Abstract
The P46 and P65 proteins of Mycoplasma hyopneumoniae are two membranous proteins carrying species-specific antigenic determinants. Based on the genomic sequence of the reference strain ATCC 25934, primers were designed for PCR amplification of the genes encoding entire P46 (1,260 bp) and P65 (1,803 bp) and N-terminally truncated P65c (1,200 bp). These primers were shown to be specific to M. hyopneumoniae since no DNA amplicons could be obtained with other mycoplasma species that commonly colonize the porcine respiratory tract. Both amplified genes were then cloned into the pGEX-4T-1 vector to be expressed in Escherichia coli cells as recombinant fusion proteins with glutathione S-transferase (GST). Prior to generation of expression constructs, TGA nonsense codons, exceptionally used for tryptophan residues by M. hyopneumoniae, had been converted to TGG codons by PCR-directed mutagenesis. Following induction by IPTG (isopropyl-β-d-thiogalactopyranoside), both GST-P46 and GST-P65c recombinant fusion proteins were recovered by disrupting transformed cells by sonication, purified by affinity chromatography, and then cut with thrombin to release the P46 and P65c moieties. The enriched E. coli-expressed P46 and P65c proteins were used to immunize female BALB/c mice for the generation of anti-P46 and anti-P65c monoclonal antibodies (MAbs). The polypeptide specificities of MAbs obtained was confirmed by Western blotting with cell lysates prepared from the homologous strain. Cross-reactivity study of the anti-P46 and anti-P65c MAbs towards two other M. hyopneumoniae reference strains (ATCC 25095 and J strains) and Quebec field strains that had been isolated in culture, suggested that the MAbs obtained against both membranous proteins were directed against highly conserved species-specific epitopes. No reactivity to other mycoplasma species tested was demonstrated. Clinical signs and lesions suggestive of enzootic pneumonia were reproduced in specific-pathogen-free pigs that had been inoculated intratracheally with a virulent Quebec field strain (IAF-DM9827) of M. hyopneumoniae. Both anti-P46 and anti-P65c MAbs permitted effective detection by indirect immunofluorescence and indirect immunoperoxidase assay of M. hyopneumoniae in, respectively, frozen and formalin-fixed, paraffin-embedded lung sections from pigs that were killed after the 6- to 7-week observation period.
Mycoplasma hyopneumoniae is the causative agent of enzootic pig pneumonia, a disease found on pig farms worldwide and characterized by high morbidity and low mortality rates (15, 22). Coughing is the main clinical sign, but retarded growth and poor food conversion may result in considerable economic losses (22, 26). This microorganism predisposes pigs to secondary infections that increase the mortality rates, such as infections by porcine reproductive and respiratory syndrome virus and swine influenza virus (33)
The diagnosis of M. hyopneumoniae is usually done by PCR, cultivation of the organism in enriched Friis medium, or immunofluorescence tests performed on frozen thin lung sections (1, 3, 5, 6, 17, 19, 25). The culture of this fastidious bacteria and its identification may take up to 1 month. Contamination with Mycoplasma hyorhinis and Mycoplasma flocculare, both considered to be nonpathogenic species, is frequently observed. These less fastidious nonpathogenic species may often overgrow M. hyopneumoniae in primary isolation attempts (20), from which arises the necessity to discriminate among porcine mycoplasmas that have a respiratory tropism. Moreover, the overall efficacy of serological detection methods, such as enzyme-linked immunosorbent assays (ELISAs), is often hampered because of antigenic cross-reactions that exist between M. hyopneumoniae, M. flocculare, and M. hyorhinis. (2, 18).
The M. hyopneumoniae genome codes for several immunodominant proteins, among which are the P36 cytosolic protein; the P46, P65, and P74 membranous proteins; and the P97 adhesin. These proteins are known to trigger early specific antibody responses in postweaning and growing pigs following acute or initial infection with M. hyopneumoniae (11, 14, 19). The corresponding open reading frames (ORFs) are 1,260 bp for P46 surface lipoprotein and 1,803 bp for P65 lipid-modified amphiphilic surface protein. Sequence analysis of P45- and P65-encoding genes revealed the presence of, respectively, three and one translation termination or nonsense UGA codons, which are exceptionally used for tryptophan residues, in addition to TGG in several mycoplasma genes (14).
The indirect immunofluorescence (IIF) assay is still widely used for diagnosis of M. hyopneumoniae since it is a rapid and convenient technique for detection of specific antigens in lung tissues. However, in frozen tissue sections, microstructures are most frequently broken and difficult to recognize, and the use of polyclonal antisera may result in nonspecific detection of other pathogens, namely, M. flocculare and M. hyorhinis. On the other hand, the use of MAbs increases the specificity of serological and immunohistochemical antigen detection tests (1, 18, 23).
This paper describes site-directed mutagenesis of TGA codons of the P46 and P65 genes into TGG codons by overlapping PCRs. The modified P46-encoding gene, as well as the C-terminal portion of the modified P65 encoding gene, was cloned in a procaryotic plasmid vector to allow expression of the entire P46 and N-terminally truncated P65c membranous lipoproteins, in genetically transformed Escherichia coli cells, as recombinant fusion proteins with glutathione S-transferase (GST). The production and characterization of specific anti-P46 and anti-P65c MAbs are also described, as well as their potential application for the specific immunodetection of M. hyopneumoniae authentic membranous proteins by IIF and streptavidin-biotin immunoperoxidase assays using frozen or paraffin-embedded lung sections, respectively. The immunogenicity of the recombinant fusion proteins was also investigated in pigs.
(This report was taken in part from a dissertation to be submitted by K. Cheikh Saad Bouh to the INRS-Institut Armand-Frappier, in partial fulfillment of the requirements for the Ph.D. degree.)
MATERIALS AND METHODS
Microorganisms and growth conditions.
The ATCC 25934 strain of M. hyopneumoniae was obtained from the American Type Culture Collection (ATCC), Manassas, Va., and used as the reference strain in this study. Other mycoplasma strains including the reference ATCC 25095 and J strains of M. hyopneumoniae, M. flocculare (ATCC 27399), Mycoplasma arginini (ATCC 23838), M. hyorhinis (ATCC 17981), and Acholeplasma laidlawii (ATCC 23206) were also obtained from the ATCC and used in comparative antigenic studies. Mycoplasma hyosynoviae was kindly obtained from Claude Montpetit, Ministère de l'Agriculture des Pêcheries et de l'Alimentation du Québec. All available strains were grown in modified Friis medium (12) containing mycoplasma culture-tested free 20% horse serum (Gibco-BRL, New Zealand), 5% fresh yeast extract (Gibco-BRL), methicillin (0.15 mg/ml; Sigma-Aldrich, Oakville, Ontario, Canada), bacitracin (0.15 mg/ml; Sigma-Aldrich) and thallium acetate (0.08 mg/ml; Sigma-Aldrich). The cells were harvested by centrifugation at 12,000 × g for 30 min at 4°C, washed three times and suspended in 0.1 M phosphate-buffered saline (PBS), pH 7.4.
DNA extraction and PCR conditions.
Genomic DNA from M. hyopneumoniae was extracted and purified, as previously described (6). The oligonucleotide primers used for enzymatic amplification of the entire ORFs of the P46 (1,260-bp) and P65 (1,803-bp) genes of M. hyopneumoniae were selected from the previously published DNA sequences of the ATCC 25934 strain (GenBank accession no. D16682 and U50209, respectively). Sequences of the forward primers for specific amplification of the P46 and P65 encoding genes, P46BamH1 and FSLP65, were 5′-ACCGGATCCATGAAAAAAATGCTTAGAAAAAAATT-3′ and 5′-GGCCGGGAATTCATGGCAAAAGAAATCATTTTA-3′, respectively, and those of the reverse primers, P46Sal1 and R2SP65, were 5′-CCCGTCGACTTAGGCATCAGGATTATCAAC-3′ and 5′GGGCCGGTCGACTTAATCCTGCTTGATTTCAGCATC-3′, respectively.
Sequence analyses for the selection of primers were performed using the McVector (version 3.5; International Biotechnologies) and Gene Works (version 2.2; Intelligenetics Inc., Mountain View, Calif.) programs. The oligonucleotide primers were synthesized in an automated Gene Assembler DNA synthesizer (Pharmacia LKB). The PCR protocol used for amplification of the P46 and P65 genes was essentially similar to that described previously (6). The amplification reactions were performed in a DNA Engine thermo-cycler (model PTC-100 with hot bonnet; MJ Research). To overcome the error rate of the Taq DNA polymerase, the Vent DNA polymerase was used for the amplification reaction, and clones from at least three different PCR events were sequenced. Aliquots of 10 μl of the amplified products were analyzed by electrophoresis on 1% agarose gels (Boehringer Mannheim) in TAE buffer (40 mM Tris-acetate [pH 8.5], 2 mM EDTA) in the presence of ethidium bromide at 100 V for 1 h and viewed under UV illumination. Sequencing of cDNA clones was performed on both strands by the dideoxynucleotide chain termination modified method (27), using T7 DNA polymerase with an automated laser fluorescent DNA sequencer (Pharmacia LKB).
Directed mutagenesis of TGA codons by PCR.
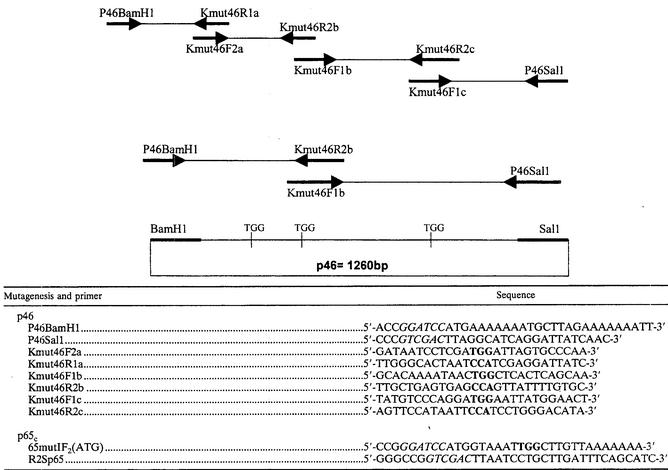
The replacement of nonsense TGA codons by TGG codons (tryptophan) within the P46 and P65 genes was performed using overlapping sense and antisense oligonucleotide primers in PCR assays done with cDNA clones that have been obtained from previous amplification reactions. The location in the targeted gene and size of expected modified DNA fragments generated with different primer pairs are depicted in Table 1. The DNA fragments obtained following directed mutagenesis of TGA codons in TGG codons were then ligated by PCR using previously described primer pairs used to amplify the entire p46 encoding gene (1,260 bp) but only the C-terminal region of P65 gene (1,200 bp) (P65c). For directional cloning, two restriction sites (BamHI and SalI) for the p46 gene and two other (BamHI and SalI) for the P65c N-terminally truncated gene were added at the 5′and 3′ends of the oligonucleotide primers. After amplification of expected DNA fragments, all the constructs were sequenced to confirm that changes from A to G occurred at the third position of the targeted codons.
TABLE 1.
Primers used for p46 and p65c mutagenesisa
The diagram shows an example of TGA mutagenesis.
Cloning, procaryotic expression, and purification of GST-P46 and GST-P65c recombinant fusion proteins.
The amplified products were purified using the GFX purification kit (Pharmacia), digested with BamHI and SalI for P46 or EcoRI and SalI for P65c, and finally ligated into a similarly digested pGEX-4T1 procaryotic vector, according to the manufacturer's instructions (Pharmacia). The recombinant plasmids were used to transform competent E. coli cells, strain HB101, to obtain more copies of recombinant plasmids and for a better efficacy of ligation. Then, appropriate clones were used to transform BL21(DE3) cells (Gibco-BRL) for a more intense expression of the GST-P46 and GST-P65c recombinant fusion proteins. Briefly, overnight cultures of transformed bacteria were diluted 1:10 in a total volume of 120 ml of Luria-Bertani medium containing ampicillin (100 μg/ml) and incubated at 37°C with shaking (250 rpm) to A600 values of 0.6 to 0.8. Protein expression was induced by the addition of 0.1 to 0.5 mM isopropyl-β-d-thiogalactopyranoside (IPTG), and after a 5-h incubation period at 28°C with vigorous shaking (250 rpm), the cells were pelleted by centrifugation at 8,000 × g and then resuspended in 6.25 ml of ice-cold 0.1 M PBS (pH 7.4) containing 1 mM phenylmethylsulfonyl fluoride (Boehringer Mannheim). Transformed cells were disrupted by sonication, and Triton X-100 (Sigma-Aldrich) was added to a final concentration of 1%, followed by a 30-min incubation period at room temperature to aid the solubilization process. The soluble recombinant fusion proteins were purified by affinity chromatography on glutathione-Sepharose 4B beads (Pharmacia) and either eluted with reduced glutathione (20 mM glutathione, 50 mM Tris-HCl[pH 8.0], 120 mM NaCl) (27) or directly cleaved by the addition of 18 U of thrombin followed by a subsequent overnight incubation period to obtain the P46 and P65c moieties.
Production and characterization of MAbs to P46 and P65c membranous proteins.
In vivo experiments in mice were approved by the INRS-IAD Animal Care Committee under the supervision of the Canadian Association for Animal Care. Female BALB/c mice were immunized with 20 μg of affinity-purified GST-P46 and GST-P65c recombinant fusion proteins mixed with Freund's complete or incomplete adjuvant for the first and second injections, respectively. Intraperitoneal injections were given at 2-week intervals, followed by an intraperitoneal dose of the antigen without adjuvant 3 days prior to the fusion experiment. The fusion protocol for sensitized splenocytes with SP2/O-Ag14 myeloma cells was essentially similar to that previously described (7). Hybrid cells were cultured in hypoxanthine-aminopterine-thymidine medium containing l5% fetal calf serum. Hybridoma supernatants were screened for the presence of anti-P46 and anti-P65c antibodies by an indirect ELISA as described elsewhere (7). The use of GST-coated plates permitted the elimination of all clones directed against GST. The optimal GST-P46 and GST-P65c antigen concentration coated in the wells of a 96-well microplates was determined by checkerboard titration and corresponded to 0.15 μg of protein/well. In the indirect ELISA, the washing buffer consisted of 0.1 M PBS (pH 7.4) containing 0.05% Tween 80, and the saturation or dilution buffer consisted of 0.1 M PBS (pH 7.4) containing 0.05% Tween 80 supplemented with 5% goat serum and 3% skim milk (BLOTTO). The secreting hybridoma cells were subcloned twice by serial dilutions, and immunoglobulin (Ig) isotyping was done using a commercial enzyme immunoassay (Boehringer Mannheim). Ascitic fluids containing anti-P46 or anti-P65c MAbs were obtained by intraperitoneal injection of 1 × 106 to 2 × 106 cloned hybrid cells into 16-week-old female BALB/c mice that had been primed 14 days before with 0.5 ml of pristane (2,6,10,14-tetramethyl pentadecane; Sigma-Aldrich).
Western immunoblotting.
Mycoplasma proteins separated by electrophoresis using sodium dodecyl sulfate-12% polyacrylamide gel were electrotransferred to nitrocellulose membranes (0.45-μm pore size; Xymotech) for 1 h at 100 V, as previously described (24). Membranes were blocked for 2 h in PBS buffer (pH 7.4) containing 0.5% Tween 80, 5% goat serum, and 3% skim milk and cut into 2- to 3-mm-wide strips. Each strip was incubated for 1 h at room temperature in the presence of either a 1:200 to 1:1,000 dilution of porcine hyperimmune serum, or a 1:1,000 dilution of mouse ascitic fluid in the blocking buffer. The immune reactions were revealed following a 30-min incubation into a 1:2,000 dilution of the appropriate peroxidase-labeled anti-IgG conjugate, followed by a final incubation period in the enzyme substrate solution, as previously described (7).
Immunogenicity of E. coli-expressed proteins in pigs.
In vivo experiments in pigs were approved by the INRS-IAD Animal Care Committee under the supervision of the Canadian Association for Animals. E. coli-expressed GST-P46 and GST-P65c proteins were used to immunize eight 4- to 5-week-old specific-pathogen-free (SPF) piglets obtained from a breeding farm located in Southern Quebec, Canada. The breeding stock and piglets were tested and proven to be seronegative for porcine reproductive and respiratory syndrome virus, encephalomyocarditis virus, porcine parvovirus, haemagglutinating encephalomyelitis virus, transmissible gastroenteritis virus, and M. hyopneumoniae. They were found to be seronegative to M. hyopneumoniae by the routinely used commercial blocking ELISA.(Dako) for detection of antibodies to a P74 membranous protein and were found to be free of the agent by multiplex P36-P46 PCR done on nasal swabs (6). Two piglets were used as negative controls since they were inoculated with PBS only. The six other piglets were first inoculated intramuscularly with a mixture of 50 μg of GST-P46 or GST-P65c or both recombinant fusion proteins poured in 200 μl of a 1:4 suspension of PBS-vegetable oil adjuvant (MontanideIMS 1313; SEPPIC). A booster dose was given at postinfection day (PID) 21. Piglets were bled at PID 14, 21, and 28. Presence of antibodies to the GST-P46 or GST-P65c or to both membranous authentic proteins was confirmed by indirect ELISA using purified recombinant fusion proteins or lysates of authentic membranous proteins as antigens. The use of GST-coated plates permitted the confirmation of the reactivities of pigs sera against recombinants proteins.
Experimental infection of SPF pigs.
Six crossbred F1 (Landrace × Yorkshire) castrated SPF pigs (5 to 6 weeks old) were obtained from the aforementioned breeding farm. Experimental inoculation of pigs by the intratracheal route was conducted as described previously (7). The animals were separated in two groups, consisting of two control pigs that received only fresh Friis medium whereas the remaining four pigs received an identical volume (8 ml) of a culture of the virulent IAF-DM9827 field strain of M. hyopneumoniae, corresponding to an infectious dose of 107 color-changing units/ml. Both groups of pigs were allocated to separate rooms in facilities equipped with a microorganism-free filtered in-flowing and out-flowing air system. The animals were monitored clinically and serologically for a 7-week period and then euthanized. Their lungs were aseptically collected and processed for histopathology, PCR, and cultivation attempts in modified Friis medium.
Microscopic analysis of tissue sections.
Thin sections (5 μm thick) of formalin-fixed, paraffin-embedded tissues from the lungs of experimentally infected pigs were routinely processed for hematoxylin-eosin staining, as previously described (7).
IIF staining.
Thin frozen lung sections with typical lesions of enzootic pneumonia were mounted on glass slides and fixed with 100% ice-cold acetone. Once the slides were dried, they were incubated 1 h at 37°C with 100 μl of anti-P46 or anti-P65c MAbs at a dilution of 1:100, washed in PBS, and reacted similarly with fluorescein-conjugated goat anti-mouse IgA-IgG-IgM (heavy and light chain) (Kirkegaard & Perry Laboratories, Gaithersburg, Md.) diluted 1:50 in PBS. After a further washing step, the fluorescent reaction was observed under a UV microscope (Leica, Leitz Wetzlar, Germany).
Immunoperoxidase assay.
Paraffin-embedded sections were deparaffinized by immersing slides twice in toluene for 2 min and rehydrated in 100, 95, 80, and 70% ethyl alcohol, respectively. They were then immersed in distilled water and treated with 3% aqueous hydrogen peroxide for 30 min to inactivate endogenous peroxidases. After several washes in PBS (pH 7.4), they were treated for 15 min at 37°C with 0.05% protease (protease XIV; Sigma) diluted in preheated PBS (37°C). The slides were washed consecutively three times in preheated (37°C) PBS for 5 min and once in cold (4°C) PBS for 5 min. The MAbs directed against P46 or P65c, as well as negative porcine serum, were diluted 1:100 in BLOTTO buffer and then poured on the slides. Following an incubation period of 90 min at 37°C, slides were washed three times in PBS for 5 min and then 5 min in blocking buffer which consisted of PBS containing 1% goat serum. Biotinylated sheep anti-mouse Ig (Boehringer Mannheim) or rabbit anti-pig IgG (Sigma) at a dilution of 1:1,000 was poured on the slides, and then the slides were incubated for 30 min at 37°C. Slides were then washed three times in PBS for 5 min and reacted with peroxidase-conjugated streptavidin (Sigma-Aldrich) diluted 1:1,000 in PBS-1% goat serum, for 30 min at room temperature. Following three other washes of 5 min in PBS, sections were incubated with 3,3′-diaminobenzidine tetrahydrochloride (DAB) for 10 min at room temperature and finally rinsed in distilled water for 10 min. Counterstaining was done with hematoxylin. After dehydration, lung sections were covered with mounting medium and coverslips.
RESULTS
PCR amplification and directed mutagenesis of TGA codons of the P46 and P65 encoding genes.
Based on the reported sequences of the P46 (1,260 bp) and P65 (1,803 bp) membranous protein genes of M. hyopneumoniae (strain ATCC 25934), oligonucleotide primers were designed so as to permit amplification of the entire ORFs of the targeted genes. The primer pairs P46BamH1-P46Sal1 and FSLP65-R2SP65 yielded DNA fragments of the expected size when PCR was performed with extracted genomic DNA from the homologous strain of M. hyopneumoniae (Fig. 1). Both primers pairs were also tested for their capacity to amplify DNA fragments from theP46 and P65 genes of two other reference strains of M. hyopneumoniae (ATCC 25934 and ATCC 25095). Expected PCR amplification products from the P46 and P65 genes were obtained with both heterologous strains. No reactivity was obtained when the PCR was performed with genomic DNA extracted from M. hyorhinis and A. laidlawii species that commonly infect pigs (Fig. 1). Sequencing analyses confirmed the identity of the two genes.
FIG. 1.
PCR amplification of the ORF encoding the P46 and P65 membranous proteins of M. hyopneumoniae (strain ATCC 25934). Lanes 1 and 2 contain 1,260- and 1,803-bp DNA amplicons corresponding to the entire P46 and P65 gene, which were obtained by PCR using the oligonucleotide primer pairs P46BamH1-P46Sal1 and FSLP65-R2SP65, respectively. As controls M. hyorhinis and A. laidlawii were used, respectively. Lanes 3 and 4 contain P46 primers; lanes 5 and 6 contain P65 primers. Lane M, molecular sizes of fragments of the 1-kb Plus DNA ladder.
For several Mycoplasma genes, including M. hyopneumoniae, TGA codons are translated as tryptophan residues rather than corresponding to translational stop signals as in mammalian and other bacterial cells. For M. hyopneumoniae, the ORFs coding for P46 (1,260 bp) and P65 (1,803 bp) possess three and one TGA codons, respectively. Therefore, DNA fragments within both targeted genes of the reference ATCC 25934 strain of M. hyopneumoniae were amplified by PCR using overlapping oligonucleotide primers that were specially designed so as to permit replacement of the TGA codons in TGG in the DNA fragments (Table 1). The DNA fragments were then appropriately ligated, and the complete modified genes were amplified using primers previously used to amplify the entire P46 and P65 encoding genes. Further sequencing analysis confirmed that the three TGA codons located at positions 208, 301, and 760 of the 1,260-bp P46-encoding gene and at position 631 of the 1,803-bp P65-encoding gene had been replaced by TGG codons.
Cloning, procaryotic expression, and purification of GST-P46 and GST-P65c recombinant fusion proteins.
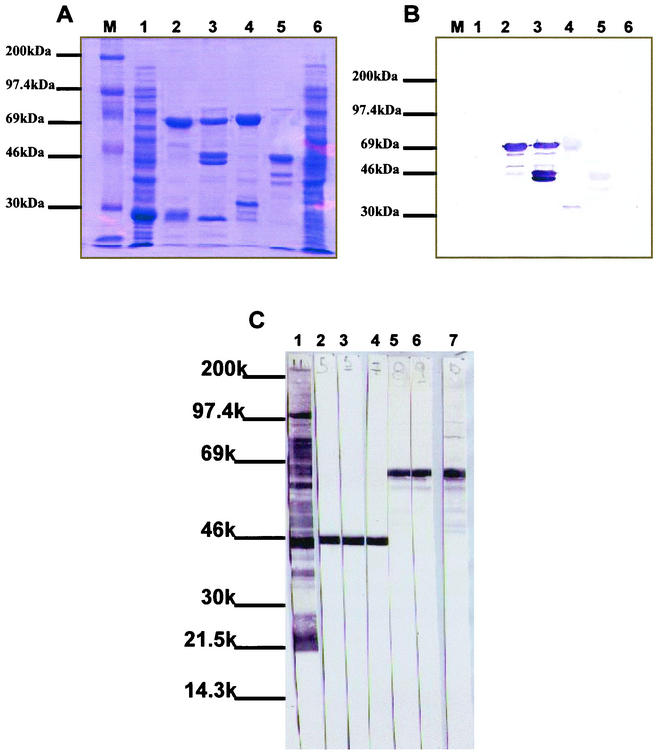
The mutated P46 and P65 encoding genes were ligated into the procaryotic pGEX-4T1 vector (Pharmacia) and used to transform competent E. coli BL21(DE3) cells to produce recombinant proteins fused to GST. The mutated constructs were cloned in pGEX-4T1 by using restriction sites BamHI and SalI for the P46 gene or EcoRI and SalI for an N-terminally truncated P65 (P65c) gene fragment, and the procaryotic pGEX-4T1 vector was digested by the same combination of restriction enzymes, respectively. Ligation steps were conducted overnight at 14°C. The transformed competent E. coli cells were then induced by the addition of IPTG in the culture medium to express the fusion proteins GST-P46 and GST-P65c. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis of sonicated lysates of IPTG-induced transformed E. coli cells revealed the presence of two additional 72- and 70-kDa protein species. Those proteins species could not be detected either in lysates prepared from noninduced bacteria or in lysates prepared from nontransformed bacteria. The Mrs of both recombinant proteins, as estimated from the electrophoretic migration profiles, corresponded to the Mrs determined from the deduced amino acid sequences of the PCR-amplified P46 entire gene or C-terminal moieties of the P65 protein to which was fused the GST protein (Mr = 26,000).
Following bulk purification of the induced sonicates on glutathione-Sepharose beads, 1.8 to 2.5 mg of recombinant fusion protein were usually recovered from a culture of 500 ml of IPTG-induced and transformed bacteria (Fig. 2A).
FIG. 2.
Expression of recombinant P46 and P65c and Western blotting patterns of specific monoclonal antibodies. (A) Expression of the GST-P46 and GST-P65c recombinant fusion proteins in E. coli. Lane 1, IPTG-induced GST recovered from pGEX-4T1-transformed bacteria (GST = 26 kDa); lane 2, purified GST-P46 recombinant fusion protein (72 kDa); lane 3, cleaved recombinant GST-P46 fusion protein (46 kDa); lane 4, purified GST-P65c recombinant fusion protein (70 kDa); lane 5, cleaved GST-P65c recombinant fusion protein (44 kDa); lane 6, noninduced recombinant bacteria; lane M, Rainbow molecular mass markers. (B) Polypeptide specificities of pooled MAbs produced against E. coli-expressed P46 and P65c recombinant proteins. The reactivities of pooled MAbs were tested against the fusion and cleaved recombinant proteins. Lane 1, IPTG-induced pGEX-4T1-transformed bacteria (GST = 26 kDa); lane 2, purified GST-P46 recombinant fusion protein (72 kDa); lane 3, cleaved recombinant GST-P46 fusion protein (46 kDa); lane 4, purified GST-P65c recombinant fusion protein (70 kDa); lane 5, cleaved GST-P65c recombinant fusion protein (44 kDa); lane 6, noninduced recombinant bacteria; lane M, Rainbow molecular weight markers. (C) Reactivities of the anti-P46 and anti-P65c MAbs as determined by Western blotting using whole M. hyopneumoniae lysate as antigenic preparation. The figure illustrates the reactivity patterns as follows: lane 1, polyclonal antiserum to M. hyopneumoniae; lane 2, MAb 7D3-E9 (anti-P46); lane 3, MAb 7D3-C11 (anti-P46); lane 4, MAb 6A4-G9 (anti-P46); lane 5, MAb 4D11-C11 (anti-P65c); lane 6, MAb 1D3-C6 (anti-P65c); lane 7, MAb 4D11-G8 (anti-P65c).
Species specificity of anti-P46 and anti-P65c MAbs.
From two fusion experiments with spleen cells of hyperimmunized mice, a total of 30 hybridoma cell lines secreting anti-P46 MAbs and 26 hybridoma cell lines secreting anti-P65c MAbs could be established. From these hybridomas, eight secreted anti-P46 MAbs and seven secreted anti-P65c MAbs that reacted intensively against homologous proteins (Fig. 2B). These secreting hybridomas were subcloned and either maintained in cultures or used to produce ascitic fluids in pristane-primed mice. MAbs that were secreted from the established hybridomas were tested for their polypeptide and species specificity by Western blotting against complete antigenic preparations of three reference strains of M. hyopneumoniae (ATCC 25934, ATCC 25095, and J strains) and that of other porcine mycoplasma species, including A. laidlawii. All of the MAbs obtained from established hybridomas reacted very specifically toward the three reference strains of M. hyopneumoniae (Fig. 2C) and showed no reactivity toward other mycoplasma species tested (data not shown). The anti-P65c MAbs were all determined to be of the IgG1 isotype, whereas six of the eight identified anti-P46 MAbs were found to be IgG1 antibodies and two were more likely of the IgG2a isotype
Immunogenicity of E. coli-expressed GST-P46 and GST-P65c recombinant fusion proteins.
By indirect ELISA, using recombinant protein as antigen, seroconversion to M. hyopneumoniae was demonstrated for sera collected from pigs which had received one injection with GST-P46 or GST-P65c or both of these recombinant fusion proteins prepared in a vegetable oil adjuvant. Interestingly, within 14 days after the first injection, specific IgG antibody titers higher than 1:10,000 could be detected by ELISA to both membranous proteins that have been injected individually to the pigs. The sera showed no cross-reactivity against heterologous proteins. On the other hand, when the proteins were injected simultaneously to the pigs, the reactivity to P46 was detected by indirect ELISA 2 weeks (PID 14) earlier than that directed to P65c, for which antibody titers higher than 1:10,000 were not detectable before the second dose of the antigenic preparation was given to the pigs (Table 2). Indeed, when the proteins were injected simultaneously, no reactivity was detected 21 days after first injection of the mixture of both recombinant fusion proteins, and A450 values higher than 1.0 were not reached until PID 42 or 2 to 3 weeks following the booster injection. Western blotting experiments conducted with sera collected at PID 42 (dilution, 1:100) also confirmed their reactivities against the authentic proteins of M. hyopneumoniae.
TABLE 2.
Absorbance values (A450) of serum (dilution, 1:8,000) from pigs immunized with recombinant proteinsa
| Pig inoculated (immunization) |
A450 determined with recombinant protein on:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| D0
|
PID 14
|
PID 21
|
PID 42
|
|||||
| P46 | P65c | P46 | P65c | P46 | P65c | P46 | P65c | |
| 55 (GST − P46) | 0 | 0 | 0.5 | 0 | 0.6 | 0 | 1.6 | 0 |
| 52 (GST − P46) | 0 | 0 | 0.8 | 0 | 0.6 | 0 | 1.4 | 0.5 |
| 53 (GST − P65c) | 0 | 0 | 0 | 1.5 | 0 | 1.0 | 0 | 2.0 |
| 57 (GST − P65c) | 0 | 0 | 0 | 0.5 | 0 | 1.0 | 0 | 1.2 |
| 56 (P46+P65c) | 0 | 0 | 0.5 | 0 | 0.95 | 0 | 2.0 | 1.5 |
| 58 (P46+P65c) | 0 | 0 | 0.5 | 0 | 1.0 | 0 | 0.9 | 0.8 |
| Control 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Control 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Pigs were immunized by injection of individual or mixed P46 or P65c recombinant proteins, and A450 was determined by ELISA using either recombinant protein as the antigen.
Clinical and pathological findings in experimentally infected pigs.
Weaned pigs that were infected with the Quebec field strain IAF-DM9827 of M. hyopneumoniae did not manifest clinical signs until 3 to 4 weeks postinoculation. Then, from the 6th to 7th week postinfection, coughing could be elicited by exercising infected pigs around the pen; coughing occurred with greater frequency in the period immediately following the exercise. At this time, the infected pigs were also apatic, reacted less to their environment, and preferred to lay on the ground rather than stand. Compared to the pigs in the control group, the infected pigs also showed a drop in feed consumption, as judged by the quantity of feed remaining in the pens, which were changed twice a day. Rectal temperatures were not taken during this study; body weights and changes in hemograms were also not monitored.
Ìnfected pigs that were necropsied at day 42 or 49 p.i. had gross lesions that were confined to the respiratory tract and thoracic cavity. The lung lesions were confined almost entirely to the apical and cardiac lobes and were clearly demarcated from the normal lung tissue. Plum-colored or greyish areas of consolidation resembling lymphoid tissues were scattered along the ventral borders of the lobes. The mediastinal lymph nodes were enlarged and congested. Minor (25 ml) to large (≥100 ml) amounts of bloody fluid could also be demonstrated within the thoracic cavity and pericardium of two infected pigs. M. hyopneumoniae infection of the lungs and upper respiratory conducts was confirmed by a single P36 PCR assay (6)
Microscopic lesions and immunohistopathology.
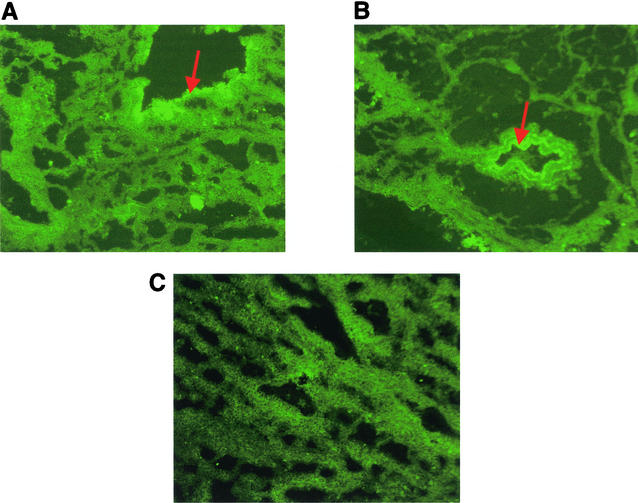
At PID 42, the microscopic lesions observed were confined to the thoracic and cardiac lobes of lung of the infected pigs. The plum-colored and consolidated areas of the infected lungs corresponded to mild to severe characteristic perivascular and peribronchiolar lymphomononuclear nodules of infiltration, often compressing the lumen of the bronchioles. Hyperplasia of the epithelial cells of the affected bronchioles was observed in all four infected pigs, but also, in two pigs the lumen of their bronchioles was completely filled with an infiltrate consisting of cell debris and numerous lymphomononuclear cells. Thin frozen lung sections mounted on glass slides and fixed with 100% ice cold acetone were first tested for the presence of M. hyopneumoniae antigens using pools of either two to three of the anti-P46 or anti-P65c MAbs enriched ascitic fluids, and then reacted with fluorescein isothiocyanate-conjugated goat anti-mouse IgG (Boehringer Mannheim) diluted 1:50 in PBS. With both types of MAbs, specific fluorescent cells were usually observed lining the bronchiolar epithelium. The morphology and delimitation of bronchiolar epithelial cells were more easily recognized when slides were incubated with the pool of anti-P46 MAbs (Fig. 3A) compared to the pool of anti-P65c MAbs (Fig. 3B). No such fluorescence was observed when lung sections were incubated with normal mouse serum (Fig. 3C).
FIG. 3.
Immunofluorescent staining by anti-P46 (A) and anti-P65c (B) MAbs of frozen sections of lungs from experimentally infected pigs. Staining revealed fluorescence lining the bronchiolar epithelium, as indicated by arrows.
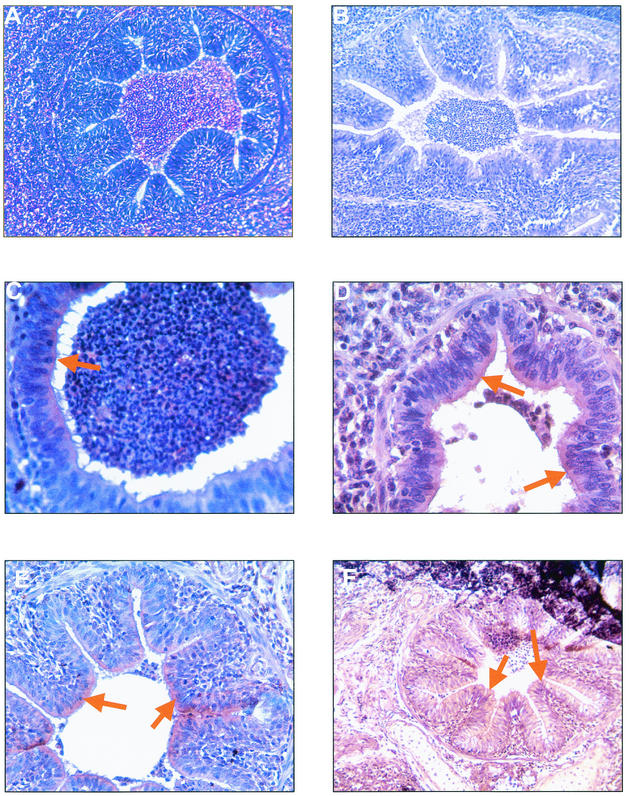
On the other hand, immunohistochemical detection of M. hyopneumoniae in lungs tissues of the four experimentally infected pigs could be achieved by immunoperoxidase staining. Both pools of anti-P46 or anti-P65c MAbs and homologous porcine hyperimmune serum, permitted effective detection by indirect immunoperoxidase assay of M. hyopneumoniae-specific antigens in formalin-fixed, paraffin-embedded lung sections from pigs that were killed after the 6- to 7-week observation period. In comparison with the IIF method on frozen lung sections, the morphology of lung tissues was much more preserved, such that positive staining was often associated with the typical histopathological lesions described above. Positive staining associated with the presence of specific M. hyopneumoniae antigens was localized as a diffused brownish labeling in the cytoplasm, but more intensively on the surface, of the epithelial cells of large airways such as bronchioles and bronchi, but no staining could be observed of the endothelial cells of alveolar ducts and alveoli. Positively stained hyperplasic epithelial cells of the thoracic bronchioles was observed, mainly following incubation with anti-P46 MAbs, but in all cases, there was no staining of infiltrated lymphomononuclear cells (Fig. 4C and D). Only debris of epithelial cells appeared to be stained in the exudate presented in the lumen of bronchoalveolar ducts. The staining reaction with anti-P65c MAbs was more diffused (Fig. 4E and F). Thin sections of lung tissues from control pigs were negative for the presence of specific antigens, as well as lung sections from experimentally infected pigs that had been incubated with negative mouse sera (Fig. 4B).
FIG. 4.
Histological and immunohistochemical findings as observed in the lungs of experimentally infected SPF pigs. (A) Peribronchiolar and perivascular accumulation of mononuclear cells with a mild hyperplasia of the bronchiolar epithelium (HE staining). (B to F) Immunoperoxidase staining by negative mouse serum (B) or by anti-P46 (C and D) and anti-P65c (E and F) MAbs of paraffin-embedded sections of lungs from experimentally infected pigs revealed staining patterns similar to that observed following immunofluorescence as indicated by arrows. Magnification, ×18 (A, B, E, and F) and ×36 (C and D).
DISCUSSION
M. hyopneumoniae is a fastidious microorganism that is difficult to isolate in culture (1) from clinical specimens on a routine basis, and as the only recognized etiological agent of enzootic pneumonia in pigs, it shares some antigenic determinants with less virulent species, such as M. hyorhinis and M. flocculare (10). Both of these properties have hampered the development of accurate diagnostic tests necessary to the establishment of control epidemiological programs. The inefficacy of inactivated or killed vaccines (bacterins) to prevent pig infection by M. hyopneumoniae, as the result of a poor stimulation of the specific mucosal immunity, makes this pig infectious disease a chronic, costly, and uncontrolled pathological entity with a worldwide distribution. Consequently, there is a need for standardized sources of purified proteins carrying most predominant antigenic determinants and of specific antibodies directed against these proteins for the development of species-specific and accurate diagnostic assays for field strain identification and detection of specific circulating and mucosal antibodies.
Membranous lipoproteins, particularly the P46, P65, and P97 proteins, have been demonstrated to carry species-specific antigenic determinants (34), but their association with a protective immune response is still to be investigated (31).
In the present study, the P46 and P65 membranous proteins of M. hyopneumoniae could successfully be expressed in E. coli, provided that TGA stop codons were replaced with TGG using directed PCR mutagenesis. Moreover, the procaryotic pGEX-4T-1 expression system has been shown to easily and rapidly produce large quantities of pure proteins (25, 29), and because of the presence of a thrombin protease recognition site downstream of the GST coding sequences, this allows cleavage of the desired protein from the fusion partner. Different incubation temperatures and concentrations of IPTG used for the induction can be tested to increase the level of production of the recombinant protein and to avoid its accumulation in the form of inclusion bodies (25). Another advantage of this expression system is that GST protein is not present in E. coli; hence, pig sera should not possess any antibodies that would react against this protein and interfere with data obtained from serological tests.
The E. coli-expressed recombinant proteins displayed the antigenicity of the authentic proteins being recognized by convalescent pig sera by Western blotting and ELISA. The immunogenicity of both authentic proteins was preserved since following injection of mice and SFP pigs, both species produced antibodies that specifically reacted to the authentic P46 and P65 proteins by Western blotting. Furthermore, MAbs generated following fusion experiments with mice hyperimmunized against the recombinant proteins also reacted specifically to the authentic proteins of reference and field isolates of M. hyopneumoniae, but not against proteins of other Mycoplasma species. Therefore, for diagnosis purpose, the anti-P46 and anti-P65c MAbs could be used for the final identification of M. hyopneumoniae field strains isolated in culture.
IIF on frozen tissue sections is probably the most common diagnostic tool used for the detection of M. hyopneumoniae in tissues of infected pigs (23, 30; Feenstra et al., Proc. 13th Int. Pig Vet. Soc. Congr.), but the streptavidin-biotin immunoperoxidase techniques have many advantages compared to cultivation methods; they are rapid and sensitive antigen detection tests, and contrary to IFF, they permit the simultaneous quantification of damages or lesions caused by M. hyopneumoniae in the lungs tissues and upper respiratory tract airways. However, polyclonal antibodies are still currently used in the IIF and immunoperoxidase tests (1, 22, 23, 30; Feenstra et al., Proc. 13th Int. Pig Vet. Soc. Congr.). Consequently, false-positive results may arise due to cross-reactions that exist between pathogenic (M. hyopneumoniae and M. hyosynoviae) and non- or less virulent (M. flocculare and M. hyorhinis) mycoplasma species (10). Therefore, to eliminate misinterpretation due to nonspecific immunolabeling, the use of MAbs which react to specific immunodominant proteins of M. hyopneumoniae is suggested. Recently, we demonstrated that MAbs raised against the species-specific P36 cytosolic protein, and its encoding gene, may be considered for early and specific diagnosis of M. hyopneumoniae infection by PCR and IIF on frozen lung sections (6, 7). However, no characteristic immunolabeling pattern could really be defined using anti-P36 MAbs. Herein, anti-P46 and anti-P65c MAbs could be applied for the specific diagnosis of M. hyopneumoniae infection by IIF on frozen lung sections and by indirect immunoperoxidase on formalin-fixed, paraffin-embedded lung sections from pigs experimentally infected with a virulent field strain of M. hyopneumoniae. The great advantage of the immunoperoxidase labeling technique is that one can easily interpret the pathological lesions in term of cells infected by the microorganism and type of inflammatory cells involved, since the morphology of lung tissues is well preserved and the counterstaining method allows histopathological diagnosis. Positive staining associated with the presence of specific M. hyopneumoniae antigens showed that the infection was mainly localized on the surface of epithelial cells of the bronchi and bronchioles. The immune response involved infiltration of the surrounding interstitial tissue by lymphomononuclear cells, which are noninfected by this virulent agent. M. hyopneumoniae has not been reported as a tissue invader, but rather it is considered to be an extracellular pathogen which associates very intimately with the ciliated epithelial cells of the porcine lower respiratory tract (22, 26), which is in agreement with the immunofluorescence and immunoperoxidase patterns obtained in the present study. We are currently evaluating the anti-P46 and anti-P65c MAbs for their potential use in a specific and sensitive blocking ELISA for detection of antibodies in pigs.
Acknowledgments
We thank L. Wilson and N. Sawyer for their excellent technical assistance. Particular thanks go to C.A. Gagnon, INRS-Institut Armand-Frappier, for his help in experimental infections and necropsies. We also thank D. Larochelle, Laboratoire de pathologie animale, Ministère de l'Agriculture, des Pêcheries et de l'Alimentation du Québec, Ste-Foy, Québec, Canada, for the preparation of histologic sections.
This work was partly supported by the Conseil de Recherches en Pêche et Agro-Alimentaire du Quebec (grant 4600); the Quebec Federation of Swine Producers; and Biovet Inc., St-Hyacinthe, Quebec, Canada.
REFERENCES
- 1.Amanfu, W., C. N. Weng, R. F. Ross, and H. J. Barnes. 1984. Diagnosis of mycoplasmal pneumonia of swine: sequential study by direct immunofluorescence. Am. J. Vet. Res. 45:1349-1352. [PubMed] [Google Scholar]
- 2.Armstrong, C. H., M. J. Freeman, and L. Sands-Freeman. 1987. Cross-reactions between Mycoplasma hyopneumoniae and Mycoplasma flocculare: practical implications for the serodiagnosis of mycoplasmal pneumonia of swine. Isr. J. Med. Sci. 23:654-656. [PubMed] [Google Scholar]
- 3.Blanchard, B., M. Kobisch, J. M. Bove, and C. Saillard. 1996. Polymerase chain reaction for Mycoplasma detection in tracheobronchiolar washings from pigs. Mol. Cell. Probes 10:15-22. [DOI] [PubMed] [Google Scholar]
- 4.Brooks, E., and D. Faulds. 1989. The Mycoplasma hyopneumoniae 74,5kD antigen elicits neutralizing antibodies and shares sequence similarity with heat-shock proteins. Vaccine 89:265-269. [Google Scholar]
- 5.Calsamaglia, M., C. Pijoan, and A. Trigo. 1999. Application of a nested polymerase chain reaction assay to detect Mycoplasma hyopneumoniae from nasal swabs. J. Vet. Diagn. Investig. 11:246-251. [DOI] [PubMed] [Google Scholar]
- 6.Caron, J., M. Ouardani, and S. Dea. 2000. Detection and differentiation of Mycoplasma hyopneumoniae and Mycoplasma hyorhinis by PCR amplification of the p36 and p46 genes. J. Clin. Microbiol. 38:1390-1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caron, J., N. Sawyer, B. Ben Abdel Moumen, K. Cheikh Saad Bouh, and S. Dea. 2000. Species-specific monoclonal antibodies to Escherichia coli-expressed p36 cytosolic protein of Mycoplasma hyopneumoniae. Clin. Diagn. Lab. Immunol. 7:528-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dea, S., R. Bilodeau, R. Sauvageau, and G. P. Martineau. 1991. Outbreaks of respiratory and reproductive problems associated with encephalomyocarditis virus in Quebec pig farms. J. Vet. Diagn. Investig. 3:275-282. [DOI] [PubMed] [Google Scholar]
- 9.Feld, N. C., P. Qvist, P. Ahrens, N. F. Friis, and A. Meyling. 1992. A monoclonal blocking ELISA detecting serum antibodies to Mycoplasma hyopneumoniae. Vet. Microbiol. 30:35-46. [DOI] [PubMed] [Google Scholar]
- 10.Freeman, M. J., C. H. Armstrong, L. L. Freeman-Sands, and M. Lopez-Osuna. 1984. Serological cross-reactivity of porcine reference antisera to Mycoplasma hyopneumoniae, M. flocculare, M. hyorhinis and M. hyosynoviae indicated by the enzyme-linked immunosorbent assay, complement fixation and indirect hemagglutination tests. Can. J. Comp. Med. 48:202-207. [PMC free article] [PubMed] [Google Scholar]
- 11.Frey, J., A. Haldimann, M. Kobisch, and J. Nicolet. 1994. Immune response against the L-lactate dehydrogenase of Mycoplasma hyopneumoniae in enzootic pneumonia of swine. Microb. Pathog. 17:313-322. [DOI] [PubMed] [Google Scholar]
- 12.Friis, N. F. 1973. The pathogenicity of Mycoplasma flocculare. Acta Vet. Scand. 14:344-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frydenberg, J., K. Lind, and P. C. Hu. 1987. Cloning of Mycoplasma pneumoniae DNA and expression of P1-epitopes in Escherichia coli. Isr. J. Med. Sci. 23:759-762. [PubMed] [Google Scholar]
- 14.Futo, S., Y. Seto, M. Okada, S. Sato, T. Suzuki, K. Kawai, Y. Imada, and Y. Mori. 1995. Recombinant 46-kilodalton surface antigen (P46) of Mycoplasma hyopneumoniae expressed in Escherichia coli can be used for early specific diagnosis of mycoplasmal pneumonia of swine by enzyme-linked immunosorbent assay. J. Clin. Microbiol. 33:680-683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goodwin, R. F. W., A. P. Pomeroy, and P. Whittlestone. 1965. Production of enzootic pneumonia in pigs with mycoplasma. Vet. Rec. 77:1247-1249. [Google Scholar]
- 16.Haldimann, A., J. Nicolet, and J. Frey. 1993. DNA sequence determination and biochemical analysis of the immunogenic protein p36, the lactate dehydrogenase (LDH) of Mycoplasma hyopneumoniae. J. Gen. Microbiol. 139:317-323. [DOI] [PubMed] [Google Scholar]
- 17.Harasawa, R., K. Koshimizu, O. Takeda, T. Uemori, K. Asada, and I. Kato. 1991. Detection of Mycoplasma hyopneumoniae by the ploymerase chain reaction. Mol. Cell. Probes 5:103-109. [DOI] [PubMed] [Google Scholar]
- 18.Kim, M. F., M. B. Heidari, S. J. Stull, M. A. McIntosh, and K. S. Wise. 1990. Identification and mapping of an immunogenic region of Mycoplasma hyopneumoniae P65 surface lipoprotein expressed in Escherichia coli from a cloned genomic fragment. Infect. Immun. 58:2637-2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klinkert, M. Q., R. Herrmann, and H. Schaller. 1985. Surface proteins of Mycoplasma hyopneumoniae identified from an Escherichia coli expressed plasmid library. Infect. Immun. 49:329-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kobish, M., and N. F. Friis. 1996. Swine mycoplasmoses. Rev. Sci. Off. Int. Epizoot. 15:1569-1606. [DOI] [PubMed] [Google Scholar]
- 21.Maes, D., M. Verdonck, H. Deluyker, and A. de Kruif. 1996. Enzootic pneumonia in pigs. Vet. Q. 18:104-109. [DOI] [PubMed] [Google Scholar]
- 22.Maré, C. J., and W. P. Switzer. 1965. Mycoplasma hyopneumoniae, a causative agent of virus pig pneumonia. Vet. Med. 60:841-845. [PubMed] [Google Scholar]
- 23.Mori, Y., T. Hamaoka, and S. Sato. 1987. Use of monoclonal antibody in an enzyme-linked immunosorbent assay (ELISA) for the detection of antibodies against Mycoplasma hyopneumoniae. Isr. J. Med. Sci. 23:657-662. [PubMed] [Google Scholar]
- 24.Mori, Y., T. Hamaoka, S. Sato, and S. Takeuchi. 1988. Immunoblotting analysis of antibody response in swine experimentally inoculated with Mycoplasma hyopneumoniae. Immunol. Immunopathol. 19:239-250. [DOI] [PubMed] [Google Scholar]
- 25.Nakano, H., T. Yamazaki, M. Ikeda, H. Masai, S. Miyatake, and T. Saito. 1993. Purification of glutathione S-transferase fusion proteins as a non-degraded form by using a protease-negative E. coli strain, AD202. Nucleic Acids Res. 22:543-544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ross, R. F. 1992. Mycoplasmal diseases, p. 537-551. In A. D. Leman, B. Straw, W. Mengeling, S. D'Allaire, and D. Taylor (ed.), Diseases of swine, 7th ed. Iowa State University Press, Ames.
- 27.Sanger, N. S., S. Nicklen, and A. R. Coulson. 1977. DNA sequencing with chain termination inhibitors. Proc. Natl. Acad. Sci. USA 74:5463-5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 29.Smith, D. B., and K. S. Johnson. 1988. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene 67:31-40. [DOI] [PubMed] [Google Scholar]
- 30.Stipkovits, L., J. Nicolet, A. Haldimann, and J. Frey. 1991. Use of antibodies against the p36 protein of Mycoplasma hyopneumoniae for the identification of M. hyopneumoniae strains. Mol. Cell. Probes 5:451-457. [DOI] [PubMed] [Google Scholar]
- 31.Strasser, M., J. Frey, G. Bestetti, M. Kobisch, and J. Nicolet. 1991. Cloning and expression of a novel species-specific early immunogenic 36-kilodalton of Mycoplasma hyopneumoniae in Escherichia coli. Infect. Immun. 59:1217-1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thacker, E. L., P. G. Halbur, R. F. Ross, R. Thanawongnuwech, and B. J. Thacker. 1999. Mycoplasma hyopneumoniae potentiation of porcine reproductive and respiratory syndrome virus-induced pneumonia. J. Clin. Microbiol. 37:620-627. [DOI] [PMC free article] [PubMed]
- 33.Thacker, E. L., B. J. Thacker, and B. H. Janke. 2001. Interaction between Mycoplasma hyopneumoniae and swine influenza virus. J. Clin. Microbiol. 39:2525-2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trevino, L. B., W. G. Haldenwang, and J. B. Baseman. 1986. Expression of Mycoplasma pneumoniae antigens in Escherichia coli. Infect. Immun. 53:129-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang, Q., T. Young, and R. F. Ross. 1995. Identification and characterization of a Mycoplasma hyopneumoniae adhesin. Infect. Immun. 63:1013-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]