Abstract
Background
Mandatory reporting of HIV infection to public health authorities, although now common, may deter people from undergoing testing. We examined HIV testing frequency in Alberta before and after mandatory reporting was implemented. We also examined the effect on testing rates among pregnant women when Alberta adopted an opt-out approach to prenatal HIV screening.
Methods
Using data from the Provincial Laboratory for Public Health, we determined the number of HIV tests done between Jan. 1, 1993, and Dec. 31, 2000, for males and females in Alberta. We used data from the Canadian Blood Services laboratories to obtain the number of tests conducted as part of the opt-out prenatal HIV testing program. Reporting of HIV infection became mandatory on May 1, 1998, and opt-out prenatal HIV testing was introduced on Sept. 1, 1998.
Results
Among males, the average annual percent increase in the number of HIV tests was 4.0% for the period before mandatory testing, as compared with 4.3% for the period after mandatory reporting was implemented; the difference in yearly trend was significant (p < 0.001). Among females, the average annual percent increase in the number of HIV tests was 9.2% for the period before mandatory reporting. In the month immediately following the adoption of opt-out prenatal HIV testing, the rate increased by 28%. Between 1999 and 2000, the average annual percent increase in the number of HIV tests among females was 1.4%.
Interpretation
The introduction of mandatory reporting of HIV infection did not appear to have a deterrent effect on rates of HIV testing. The implementation of an opt-out prenatal HIV testing policy resulted in a dramatic increase in the number of females being tested for HIV infection.
Reporting of HIV infection to facilitate appropriate follow-up for infected people, to counsel them about their infectiousness to others and to identify partners who may be unaware of their exposure is now widely recommended.1 However, there is concern that such disclosure policies may deter people from seeking HIV testing. The Alberta Medical Association and some community-based AIDS organizations in Alberta expressed concern that mandatory reporting of HIV infection could adversely effect voluntary, informed HIV testing and counselling.2,3 At the same time, it was recognized that a mandatory reporting system could ensure better tracking of a serious medical problem and provide more accurate HIV statistics at a local level for program planning and distribution of funds.3,4
A review of HIV testing rates in publicly funded counselling and testing programs in five US states showed no decrease in rates after name-based reporting was implemented.5 Results of a study involving injection drug users indicated that such reporting might cause them to delay testing.6 Other studies have shown that fear of receiving a positive test result rather than fear of mandatory reporting was the most important deterrent to testing.7,8,9 However, all these studies dealing with nominal reporting were limited in that they focused on specific groups rather than an entire population.
AIDS became a notifiable disease under public health legislation in Alberta in 1983. Testing for HIV infection was introduced in the province in 1985. On May 1, 1998, a positive HIV test result was deemed to be a notifiable condition. Notification of HIV infection requires the Provincial Laboratory for Public Health (which conducts all confirmatory HIV testing in the province) and physicians caring for HIV-infected people to report all newly diagnosed cases of HIV infection to the provincial medical health officer at Alberta Health and Wellness through the regional medical officers of health.10 Although the medical officers of health may receive nominal reports in order to facilitate necessary follow-up of HIV cases, it is not required to report nominally to Alberta Health and Wellness. Thus, since May 1, 1998, all people with a newly diagnosed HIV infection have been reported to the public health officials for follow-up, as appropriate.
As in other jurisdictions across Canada, prenatal testing for HIV infection had been performed inconsistently in Alberta, with the result that children continued to be born to HIV-infected women who had not received prophylactic treatment to prevent vertical transmission.11 Therefore, on Sept. 1, 1998, Alberta adopted an opt-out strategy for prenatal testing for HIV infection. Under this policy, HIV testing is done routinely for all pregnant women seeking prenatal care unless they specifically choose not to be tested.12 Serum samples are screened at the Canadian Blood Services laboratories in Edmonton and Calgary, and confirmatory tests are conducted at the Provincial Laboratory for Public Health.
We thus had an opportunity to evaluate the effect of mandatory reporting of HIV infection and routine screening for prenatal HIV infection on requests for HIV testing in Alberta.
Methods
We obtained data from the Provincial Laboratory for Public Health on the number of males and females in Alberta who were tested for HIV infection between Jan. 1, 1993, and Dec. 31, 2000. We obtained the number of females screened for HIV infection between Sept. 1, 1998, and Dec. 31, 2000, under the opt-out prenatal HIV screening program from the Canadian Blood Services laboratories, and the number of confirmed positive test results from the Provincial Laboratory for Public Health. To the extent possible, for people who had more than 1 positive test result (i.e., repeat testing), we included only the first test result.
We calculated average annual percent increases in the number of HIV tests performed before and after mandatory reporting was instituted. We used simple linear regression analysis to assess trends and 2-sided p values to determine whether the trends were significant. Given the short period between the introduction of mandatory reporting of HIV infection and of the opt-out prenatal HIV testing policy, for females we also included the actual number of tests conducted between April and December 1998.
Results
Among males, the average annual percent increase in the number of HIV tests for the period before mandatory reporting (Jan. 1, 1993, to Apr. 30, 1998) was 4.0%. The corresponding increase for the period after mandatory reporting was introduced (May 1, 1998, to Dec. 31, 2000) was 4.3% (Fig. 1). The trend was significantly greater in the period after the introduction of mandatory reporting (p < 0.001). Among females, the average annual percent increase in the number of HIV tests was 9.2% for the period before mandatory reporting. During April 1998, 3706 HIV tests were performed among females. The number of tests performed per month during the 4-month period immediately following the introduction of mandatory reporting was 4127, 4360, 4185 and 4160 respectively. During September 1998, immediately following the introduction of the opt-out prenatal HIV screening policy, 5339 HIV tests were performed, about 28% greater than the number performed the preceding month. The number of HIV tests performed per month between Oct. 1 and Dec. 31, 1998, was 5501, 5155 and 4652 respectively (Fig. 1). Between 1999 and 2000, the average annual percent increase in the number of HIV tests performed among females was 1.4%.
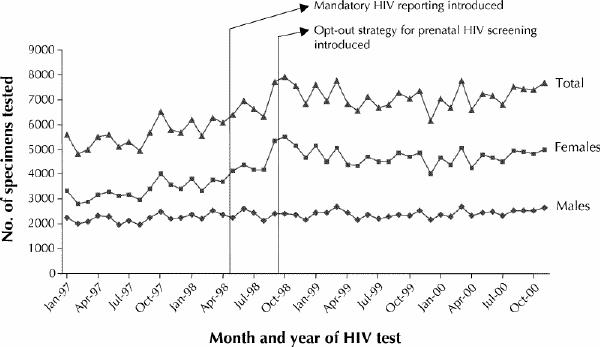
Fig. 1: Total numbers of HIV tests performed in Alberta during 1997–2000, by sex and by month and year of test.
Of the 20 396 women eligible for prenatal HIV testing during the first 4 months after the opt-out prenatal screening policy was introduced, 4.7% declined to be tested. During the entire next year (1999), 3.3% declined testing, and in 2000, 1.7% declined testing. The number of women who were found to be HIV-positive on prenatal testing was 11 (rate 2.4 per 10 000) in 1999 and 15 (rate 3.3 per 10 000) in 2000.
Interpretation
The utility of mandatory reporting of HIV infection for surveillance purposes is well documented.1 However, a recent review of legislation on HIV testing among 121 of the 191 member states of the World Health Organization indicated that only 31 countries, representing 43% of the world's population, report HIV infection.13 Even in countries where HIV infection is notifiable, measures for reporting vary. In Canada, HIV infection is reportable (nominally or nonnominally) in every province and territory except British Columbia, which is reassessing its policy.14 HIV infection is reportable in 34 of the 50 US states.15
Mandatory reporting — even nonnominal — of HIV infection remains a controversial issue largely owing to concerns about potential personal and social problems related to breaches in confidentiality. Our study shows a clear trend toward increased HIV testing in Alberta despite the introduction of mandatory reporting of HIV infection to public health authorities. Our results are consistent with other studies showing that reporting of HIV infection does not provide a distinct disincentive to testing.5,6,7,8,9 However, these other studies dealt largely with name-based reporting and were conducted in specific populations at risk of HIV infection rather than in the general population.
In addition, we found a dramatic and sustained increase in rates of prenatal HIV testing after the opt-out policy was introduced. Similar trends have been reported in the United Kingdom and the United States.16,17,18,19 In those countries, the proportion of women tested for HIV infection increased from 33%–74% under an opt-in policy (HIV testing is offered by the family physician or obstetrician and is done only once the physician has formally obtained informed consent) to 81%–88% under an opt-out policy. Given the high achievable coverage rate through an opt-out policy, and with it the enhanced likelihood of preventing vertical transmission, Bitnun and colleagues20 recently advocated the adoption of routine opt-out prenatal HIV testing throughout Canada.
Our finding of an increased rate of positive test results among pregnant women between 1999 and 2000 is cause for concern. The increase may have been due to a real change in incidence among women or may have been a result of better coverage of women at higher risk of HIV infection (e.g., sex workers). The lack of information on the laboratory test requisition forms relating to exposure categories and associated risk factors made it difficult to determine the effect of the opt-out policy on coverage of people at high risk. These data would also have been useful to determine the effect of mandatory reporting on testing in these groups.
Given that the population of Alberta is 2.7 million, the increase in population of 100 000 between 1998 and 2000 (Statistics Canada data) is unlikely to have contributed meaningfully to the number of people being tested for HIV infection in the province. However, we were unable to determine the number of pregnant women who were tested for HIV infection before the routine prenatal program was introduced, because, before September 1998, information on whether the tests were for prenatal HIV screening was not provided consistently. Thus, we could not ascertain the effect that mandatory reporting had on testing among women outside the opt-out prenatal HIV testing program.
Last, although we attempted to eliminate from the data set data for people who were undergoing repeat HIV testing because they were being followed for a positive test result, this was incomplete. There are a few anonymous test sites in Alberta, and people at those sites could not be identified. However, we believe that anonymous testing is requested infrequently. One of the 2 main STD clinics in the province has had only 23 requests for anonymous HIV testing since May 1998, when HIV infection became notifiable (Dr. Ronald R. Read, Medical Director, STD Clinic, Calgary: personal communication, 2002).
Our finding that mandatory reporting of HIV infection has not adversely affected the number of HIV tests done in the province is reassuring. It is unclear, however, whether reporting has had a differential effect by deterring people who may be at higher risk of HIV infection, such as men who have sex with men, injection drug users and recent immigrants to Canada, from undergoing testing and seeking treatment and prevention services. Surveys of men who have sex with men, injection drug users and ethnic minorities have indicated that these groups may be unwilling to undergo testing if positive results are reported nominally to health authorities.21,22,23,24,25 However, definitive studies have yet to be published on the effect of nonnominal reporting of HIV infection in these groups. There is some evidence that, at least among injection drug users in Alberta, nonnominal reporting of positive HIV test results to public health officials has not been a deterrent to testing (J.K.P., unpublished data, 2002). Continued monitoring and evaluation are required to ensure that surveillance policies do not adversely affect HIV testing.
β See related article page 707
Acknowledgments
We acknowledge Rhonda Gordon and Ross McWhirter for extracting the HIV testing data from the Provincial Laboratory for Public Health database, and Joan Cockroft, Stella Basten, Michelle Knol, Jean Ashdown and Lynda Edwards for providing access to the Canadian Blood Services prenatal data. Our thanks to Dr. Donald Sutherland, Health Canada, for critically reviewing an earlier version of the manuscript and to Dena Schanzer, Health Canada, for advice on trend analyses.
Footnotes
This article has been peer reviewed.
Contributors: Dr. Jayaraman was primarily responsible for conducting the study, including the analysis presented here, and for writing the first draft. Drs. Preiksaitis and Larke conceived the project, provided consultation and contributed to revisions of the manuscript.
Competing interests: None declared.
Correspondence to: Dr. Gayatri C. Jayaraman, Field Surveillance Officer and Senior Epidemiologist, Health Canada, Provincial Laboratory for Public Health, 3030 Hospital Dr. NW, Calgary AB T2N 4W4; fax 403 283-0142; gayatri_jayaraman@hc-sc.gc.ca
References
- 1.Valdiserri RO, Janssen, RS, Buehler JW, Fleming PL. The context of HIV/AIDS surveillance. J Acquir Immune Defic Syndr 2000;25(Suppl 2):S97-104. [DOI] [PubMed]
- 2.Bray D. Province wants HIV cases ID'd. Doctors told to reveal patients' names. Edmonton Sun 1998 Feb 10:5.
- 3.Johnson B. AIDS group OK with tracking HIV patients. Need for reliable statistics cited. Edmonton Sun 1998 Feb 11:16.
- 4.MacDonald J. Names of sex partners sought to track new HIV cases. Edmonton Journal 1998 Feb 8;Sect A:7.
- 5.Nakashima AK, Horsely R, Frey RL, Sweeney PA, Weber JT, Fleming PL, et al. Effect of HIV reporting by name on use of HIV testing in publicly funded counseling and testing programs. JAMA 1998;280:1421-6. [DOI] [PubMed]
- 6.Hecht FM, Chesney MA, Lehman JS, Osmond D, Vranizan K, Colman S, et al. Does HIV reporting by name deter testing? AIDS 2000;14:1801-8. [DOI] [PubMed]
- 7.Woods WJ, Dilley JW, Lihatsh T, Sabatino J, Adler B, Rinaldi J, et al. Name-based reporting of HIV-positive test results as a deterrent to testing. Am J Public Health 1999;89:1097-100. [DOI] [PMC free article] [PubMed]
- 8.Godin G, Myers T, Lambert J, Calzavara L, Locker D. Understanding the intention of gay and bisexual men to take the HIV antibody test. AIDS Educ Prev 1997;9:31-41. [PubMed]
- 9.Osmond DH, Bindman AB, Vranizan K, Lehman JS, Hecht FM, Keane D, et al. Name-based surveillance and public health interventions for persons with HIV infection. Multistate Evaluation of Surveillance for HIV Study Group. Ann Intern Med 1999;131:775-9. [DOI] [PubMed]
- 10.HIV to become a notifiable disease in Alberta [news release]. Edmonton: Government of Alberta; 1998 Feb 25. Available: www.gov.ab.ca/acn/199802/5914.html (accessed 2003 Feb 12).
- 11.Robinson JL, Lee BE. Prevention of perinatal transmission of HIV infection. CMAJ 2000;163(7):831-2. [PMC free article] [PubMed]
- 12.Alberta Routine Prenatal HIV Screening Program: final evaluation report prepared for Alberta Medical Association and Alberta Health and Wellness. Edmonton: Howard Research and Instructional Systems; 2001. Available: www.albertadoctors.org/resources/womens/hiv.html (accessed 2003 Feb 12).
- 13.D'Amelio R, Tuerlings E, Perito O, Biselli R, Natalicchio S, Kingma S. A global review of legislation on HIV/AIDS: the issue of HIV testing. J Acquir Immune Defic Syndr 2001;28:173-9. [DOI] [PubMed]
- 14.Division of HIV/AIDS Epidemiology and Surveillance, Centre for Infectious Disease Prevention and Control, Health Canada. HIV infection reporting in Canada. HIV/AIDS Epi Update series. Ottawa: Health Canada; 2002. p. 9-11. Available: www.hc-sc.gc.ca/pphb-dgspsp/publicat/epiu-aepi/hiv-vih/hivrep_e.html (accessed 2003 Feb 12).
- 15.HIV/AIDS surveillance report. Atlanta: US Centers for Disease Control and Prevention; 2001. Available: www.cdc.gov/hiv/stats/hasrlink.htm (accessed 2003 Feb 12).
- 16.Stringer EM, Stringer JS, Cliver SP, Goldenberg RL, Goepfert AR. Evaluation of a new testing policy for human immunodeficiency virus to improve screening rates. Obstet Gynecol 2001;98(6):1104-8. [DOI] [PubMed]
- 17.Lo B, Wolf S, Sengupta S. Ethical issues to early detection of HIV infection to reduce vertical transmission. J Acquir Immune Defic Syndr Hum Retrovirol 2000;25:S136-43. [DOI] [PubMed]
- 18.Simpson WM, Johnstone ED, Goldbert DJ, Gormley SM, Hart GJ. Antenatal HIV testing: assessment of a routine, voluntary approach. BMJ 1999;318:1660-1. [DOI] [PMC free article] [PubMed]
- 19.Blott M, Yearwood J, Gerval M, Welch J, Zuckerman M. Routine antenatal HIV testing is acceptable to women. BMJ 1999;319:1069-70. [PubMed]
- 20.Bitnun A, King SM, Arneson C, Read SE. Failure to prevent perinatal HIV infection [letter]. CMAJ 2002;166(7):904-5. [PMC free article] [PubMed]
- 21.Fehrs LJ, Fleming D, Foster LR, McAlister RO, Fox V, Modesitt S, et al. Trial of anonymous versus confidential human immunodeficiency virus testing. Lancet 1988;2:379-82. [DOI] [PubMed]
- 22.Kegeles SM, Coates TG, Lo B. Mandatory reporting of HIV testing would deter men from being tested [letter]. JAMA 1989;261:1275-6. [DOI] [PubMed]
- 23.Hirano D, England B, Hoff C, Helmstadter L, Boyd D, Fickes M, et al. Reporting HIV infections [letter]. J Acquir Immune Defic Syndr 1994;7:417-8. [PubMed]
- 24.Fordyce EJ, Sambula S, Stoneburner R. Mandatory reporting of human immunodeficiency virus testing would deter blacks and Hispanics from being tested [letter]. JAMA 1989;262:349. [PubMed]
- 25.Tharao E, Calzavara L, Myers T. To test or not to test: factors influencing HIV testing in east African communities living in Toronto [lecture]. Tenth Annual Canadian Conference on HIV/AIDS Research; 2001 May 31–June 3; Toronto.


