PFR but also clearly establish the utility of RNAi for determining the function of essential genes, for which conventional gene knockouts are not possible.
Interestingly, loss of PFRA also causes mislocalization of PFRC, which is deposited in the distal tip of the mature flagellum (6). Further analysis of PFRA-deficient and PFR1 PFR2 mutants has provided important information about the processes of flagellum biogenesis in trypanosomes (2, 5,45). This process appears to be related to intraflagellar transport (IFT), a motility process that provides a means for delivering axonemal subunits from the cytoplasm to the tip of the elongating axoneme (65). IFT was first discovered in flagella of the green alga C. reinhardtii (38, 64), where large IFT particles composed of an estimated 16 polypeptides (12, 59, 65) are transported bidirectionally along the flagellar axoneme between the outer doublet microtubules and the flagellar membrane (37, 65). These particles are hypothesized to carry axonemal subunits to the flagellum tip (anterograde movement), where they drop off their cargo, and then return to the cytoplasm (retrograde movement) to be reutilized (65). The identities of some IFT particle proteins in C. reinhardtii have been determined (12, 55, 56, 65), and mutations in the corresponding genes cause defects in flagellar assembly (8, 16, 54, 55, 65). IFT is dependent upon members of the kinesin (anterograde transport) (37) and dynein (retrograde transport) (56) families of molecular motors. Although the precise functions of individual IFT particle proteins are not known, the process is conserved in other eukaryotes, e.g., Caenorhabditis elegans (12) and mammals (54, 55), and the reader is referred to reference 65 for a detailed review of IFT in these organisms. Genes for putative homologues of IFT components are present in the T. brucei genome database, which can be accessed at http://www.sanger.ac.uk/Projects/T_brucei/(24). Direct analysis of IFT in trypanosomes promises to be a very exciting area of future investigation.
Flagellum attachment zone
In contrast to the situation in most flagellated cells, the flagellum of T. brucei is attached along its length to the cell body in a specialized cytoskeleton-membrane domain, the FAZ (Fig. 1C) (26, 69, 78, 80). The FAZ extends from the flagellar pocket to the anterior end of the cell, and within this region the flagellar membrane and plasma membrane are held in close juxtaposition by desmosome-like adhesion junctions (26, 69, 78, 80). The distal tip of the flagellum extends slightly beyond the anterior end of the cell, and the length of this “free” flagellar segment is different in different developmental stages.
The cytoplasmic side of the FAZ is defined by an electron-dense filament of unknown composition that subtends the plasma membrane and runs parallel to the long axis of the cell (26). Immediately to the left of this FAZ filament, as seen looking toward the anterior end of the cell, is a quartet of specialized microtubules that are associated with a membranous tubule. These four microtubules fractionate with the FAZ and flagellar cytoskeleton upon extraction with detergent and Ca2+, conditions that depolymerize the other microtubules of the subpellicular corset (36, 62). The function of this set of FAZ microtubules and the significance of their association with the membranous tubule are not known. Attachment of the flagellum to the cell body is mediated by a network of thin filaments that provide a physical link between the FAZ filament in the cytoplasm and the PFR and axoneme in the flagellum. These connecting filaments are assembled into regularly spaced, 25-nm-diameter attachment complexes that resemble desmosomes of mammalian cells and have a center-to-center period of 95 nm (26, 80). Transverse transmission electron microscopy sections through the FAZ show that there is extensive contact between the flagellar and plasma membranes outside the direct connections composed of these cytoskeletal filaments (26, 80). The nature of these membrane contacts and the composition of the cytoskeletal attachments are unknown.
The trypanosome flagellum and FAZ have been the subject of detailed ultrastructural analysis for some 40 years (66, 69, 78, 80). However, aside from the major structural proteins of the PFR and axoneme, little is known about the identities and characteristics of proteins that mediate flagellum attachment. Although antibodies raised against trypanosome cytoskeleton preparations have revealed a number of proteins that are localized to the FAZ (26, 35, 36), the identities and functions of these proteins are largely unknown. Recently, two proteins have been demonstrated experimentally to function in flagellum attachment in trypanosomes: GP72/FLA1 (13, 18, 40) and trypanin (33).
GP72 is a 72-kDa membrane-associated glycoprotein from T. cruzi that was originally identified as an immunodominant surface antigen (72). Indirect immunofluorescence localization studies indicate that GP72 in T. cruzi and FLA1, a GP72 homologue in T. brucei (51), are enriched along the flagellum and FAZ (18, 27, 51). Cross and colleagues (13) used conventional gene disruption to delete both alleles of the T. cruzi GP72 gene. The resultant GP72-null mutants exhibited a dramatic phenotype in which the flagellum is completely detached from the cell, except at the flagellar pocket (13, 18). GP72-null cells are viable in culture but display impaired motility and sediment to the bottom of the culture flask (13, 18). Viability was severely reduced in the insect vector, but no difference was observed in infection of cultured mammalian cells relative to that by wild-type parasites (18). These results provided the first demonstration that flagellum attachment is required for normal cell motility in trypanosomes.
Efforts to delete both alleles of the gene encoding the GP72 homologue, FLA1, in T. brucei were unsuccessful, suggesting that it is an essential gene (51). Once again, RNAi provided a means to overcome this problem. By using RNAi to block FLA1 expression, LaCount et al. (40) showed that loss of FLA1 causes a flagellum detachment and cell motility phenotype similar to that seen in GP72-null T. cruzi mutants. Importantly, the authors went on to show that loss of FLA1 also blocks cytokinesis, thus confirming that the FLA1 gene is essential (39). Expression of GP72 in FLA1-deficient T. brucei mutants does not rescue the flagellum attachment or cytokinesis defect (39). Therefore, despite the sequence similarity between GP72 and FLA1, these two proteins are not functionally interchangeable. Interestingly, ectopic expression of the T. cruzi GP72 gene in T. brucei causes flagellum detachment but does not block cytokinesis (39). The ability of GP72 to interfere with one FLA1 function (flagellum attachment), but not another (cytokinesis), suggests that the flagellum attachment and cytokinesis functions of FLA1 might be separable. A more complete understanding of FLA1 function will require further investigation. Of particular interest will be more precise localization of the protein in wild-type cells and ultrastructural analysis of FLA1-deficient mutants. Investigation of FLA2 (39), encoded by a gene related to FLA1, should also prove very informative.
The observation that PFRA and FLA1 are essential in T. brucei, but that the corresponding proteins in L. mexicana (PFR2) and T. cruzi (GP72) are dispensable, is intriguing and suggests that the PFR and FAZ participate in processes that are linked to cell division in T. brucei. Indeed, these results lend further support to the hypothesis that, in addition to their roles in cell motility, the T. brucei flagellum and FAZ provide important positional and directional information for cytokinesis and cell morphogenesis (26, 47, 50, 63).
Trypanin is a 54-kDa coiled-coil protein that is associated with the detergent- and calcium-insoluble flagellar fraction of the T. brucei cytoskeleton (28, 33). Biochemical fractionation studies demonstrate that trypanin is an integral component of the flagellar cytoskeleton (28), and indirect immunofluorescence studies demonstrate that the protein is localized along the flagellum and FAZ (33). The precise position of trypanin within this region awaits characterization by immunoelectron microscopy. Procyclic trypanosomes depleted of trypanin through RNAi exhibit a remarkable cell motility defect (33). Specifically, these mutants are completely incapable of directional cell motility. The vigorous motility of wild-type trypanosomes enables them to travel long distances at velocities as high as 20 μm/s (Fig. 2) (5, 33). In contrast, trypanin-deficient mutants spin and tumble uncontrollably, remaining primarily in one location or occasionally moving backward (33). The most striking aspect of this motility defect is that trypanin-deficient cells are not paralyzed. Rather, they have an actively beating flagellum but can no longer harness flagellar beat to drive productive cell motility. Thus, without inhibiting cell motion per se, loss of trypanin prevents directional cell motility, i.e., the ability to move from point A to point B.
FIG. 2.
Trypanin is required for directional cell motility. (A) Time-lapse video microscopy of trypanin-postive and trypanin-deficient trypanosomes. Elapsed time is shown in seconds, and the midpoint of each cell at time zero (white arrows) and at each successive time point (black arrows) is indicated. (B) Cartoon diagram depicting the typical cell motion of wild-type (WT) and trypanin mutant trypanosomes. Relative cell motion is indicated with an arrow, and the rotational axis of trypanin-deficient cells is indicated by a black dot or a vertical dotted line. (Reprinted from reference 33 with permission of the publisher.)
Evidence for trypanin's involvement in flagellum attachment came from examination of whole cells by scanning electron microscopy (33), which revealed a partially detached flagellum in ∼30% of trypanin-deficient cells. Similar regions of flagellum detachment are observed in wild-type cells, but at a much lower frequency, ∼10%. The extent of flagellum detachment is relatively minor in intact cells but becomes more pronounced and more widespread (∼60% of mutant cells versus 10% of wild-type cells) when cellular membranes are removed by detergent extraction (33). Time course experiments demonstrated that flagellum detachment parallels the loss of trypanin protein and the loss of cell motility. In transmission electron microscopy analysis of detergent-extracted trypanin-deficient cytoskeletons, the FAZ lacks the highly structured organization seen in wild-type cells. However, prior to detergent extraction, these structures appear unperturbed. This suggests that trypanin participates in the direct coupling of the flagellar cytoskeleton to the subpellicular cytoskeleton and that additional interaction between the flagellar and plasma membranes contributes to the overall stability of the complex. In the absence of trypanin, the cytoskeleton connection is destabilized, though not completely destroyed, and subsequent removal of the membrane connection leads to complete disruption of the attachment complex. This interpretation is consistent with earlier models for a bipartite attachment complex, consisting of both weak (membrane) and strong (cytoskeletal) components (27, 78).
Studies on GP72/FLA1 and trypanin demonstrate that the integrity of flagellum attachment complexes must be maintained for normal cell motility in T. brucei. As more of the genes for proteins involved in flagellum attachment are isolated, similar approaches, together with assays for protein-protein interactions, will make it possible to determine how these proteins function individually and collectively in flagellum attachment and cell motility. The existence of motility-deficient mutants will also provide an opportunity to investigate the relationship between cell motility, disease pathogenesis, and parasite development in the mammalian host and insect vector.
SUMMARY
Flagellum-mediated cell motility has captured the attention and imagination of biologists for more than 300 years (68). Trypanosomes provide an excellent experimental system for studying cell motility. These parasites are easily cultured in semidefined medium, and the ultrastructure of their flagellar apparatus and other cellular structures have been extensively characterized. Importantly, several state-of-the-art tools for molecular genetic manipulation of trypanosomes are now available. In particular, inducible silencing of gene expression via RNAi provides an extremely powerful method for determining the function of essential genes. As discussed above, these approaches have allowed the first glimpse of function for several prominent and enigmatic components of the trypanosome flagellar apparatus. Ongoing genome projects for T. brucei and related kinetoplastid parasites (17) will further enhance the utility of these organisms as experimental systems. In addition to presenting a fascinating biological phenomenon, cell motility plays an important role in the pathogenesis of infectious disease. In the case of trypanosomes and other protozoan pathogens, we are only now beginning to understand the nature of this relationship, and further study of both the biological and mechanistic aspects of cell motility are necessary before we can accurately describe the relationship between parasite and host.
Acknowledgments
Work in my laboratory is supported by an NIH Research Scholar Development Award (AI01762) and NIH grant AI052348-01.
REFERENCES
- 1.Balber, A. E. 1990. The pellicle and the membrane of the flagellum, flagellar adhesion zone, and flagellar pocket: functionally discrete surface domains of the bloodstream form of African trypanosomes. Crit. Rev. Immunol. 10:177-201. [PubMed] [Google Scholar]
- 2.Bastin, P., K. Ellis, L. Kohl, and K. Gull. 2000. Flagellum ontogeny in trypanosomes studied via an inherited and regulated RNA interference system. J. Cell Sci. 113:3321-3328. [DOI] [PubMed] [Google Scholar]
- 3.Bastin, P., K. R. Matthews, and K. Gull. 1996. The paraflagellar rod of Kinetoplastida: solved and unsolved questions. Parasitol. Today 12:302-307. [DOI] [PubMed] [Google Scholar]
- 4.Bastin, P., T. J. Pullen, F. F. Moreira-Leite, and K. Gull. 2000. Inside and outside of the trypanosome flagellum: a multifunctional organelle. Microbes Infect. 2:1865-1874. [DOI] [PubMed] [Google Scholar]
- 5.Bastin, P., T. J. Pullen, T. Sherwin, and K. Gull. 1999. Protein transport and flagellum assembly dynamics revealed by analysis of the paralysed trypanosome mutant snl-1. J. Cell Sci. 112:3769-3777. [DOI] [PubMed] [Google Scholar]
- 6.Bastin, P., T. Sherwin, and K. Gull. 1998. Paraflagellar rod is vital for trypanosome motility. Nature 391:548.. [DOI] [PubMed] [Google Scholar]
- 7.Bray, D. 2001. Cell movements: from molecules to motility. Garland Publishing, New York, N.Y.
- 8.Brazelton, W. J., C. D. Amundsen, C. D. Silflow, and P. A. Lefebvre. 2001. The bld1 mutation identifies the Chlamydomonas osm-6 homolog as a gene required for flagellar assembly. Curr. Biol. 11:1591-1594. [DOI] [PubMed] [Google Scholar]
- 9.Brokaw, C. 1966. Effects of increased viscosity on the movements of some invertebrate spermatozoa. J. Exp. Biol. 45:113-139. [DOI] [PubMed] [Google Scholar]
- 10.Cachon, J., M. Cachon, M.-P. Cosson, and J. Cosson. 1988. The paraflagellar rod: a structure in search of a function. Biol. Cell 63:169-181. [Google Scholar]
- 11.Clayton, C. E. 1999. Genetic manipulation of kinetoplastida. Parasitol. Today 15:372-378. [DOI] [PubMed] [Google Scholar]
- 12.Cole, D. G., D. R. Diener, A. L. Himelblau, P. L. Beech, J. C. Fuster, and J. L. Rosenbaum. 1998. Chlamydomonas kinesin-II-dependent intraflagellar transport (IFT): IFT particles contain proteins required for ciliary assembly in Caenorhabditis elegans sensory neurons. J. Cell Biol. 141:993-1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cooper, R., A. R. de Jesus, and G. A. Cross. 1993. Deletion of an immunodominant Trypanosoma cruzi surface glycoprotein disrupts flagellum-cell adhesion. J. Cell Biol. 122:149-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cosson, J. 1996. A moving image of flagella: news and views on the mechanisms involved in axonemal beating. Cell. Biol. Int. 20:83-94. [DOI] [PubMed] [Google Scholar]
- 15.Cosson, M. P., J. Cosson, F. Andre, and R. Billard. 1995. cAMP/ATP relationship in the activation of trout sperm motility: their interaction in membrane-deprived models and in live spermatozoa. Cell Motil. Cytoskeleton 31:159-176. [DOI] [PubMed] [Google Scholar]
- 16.Deane, J. A., D. G. Cole, E. S. Seeley, D. R. Diener, and J. L. Rosenbaum. 2001. Localization of intraflagellar transport protein IFT52 identifies basal body transitional fibers as the docking site for IFT particles. Curr. Biol. 11:1586-1590. [DOI] [PubMed] [Google Scholar]
- 17.Degrave, W. M., S. Melville, A. Ivens, and M. Aslett. 2001. Parasite genome initiatives. Int. J. Parasitol. 31:532-536. [DOI] [PubMed] [Google Scholar]
- 18.de Jesus, A. R., R. Cooper, M. Espinosa, J. E. Gomes, E. S. Garcia, S., Paul, and G. A. Cross. 1993. Gene deletion suggests a role for Trypanosoma cruzi surface glycoprotein GP72 in the insect and mammalian stages of the life cycle. J. Cell Sci. 106:1023-1033. [DOI] [PubMed] [Google Scholar]
- 19.Dessens, J. T., A. L. Beetsma, G. Dimopoulos, K. Wengelnik, A. Crisanti, F. C. Kafatos, and R. E. Sinden. 1999. CTRP is essential for mosquito infection by malaria ookinetes. EMBO J. 18:6221-6227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dobrowolski, J. M., V. B. Carruthers, and L. D. Sibley. 1997. Participation of myosin in gliding motility and host cell invasion by Toxoplasma gondii. Mol. Microbiol. 26:163-173. [DOI] [PubMed] [Google Scholar]
- 21.Dobrowolski, J. M., and L. D. Sibley. 1996. Toxoplasma invasion of mammalian cells is powered by the actin cytoskeleton of the parasite. Cell 84:933-939. [DOI] [PubMed] [Google Scholar]
- 22.Dupuis-Williams, P., A. Fleury-Aubusson, N. G. de Loubresse, H. Geoffroy, L. Vayssie, A. Galvani, A. Espigat, and J. Rossier. 2002. Functional role of epsilon-tubulin in the assembly of the centriolar microtubule scaffold. J. Cell Biol. 158:1183-1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dutcher, S. K. 1995. Flagellar assembly in two hundred and fifty easy-to-follow steps. Trends Genet. 11:398-404. [DOI] [PubMed] [Google Scholar]
- 24.El-Sayed, N. M., P. Hegde, J. Quackenbush, S. E. Melville, and J. E. Donelson. 2000. The African trypanosome genome. Int. J. Parasitol. 30:329-345. [DOI] [PubMed] [Google Scholar]
- 25.Gibbons, I. R. 1995. Dynein family of motor proteins: present status and future questions. Cell Motil. Cytoskeleton 32:136-144. [DOI] [PubMed] [Google Scholar]
- 26.Gull, K. 1999. The cytoskeleton of trypanosomatid parasites. Annu. Rev. Microbiol. 53:629-655. [DOI] [PubMed] [Google Scholar]
- 27.Haynes, P. A., D. G. Russell, and G. A. Cross. 1996. Subcellular localization of Trypanosoma cruzi glycoprotein Gp72. J. Cell Sci. 109:2979-2988. [DOI] [PubMed] [Google Scholar]
- 28.Hill, K. L., N. R. Hutchings, P. M. Grandgenett, and J. E. Donelson. 2000. T lymphocyte triggering factor of African trypanosomes is associated with the flagellar fraction of the cytoskeleton and represents a new family of proteins that are present in several divergent eukaryotes. J. Biol. Chem. 275:39369-39378. [DOI] [PubMed] [Google Scholar]
- 29.Holwill, M. E. 1974. Some physical aspects of the motility of ciliated and flagellated microorganisms. Sci. Prog. 61:63-80. [PubMed] [Google Scholar]
- 30.Holwill, M. E. J. 1965. Deformation of erythrocytes by trypanosomes. Exp. Cell Res. 37:306-311. [DOI] [PubMed] [Google Scholar]
- 31.Holwill, M. E. J. 1965. The motion of Strigomona oncopelti. J. Exp. Biol. 42:125-137. [Google Scholar]
- 32.Hungerglaser, I., and T. Seebeck. 1997. Deletion of the genes for the paraflagellar rod protein PFR-A in Trypanosoma brucei is probably lethal. Mol. Biochem. Parasitol. 90:347-351. [DOI] [PubMed] [Google Scholar]
- 33.Hutchings, N. R., J. E. Donelson, and K. L. Hill. 2002. Trypanin is a cytoskeletal linker protein and is required for cell motility in African trypanosomes. J. Cell Biol. 156:867-877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jahn, T. L., and E. C. Bovee. 1968. Locomotion of blood protists, p. 393-436. In D. Weinman and M. Ristic (ed.), Infectious blood diseases of man and animals, vol. 1. Academic Press, London, England.
- 35.Kohl, L., and K. Gull. 1998. Molecular architecture of the trypanosome cytoskeleton. Mol. Biochem. Parasitol. 93:1-9. [DOI] [PubMed] [Google Scholar]
- 36.Kohl, L., T. Sherwin, and K. Gull. 1999. Assembly of the paraflagellar rod and the flagellum attachment zone complex during the Trypanosoma brucei cell cycle. J. Eukaryot. Microbiol. 46:105-109. [DOI] [PubMed] [Google Scholar]
- 37.Kozminski, K. G., P. L. Beech, and J. L. Rosenbaum. 1995. The Chlamydomonas kinesin-like protein FLA10 is involved in motility associated with the flagellar membrane. J. Cell Biol. 131:1517-1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kozminski, K. G., K. A. Johnson, P. Forscher, and J. L. Rosenbaum. 1993. A motility in the eukaryotic flagellum unrelated to flagellar beating. Proc. Natl. Acad. Sci. USA 90:5519-5523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.LaCount, D. J., B. Barrett, and J. E. Donelson. 2002. Trypanosoma brucei FLA1 is required for flagellum attachment and cytokinesis. J. Biol. Chem. 277:17580-17588. [DOI] [PubMed] [Google Scholar]
- 40.LaCount, D. J., S. Bruse, K. L. Hill, and J. E. Donelson. 2000. Double-stranded RNA interference in Trypanosoma brucei using head-to-head promoters. Mol. Biochem. Parasitol. 111:67-76. [DOI] [PubMed] [Google Scholar]
- 41.LaCount, D. J., and J. E. Donelson. 2001. RNA interference in African trypanosomes. Protist 152:103-111. [DOI] [PubMed] [Google Scholar]
- 42.Lindemann, C. B., and K. S. Kanous. 1997. A model for flagellar motility. Int. Rev. Cytol. 173:1-72. [DOI] [PubMed] [Google Scholar]
- 43.Luck, D., G. Piperno, Z. Ramanis, and B. Huang. 1977. Flagellar mutants of Chlamydomonas: studies of radial spoke-defective strains by dikaryon and revertant analysis. Proc. Natl. Acad. Sci. USA 74:3456-3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maga, J. A., and J. H. LeBowitz. 1999. Unravelling the kinetoplastid paraflagellar rod. Trends Cell Biol. 9:409-413. [DOI] [PubMed] [Google Scholar]
- 45.Maga, J. A., T. Sherwin, S. Francis, K. Gull, and J. H. LeBowitz. 1999. Genetic dissection of the Leishmania paraflagellar rod, a unique flagellar cytoskeleton structure. J. Cell Sci. 112:2753-2763. [DOI] [PubMed] [Google Scholar]
- 46.Manson, M. D., J. P. Armitage, J. A. Hoch, and R. M. Macnab. 1998. Bacterial locomotion and signal transduction. J. Bacteriol. 180:1009-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moreira-Leite, F. F., T. Sherwin, L. Kohl, and K. Gull. 2001. A trypanosome structure involved in transmitting cytoplasmic information during cell division. Science 294:610-612. [DOI] [PubMed] [Google Scholar]
- 48.Morris, J. C., Z. Wang, M. E. Drew, and P. T. Englund. 2002. Glycolysis modulates trypanosome glycoprotein expression as revealed by an RNAi library. EMBO J. 21:4429-4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moss, B., and B. M. Ward. 2001. High-speed mass transit for poxviruses on microtubules. Nat. Cell Biol. 3:E245-E246. [DOI] [PubMed] [Google Scholar]
- 50.Ngo, H., C. Tschudi, K. Gull, and E. Ullu. 1998. Double-stranded RNA induces mRNA degradation in Trypanosoma brucei. Proc. Natl. Acad. Sci. USA 95:14687-14692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nozaki, T., P. A. Haynes, and G. A. Cross. 1996. Characterization of the Trypanosoma brucei homologue of a Trypanosoma cruzi flagellum-adhesion glycoprotein. Mol. Biochem. Parasitol. 82:245-255. [DOI] [PubMed] [Google Scholar]
- 52.Opitz, C., and D. Soldati. 2002. ′The glideosome': a dynamic complex powering gliding motion and host cell invasion by Toxoplasma gondii. Mol. Microbiol. 45:597-604. [DOI] [PubMed] [Google Scholar]
- 53.Ottemann, K. M., and J. F. Miller. 1997. Roles for motility in bacterial-host interactions. Mol. Microbiol. 24:1109-1117. [DOI] [PubMed] [Google Scholar]
- 54.Pazour, G. J., S. A. Baker, J. A. Deane, D. G. Cole, B. L. Dickert, J. L. Rosenbaum, G. B. Witman, and J. C. Besharse. 2002. The intraflagellar transport protein, IFT88, is essential for vertebrate photoreceptor assembly and maintenance. J. Cell Biol. 157:103-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pazour, G. J., B. L. Dickert, Y. Vucica, E. S. Seeley, J. L. Rosenbaum, G. B. Witman, and D. G. Cole. 2000. Chlamydomonas IFT88 and its mouse homologue, polycystic kidney disease gene tg737, are required for assembly of cilia and flagella. J. Cell Biol. 151:709-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pazour, G. J., C. G. Wilkerson, and G. B. Witman. 1998. A dynein light chain is essential for the retrograde particle movement of intraflagellar transport (IFT). J. Cell Biol. 141:979-992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pepin, J., and J. E. Donelson. 1999. African trypanosomiasis (sleeping sickness), p. 774-784. In R. Guerrant et al. (ed.), Tropical infectious diseases: principles, pathogens and practice, vol. 1. Churchill Livingstone, Edinburgh, Scotland.
- 58.Pinder, J., R. Fowler, L. Bannister, A. Dluzewski, and G. H. Mitchell. 2000. Motile systems in malaria merozoites: how is the red blood cell invaded? Parasitol. Today 16:240-245. [DOI] [PubMed] [Google Scholar]
- 59.Piperno, G., and K. Mead. 1997. Transport of a novel complex in the cytoplasmic matrix of Chlamydomonas flagella. Proc. Natl. Acad. Sci. USA 94:4457-4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Porter, M. E., and W. S. Sale. 2000. The 9 + 2 axoneme anchors multiple inner arm dyneins and a network of kinases and phosphatases that control motility. J. Cell Biol. 151:F37-F42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ridgley, E., P. Webster, C. Patton, and L. Ruben. 2000. Calmodulin-binding properties of the paraflagellar rod complex from Trypanosoma brucei. Mol. Biochem. Parasitol. 109:195-201. [DOI] [PubMed] [Google Scholar]
- 62.Robinson, D., P. Beattie, T. Sherwin, and K. Gull. 1991. Microtubules, tubulin, and microtubule-associated proteins of trypanosomes. Methods Enzymol. 196:285-299. [DOI] [PubMed] [Google Scholar]
- 63.Robinson, D. R., T. Sherwin, A. Ploubidou, E. H. Byard, and K. Gull. 1995. Microtubule polarity and dynamics in the control of organelle positioning, segregation, and cytokinesis in the trypanosome cell cycle. J. Cell Biol. 128:1163-1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rosenbaum, J. L., D. G. Cole, and D. R. Diener. 1999. Intraflagellar transport: the eyes have it. J. Cell Biol. 144:385-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rosenbaum, J. L., and G. B. Witman. 2002. Intraflagellar transport. Nat. Rev. Mol. Cell Biol. 3:813-825. [DOI] [PubMed] [Google Scholar]
- 66.Rudzinska, M. A., and K. Vickerman. 1968. The fine structure, p. 217-306. In D. Weinman and M. Ristic (ed.), Infectious blood diseases of man and animals, vol. 1. Academic Press, London, England.
- 67.Santrich, C., L. Moore, T. Sherwin, P. Bastin, C. Brokaw, K. Gull, and J. H. LeBowitz. 1997. A motility function for the paraflagellar rod of Leishmania parasites revealed by PFR-2 gene knockouts. Mol. Biochem. Parasitol. 90:95-109. [DOI] [PubMed] [Google Scholar]
- 68.Satir, P. 1995. Landmarks in cilia research from Leeuwenhoek to us. Cell Motil. Cytoskeleton 32:90-94. [DOI] [PubMed] [Google Scholar]
- 69.Sherwin, T., and K. Gull. 1989. The cell division cycle of Trypanosoma brucei brucei: timing of event markers and cytoskeletal modulations. Philos. Trans. R. Soc. Lond. Ser. B 323:573-588. [DOI] [PubMed] [Google Scholar]
- 70.Sibley, L. D., and N. W. Andrews. 2000. Cell invasion by un-palatable parasites. Traffic 1:100-106. [DOI] [PubMed] [Google Scholar]
- 71.Silflow, C. D., and P. A. Lefebvre. 2001. Assembly and motility of eukaryotic cilia and flagella. Lessons from Chlamydomonas reinhardtii. Plant Physiol. 127:1500-1507. [PMC free article] [PubMed] [Google Scholar]
- 72.Snary, D., M. A. Ferguson, M. T. Scott, and A. K. Allen. 1981. Cell surface antigens of Trypanosoma cruzi: use of monoclonal antibodies to identify and isolate an epimastigote specific glycoprotein. Mol. Biochem. Parasitol. 3:343-356. [DOI] [PubMed] [Google Scholar]
- 73.Stock, J. B., and M. G. Surette. 1996. Chemotaxis, p. 1103-1129. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology. American Society for Microbiology, Washington, D.C.
- 74.Sugrue, P., M. R. Hirons, J. U. Adam, and M. E. Holwill. 1988. Flagellar wave reversal in the kinetoplastid flagellate Crithidia oncopelti. Biol. Cell 63:127-131. [DOI] [PubMed] [Google Scholar]
- 75.Tetley, L., and K. Vickerman. 1985. Differentiation in Trypanosoma brucei: host-parasite cell junctions and their persistence during acquisition of the variable antigen coat. J. Cell Sci. 74:1-19. [DOI] [PubMed] [Google Scholar]
- 76.Ullu, E., A. Djikeng, H. Shi, and C. Tschudi. 2002. RNA interference: advances and questions. Philos. Trans. R. Soc. Lond. Ser. B 357:65-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Van Den Abbeele, J., Y. Claes, D. van Bockstaele, D. Le Ray, and M. Coosemans. 1999. Trypanosoma brucei spp. development in the tsetse fly: characterization of the post-mesocyclic stages in the foregut and proboscis. Parasitology 118:469-478. [DOI] [PubMed] [Google Scholar]
- 78.Vickerman, K. 1969. On the surface coat and flagellar adhesion in trypanosomes. J. Cell Sci. 5:163-193. [DOI] [PubMed] [Google Scholar]
- 79.Vickerman, K. 1985. Developmental cycles and biology of pathogenic trypanosomes. Br. Med. Bull. 41:105-114. [DOI] [PubMed] [Google Scholar]
- 80.Vickerman, K., and T. M. Preston. 1976. Comparative cell biology of the Kinetoplastid flagellates, p. 35-130. In W. H. R. Lumsden and D. A. Evans (ed.), Biology of the Kinetoplastida, vol. 1. Academic Press, London, England.
- 81.Vickerman, K., L. Tetley, K. A. Hendry, and C. M. Turner. 1988. Biology of African trypanosomes in the tsetse fly. Biol. Cell 64:109-119. [DOI] [PubMed] [Google Scholar]
- 82.Walker, P. J., and J. C. Walker. 1963. Movement of trypanosome flagella. J. Protozool. 10(Suppl.):32. [Google Scholar]
- 83.Walker, P. J. 1961. Organization of function in trypanosome flagella. Nature 189:1017-1018. [DOI] [PubMed] [Google Scholar]
- 84.Webster, P., and D. G. Russell. 1993. The flagellar pocket of trypanosomatids. Parasitol. Today 9:201-206. [DOI] [PubMed] [Google Scholar]
- 85.Welburn, S. C., and I. Maudlin. 1999. Tsetse-trypanosome interactions: rites of passage. Parasitol Today 15:399-403. [DOI] [PubMed] [Google Scholar]
- 86.Wu, Y., J. Deford, R. Benjamin, M. G. Lee, and L. Ruben. 1994. The gene family of EF-hand calcium-binding proteins from the flagellum of Trypanosoma brucei. Biochem. J. 304:833-841. [DOI] [PMC free article] [PubMed] [Google Scholar]