Abstract
To differentiate scrub typhus from other acute febrile diseases, a rapid and reliable serological diagnosis is important. We developed an immunoglobulin M (IgM) capture enzyme-linked immunosorbent assay (ELISA) for diagnosis of recent Orientia tsutsugamushi infections in humans. The 56-kDa major outer membrane protein of O. tsutsugamushi is well known as the most immunodominant antigen in scrub typhus. The test is based on the use of the biotinylated recombinant 56-kDa protein of O. tsutsugamushi Boryong, Bor56, which was expressed as a fusion protein with a maltose-binding protein in Escherichia coli. In the test, the serum IgM antibodies were captured by anti-human IgM antibodies coated onto a microtiter plate. The captured IgM antibodies were revealed through sequential addition of biotinylated Bor56 antigen and peroxidase-conjugated streptavidin to the plate. The IgM capture ELISA was compared with the immunofluorescence antibody assay (IFA) by testing 176 serum samples from patients with diagnosed cases of rickettsial disease and patients with other acute febrile diseases. Of the 81 IgG IFA-positive samples, 78 tested positive (sensitivity, 96.3%) and all 31 IgM IFA-positive samples tested positive (sensitivity, 100%) by the IgM capture ELISA. The specificity of the IgM capture ELISA was 99%, and 1 of the 95 IFA-negative samples was positive in the assay. These results strongly suggest that IgM capture ELISA using the recombinant Bor56 antigen is a reliable and detailed method for the detection of early O. tsutsugamushi infection.
Scrub typhus, caused by Orientia tsutsugamushi, has become an important acute febrile illness in the Korea, Japan, and elsewhere in the Asia-Pacific region (4, 17, 20, 21). The disease is characterized by fever, rash, headache, eschar, pneumonitis, meningitis, and disseminated intravascular coagulation, leading to severe systemic multiorgan failure in untreated cases (15, 17, 21). Diagnosis of scrub typhus based on the clinical presentation and the history of a patient is difficult because the clinical symptoms and signs resemble those of many other febrile diseases such as murine typhus, leptospirosis, and hemorrhagic fever with renal syndrome. Serological testing by using indirect immunofluorescence antibody assay (IFA) (2, 3), passive hemagglutination assay (13), indirect immunoperoxidase test (25), and enzyme-linked immunosorbent assay (ELISA) (8, 11, 23) is a useful aid in the diagnosis of scrub typhus. However, obtaining results from these tests may require paired serum samples or the propagation of rickettsiae in tissue cultures. Furthermore, many of these tests are technically demanding, making them difficult to apply reproducibly, and are often poor measures of the presence of the early antibody immunoglobulin M (IgM). Clearly, the need for a more rapid and efficient diagnostic tool is evident.
Recently published studies show that IgM capture ELISA is a sensitive method and can be used for the diagnosis of early disease phases (16, 26). Serological confirmation by the test can be made with a single serum specimen during any phase of the acute disease and early convalescence, except for the first 2 to 3 days after the onset of the disease. The 56-kDa outer membrane protein is an abundant protein in O. tsutsugamushi, and its function is still unknown (18). Recently, a recombinant 56-kDa protein from O. tsutsugamushi was shown to be suitable for diagnosis of scrub typhus by ELISA, passive hemagglutination assay, and rapid-flow assay (6, 7, 12, 13).
The objective of this study was to develop a reliable diagnostic method that can detect early O. tsutsugamushi infection. Here we describe the development of a rapid and reliable IgM capture ELISA based on the recombinant 56-kDa protein and a comparison of this test and IFA.
MATERIALS AND METHODS
Serum specimens.
Human sera were obtained in September to December 1997 from 176 patients with a febrile illness. Serum specimens were selected on the basis of a positive or negative result for O. tsutsugamushi by IFA. The sera were stored at −70°C until used. These sera were assayed and used for a comparison of the IFA and IgM capture ELISA methods.
IFA.
The IFA was performed using classical methods (2). Briefly, O. tsutsugamushi strains Boryong, Gilliam, Karp, and Kato were cultured, in a humidified 5% CO2 atmosphere at 37°C, on confluent mouse L929 cells in Dulbecco's modified Eagle's medium (Gibco BRL, Grand Island, N.Y.) containing 5% fetal bovine serum (Gibco BRL), 0.4 μg of daunomycin per ml, 100 μg of streptomycin per ml, 100 U of penicillin per ml, and 2 mM l-glutamine. When more than 90% of the cells were infected, the cells were harvested and washed with 0.01 M phosphate-buffered saline (PBS) (pH 7.2). Then Teflon-coated spot slides were coated with Orientia-infected L929 cells, which were fixed with cold acetone for 10 min. The slides were stored at −70°C in a freezer until used. Twofold serially diluted (1:20 to 1:2,560 in PBS) patient sera were added to the antigen-coated spot on the slide (10 μl per spot), and the slide was incubated for 30 min in a moist chamber at room temperature. Fluorescein isothiocyanate-conjugated antibodies, anti-human IgM (F0203; Dako, Copenhagen, Denmark) and anti-human IgG (F0202; Dako), diluted 1:200 in PBS, were used as secondary antibodies. The stained slides were examined at ×400 magnification with a fluorescence microscope (Axioscope; Karl Zeiss, Oberkochen, Germany). Endpoint titers against individual antigens were represented as the reciprocal of the highest serum dilution at which rickettsiae exhibited 1+ fluorescence with any one of the four strains. Because single serum titers of ≥1:64 have been suggested to be diagnostic for typhus infections (14), we selected IgG and IgM IFA cutoff values of ≥1:80.
Preparations of biotin-labeled recombinant Bor56 antigen.
Preparation of the O. tsutsugamushi Boryong recombinant 56-kDa protein (Bor56) was described previously (12). Briefly, bacteria containing the recombinant expression vector were grown in Luria-Bertani broth supplemented with ampicillin (100 μg/ml; Sigma, St. Louis, Mo.). Protein expression was induced by the addition of 0.2 mM isopropyl-β-d-thiogalactopyranoside (IPTG; Sigma). After additional incubation, the bacteria were harvested and sonicated using a ultrasonic liquid processor (model XL 2020; Misonix Inc., New York, N.Y.). Cell debris was removed by centrifugation. Then the recombinant protein was purified from the supernatant by amylose affinity column chromatography (New England Biolabs, Beverly, Mass.). The purified protein was analyzed using electrophoresis on a sodium dodecyl sulfate-10% polyacrylamide gel. The purified Bor56 proteins were covalently conjugated to biotin as described by the manufacturers (Roche Diagnostics GmbH, Mannheim, Germany). The Bor56 protein concentration was adjusted to 1 mg/ml, and the protein was labeled with d-biotinoyl-e-aminocaproic acid-N-hydroxysuccinimide ester (biotin-7-NHS [Roche]) (1:10 molar ratio) for 2 h at room temperature. To remove excess biotin-7-NHS, a Sephadex G-25 column (Pharmacia LKB, Uppsala, Sweden) was used. Biotin-labeled Bor56 proteins were used at concentrations from 0.25 to 2 μg/ml in the IgM capture ELISA.
Determination of the working concentrations of reagents for IgM capture ELISA.
To develop the IgM capture ELISA, the optimal dilutions of the reagent were determined using a checkerboard titration method. The pooled sera from 10 healthy donors (IFA negative) and 10 patients with scrub typhus (IFA positive) were diluted to 1:50 in PBS and were added to wells coated with anti-human IgM antibodies (μ-chain specific) (A0425; Dako) at various concentrations. After incubation for 1 h at 37°C followed by five washing steps with PBST (PBS containing 0.05% [vol/vol] Tween 20), various dilutions of the biotin-labeled recombinant Bor56 proteins were added to wells. The plates were incubated for 1 h at 37°C and washed. Streptavidin-peroxidase (P0397; Dako) at various dilutions were added to the wells, which were incubated for 30 min at 37°C and then washed. The substrate was a peroxidase substrate buffer containing o-phenylenediamine (0.4 mg/ml in 0.1 M citrate-phosphate buffer [pH 5.0]). The optimum concentrations of reagents were determined based on a ratio of positive to negative sera (P/N) of >1.0 in the positive control group. The optimal dilutions of the reagents were selected and used in the IgM capture ELISA.
IgM capture ELISA.
The IgM capture ELISA method is based on the capture of IgM antibodies in sera, demonstrated by the sequential addition of a biotin-labeled Orientia antigen and peroxidase-conjugated streptavidin. A 96-well Maxisorp microtiter plate (no. 439454; Nalge Nunc International) was sensitized by adding anti-human IgM in 100 μl of 0.05 M bicarbonate buffer (pH 9.6) to the wells and incubating the plate at 37°C for 18 h. The plate was washed with 250 μl of PBS and incubated with 200 μl of 3% bovine serum albumin (Sigma) at 37°C for 2 h. The serum samples diluted at 1:50 in PBS were added to the well at 100 μl per well by duplication. The plate was incubated at 37°C for 1 h and then washed five times with 250 μl of PBST. The biotin-labeled recombinant Bor56 proteins at the predetermined concentration (1 μg/ml) in 100 μl of PBS were added to the wells. The plate was then incubated for 1 h at 37°C and washed as described above. Then streptavidin-peroxidase at a predetermined dilution of 1:5,000 in 100 μl of PBS per well was added. The plate was incubated for another 30 min and then washed as described above. Finally, 100 μl of peroxidase substrate buffer was added. After the final incubation (at room temperature for 10 min), the substrate reaction was stopped by addition of 50 μl of 2 N sulfuric acid to each well. The resultant color change was quantified by reading the optical density at 490 nm (OD490) using an MR700 reader (Dynatech Laboratories Inc., Torrance, Calif.).
RESULTS
IFA.
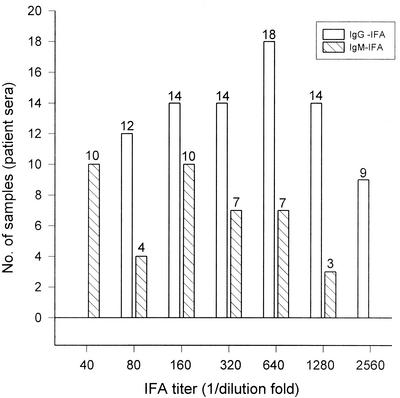
We evaluated IgM- and IgG-specific reactivity against O. tsutsugamushi Boryong, Gilliam, Karp, and Kato in 176 sample sera and found that 81 sera were positive (titer, ≥1:80). The IFA values (IgG and IgM) against O. tsutsugamushi strain Boryong in patient sera are shown in Fig. 1. The IgG-positive sera included 81 samples, and the IgM-positive sera included 31 samples.
FIG. 1.
Distribution of IFA titers of serum samples from patients. Samples were determined as scrub typhus positive by having IgG or IgM IFA titers of ≥1:80, which is considered significant. The IgG-positive sera consisted of 81 samples, and the IgM-positive sera consisted of 31 samples. O. tsutsugamushi Boryong was used as an IFA antigen.
Purification and labeling of recombinant Bor56 antigen.
The recombinant Boryong 56-kDa protein was purified by amylose affinity column chromatography. The yield of the protein purification was 10 mg per liter of bacterial culture. Protein analysis showed a single band by electrophoresis on a sodium dodecyl sulfate-10% polyacrylamide gel (data not shown). The purified protein was labeled with biotin. Through the one-step purification of biotin-labeled antigen, a total of 600 μg of labeled protein (concentration, 350 μg/ml) was recovered from 1 mg of protein in a reaction mixture.
Determination of the working concentration of anti-human IgM antibody, biotin-labeled Bor56 antigens, and streptavidin-peroxidase for IgM-capture ELISA.
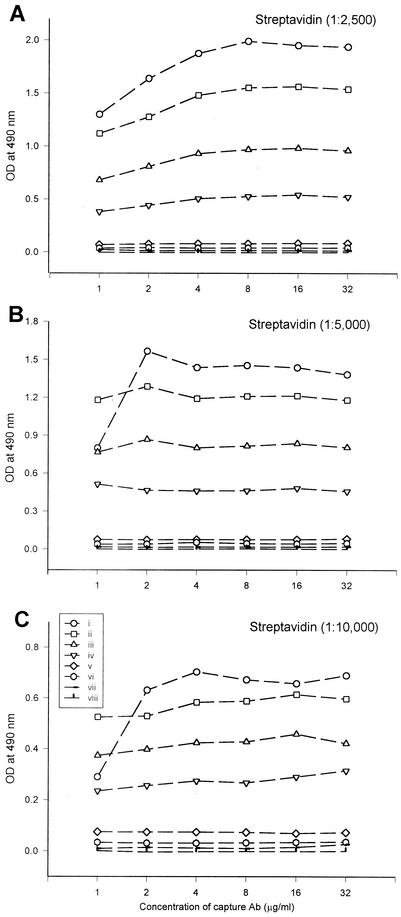
The working dilution concentrations of the capture antibody (anti-human IgM), biotin-labeled Bor56 antigen, and streptavidin-peroxidase were determined for the development of IgM capture ELISA. The pooled sera from 10 healthy donors (IFA negative) and 10 patients with scrub typhus (IFA positive) were diluted 1/50 in PBS and added to anti-human IgM-coated wells to determine the IgM capture ELISA conditions. The optimum concentrations of reagents were determined based on a P/N ratio of >1.0 among the positive-serum control group. The point when the P/N ratio was highest for scrub typhus patient sera and lowest for the healthy group was set at 1 μg of capture antibody per liter, 1 μg of biotin-labeled antigen per liter, and 1:5,000-diluted streptavidin-peroxidase (Fig. 2). Thus above dilutions were used following IgM capture ELISA.
FIG. 2.
Optimal conditions of anti-human IgM antibody, biotin-labeled Bor56 antigen, and streptavidin-peroxidase for the IgM capture ELISA. The optimal concentrations of the reagent were determined in an IgM capture ELISA as follows. Anti-human IgM antibodies (capture Ab) were coated at various concentrations (1 to 32 μg/ml) onto the microplate wells, and then a positive or negative serum, biotinylated Bor56 protein, and streptavidin-peroxidase were added to the wells. The following procedures were the same as those of normal ELISA. Control sera: i to iv, positive serum; v to viii, negative serum. Various concentrations of biotinylated Bor56 protein used in the assay: i and v, 2 μg/ml; ii and vi, 1 μg/ml; iii and vii, 0.5 μg/ml; iv and viii, 0.25 μg/ml. Various dilutions of streptoavidin-peroxidase were used: 1:2,500 (A), 1:5,000 (B), and 1:10,000 (C).
Determination of cutoff values in IgM capture ELISA.
Cutoff values were determined using 95 IFA-negative sera. The mean OD490 of these sera in the IgM capture ELISA was 0.06. The cutoff value was defined as the mean OD490 + 3 standard deviations (3 Stdev. = 0.129). Therefore, when the OD490 of a serum sample is greater than 0.2, the sample is considered to be positive in the IgM capture ELISA.
Determination of the specificity and sensitivity of the IgM capture ELISA.
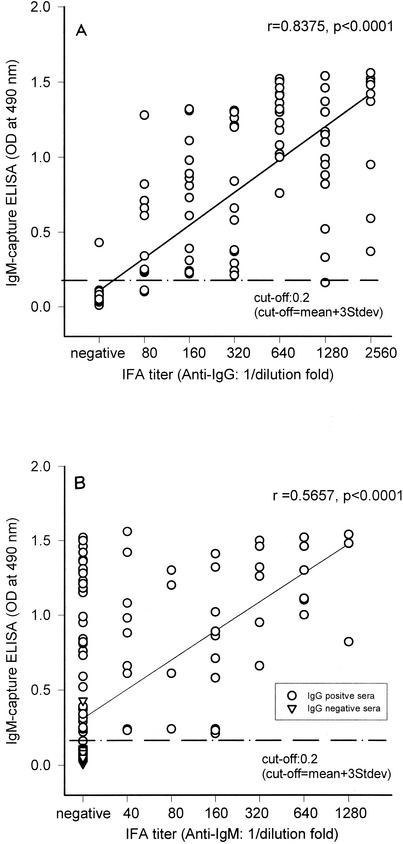
The sensitivity and specificity of the IgM capture ELISA were determined by testing 176 sera in comparison with the IFA. Of the 81 IgG-IFA positive samples, 78 were positive and 3 were below the cutoff value of the IgM capture ELISA (Fig. 3A). All 31 IgM IFA-positive sera were positive in the IgM capture ELISA (Fig. 3B). One of the 95 IFA-negative sera was positive in the IgM capture ELISA. Therefore, the sensitivity of the IgM capture ELISA versus IgG IFA and IgM IFA was 96.3 and 100%, respectively. The specificity of the test was 99%. The comparisons of IgM capture ELISA OD490 values to the IFA titer of positive sera are presented in Fig. 3. Spearman's correlation analysis was performed to compare the results of the IgM capture ELISA with those of IFA for individual sera. The number of dots in the IgM capture ELISA significantly correlated with the IgG IFA results (r = 0.8375, P < 0.0001, n = 176) but not with the IgM IFA results (r = 0.5657, P < 0.0001, n = 176).
FIG. 3.
Comparison of the IgM capture ELISA and the IFA. The reactivity of the serum samples (n = 176) was assessed by IFA and ELISA and plotted for IgG IFA versus IgM capture ELISA (A) and IgM IFA versus IgM capture ELISA (B). Each circle describes the reactivity of one serum specimen. The cutoff value was determined on the basis of a seronegative serum sample + 3 standard deviations (Stdev) and is shown by dashed lines. Correlations between serum reactivity and IgM capture versus IgG-IFA (A) and IgM capture versus IgM-IFA (B) are also shown.
DISCUSSION
The 56-kDa protein is located on the outer membrane of O. tsutsugamushi. The protein comprises 10 to 15% of the total bacterial cellular protein contents (10, 18), and most sera from scrub typhus patients recognize this O. tsutsugamushi protein in multiple strains (9, 12, 13, 19), although some sera react predominantly with a 56-kDa polypeptide antigen from a single strain (19). Several antigenic varieties of O. tsutsugamushi were isolated and were distinguishable from each other through serological cross-testing (1, 5, 19). The serological variations of the bacteria depend on the antigenicity of an immunodominant 56-kDa major surface protein (9, 10, 12, 22, 24), which contains the group-specific and strain-specific epitopes. Because the 56-kDa protein was highly reactive with patient sera for its antigenicity and abundances, it could be the first antigen candidate for use in the diagnosis of O. tsutsugamushi disease. Previous work in our laboratory and others has shown that the recombinant 56-kDa proteins are suitable diagnostic antigens for scrub typhus in ELISA (6, 7, 12, 13). There are at least four serotypes of O. tsutsugamushi in Korea, of which serotype Boryong is the most dominant (11). Therefore, we have indicated the use of the 56-kDa protein (Bor56) from the Boryong serotype fused with maltose-binding protein in Escherichia coli as a diagnostic antigen.
Due to the nature of IFA and ELISA, they are not able to differentiate easily between IgG and IgM antibodies. However an IgM capture assay can detect IgM antibodies from a serum sample and also eliminate false-positive reactions due to rheumatoid factor (RF), which is not an important problem for IgG and IgA assays. In this study, we developed an IgM capture ELISA using a streptavidin-biotin system for the detection of recent scrub typhus infection by targeting the IgM antibodies against the Bor56 protein. A streptavidin-biotin system was used to increase the sensitivity of the assay by using its property of signal amplification. A biotinylated Bor56 antigen used in the assay was found to be stable for at least 6 months at −20°C.
When results from the 176 serum samples were used to determine the performance values of the assay, overall sensitivity and specificity results were 96.3 and 99%, respectively, for the IgM capture ELISA versus IgG IFA and 100 and 99%, respectively (using 95 IgG IFA-negative sera), for the IgM capture ELISA versus IgM-IFA. These data suggest that the assay is more reliable than previously studied assays, which showed sensitivities of 87 to 100% and specificities of 87.7 to 100% (6, 7, 8, 11, 23). Of the 81 IgG IFA-positive sera, 78 were positive by IgM capture ELISA and 3 were negative. The three IgM capture ELISA-negative responses among the Boryong IgG IFA-positive serum samples were confirmed by IgG and IgM IFA with other O. tsutsugamushi serotypes: Gilliam, Karp, and Kato. One serum sample responded positively to all four strains, including Boryong by IgG IFA (Gilliam, 1:2,560; Karp, 1:2,560; Kato, 1:2,560; Boryong, 1,280), while it responded negatively to all four strains by IgM IFA. The other two sera were negative to all serotypes by IgG and IgM IFA, except for Boryong by IgG IFA (1:80). It is presumed that the sera were obtained from patients who had been infected in the past and/or had already convalesced from scrub typhus. All 30 IgM IFA sera from the 81 IgG-IFA-positive sera were IgM capture ELISA positive. The IgM capture ELISA could also detect IgM antibodies in the 48 IgM IFA-negative sera from the 81 total serum samples. These results confirm that IgM capture ELISA can detect IgM antibodies in a serum sample without any hindrance by IgG and/or RF. We found that the number of dots in the IgM capture ELISA correlated significantly with the IgG IFA results (r = 0.8375 [Fig. 3A]) but not with the IgM IFA results (r = 0.5657 [Fig. 3B]). These data also showed that the IgM capture technique cannot be directly compared to an indirect IgM assay but should be considered preferable, mainly due to the potential problem of false-positive reactions as a result of RF and false-negative reactions as a result of IgG.
This study clearly demonstrates that the IgM capture ELISA is a reliable and useful diagnostic method for early detection of scrub typhus. It also makes it possible to test a large number of sera at a time.
Acknowledgments
This work was supported by Korea Research Foundation grant KRF-1997-021-F00102.
REFERENCES
- 1.Bourgeois, A. L., J. G. Olson, R. C. Fang, J. Huang, C. L. Wang, L. Chow, D. Bechthold, D. T. Dennis, J. C. Coolbaugh, and E. Weiss. 1982. Humoral and cellular responses in scrub typhus patients reflecting primary infection and reinfection with Rickettsia tsutsugamushi. Am. J. Trop. Med. Hyg. 31:532-540. [DOI] [PubMed] [Google Scholar]
- 2.Bozeman, G. W., and B. L. Elisberg. 1963. Serological diagnosis of scrub typhus by indirect immunofluorescence. Proc. Soc. Exp. Biol. Med. 112:568-573. [DOI] [PubMed] [Google Scholar]
- 3.Brown, G. W., A. Shirai, C. Rogers, and M. G. Groves. 1983. Diagnostic criteria for scrub typhus: probability values for immunofluorescent antibody and Proteus OKK agglutinin titers. Am. J. Trop. Med. Hyg. 32:1101-1107. [DOI] [PubMed] [Google Scholar]
- 4.Chang, W. H. 1995. Current status of tsutsugamushi disease in Korea. J. Korean Med. Sci. 10:227-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang, W. H., J. S. Kang, W. K. Lee, M. S. Choi, and J. H. Lee. 1990. Serological classification by monoclonal antibodies of Rickettsia tsutsugamushi isolated in Korea. J. Clin. Microbiol. 28:685-688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ching, W. M., H. Wang, C. Eamsila, D. J. Kelly, and G. A. Dasch. 1998. Expression and refolding of truncated recombinant major outer membrane protein antigen (r56) of Orientia tsutsugamushi and its use in enzyme-linked immunosorbent assays. Clin. Diagn. Lab. Immunol. 5:519-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ching, W. M., D. Rowland, Z. Zhang, A. L. Bourgenois, D. Kelly, G. A. Dasch, and P. L. Devine. 2001. Early diagnosis of scrub typhus with a rapid flow assay using recombinant major outer membrane protein antigen (r56) of Orientia tsutsugamushi. Clin. Diagn. Lab. Immunol. 5:409-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dasch, G. A., S. Halle, and A. L. Bourgeous. 1979. Sensitive microplate enzyme-linked immunosorbent assay for detection of antibodies against the scrub typhus rickettsia, Rickettsia tsutsugamushi. J. Clin. Microbiol. 9:38-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eisemann, C. S., and J. V. Osterman. 1981. Antigens of scrub typhus rickettsiae: separation by polyacrylamide gel electrophoresis and identification by enzyme-linked immunosorbent assay. Infect. Immun. 32:525-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanson, B. 1985. Identification and partial characterization of Rickettsia tsutsugamushi major protein immunogens. Infect. Immun. 50:603-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kelly, D. J., P. W. Wong, E. Gan, T. C. Chan, D. Cowan, and G. E. Lewis, Jr. 1990. Multi-laboratory evaluation of a scrub typhus diagnostic kit. Am. J. Trop. Med. Hyg. 43:301-307. [DOI] [PubMed] [Google Scholar]
- 12.Kim, I. S., S. Y. Seong, S. G. Woo, M. S. Choi, and W. H. Chang. 1993. High-level expression of a 56-kilodalton protein gene (bor56) of Rickettsia tsutsugamushi Boryong and its application to enzyme-linked immunosorbent assays. J. Clin. Microbiol. 31:598-605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim, I. S., S. Y. Seong, S. G. Woo, M. S. Choi, J. S. Kang, and W. H. Chang. 1993. Rapid diagnosis of scrub typhus by a passive hemagglutination assay using recombinant 56-kilodalton polypeptides. J. Clin. Microbiol. 31:2057-2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McDade, J. E., and D. B. Fishbein. 1988. Rickettsiaceae: the Rickettsiae, p. 864-890. In E. H. Lennette, P. Halonen, and F. A. Murphy (ed.), Laboratory diagnosis of infectious diseases, principles and practice, vol. II. Viral, rickettsial, and chlamydial diseases. Springer-Verlag, New York, N.Y.
- 15.Kuroda, T., S. Suzuki, and M. Konno. 1991. A case of scrub typhus with disseminated intravascular coagulation, meningitis and pulmonary fibrosis. Nippon Naika Gakkai Zasshi 80:1816-1817. [PubMed] [Google Scholar]
- 16.Nawa, M., T. Takasaki, K. I. Yamada, T. Akatsuka, and I. Kurane. 2001. Development of dengue IgM-capture enzyme-linked immunosorbent assay with higher sensitivity using monoclonal detection antibody. J. Virol. Methods 92:65-70. [DOI] [PubMed] [Google Scholar]
- 17.Oaks, S. C., Jr., R. L. Ridgway, A. Shiral, and J. C. Twartz. 1983. Scrub typhus. Bull. Inst. Med. Res. 21:25-33.
- 18.Ohashi, N., A. Tamura, M. Ohta, and K. Hayashi. 1989. Purification and partial characterization of a type-specific antigen of Rickettsia tsutsugamushi. Infect. Immun. 57:1427-1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ohashi, N., A. Tamura, and T. Suto. 1988. Immunoblotting analysis of anti-rickettsial antibodies produced in patients of Tsutsugamushi disease. Microbiol. Immunol. 32:1085-1092. [DOI] [PubMed] [Google Scholar]
- 20.Rapmund, G. 1984. Rickettsial diseases of the Far East: new perspectives. J. Infect. Dis. 149:330-338. [DOI] [PubMed] [Google Scholar]
- 21.Seong, S. Y., M. S. Choi, and I. S. Kim. 2001. Orientia tsutsugamushi infection: review and immune responses. Microbes Infect. 3:11-21. [DOI] [PubMed] [Google Scholar]
- 22.Stover, C. K., D. P. Maana, J. M. Carter, B. A. Roe, E. Mardis, and E. V. Oaks. 1990. The 56-kilodalton major protein antigen of Rickettsia tsutsugamushi: molecular cloning and sequence analysis of sta56 gene precise identification of a strain-specific epitope. Infect. Immun. 58:2076-2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suwanabun, N., C. Chouriyagune, C. Eamsila, P. Watcharapichat, G. Dasch, R. S. Howard, and D. J. Kelly. 1997. Evaluation of an enzyme-linked immunosorbent assay in Thai scrub typhus patients. Am. J. Trop. Med. Hyg. 56:38-43. [DOI] [PubMed] [Google Scholar]
- 24.Tamura, A., N. Ohashi, H. Urakami, K. Takahashi, and M. Oyanagi. 1985. Analysis of polypeptide composition and antigenic components of Rickettsia tsutsugamushi by polyacrylamide gel electrophoresis and immunoblotting. Infect. Immun. 48:671-675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamamoto, S., and Y. Minamishima. 1982. Serodiagnosis of tsutsugamushi fever (scrub typhus) by the indirect immunoperoxidase technique. J. Clin. Microbiol. 15:1128-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou, E. M., D. Ridd, J. Riva, L. Fernando, and A. Clavijo. 2001. Development and evaluation of an IgM-capture ELISA for detection of recent infection with bluetongue viruses in cattle. J. Virol. Methods 91:175-182. [DOI] [PubMed] [Google Scholar]