Abstract
The activation of coagulation has been shown to contribute to proinflammatory responses in animal and in vitro experiments. Here we report that the activation of coagulation in healthy human subjects by the administration of recombinant factor VIIa also elicits a small but significant increase in the concentrations of interleukin 6 (IL-6) and IL-8 in plasma. This increase was absent when the subjects were pretreated with recombinant nematode anticoagulant protein c2, the inhibitor of tissue factor-factor VIIa.
The activations of coagulation and inflammation during sepsis appear to be linked in a bimodal way. While cytokines are involved in the procoagulant state that follows endotoxemia or severe infection, recent studies have shown that activated coagulation factors in turn are capable of eliciting a proinflammatory response (9). Ex vivo clotting of human blood stimulates interleukin 8 (IL-8) and IL-6 production by monocytes and endothelial cells (13, 14). Recent in vitro studies have shown that several coagulation factors, such as factor VIIa, factor Xa, and thrombin, can activate cells directly and provoke a variety of proinflammatory responses (9). In this study we sought to determine whether activation of coagulation in vivo might also elicit a proinflammatory response. We therefore measured the cytokine response following the generation of thrombin by the intravenous administration of factor VIIa to healthy human subjects with and without pretreatment with recombinant nematode anticoagulant protein c2 (rNAPc2) (Corvas Inc., San Diego, Calif.), a potent inhibitor of the factor VIIa-tissue factor complex (2). Previous studies have shown that the infusion of recombinant factor VIIa (rVIIa) leads to factor Xa-dependent thrombin formation in chimpanzees (21) as well as in humans (10). The study was performed as a double-blind, randomized, placebo-controlled crossover study of six healthy male volunteers (21 to 26 years of age). Treatment consisted of an intravenous bolus injection of 90 μg of rVIIa (NovoSeven; NovoNordisk, Copenhagen, Denmark) per kg of body weight 4 h after the subjects received either rNAPc2 at a dose of 3.5 μg/kg as a single subcutaneous injection or a placebo. Assays for prothrombin activation fragment F1+2, factor X activation peptide, and the levels of factor VIIa, tumor necrosis factor (TNF), IL-6, IL-8, soluble TNF receptor type 1 (sTNF-R1), IL-1 receptor antagonist (IL-1ra) and sE-selectin in plasma were performed according to the instructions of the manufacturer and were described previously (8, 10, 18). Detection limits were 0.4 pmol/ml, 8.2 pg/ml, 3.2 ng/ml, 82 pg/ml, and 140 pg/ml for factor VIIa, TNF, sE-selectin, IL-1ra, and sTNF-R1, respectively.
Values are given as means ± standard errors of the means. Differences in the results between the two treatment groups were tested by repeated-measurement analysis of variance.
The coagulant response to rVIIa and the anticoagulant effect of the factor VIIa-tissue factor inhibitor rNAPc2 have been described in detail in another report (10). We found that the levels of factor VIIa in plasma were below the limit of detection prior to the administration of rVIIa. Peak levels of 23.7 ± 2.4 nmol per liter of plasma were reached at 30 min after its administration. Pretreatment with rNAPc2 had no effect on the levels of factor VIIa in plasma (data not shown). As previously described in detail, the administration of rVIIa resulted in the substantial activation of thrombin generation, as reflected by a 3.6-fold increase in F1+2 levels to 4.0 ± 0.3 nmol/liter. Pretreatment with rNAPc2 attenuated thrombin generation (peak levels, 3.2 ± 1.5 nmol/liter; P < 0.05) (10). Maximal levels of factor X activation peptide in plasma were reached at 60 min following the administration of rVIIa. Pretreatment with rNAPc2 resulted in lower rVIIa-induced peak levels of factor X activation peptide than those resulting from the injection of rVIIa alone (from 116 ± 6 to 387 ± 6 pmol/liter after the administration of rVIIa-placebo and from 87 ± 9 pmol/liter to 302 ± 25 pmol/liter after the administration of rVIIa preceded by that of rNAPc2;, P < 0.05 for the difference in the results between the treatment groups).
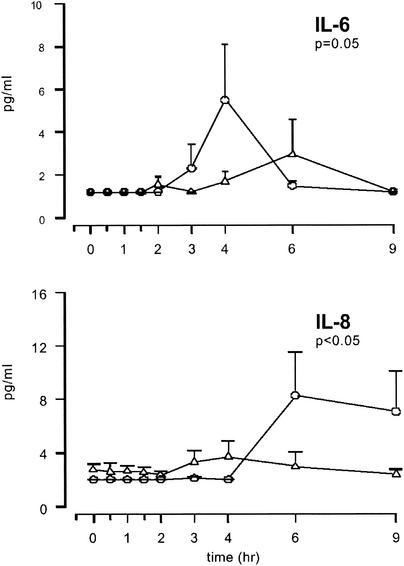
Plasma IL-6 levels increased from below the limit of detection (1.2 pg/ml) at the baseline to 5.5 ± 2.5 pg/ml at 4 h after the administration of rVIIa and to 2.9 ± 1.6 pg/ml at 6 h after the administration of rVIIa preceded by that of rNAPc2 (P = 0.05 for the difference in the results between the treatment groups) (Fig. 1). The levels of IL-8 increased from <2 pg/ml to 8.3 ± 3.3 pg/ml at 6 h after the administration of rVIIa and to 3.7 ± 1.1 pg/ml at 4 h after the administration of rVIIa-rNAPc2 (P < 0.05 for the difference in the results between the treatment groups). In contrast, the levels in plasma of TNF, the cytokine inhibitors sTNF-R1 and IL-1ra, and sE-selectin (a marker for endothelial-cell activation) were not influenced by the administration of rVIIa (data not shown), but the scope of this study is clearly insufficient to exclude such an effect.
FIG. 1.
Mean concentrations of IL-6 and IL-8 in plasma after the administration of a placebo and rVIIa (circles) or rNAPc2 and rVIIa (triangles). Error bars indicate the standard errors of the means. P values indicate the differences in results of the two experiments as determined by repeated-measurement analysis of variance.
This is the first study to show that the activation of coagulation can induce the release of cytokines in humans. Pretreatment with the factor VIIa-tissue factor inhibitor rNAPc2 decreased rVIIa-induced thrombin generation and prevented an increase in circulating IL-6 and IL-8. Consistent with our observation that release of IL-6 and IL-8 is stimulated by the rVIIa-induced activation of coagulation, in vitro studies with human whole blood revealed that activation of coagulation stimulates the production of IL-8 and, to a lesser extent, IL-6, but not TNF (13). Furthermore, in primate models of sepsis, the levels of IL-6 and IL-8, but not TNF, were significantly attenuated by anticoagulant interventions with tissue factor pathway inhibitor (6) or antithrombin (19). Several coagulation factors could potentially contribute to the release of IL-6 and IL-8. The administration of rVIIa resulted in thrombin generation, as reflected by increased levels of F1+2. Thrombin has been shown to stimulate the release of IL-1, IL-6, IL-8, TNF, and monocyte chemotactic protein 1by monocytes and endothelial cells (5, 11, 12, 14, 15, 17, 22), probably because of its catalytic activity (1, 14) and because of the cleavage of cell surface protease-activated receptors (16). Another candidate for eliciting an inflammatory response is factor Xa. Factor Xa, by binding to effector cell protease receptor 1 and independently of thrombin, has been found to trigger acute inflammatory responses in animal experiments (4). Factor Xa was shown to stimulate cultured human endothelial cells to produce IL-6, IL-8, monocyte chemotactic protein 1, and the adhesion molecules sE-selectin, intercellular adhesion molecule 1, and vascular cell adhesion molecule 1 by a mechanism independent of thrombin and effector cell protease receptor 1 (20). Indeed, a prolonged increase of factor X activation peptide was found in our study following the administration of rVIIa. Finally, factor VIIa itself could contribute to the observed increase in cytokine levels. It has been shown that factor VIIa, independently of other coagulation proteins, can induce proinflammatory changes in mononuclear cells (7), possibly by the activation of protease-activated receptor 2 (PAR2) and to a lesser degree PAR1 (3). In the presence of factor Xa, low picomolar concentrations of factor VIIa caused robust signaling in cells expressing tissue factor and PAR2 (3). It is worth noting that these concentrations are much lower than the nanomolar concentrations of factor VIIa that were measured in our experiments after the administration of rVIIa. Our observations indicate that activation of the coagulation response in humans can elicit cytokine release. Clearly, this inflammatory response is small, with IL-6 and IL-8 levels below 10 pg/ml. This limited response could be caused by the short-term and relatively modest effect of the intravenous bolus injection of rVIIa on thrombin generation. A more prolonged activation of coagulation could potentially have more influence on inflammatory responses. Furthermore, coagulation could have a more profound effect on other inflammatory cascades when these are already activated to some extent by a common stimulus, such as bacterial infection or endotoxin. Alternatively, the limited effect of rVIIa on the cytokine response could be explained by the absence of the expression of its cofactor, tissue factor, on the circulating cells of healthy subjects. In situations with increased tissue factor expression on circulating blood cells, as during severe sepsis, the activation of coagulation could potentially induce a larger proinflammatory response. However, in the mild inflammation model of endotoxemia in healthy humans, in which the endotoxin-induced activation of coagulation was prevented by the administration of tissue factor pathway inhibitor, this inhibition of thrombin generation did not affect the cytokine response (8). Thus, it can be argued that the magnitude of the inflammatory response following the activation of coagulation could be higher in different situations. On the other hand, the possibility cannot be excluded that the inflammatory response is exclusively the result of very high, supraphysiological levels of factor VIIa and thus has limited physiological relevance. Further studies are planned to determine whether the coagulation-initiated inflammatory response may be quantitatively relevant in clinical situations, like sepsis, with disseminated intravascular coagulation.
In conclusion, we demonstrated that the activation of coagulation by the administration of recombinant factor VIIa can elicit both IL-6 and IL-8 responses in healthy human subjects. This cytokine response can be prevented by attenuating the activation of coagulation with the inhibitor of factor VIIa-tissue factor, rNAPc2.
REFERENCES
- 1.Anrather, D., M. T. Millan, A. Palmetshofer, S. C. Robson, C. Geczy, A. J. Ritchie, F. H. Bach, and B. M. Ewenstein. 1997. Thrombin activates nuclear factor-kappaB and potentiates endothelial cell activation by TNF. J. Immunol. 159:5620-5628. [PubMed] [Google Scholar]
- 2.Bergum, P. W., A. Cruikshank, S. L. Maki, C. R. Kelly, W. Ruf, and G. P. Vlasuk. 2001. Role of zymogen and activated factor X as scaffolds for the inhibition of the blood coagulation factor VIIa-tissue factor complex by recombinant nematode anticoagulant protein c2. J. Biol. Chem. 276:10063-10071. [DOI] [PubMed] [Google Scholar]
- 3.Camerer, E., W. Huang, and S. R. Coughlin. 2000. Tissue factor- and factor X-dependent activation of protease-activated receptor 2 by factor VIIa. Proc. Natl. Acad. Sci. USA 97:5255-5260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cirino, G., C. Cicala, M. Bucci, L. Sorrentino, G. Ambrosini, G. DeDominicis, and D. C. Altieri. 1997. Factor Xa as an interface between coagulation and inflammation. Molecular mimicry of factor Xa association with effector cell protease receptor-1 induces acute inflammation in vivo. J. Clin. Investig. 99:2446-2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colotta, F., F. L. Sciacca, M. Sironi, W. Luini, M. J. Rabiet, and A. Mantovani. 1994. Expression of monocyte chemotactic protein-1 by monocytes and endothelial cells exposed to thrombin. Am. J. Pathol. 144:975-985. [PMC free article] [PubMed] [Google Scholar]
- 6.Creasey, A. A., A. C. Chang, L. Feigen, T. C. Wun, F. B. J. Taylor, and L. B. Hinshaw. 1993. Tissue factor pathway inhibitor reduces mortality from Escherichia coli septic shock. J. Clin. Investig. 91:2850-2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cunningham, M. A., P. Romas, P. Hutchinson, S. R. Holdsworth, and P. G. Tipping. 1999. Tissue factor and factor VIIa receptor/ligand interactions induce proinflammatory effects in macrophages. Blood 94:3413-3420. [PubMed] [Google Scholar]
- 8.de Jonge, E., P. E. P. Dekkers, A. A. Creasey, C. E. Hack, S. K. Paulson, A. Karim, J. Kesecioglu, M. Levi, S. J. van Deventer, and T. van der Poll. 2000. Tissue factor pathway inhibitor (TFPI) dose-dependently inhibits coagulation activation without influencing the fibrinolytic and cytokine response during human endotoxemia. Blood 95:1124-1129. [PubMed] [Google Scholar]
- 9.Esmon, C. T. 2000. Introduction: are natural anticoagulants candidates for modulating the inflammatory response to endotoxin? Blood 95:1113-1116. [PubMed] [Google Scholar]
- 10.Friederich, P. W., M. Levi, K. A. Bauer, G. P. Vlasuk, W. E. Rote, D. Breederveld, T. Keller, M. Spataro, S. Barzegar, and H. R. Buller. 2001. The ability of recombinant factor VIIa to generate thrombin during inhibition of tissue factor in human subjects. Circulation 103:2555-2559. [DOI] [PubMed] [Google Scholar]
- 11.Grandaliano, G., A. J. Valente, and H. E. Abboud. 1994. A novel biologic activity of thrombin: stimulation of monocyte chemotactic protein production. J. Exp. Med. 179:1737-1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoffman, M., and S. T. Cooper. 1995. Thrombin enhances monocyte secretion of tumor necrosis factor and interleukin-1 beta by two distinct mechanisms. Blood Cells Mol. Dis. 21:156-167. [DOI] [PubMed] [Google Scholar]
- 13.Johnson, K., L. Aarden, Y. Choi, E. De Groot, and A. Creasey. 1996. The proinflammatory cytokine response to coagulation and endotoxin in whole blood. Blood 87:5051-5060. [PubMed] [Google Scholar]
- 14.Johnson, K., Y. Choi, E. DeGroot, I. Samuels, A. Creasey, and L. Aarden. 1998. Potential mechanisms for a proinflammatory vascular cytokine response to coagulation activation. J. Immunol. 160:5130-5135. [PubMed] [Google Scholar]
- 15.Jones, A., and C. L. Geczy. 1990. Thrombin and factor Xa enhance the production of interleukin-1. Immunology 71:236-241. [PMC free article] [PubMed] [Google Scholar]
- 16.Kahn, M. L., M. Nakanishi-Matsui, M. J. Shapiro, H. Ishihara, and S. R. Coughlin. 1999. Protease-activated receptors 1 and 4 mediate activation of human platelets by thrombin. J. Clin. Investig. 103:879-887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kranzhofer, R., S. K. Clinton, K. Ishii, S. R. Coughlin, J. W. Fenton II, and P. Libby. 1996. Thrombin potently stimulates cytokine production in human vascular smooth muscle cells but not in mononuclear phagocytes. Circ. Res. 79:286-294. [DOI] [PubMed] [Google Scholar]
- 18.Leeuwenberg, J. F., E. F. Smeets, J. J. Neefjes, M. A. Shaffer, T. Cinek, T. M. Jeunhomme, T. J. Ahern, and W. A. Buurman. 1992. E-selectin and intercellular adhesion molecule-1 are released by activated human endothelial cells in vitro. Immunology 77:543-549. [PMC free article] [PubMed] [Google Scholar]
- 19.Minnema, M. C., A. C. Chang, P. M. Jansen, Y. T. Lubbers, B. M. Pratt, B. G. Whittaker, F. B. Taylor, C. E. Hack, and B. Friedman. 2000. Recombinant human antithrombin III improves survival and attenuates inflammatory responses in baboons lethally challenged with Escherichia coli. Blood 95:1117-1123. [PubMed] [Google Scholar]
- 20.Senden, N. H. M., T. M. A. A. Jeunhomme, J. W. M. Heemskerk, R. Wagenvoord, C. van 't Veer, H. Coenraad Hemker, and W. A. Buurman. 1998. Factor Xa induces cytokine production and expression of adhesion molecules by human umbilical vein endothelial cells. J. Immunol. 161:4318-4324. [PubMed] [Google Scholar]
- 21.ten Cate, H., K. A. Bauer, M. Levi, T. S. Edgington, R. D. Sublett, S. Barzegar, B. L. Kass, and R. D. Rosenberg. 1993. The activation of factor X and prothrombin by recombinant factor VIIa in vivo is mediated by tissue factor. J. Clin. Investig. 92:1207-1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ueno, A., K. Murakami, K. Yamanouchi, M. Watanabe, and T. Kondo. 1996. Thrombin stimulates production of interleukin-8 in human umbilical vein endothelial cells. Immunology 88:76-81. [DOI] [PMC free article] [PubMed] [Google Scholar]