Abstract
Serological assays are valuable tools for studies of the epidemiology of human papillomaviruses (HPVs). The efficacy of a less invasive oral-fluid assay for detection of HPV antibodies was examined. Matched serum, saliva, and oral mucosal transudate (OMT) specimens collected from 150 human immunodeficiency virus-seropositive patients were tested for immunoglobulin G antibodies against HPV-6 and HPV-11 combined (HPV-6/11) and HPV-16 capsids. Antibodies to HPV were detected in both types of oral specimens. Seroprevalence rates were 55% for HPV-6/11 and 37% for HPV-16, whereas oral prevalence rates were significantly lower (for HPV-6/11 in saliva, 31%, and in OMT, 19%; for HPV-16 in saliva, 19%, and in OMT, 17%). HPV antibody detection in OMT more accurately reflected the presence of antibodies in serum than did HPV antibody detection in saliva. More stringent saliva assay cutpoints yielded stronger associations between oropositivity and seropositivity; less stringent OMT cutpoints yielded stronger associations between oropositivity and seropositivity. Although HPV antibodies were detected in oral fluids, further optimization of the assay is necessary before oral-fluid testing can be implemented as a reliable alternative to serum testing for HPV.
Human papillomavirus (HPV) infection is the most common viral sexually transmitted disease. Infection with low-risk HPV types is associated with genital warts, while infection with high-risk HPV types is associated with anogenital malignancies (43). Research on HPV has been hindered by the difficulties encountered in establishing culture systems for the virus. To circumvent this problem, researchers have produced viral capsids of various types of HPV in vaccinia virus, yeast, and baculovirus expression systems (21, 24, 29, 44). These viral capsids appear identical to natural viral capsids (19) and have been used extensively in serological assays. The serological response (mainly immunoglobulin G [IgG]) persists over time and is associated with the cumulative number of sex partners over a lifetime and not with the recent number of sex partners. Therefore, seropositivity is thought to be a marker of past infection (10).
The persistence and type specificity of the serological response to HPV has made HPV serology a useful epidemiological tool. By using serological assays, researchers have been able to follow trends in HPV infection rates in various populations and to establish risk factors for HPV infection and HPV-related disease (6, 20, 28, 41). Unfortunately, serological assays require the invasive procedure of venipuncture. An attractive alternative to venipuncture is oral sampling, which provides the advantages of convenience, prevention of accidental transmission of blood-borne pathogens, the ease of use in pediatric and geriatric populations, and the potential for self-testing by patients in their own homes (15).
Saliva has frequently been used as a specimen for antibody detection due to the ease and simplicity of its collection. Antibodies to Helicobacter pylori, Schistosoma mansoni egg antigen, Epstein-Barr virus, and human immunodeficiency virus (HIV) have been detected in saliva specimens (2, 8, 12, 33, 38, 45, 52, 55); however, saliva may be less than ideal for antibody detection. Saliva contains salivary gland secretions as well as sloughed cells, bacteria, mucin, and mucosal transudate (13). Bacteria produce proteases that can degrade the antibodies present in the sample, and mucins make saliva difficult to pipette (13).
An alternative to saliva is oral mucosal transudate (OMT), an IgG-rich serous fluid that seeps across mucosal surfaces in the oral cavity (13). OMT can be collected with the OraSure device (OraSure Technologies, Inc.), which consists of a pad designed to enhance the flow of serum transudate through the gums and a transport vial containing a medium supplemented with antibacterial agents to inhibit growth of oral flora during transport. Antibodies to HIV can be assayed by using the OraSure collection device, and these antibodies reflect serum antibody status (13). The OraSure specimen has also been used to measure antibodies to parvovirus B19 and hepatitis A, B, and C viruses (9, 51). Antibodies to HPV have previously been detected in OMT (36).
The purpose of this study was to examine the efficacy of an oral-specimen assay for HPV antibodies as a noninvasive alternative to serological testing. HIV-positive individuals were chosen to participate in this study due to the higher HPV infection rates in this population (34, 48, 49). We demonstrate that HPV-specific IgG antibodies are detectable in oral fluids and that their presence weakly correlates with the presence of antibodies in serum. Further development of this oral-fluid HPV antibody assay could lead to a safe, noninvasive epidemiological tool for the study of HPVs.
(A portion of this work was presented previously [J. E. Lewis, I. V. Snowhite, J. Slavinsky III, and M. E. Hagensee, Abstr. 18th Ann. Int. Papillomavirus Conf., Barcelona, Spain, poster abstr. 145, 2000]).
MATERIALS AND METHODS
Patient population and study design.
Study participants were men and women receiving routine blood draws at the HIV outpatient program at the Medical Center of Louisiana, New Orleans. The study protocol was approved by the Institutional Review Board of the Louisiana State University Health Sciences Center prior to initiation. Informed consent was obtained followed by collection of (i) whole saliva (participants were asked to expectorate approximately 5 ml of saliva), (ii) OMT (collected by holding the OraSure swab between the buccal mucosa and gingiva for approximately 2 min), and (iii) one tube of venous blood. A total of 150 subjects were enrolled in the study. The majority of the population was African American (59%), male (72%), and over the age of 35 years (63%).
In order to determine optimal collection, processing, and testing protocols for the detection of HPV antibodies in oral specimens, two study cohorts were established. Subjects in the first cohort (n = 100) were instructed to provide the saliva sample first, followed by passively holding the OraSure pad between the cheek and gums for approximately 2 min. The samples were frozen at −20°C immediately upon arrival at the laboratory and were thawed and processed just before testing. Subjects in the second cohort (n = 50) were instructed to vigorously rub the gums with the OraSure pad first and then to hold it passively between the cheek and gums for approximately 2 min. Subjects were asked to provide the saliva specimen following collection of the OMT specimen. The samples were processed fresh and then frozen at −20°C until tested.
Sample processing.
Blood tubes were centrifuged at 2,400 × g in a clinical centrifuge, and sera were aliquoted and stored at 4°C until testing at a later date. Saliva was centrifuged at 1,800 × g for 5 min at 4°C, and supernatants were collected. OraSure collection devices were centrifuged at 2,400 × g in a clinical centrifuge for 20 min to draw the OMT fluid from the collection pad.
Sample analysis.
Total volumes were recorded for oral samples (OMT and saliva) after processing. Oral samples were tested for total protein concentration by using a bicinchoninic acid protein concentration assay (Pierce) in accordance with the manufacturer's protocol. The protein concentration of samples was determined by using standard curves derived from the means of duplicate wells of standard dilutions. Each plate of samples was analyzed against duplicate standards tested on the same plate. The blood content of oral samples was assessed by measurement of heme with Hemastix (Bayer Corp.) detection strips, and results were recorded on a scale from 0 (negative) to 4 (highly positive).
Total IgG.
Oral samples were tested for total IgG concentration by capture enzyme-linked immunosorbent assay (ELISA). Immulon-2 microtiter plates (Dynex Technologies) were coated with goat anti-human whole-molecule IgG at 5 μg/ml (Sigma Diagnostics, Inc.) overnight. The plates were washed with phosphate-buffered saline (PBS) and blocked with PBS plus 10% goat serum. Samples were added in triplicate at appropriate dilutions (saliva, 1:10; OMT, 1:100) in blocking buffer and incubated for 1 h at 37°C. The plates were washed, secondary antibody (goat anti-human whole-molecule IgG conjugated to horseradish peroxidase; Sigma) was added at a dilution of 1:30,000 in blocking buffer, and the plates were incubated for 1 h at room temperature. The plates were washed and developed with Sigmafast OPD (o-phenylenediamine dihydrochloride) (Sigma) for 15 min, the reaction was stopped with 1 N H2SO4, and absorbance was read on an ELISA plate reader (Bio-tek Instruments, Inc.) at 450 nm. The median value of results for the three replicates of each sample was used to calculate the concentration based on the standard curve.
HPV-specific antibodies.
Sera and oral fluids were tested for IgG antibodies to HPV types 6 and 11 combined (HPV-6/11) and for HPV type 16 (HPV-16) by type-specific capture ELISA (17). Briefly, Immulon-2 microtiter plates were coated with monoclonal antibody (H11.B2 for the HPV-6/11 assay and H16.V5 for the HPV-16 assay, graciously provided by N. Christensen, Pennsylvania State Medical Center, Hershey) optimally diluted in 0.9 M sodium carbonate buffer (pH 9.5) and incubated overnight at room temperature. The plates were washed with PBS and blocked for 1 h with Tris-buffered saline plus Tween plus 10% goat serum. HPV-6/11 or HPV-16 capsids produced in vaccinia virus expression systems (21) were added at optimal dilutions in PBS (for HPV-6/11, capsid dilution was optimized separately and the capsids were combined) (46) and incubated for 1 h at room temperature. The plates were again washed, samples were added at appropriate dilutions in blocking buffer (serum, 1:100; OMT, 1:2 or undiluted in first and second cohorts, respectively; saliva, undiluted), and plates were incubated for 1 h at 37°C. The plates were washed, and secondary antibody (goat anti-human IgG γ chain conjugated to alkaline phosphatase, [Boehringer Mannheim]) was added at a 1/1,000 dilution in blocking buffer for 1 h at 37°C. The plates were washed and developed with phosphatase substrate (Sigma Diagnostics, Inc.) for 30 min, the reaction was stopped with 1 M NaOH, and absorbance was read at 405 nm with an ELISA plate reader. Titers in serum were determined in much the same way except that serial (twofold) dilutions of serum samples were applied to plates after capsids were added to all wells.
HPV antibody reactivities for serum, saliva, and OMT samples was determined as follows. For each sample, the natural log of the median optical density (OD) value of three wells lacking capsids (background) was subtracted from the natural log of the median OD value of three wells containing capsids, thus yielding an adjusted OD (adj OD) value. A positive reaction was determined by comparing the specimen's adj OD value to a determined cutpoint. Cutpoints for serum assays were derived from the adj OD value of sera from children under the age of 10 years with no history of sexual activity. Patients were considered HPV seropositive if the adj OD values of their sera were greater than 2 standard deviations above the mean of the adj OD values of the children's sera, and by this method all values for children's samples fell below the cutpoint value. Cutpoints for oral samples were determined by using the adj OD values of oral samples collected from children under the age of 10 years. Oropositivity was defined by an adj OD value greater than 2 standard deviations above the mean of the adj OD values of the children's oral samples, with those children's samples falling above this cutpoint being excluded and the cutpoint being recalculated (4, 22, 34, 35, 37, 54). When no negative control population was available, arbitrarily selected cutpoints were used to determine oropositivity (7, 11, 14, 49, 53). Other approaches for determining assay cutpoints for oral specimens were examined, including mathematical modeling, with the assumption that the adj OD values would fall into two groups, defined as oropositive and oronegative, and a modified receiver operator curve approach (23, 40, 47). Serum antibody titers were determined by graphing the OD values obtained for each individual's dilution curve and assessing the area under this curve with GraphPad Prism software.
Statistical analysis.
Student's t test was used to compare mean volumes and the protein, heme, and IgG contents of different types of samples and between sample groups. Chi-square analysis was used to compare HPV oropositivity (determined by the arbitrary cutpoint of 0.100) to sample volume, protein concentration, blood content, and total IgG concentration. The kappa statistic (κ) was calculated to determine the association between HPV oropositivity and seropositivity for each subject by using a variety of oral-sample cutpoints. A Pearson correlation coefficient (r) was used to examine correlations between serum OD values and oral-sample OD values for individual subjects and to examine correlations between titers in serum and oral-sample OD values.
RESULTS
Characterization of oral specimens.
The oral specimens (saliva and OMT) were analyzed for total volume, protein content, heme content, and concentration of total IgG antibodies in order to define the differences between saliva and OMT. The OraSure device yielded an average volume of 1.12 ml, while an average of 2.73 ml of saliva was obtained (P < 0.0001). Heme was detected in 89% of saliva samples compared to 60% in OMT samples (P < 0.0001). While saliva samples contained a slightly higher average protein concentration than OMT samples (4.9 and 3.9 mg/ml, respectively; P = 0.04), OMT samples contained higher average quantities of total IgG than did saliva samples (16.16 and 1.1 μg/ml, respectively; P < 0.0001).
Analysis of collection and processing protocols.
Sample characteristics of the two cohorts were compared in order to determine which protocol yielded the best samples, particularly the best OMT samples, for antibody detection assays (Table 1). OMT specimens in the first cohort contained 3.44 mg of total protein per ml, while OMT specimens in the second cohort contained 4.77 mg of total protein per ml (P < 0.001). Although not significant, the average total IgG concentration in OMT specimens of the second cohort was 25.2 μg/ml compared to 14.4 μg/ml in OMT specimens of the first cohort. In addition, 72% of OMT samples in the second cohort contained detectable heme compared to 43% of samples in the first cohort (P < 0.05).
TABLE 1.
Comparison of OMT and saliva samples
| Cohort and sample | Vol (ml)a | Protein concn (mg/ml)a | Total IgG concn (μg/ml)a | Heme content (%)b |
|---|---|---|---|---|
| First | ||||
| OMT | 1.0 (0.5-1.5) | 3.44 (0.8-14.5) | 14.4 (1.4-63.4) | 1.2 (43) |
| Saliva | 2.5 (0.1-12.5) | 6.31 (0.9-40.0) | 1.1 (0-9.9) | 2.0 (61) |
| Second | ||||
| OMT | 1.2 (0.5-1.75) | 4.77 (2.0-13.0)c | 25.2 (1.0-262.3)c | 1.8 (72)c |
| Saliva | 3.2 (1.0-7.5) | 2.33 (0-8.6)c | 1.2 (0-3.2) | 2.8 (98)c |
Mean values (ranges) of sample characteristics for oral specimens are shown.
Mean Hemastix scores (0 to 4) are given, followed by the percentages of samples that were positive (score > 0).
Second cohort values (n = 50) that were statistically different from those for the first cohort (n = 100) (P < 0.05).
Ability of HPV capture ELISA to detect spiked HPV-16 antibodies in oral specimens.
In order to make certain that neither the OraSure transport medium nor saliva would inhibit the ability of the HPV capture ELISA to detect HPV antibodies, OraSure transport medium and saliva were spiked with serum known to be highly reactive for antibodies to HPV-16. A twofold dilution series of the serum in blocking buffer was tested alongside a dilution curve of the spiked oral fluids. Neither OraSure transport medium nor saliva inhibited the ability of the ELISA to detect HPV antibodies (data not shown).
HPV-specific IgG detection in an HIV-positive population.
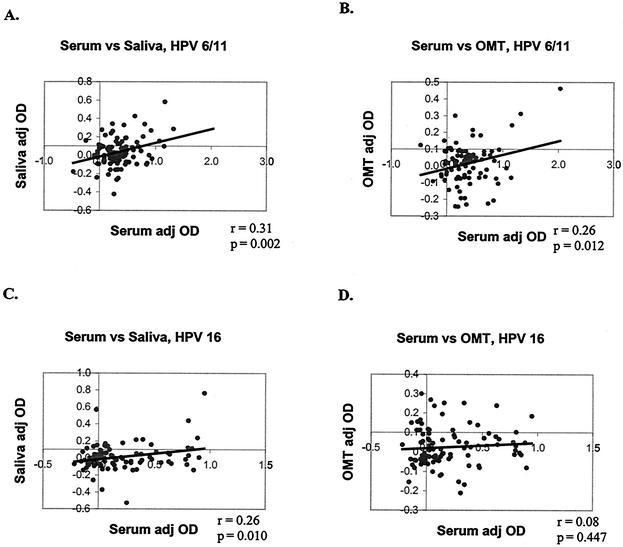
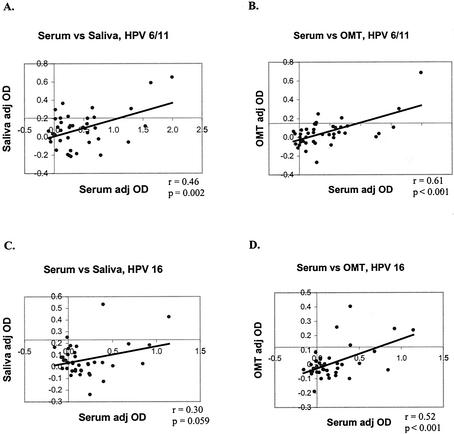
Serum, saliva, and OMT samples were tested for HPV-6/11- and HPV-16-specific IgG. Results for the two cohorts are presented separately. With the data set treated as a continuous variable, for the first cohort, the adj OD of saliva correlated with the adj OD of serum for HPV-6/11 (P = 0.002) and HPV-16 (P = 0.01) (Fig. 1A and B). The adj OD of OMT correlated with the adj OD of serum for HPV-6/11 (P = 0.01) but not for HPV-16 (P = 0.45) (Fig. 1C and D). For the second cohort, the adj OD of saliva correlated with the adj OD of serum for HPV-6/11 (P < 0.01) but not for HPV-16 (P = 0.06). The adj OD of OMT correlated with the adj OD of serum for HPV-6/11 (P < 0.001) and HPV-16 (P < 0.001) (Fig. 2).
FIG. 1.
Correlations between oral-specimen adj OD values and serum adj OD values for the first cohort. Adj OD values were treated as continuous variables, and paired samples were compared by using the Pearson correlation coefficient (r statistic). (A) HPV-6/11, saliva versus serum; (B) HPV-6/11, OMT versus serum; (C) HPV-16, saliva versus serum; (D) HPV-16, OMT versus serum.
FIG. 2.
Correlations between oral-specimen adj OD values and serum adj OD values for the second cohort. (A) HPV-6/11, saliva versus serum; (B) HPV-6/11, OMT versus serum; (C) HPV-16, saliva versus serum; (D) HPV-16, OMT versus serum.
Various methods for determining antibody reactivity cutpoints were examined in order to optimize data interpretation and analysis for oral specimens. By using a histogram technique, it was found that two distinct populations could not be readily distinguished (data not shown).
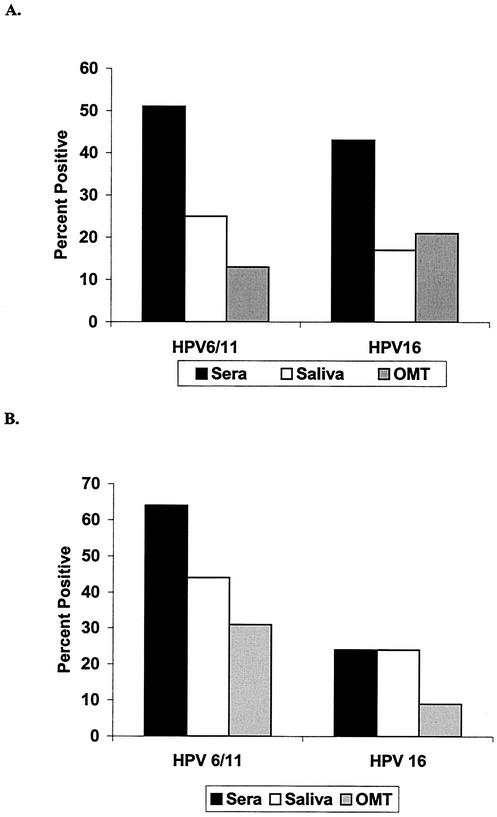
Seropositivity was then determined by using cutpoints derived from a negative control population, while oropositivity was determined by using the arbitrarily assigned cutpoint of 0.100. In the first cohort, 51% of the subjects were seropositive for HPV-6/11 and 43% were seropositive for HPV-16. When saliva samples were used, 25% of subjects were oropositive for HPV-6/11 and 17% were oropositive for HPV-16. When OMT samples were used, 13 and 21% were oropositive for HPV-6/11 and HPV-16, respectively (Fig. 3A). In the second cohort, 64% were seropositive for HPV-6/11 and 24% were seropositive for HPV-16. When saliva samples were used, 44% were oropositive for HPV-6/11 and 24% were oropositive for HPV-16. When OMT samples were used, 31 and 9% were oropositive for HPV-6/11 and HPV-16, respectively (Fig. 3B).
FIG. 3.
Prevalence of HPV-6/11- and HPV-16-specific IgG in serum and oral specimens. (A) Prevalence of HPV antibodies in samples from the first cohort (unstimulated OMT, n = 100); (B) prevalence of HPV antibodies in samples from the second cohort (stimulated OMT, n = 50). Seropositivity was defined by a negative control population. Oropositivity was defined by the arbitrarily selected cutpoint of 0.100.
With the above-determined cutpoints, κ was used to examine the associations between oropositivity and seropositivity. A κ value between 0 and 0.40 reflects poor or marginal agreement between the specimens, 0.40 ≤ κ ≤ 0.75 reflects good agreement, and a κ of >0.75 reflects excellent agreement (3). Oropositivity was not associated with seropositivity for either HPV-6/11 or HPV-16 in the specimens collected in the first cohort (all κ values were <0.10). For the second cohort, oropositivity based on saliva samples was not associated with seropositivity for either virus type; oropositivity based on OMT samples, however, more accurately reflected seropositivity for HPV-6/11 (κ = 0.35) and for HPV-16 (κ = 0.49). Due to the improved association between oropositivity and seropositivity in the second cohort, further cutpoint analysis was restricted to this cohort.
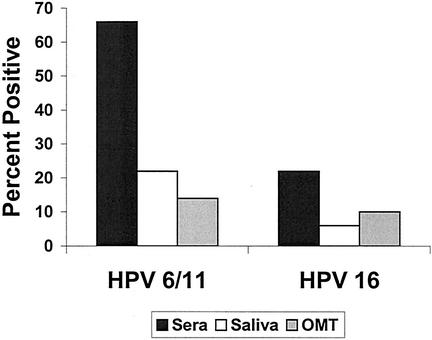
To determine a more accurate cutpoint for oral-fluid assays, saliva and OMT samples were collected from children under the age of 10 years to serve as negative controls. By using this method to analyze the samples from the second cohort, 66% were seropositive for HPV-6/11 and 22% were seropositive for HPV-16; 22 and 6% were oropositive for HPV-6/11 and HPV-16, respectively, based on saliva samples; and 14 and 10% were oropositive for HPV-6/11 and HPV-16, respectively, based on OMT samples (Fig. 4). With these cutpoints, oropositivity was not associated with seropositivity for HPV-6/11. For HPV-16, OMT sample positivity more accurately reflected seropositivity than did saliva sample positivity (κ values of 0.49 and 0.31, respectively).
FIG. 4.
Prevalence of HPV IgG in specimens when negative control cutpoints were used. Both seropositivity and oropositivity were determined by using cutpoints derived from samples collected from children under the age of 10 years. Only the samples in the second cohort (n = 50) are represented.
Finally, arbitrary serial cutpoints were selected for the oral-sample assays (modified receiver operator curves), and the associations between oropositivity (based on these cutpoints) and seropositivity (based on cutpoints derived from a negative control population) were examined by calculating the κ coefficient (Table 2). For salivary antibodies, no significant association was seen for HPV-6/11 when any assay cutpoint was used. A saliva assay cutpoint value of 0.300 yielded specificities of 100% (no seronegative subjects had falsely positive results for a saliva sample) and moderately reflected seropositivity for HPV-16 (κ = 0.31). Saliva sample positivity and seropositivity were in strongest agreement when a cutpoint value of 0.080 was used for the HPV-6/11 assay and when a cutpoint value of 0.300 was used for the HPV-16 assay.
TABLE 2.
Associations between oropositivity and seropositivity, evaluated by using serial cutpoint values, for the second cohort (n = 50)
| Virus and specimen type | Cutpoint value | κa | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|
| HPV-6/11 | ||||
| Saliva | 0.040 | 0.12 | 63 | 50 |
| 0.060 | 0.09 | 60 | 50 | |
| 0.080 | 0.21 | 60 | 64 | |
| 0.100 | 0.14 | 52 | 64 | |
| 0.205b | 0.09 | 26 | 86 | |
| 0.250 | 0.08 | 19 | 93 | |
| 0.300 | 0.13 | 19 | 100 | |
| OMT | 0.000 | 0.46 | 81 | 64 |
| 0.040 | 0.37 | 63 | 79 | |
| 0.060 | 0.23 | 48 | 79 | |
| 0.080 | 0.35 | 48 | 93 | |
| 0.100 | 0.35 | 44 | 100 | |
| 0.148b | 0.13 | 19 | 100 | |
| 0.200 | 0.11 | 15 | 100 | |
| HPV-16 | ||||
| Saliva | 0.040 | 0.03 | 44 | 59 |
| 0.060 | 0.09 | 44 | 69 | |
| 0.080 | 0.11 | 44 | 69 | |
| 0.100 | 0.21 | 44 | 78 | |
| 0.230b | 0.31 | 22 | 97 | |
| 0.250 | 0.25 | 22 | 97 | |
| 0.300 | 0.31 | 22 | 100 | |
| OMT | 0.000 | 0.31 | 67 | 72 |
| 0.040 | 0.43 | 56 | 88 | |
| 0.060 | 0.54 | 56 | 94 | |
| 0.080 | 0.54 | 56 | 94 | |
| 0.100 | 0.49 | 44 | 97 | |
| 0.121b | 0.49 | 44 | 97 | |
| 0.200 | 0.38 | 33 | 97 |
The kappa statistic (κ) was used to examine the associations between HPV oropositivity and seropositivity with different cutpoint values to define oropositivity in saliva and OMT in the second cohort.
Cutpoint values were derived from the mean plus 2 standard deviations of the adj OD values of negative controls (children's oral specimens).
When a cutpoint of 0.000 for HPV-6/11 antibodies in OMT was used, oropositivity reflected seropositivity (κ = 0.46) and yielded a sensitivity of 81% but a corresponding specificity of only 64%. With an assay cutpoint of 0.100, OMT sample positivity for HPV-6/11 moderately reflected seropositivity (κ = 0.35) and sensitivity dropped to 44% while specificity increased to 100%. For HPV-16 antibodies in OMT, oropositivity most strongly reflected seropositivity when an assay cutpoint between 0.060 and 0.080 was used; this cutpoint gave a sensitivity of 56% and a specificity of 94%. OMT sample positivity and seropositivity were in strongest agreement when a cutpoint of 0.000 was used for the HPV-6/11 assay and when a cutpoint of 0.060 was used for the HPV-16 assay.
Serum antibody titers versus oral-sample adj OD values.
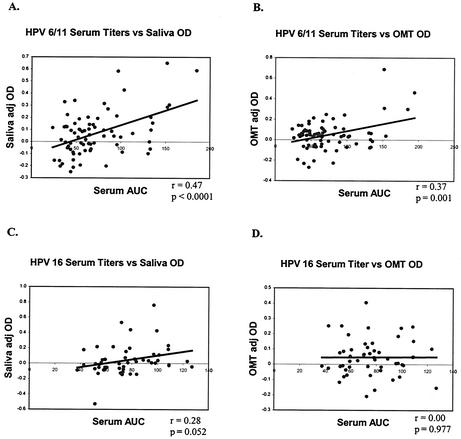
We believed that the apparently low sensitivity of the oral assays may have prevented the detection of HPV antibodies in subjects with low serum HPV antibody titers. Therefore, the relationship between serum antibody titer for all seropositive specimens (both cohorts) and the corresponding oral-specimen antibody reactivity was examined. For HPV-6/11, oral-sample adj OD values correlated with serum antibody titers (saliva, P < 0.0001; OMT, P = 0.001). This was not true, however, for HPV-16 antibodies (Fig. 5).
FIG. 5.
Correlations between serum HPV IgG titers and oral-specimen adj OD values. Seropositive samples from both cohorts were diluted twofold and tested for HPV antibody titers, and the Pearson r statistic was used to examine correlations between serum antibody titers and oral-specimen OD values. (A) HPV-6/11, saliva adj OD versus serum antibody titer, shown as the area under the curve (AUC); (B) HPV-6/11, OMT adj OD versus serum antibody titer; (C) HPV-16, saliva adj OD versus serum antibody titer; (D) HPV-16, OMT adj OD versus serum antibody titer.
DISCUSSION
Genital infection with the types of HPV that have a high risk for oncogenicity can lead to malignancy. Serological studies of HPV contribute greatly to the knowledge of prevalence and transmission of HPV as well as the risk factors for HPV-related disease. This information is valuable for guiding current efforts to develop HPV vaccines containing the most prevalent viral genotypes. Serological studies are limited by the requirement of venipuncture, which is unpleasant, invasive, and difficult to perform in some populations (e.g., the obese, the elderly, children, and intravenous drug abusers). A promising alternative to venipuncture is oral-specimen sampling.
Marais et al. (36) recently demonstrated the ability to detect HPV-specific antibodies in OMT. The data presented here are difficult to compare with those of Marais et al. due to differences in the study populations and study designs. The present study utilized a group of HIV-positive men and women from New Orleans with unknown genital HPV status; the study by Marais et al. examined South African women with cervical malignancies. This study does, however, corroborate the apparently low oral-fluid anti-HPV IgG titers reported by Marais et al.
This study is the first to thoroughly and systematically compare oral-sampling methods (saliva versus OMT) for HPV antibody detection to an established HPV serum antibody detection assay. Antibodies (IgG) to HPV-6/11 and HPV-16 could be detected in both saliva and OMT. A capture ELISA system was used to increase the probability that a detected antibody response was truly HPV specific and to increase sensitivity. By using oral specimens collected from children under the age of 10 years as negative controls, a cutpoint value could be determined. There were saliva and OMT specimens in the test population that displayed OD values above this cutpoint, indicating that these samples were most likely true positives. Although IgA antibodies are more prevalent in oral fluids than IgG, anti-HPV IgA was not examined in this study. This is because anti-HPV IgA serum responses are much more transient than anti-HPV IgG serum responses (5, 10), and so the “gold standard” serological test for HPV has been based on IgG response.
Because an OMT specimen contains systemically derived IgG, correlations between oral-fluid HPV antibody status and serum HPV antibody status were expected to be stronger with OMT samples than with saliva samples. This was true when OMT collection, processing, and testing protocols were optimal. We believe that the stimulation of the OMT was crucial to increasing the total antibodies in the specimens and that fresh processing helped greatly to retrieve the maximum specimen volume from the OraSure pad. Testing the samples in an undiluted state helped to increase the sensitivity of the assay.
HPV seropositivity rates were high in this HIV-positive population. This was expected due to the common risk factor of increased sexual partners for infection with both viruses. Oropositivity rates were generally lower than seropositivity rates regardless of the oral sample cutpoints used. This, coupled with the low sensitivity rates, indicates that the testing of oral fluids may regularly yield false negatives. Titers of antibodies in serum are generally low (assays are conducted using 1/100 dilutions of serum), and antibody titers in oral fluids appear to be even lower. A more sensitive assay, such as a chemiluminescence assay (18), might boost the detection rates of HPV antibodies in oral fluids. Alternatively, concentrating immunoglobulins in oral specimens could increase the detection of HPV-specific antibodies.
Individuals with lower serum antibody titers were expected to have correspondingly lower oral-specimen OD values, which may potentially explain the false-negative results found for some oral specimens. This proved to be true for HPV-6/11 but not for HPV-16 antibodies in both saliva and OMT. This could be due to differences in the mucosal immune responses to these divergent viral types.
Testing of oral specimens for HPV-specific antibodies is complicated by the fact that a negative control population is not yet well defined. While children serve as reasonable negative controls for HPV serological assays, strong oral antibody reactivity in children's saliva (adj OD values up to 1.00 in saliva and up to 0.300 in OMT) suggests that some children might have been exposed to HPV and thus are not appropriate negative controls for oral-specimen testing for HPV antibodies. An assessment of arbitrarily selected cutpoints for oral specimens showed that higher saliva cutpoints gave stronger associations with serum antibody status but at the expense of sensitivity of the assay. For OMT, lower cutpoints appeared to give the strongest association between oropositivity and seropositivity. Methods for determining appropriate cutpoints for oral specimens will need to be further assessed in order to improve the accuracy of the diagnosis.
Testing of saliva is further complicated by the recent findings of oral infections with HPV types generally thought to infect the genital tract (25-27, 30, 39, 42, 50), particularly in HIV-infected individuals (16, 31). Oral infection with HPV could invoke a local antibody response detectable in saliva. In this study, the oral-cavity HPV status of the patients was not tested and so the influence of oral HPV infections on the data presented here is not known. We do not believe that oral HPV infection played a significant role in the detection of antibodies in this study due to the low prevalence of oral antibodies and the relatively low titers of antibody observed in the oral specimens.
Oral-specimen HPV antibody testing is an attractive alternative to serum testing. Further analysis, however, is necessary to completely evaluate its utility as a research tool. In particular, a more thorough assessment of the usefulness of oral-specimen testing in women and the relationships between HPV oropositivity and HPV-related disease is imperative. Assay sensitivity must be improved, and cutpoint issues must be resolved. Alternatively, antibody detection in oral fluids may provide a useful tool for the study of the mucosal immune response to the virus. This study demonstrates that HPV-specific antibodies can be detected in oral specimens. These antibody responses, particularly in saliva, could be compared to antibody responses in the cervix in women in order to examine the possibility of a common mucosal immune response to HPV. Cytokine detection in oral specimens (1, 32) could be combined with oral HPV-specific antibody and DNA testing to more completely define the oral mucosal response to local HPV infection.
Acknowledgments
We thank Kristina Mire, Natalie George, and Crystal Darsum for their efforts in enrolling subjects in this study.
This work was supported by the National Institutes of Health (grant number 1R03CA81602-01) and the Doris Duke Research Foundation.
REFERENCES
- 1.Al Harthi, L., D. J. Wright, D. Anderson, M. Cohen, A. D. Matity, J. Cohn, S. Cu-Unvin, D. Burns, P. Reichelderfer, S. Lewis, S. Beckner, A. Kovacs, and A. Landay. 2000. The impact of the ovulatory cycle on cytokine production: evaluation of systemic, cervicovaginal, and salivary compartments. J. Interferon Cytokine Res. 20:719-724. [DOI] [PubMed] [Google Scholar]
- 2.Behets, F. M., B. Edidi, T. C. Quinn, L. Atikala, K. Bishagara, N. Nzila, M. Laga, P. Piot, R. W. Ryder, and C. C. Brown. 1991. Detection of salivary HIV-1-specific IgG antibodies in high-risk populations in Zaire. J. Acquir. Immune Defic. Syndr. 4:183-187. [PubMed] [Google Scholar]
- 3.Berry, C. C. 1992. The kappa statistic. JAMA 268:2513-2514. [DOI] [PubMed] [Google Scholar]
- 4.Bontkes, H. J., T. D. De Gruijl, J. Walboomers, J. Schiller, J. Dillner, T. J. Helmerhorst, R. H. Verheijen, R. J. Scheper, and C. J. L. M. Meijer. 1999. Immune responses against human papillomavirus (HPV) type 16 virus-like particles in a cohort study of women with cervical intraepithelial neoplasia. II. Systemic but not local IgA responses correlate with clearance of HPV-16. J. Gen. Virol. 80:409-417. [DOI] [PubMed] [Google Scholar]
- 5.Carter, J. J., and D. A. Galloway. 1997. Humoral immune response to human papillomavirus infection. Clin. Dermatol. 15:249-259. [DOI] [PubMed] [Google Scholar]
- 6.Carter, J. J., L. A. Koutsky, G. C. Wipf, N. D. Christensen, S.-K. Lee, J. Kuypers, N. B. Kiviat, and D. A. Galloway. 1996. The natural history of HPV-16 capsid antibodies among a cohort of university women. J. Infect. Dis. 174:927-936. [DOI] [PubMed] [Google Scholar]
- 7.Chua, K. L., F. Wiklund, P. Lenner, T. Angstrom, G. Hallmans, F. Bergman, M. Sapp, J. Schiller, G. Wadell, A. Hjerpe, and J. Dillner. 1996. A prospective study on the risk of cervical intra-epithelial neoplasia among healthy subjects with serum antibodies to HPV compared with HPV DNA in cervical smears. Int. J. Cancer 68:54-59. [DOI] [PubMed] [Google Scholar]
- 8.Crofts, N., S. Nicholson, P. Coghlan, and I. D. Gust. 1991. Testing of saliva for antibodies to HIV-1. AIDS 5:561-563. [PubMed] [Google Scholar]
- 9.Cubel, R. C. N., S. A. Oliveira, D. W. G. Brown, B. J. Cohen, and J. P. Nascimento. 1996. Diagnosis of parvovirus B19 infection by detection of specific immunoglobulin M antibody in saliva. J. Clin. Microbiol. 34:205-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dillner, J. 1999. The serological response to papillomaviruses. Semin. Cancer Biol. 9:423-430. [DOI] [PubMed] [Google Scholar]
- 11.Dillner, J., M. Lehtinen, T. Bjorge, T. Luostarinen, L. Youngman, E. Jellum, P. Koskela, R. E. Gislefoss, G. Hallmans, J. Paavonen, M. Sapp, J. T. Schiller, T. Hakulinen, S. Thoresen, and M. Hakama. 1997. Prospective seroepidemiologic study of human papillomavirus infection as a risk factor for invasive cervical cancer. J. Natl. Cancer Inst. 89:1293-1299. [DOI] [PubMed] [Google Scholar]
- 12.Frerichs, R. R., N. Silarug, N. Eskes, P. Pagcharoenpol, A. Rodklai, S. Thangsupachai, and C. Wongba. 1994. Saliva-based HIV-antibody testing in Thailand. AIDS 8:885-894. [DOI] [PubMed] [Google Scholar]
- 13.Gallo, D., R. George, J. Fitchen, A. Goldstein, and M. Hindahl. 1997. Evaluation of a system using oral mucosal transudate for HIV-1 antibody screening and confirmatory testing. JAMA 277:254-258. [PubMed] [Google Scholar]
- 14.Geijersstam, V., M. Kibur, Z. Wang, P. Koskela, E. Pukkala, J. Schiller, M. Lehtinen, and J. Dillner. 1999. Stability over time of serum antibody levels to human papillomavirus type 16. J. Infect. Dis. 177:1710-1714. [DOI] [PubMed] [Google Scholar]
- 15.George, J. R., and J. H. Fitchen. 1997. Future applications of oral fluid specimen technology. Am. J. Med. 102:21-25. [DOI] [PubMed] [Google Scholar]
- 16.Greenspan, D., A. J. Canchola, L. A. MacPhail, B. Cheikh, and J. S. Greenspan. 2001. Effect of highly active antiretroviral therapy on frequency of oral warts. Lancet 357:1411-1412. [DOI] [PubMed] [Google Scholar]
- 17.Hagensee, M. E., N. B. Kiviat, C. W. Critchlow, S. Hawes, J. Kuypers, S. Holte, and D. A. Galloway. 1997. Seroprevalence of HPV 6 and 16 capsid antibodies in homosexual men. J. Infect. Dis. 176:625-631. [DOI] [PubMed] [Google Scholar]
- 18.Hagensee, M. E., L. A. Koutsky, S. K. Lee, T. Grubert, J. Kuypers, N. B. Kiviat, and D. A. Galloway. 2000. Detection of cervical antibodies to human papillomavirus type 16 (HPV-16) capsid antigens in relation to detection of HPV-16 DNA and cervical lesions. J. Infect. Dis. 181:1234-1239. [DOI] [PubMed] [Google Scholar]
- 19.Hagensee, M. E., N. H. Olson, T. S. Baker, and D. A. Galloway. 1994. Three-dimensional structure of vaccinia virus-produced human papillomavirus type 1 capsids. J. Virol. 68:4503-4505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hagensee, M. E., J. Slavinsky, III, C. Gaffga, J. Suros, P. Kissinger, and D. H. Martin. 1999. Seroprevalence of human papillomavirus type 16 in pregnant women. Obstet. Gynecol. 94:653-658. [DOI] [PubMed] [Google Scholar]
- 21.Hagensee, M. E., N. Yaegashi, and D. A. Galloway. 1993. Self-assembly of human papillomavirus type 1 capsids by expression of the L1 protein alone or by coexpression of the L1 and L2 capsid proteins. J. Virol. 67:315-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heim, K., N. D. Christensen, R. Hoepfl, B. Wartusch, G. Pinzger, A. Zeimet, P. Baumgartner, J. W. Kreider, and O. Dapunt. 1995. Serum IgG, IgM, and IgA reactivity to human papillomavirus types 11 and 6 virus-like particles in different gynecologic patient groups. J. Infect. Dis. 172:395-402. [DOI] [PubMed] [Google Scholar]
- 23.Heino, P., C. Eklund, V. Fredriksson-Shanazarian, S. Goldman, J. T. Schiller, and J. Dillner. 1995. Association of serum immunoglobulin G antibodies against human papillomavirus type 16 capsids with anal epidermoid carcinoma. J. Natl. Cancer Inst. 87:437-440. [DOI] [PubMed] [Google Scholar]
- 24.Hofmann, K. J., J. C. Cook, J. G. Joyce, D. R. Brown, L. D. Schultz, H. A. George, M. Roslowsky, K. H. Fife, and K. U. Jansen. 1995. Sequence determination of human papillomavirus type 6a and assembly of virus-like particles in Saccharomyces cerevisiae. Virology 209:506-518. [DOI] [PubMed] [Google Scholar]
- 25.Kellokoski, J. K., S. Syrjanen, F. Chang, M. Yliskoski, and K. Syrjanen. 1992. Southern blot hybridization and PCR in detection of oral human papillomavirus (HPV) infections in women with genital HPV infections. J. Oral Pathol. Med. 21:459-464. [DOI] [PubMed] [Google Scholar]
- 26.Kellokoski, J. K., S. Syrjanen, K. Syrjanen, and M. Yliskoski. 1990. Oral mucosa changes in women with genital HPV infection. J. Oral Pathol. Med. 19:142-148. [DOI] [PubMed] [Google Scholar]
- 27.Kellokoski, J. K., S. Syrjanen, M. Yliskoski, and K. Syrjanen. 1992. Dot blot hybridization in detection of human papillomavirus (HPV) infection in the oral cavity of women with genital HPV infections. Oral Microbiol. Immunol. 7:19-23. [DOI] [PubMed] [Google Scholar]
- 28.Kirnbauer, R., N. L. Hubbert, C. M. Wheeler, T. M. Becker, D. R. Lowy, and J. T. Schiller. 1994. A virus-like particle enzyme-linked immunosorbent assay detects serum antibodies in a majority of women infected with human papillomavirus type 16. J. Natl. Cancer Inst. 86:494-499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kirnbauer, R., J. Taub, H. Greenstone, R. Roden, M. Durst, L. Gissmann, D. R. Lowy, and J. T. Schiller. 1993. Efficient self-assembly of human papillomavirus type 16 L1 and L1-L2 into virus-like particles. J. Virol. 67:6929-6936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lambropoulos, A. F., J. Dimitrakopoulos, E. Frangoulides, R. Katopodi, A. Kotsis, and D. Karakasis. 1997. Incidence of human papillomavirus 6, 11, 16, 18 and 33 in normal oral mucosa of a Greek population. Eur. J. Oral Sci. 105:294-297. [DOI] [PubMed] [Google Scholar]
- 31.Laskaris, G., M. Hadjivassiliou, and J. Stratgos. 1992. Oral signs and symptoms in 160 HIV-infected patients. J. Oral Pathol. Med. 21:120-123. [DOI] [PubMed] [Google Scholar]
- 32.Leigh, J., C. Steele, F. L. Wormley, Jr., W. Luo, R. A. Clark, W. Gallaher, and P. L. Fidel, Jr. 1998. Th1/Th2 cytokine expression in saliva of HIV-positive and HIV-negative individuals: a pilot study in HIV-positive individuals with oropharyngeal candidiasis. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 19:373-379. [DOI] [PubMed] [Google Scholar]
- 33.Luo, N., F. Kasolo, B. K. Ngwenya, H. L. du Pont, and A. Zumla. 1995. Use of saliva as an alternative to serum for HIV screening in Africa. S. Afr. Med. J. 85:156-157. [PubMed] [Google Scholar]
- 34.Marais, D., R. C. Rose, C. Lane, S. Aspinall, P. Bos, and A. L. Williamson. 2000. Seroresponses to virus-like particles of human papillomavirus types 16, 18, 31, 33, and 45 in San people of Southern Africa. J. Med. Virol. 60:331-336. [DOI] [PubMed] [Google Scholar]
- 35.Marais, D., R. C. Rose, and A. L. Williamson. 1997. Age distribution of antibodies to human papillomavirus in children, women with cervical intraepithelial neoplasia and blood donors from South Africa. J. Med. Virol. 51:126-131. [DOI] [PubMed] [Google Scholar]
- 36.Marais, D. J., J. M. Best, R. C. Rose, P. Keating, R. Soeters, L. Denny, C. M. Dehaeck, J. Nevin, P. Kay, J. A. Passmore, and A. L. Williamson. 2001. Oral antibodies to human papillomavirus type 16 in women with cervical neoplasia. J. Med. Virol. 65:149-154. [PubMed] [Google Scholar]
- 37.Marais, D. J., R. C. Rose, C. Lane, P. Kay, J. Nevin, L. Denny, R. Soeters, C. M. Dehaeck, and A. L. Williamson. 2000. Seroresponses to human papillomavirus types 16, 18, 31, 33, and 45 virus-like particles in South African women with cervical cancer and cervical intraepithelial neoplasia. J. Med. Virol. 60:403-410. [DOI] [PubMed] [Google Scholar]
- 38.Marshall, B., A. J. Howat, and P. A. Wright. 1999. Oral fluid antibody detection in the diagnosis of Helicobacter pylori infection. J. Med. Microbiol. 48:1043-1046. [DOI] [PubMed] [Google Scholar]
- 39.Mineta, H., T. Ogino, H. Amano, Y. Ohkawa, K. Araki, S. Takebayashi, and K. Miura. 1998. Human papilloma virus (HPV) type 16 and 18 detected in head and neck squamous cell carcinoma. Anticancer Res. 18:4765-4768. [PubMed] [Google Scholar]
- 40.Nonnenmacher, B., N. L. Hubbert, R. Kirnbauer, K. V. Shah, N. Munoz, F. X. Bosch, S. de Sanjose, R. Viscidi, D. R. Lowy, and J. T. Schiller. 1995. Serologic response to human papillomavirus type 16 (HPV-16) virus-like particles in HPV-16 DNA-positive invasive cervical cancer and cervical intraepithelial neoplasia grade III patients and controls from Colombia and Spain. J. Infect. Dis. 172:19-24. [DOI] [PubMed] [Google Scholar]
- 41.Nonnenmacher, B., S. Kjaer, E. Svare, J. Scott, N. Hubbert, A. van den Brule, R. Kirnbauer, J. M. M. Walboomers, D. Lowy, and J. Schiller. 1996. Seroreactivity to HPV16 virus-like particles as a marker for cervical cancer risk in high-risk populations. Int. J. Cancer 68:704-709. [DOI] [PubMed] [Google Scholar]
- 42.Palefsky, J., S. Silverman, Jr., M. Abdel-Salaam, T. E. Daniels, and J. S. Greenspan. 1995. Association between proliferative verrucous leukoplakia and infection with human papillomavirus type 16. J. Oral Pathol. Med. 24:193-197. [DOI] [PubMed] [Google Scholar]
- 43.Robinson, W., and C. Morris. 1996. Cervical neoplasia. Pathogenesis, diagnosis and management. Hematol. Oncol. Clin. N. Am. 10:1163-1176. [DOI] [PubMed] [Google Scholar]
- 44.Rose, R. C., W. Bonnez, R. C. Reichman, and R. L. Garcea. 1993. Expression of human papillomavirus type 11 L1 protein in insect cells: in vivo and in vitro assembly of viruslike particles. J. Virol. 67:1936-1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Santos, M. M., T. C. Garcia, M. Orsini, J. Disch, N. Katz, and A. Rabello. 2000. Oral fluids for the immunodiagnosis of Schistosoma mansoni infection. Trans. R. Soc. Trop. Med. Hyg. 94:289-292. [DOI] [PubMed] [Google Scholar]
- 46.Slavinsky, J., III, P. Kissinger, L. Burger, A. Boley, R. P. DiCarlo, and M. E. Hagensee. 2001. Seroepidemiology of low and high oncogenic risk types of human papillomavirus in a predominantly male cohort of STD clinic patients. Int. J. STD AIDS 12:516-523. [DOI] [PubMed] [Google Scholar]
- 47.Strickler, H. D., A. Hildesheim, R. P. Viscidi, K. V. Shah, B. Goebel, J. Drummond, D. Waters, Y. Sun, N. L. Hubbert, S. Wacholder, L. A. Brinton, C. L. Han, P. C. Nasca, R. McClimens, K. Turk, V. Devairakkam, S. Leitman, C. Martin, and J. T. Schiller. 1997. Interlaboratory agreement among results of human papillomavirus type 16 enzyme-linked immunosorbent assays. J. Clin. Microbiol. 35:1751-1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sun, X.-W., L. Kuhn, T. Ellerbrock, M. Chaisson, T. Bush, and T. Wright, Jr. 1997. Human papillomavirus infection in women infected with the human immunodeficiency virus. N. Engl. J. Med. 337:1343-1349. [DOI] [PubMed] [Google Scholar]
- 49.Svare, E., S. Kjaer, B. Nonnenmacher, A. Worm, H. Moi, R. Christensen, A. van den Brule, J. M. M. Walboomers, C. J. L. M. Meijer, N. L. Hubbert, D. R. Lowy, and J. T. Schiller. 1997. Seroreactivity to human papillomavirus type 16 virus-like particles is lower in high-risk men than in high-risk women. J. Infect. Dis. 176:876-883. [DOI] [PubMed] [Google Scholar]
- 50.Terai, M., K. Hashimoto, K. Yoda, and T. Sata. 1999. High prevalence of human papillomavirus in the normal oral cavity of adults. Oral Microbiol. Immunol. 14:201-205. [DOI] [PubMed] [Google Scholar]
- 51.Thieme, T., P. Yoshihara, S. Piacentini, and M. Beller. 1992. Clinical evaluation of oral fluid samples for diagnosis of viral hepatitis. J. Clin. Microbiol. 30:1076-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van den Akker, R., J. A. van den Hoek, W. M. van den Akker, H. Kooy, E. Vijge, G. Roosendaal, R. A. Coutinho, and A. M. van Loon. 1992. Detection of HIV antibodies in saliva as a tool for epidemiological studies. AIDS 6:953-957. [DOI] [PubMed] [Google Scholar]
- 53.van Doornum, G. J., M. Prins, A. Andersson-Ellstrom, and J. Dillner. 1998. Immunoglobulin A, G, and M responses to L1 and L2 capsids of human papillomavirus types 6, 11, 16, 18, and 33 L1 after newly acquired infection. Sex. Transm. Inf. 74:354-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Viscidi, R. P., K. L. Kotloff, B. Clayman, K. Russ, S. Shapiro, and K. V. Shah. 1997. Prevalence of antibodies to human papillomavirus (HPV) type 16 virus-like particles in relation to cervical HPV infection among college women. Clin. Diagn. Lab. Immunol. 4:122-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vyse, A. J., W. A. Knowles, B. J. Cohen, and D. W. Brown. 1997. Detection of IgG antibody to Epstein-Barr virus viral capsid antigen in saliva by antibody capture radioimmunoassay. J. Virol. Methods 63:93-101. [DOI] [PubMed] [Google Scholar]