Abstract
Peptidoglycan from Staphylococcus aureus mobilized CD66b in human neutrophils but did not upregulate surface activation markers in eosinophils. In addition, Toll-like receptor 2, implicated in the recognition of peptidoglycan, was detected on the surface of resting neutrophils but not on eosinophils. These findings suggest roles for neutrophils but not eosinophils in innate recognition of peptidoglycan.
Staphylococcus aureus is a major pathogen in endocarditis, septicemia, infectious arthritis, and nosocomial infections (9). On the other hand, S. aureus often colonizes skin and mucosal surfaces without causing disease in asymptomatic carriers (9). Therefore, it is important for the host defense to have the ability to adapt its immune response, with an increased immunosurveillance and propensity to react during states of colonization and a rapid recognition and destruction of the bacteria during invasion of tissues. The cell wall of S. aureus is composed mainly of peptidoglycan (PG) (50 to 60% by weight) (8). PG has several biological effects, such as induction of cytokines and tissue factor expression in human monocytes (12, 13). Other products derived from S. aureus, some of them acting as superantigens, have been suggested to play pathophysiologic roles in allergic inflammation (1).
Recent years of research have revealed several receptors for the innate recognition of bacterial cell wall components by the immune system. Toll-like receptor 2 (TLR-2) can recognize PG, while TLR-4 together with a combination of other molecules, among them CD14, are held responsible for the recognition of lipopolysaccharides of gram-negative bacteria (3, 16).
Neutrophils are known to play important roles in the host defense against bacterial invasion. Eosinophils, on the other hand, may be important in the defense against parasitic infestation, but this issue is yet to be settled (2, 4, 19). In addition, eosinophils are involved in diseases with allergic inflammation such as asthma (19). At present, there are conflicting data concerning the presence of TLR-2 and -4 on both neutrophils and eosinophils (14, 15).
The aim of this study was to investigate whether PG can activate human neutrophils and eosinophils. The method used has been described previously and reflects different grades of cellular activation in neutrophils and eosinophils by the detection of increased expression of surface antigens (7, 11). In addition, the surface expression of TLR-2 and -4 on neutrophils and eosinophils was investigated.
To purify cells, blood was drawn from healthy volunteers after informed consent was obtained. Neutrophils were obtained after removal of mononuclear cells by centrifugation over Ficoll-Paque (Pharmacia Biotech, Uppsala, Sweden). After lysis of the erythrocytes, the granulocytes were washed in Hanks' balanced salt solution without calcium and magnesium. The purity of neutrophils was >94%, and the viability of the cells was >99% as judged by May-Grünwald-Giemsa staining and trypan blue exclusion, respectively. Eosinophils were isolated essentially as described previously (6). Immunomagnetic beads coated with antibodies to CD16 (Miltenyi, Gladbach, Germany) were used to retrieve the neutrophils in a magnetic column, allowing the isolation of eosinophils (viability was >99% and purity was >98%; contaminating cells were lymphocytes and neutrophils).
PG was prepared from S. aureus WOOD and chemically characterized as described previously (13). Endotoxin was not detected in the PG preparation (at 100 μg/ml) as determined by the Limulus amebocyte lysate assay (detection limit, 2 pg/ml; Chromogenix, Mölndal, Sweden).
During the experiments, neutrophils or eosinophils (106/ml) were incubated in Hanks' balanced salt solution supplemented with calcium and magnesium, in the absence or presence of cytochalasin B (5 μg/ml; Sigma, St Louis, Mo.) and PG. As a positive control for the mobilization of surface markers, neutrophils were incubated with N-formyl-Met-Leu-Phe (fMLP) (1 μM; Sigma) and eosinophils were incubated with the calcium ionophore A23187 (1 μM; Sigma). After incubation, the cells were put on ice and fixed with paraformaldehyde at a final concentration of 1% (wt/vol). Thereafter, the cells were incubated with fluorescein isothiocyanate (FITC)-conjugated antibodies (CD11b, CD44, CD63, CD66b, or CD69 [DAKOPATTS, Glostrup, Denmark] or an isotype-matched FITC-conjugated irrelevant monoclonal antibody at the same concentration [Immunotech, Marseille, France]). Incubation of cells with antibodies against TLR-2 and TLR-4 (Hycult Biotechnology, Uden, The Netherlands) was followed by incubation with FITC-conjugated goat anti-mouse Fab fragments (DAKOPATTS). During flow cytometry, neutrophils and eosinophils were gated using their characteristics in side and forward scatter.
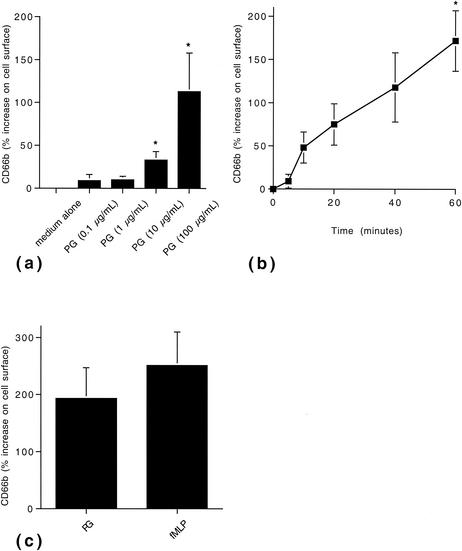
In neutrophils, PG caused a dose- and time-dependent increase in the mobilization of CD66b to the cell surface compared with controls (Fig. 1). The mobilization was enhanced in the presence of cytochalasin B compared with the response in the absence of this compound (data not shown). A spontaneous increase in CD66b, although weaker, was seen in the absence of PG. As a consequence, at each time point the mean fluorescence intensity of stimulated cells was related to that of resting cells. To compare the activating potency of PG, neutrophils were incubated with the well-characterized inducer of CD66b mobilization, fMLP, in parallel (7). PG induced mobilization of CD66b on the order of magnitude of that seen in the presence of fMLP (Fig. 1C).
FIG. 1.
PG-induced mobilization of CD66b in human neutrophils. (a) Dose-dependent mobilization of CD66b by PG. Neutrophils were incubated in the absence or presence of PG at the concentrations indicated for 40 min at 37°C. Thereafter, the surface expression of CD66b was determined by flow cytometry. The data are expressed as percent surface expression compared with cells in medium alone and are presented as means + standard errors of the means (error bars) from four independent experiments. *, P < 0.05. (b) Time-dependent mobilization of CD66b by PG. Neutrophils were incubated in the presence of PG (100 μg/ml) and investigated for their surface expression of CD66b at the time points indicated. The data are expressed as percent surface expression compared with cells in medium alone at the different time points. The data are presented as means ± standard errors of the means (error bars) from four independent experiments. (c) Comparison of PG- and fMLP-induced CD66b mobilization. Neutrophils were incubated in medium alone, in the presence of PG (100 μg/ml), or in the presence of fMLP (1 μM) for 40 min. The data are presented as means + standard errors of the means (error bars) from four independent experiments.
CD66b is a glycosyl phosphatidylinositol-anchored membrane protein belonging to the immunoglobulin-like subfamily of carcinoembryonal antigens (17). It is present in the membrane of specific and gelatinase-containing granules of neutrophils, and stimuli such as fMLP induce its mobilization to the cell surface (4, 5, 7). Cross-linking of CD66b results in cellular responses, for example, respiratory burst and increased adhesion (10). In addition, CD66b has been suggested to serve as a receptor for galectin 3 (5).
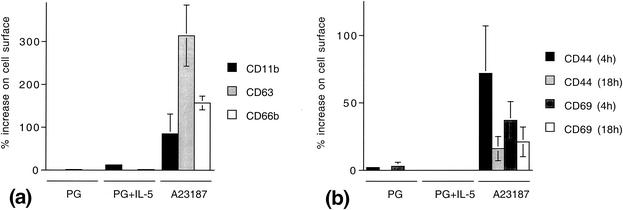
PG (100 μg/ml) did not induce significant mobilization of selected surface markers in human eosinophils—i.e., CD11b, CD63, and CD66b during 40 min of incubation or CD44 and CD69 after 4 and 18 h of incubation, respectively (Fig. 2). In addition, eosinophils were coincubated with the eosinophil-activating cytokine interleukin 5 (IL-5) (1 nM) and PG (100 μg/ml), but no increased mobilization of surface activation markers was observed. To exclude paradoxical effects from lower concentrations of PG, cells were incubated in the presence of PG at 1 and 10 μg/ml (in the absence or presence of IL-5). However, nor did these lower concentrations of PG cause mobilization of CD44 or CD69 (data not shown). As a control, some cells were stimulated with the calcium ionophore A23187 (1 μM) for 20 min, to assure that the cells were responsive and that the surface markers could indeed be mobilized.
FIG. 2.
PG does not induce mobilization of several surface activation markers in human eosinophils. (a) Mobilization of CD11b, CD63, and CD66b during short-duration incubation. Eosinophils were incubated with PG (100 μg/ml), in the presence or absence of the eosinophil-activating cytokine IL-5 (1 nM), for 40 min or in the presence of the calcium ionophore A23187 for 20 min. The data are expressed as percent surface expression compared with cells in medium alone and represent means ± standard errors of the means (error bars) from four independent experiments. (b) Mobilization of CD44 and CD69, respectively, after incubation for 4 and 18 h, respectively. The data are expressed as percent surface expression compared with cells in medium alone and represent means ± standard errors of the means (error bars) from four independent experiments.
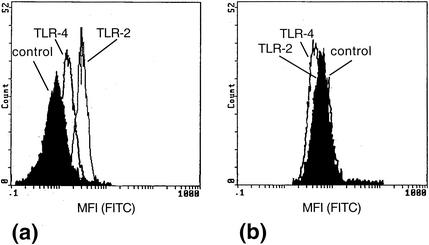
TLR-2 and, to a lesser extent, TLR-4 were detected on the surface of neutrophils by flow cytometry in the present study (Fig. 3a). The higher expression of TLR-2 and the lower expression of TLR-4 are in agreement with that reported in a recent study (13). Incubation of neutrophils with PG (100 μg/ml) for 30 min did not change the amount of TLR-2 or TLR-4 on the cell surface (data not shown). Neither TLR-2 nor TLR-4 could be detected on the surface of eosinophils (Fig. 3b), and exposure to PG (100 μg/ml) for 30 min did not result in the appearance of TLR-2 or TLR-4 on the surface of eosinophils. In a recent study, gene expression of both TLR-2 and TLR-4 was detected in eosinophils (14). However, the presence of the receptors on a protein level was not investigated. In another investigation, none of these receptors could be detected on the surface of eosinophils, nor did they respond to lipopolysaccharides while PG was not investigated (15).
FIG. 3.
Detection of TLR-2 and TLR-4 on the surface of neutrophils and eosinophils. The presence of TLR-2 and the presence of TLR-4 were detected by specific monoclonal antibodies, and secondary FITC-conjugated antibodies were detected by flow cytometry. (a) Neutrophils show a stronger signal for TLR-2 and a weaker signal for TLR-4. An isotype-matched irrelevant antibody serves as a control and represents background (black area). (b) In eosinophils, neither TLR-2 nor TLR-4 could be detected. The data shown are representative of four separate experiments.
Other cell wall components may be recognized by eosinophils. Recently, these cells were shown to respond to several exotoxins (SEA, SEB, SEC, and toxic shock syndrome toxin 1) derived from S. aureus that can serve as superantigens (18). Therefore, eosinophils may possess the ability to interact with S. aureus through innate recognition in vivo. This may be of importance during allergic inflammation (1).
In conclusion, the present study suggests roles for neutrophils but not eosinophils in innate recognition of bacterial PG.
Acknowledgments
This work was supported by grants from the Bengt Ihre Foundation, the Greta & Johan Kock Foundations, the Swedish Asthma and Allergy Association's Research Foundation, the Th. C. Berg Foundation, the Magnus Bergvall Foundation, and the Alfred Österlund Foundation.
REFERENCES
- 1.Bachert, C., P. Gevaert, and P. van Cauwenberge. 2002. Staphylococcus aureus superantigens and airway disease. Curr. Allergy Asthma Rep. 2:252-258. [DOI] [PubMed] [Google Scholar]
- 2.Behm, C. A., and K. S. Ovington. 2000. The role of eosinophils in parasitic helminth infections: insights from genetically modified mice. Parasitol. Today 16:202-209. [DOI] [PubMed] [Google Scholar]
- 3.Beutler, B. 2000. Tlr 4: central component of the sole mammalian LPS sensor. Curr. Opin. Immunol. 12:20-26. [DOI] [PubMed] [Google Scholar]
- 4.Borregaard, N., and J. B. Cowland. 1997. Granules of the human neutrophilic polymorphonuclear leukocyte. Blood 89:3503-3521. [PubMed] [Google Scholar]
- 5.Feuk-Lagerstedt, E., E. T. Jordan, H. Leffler, C. Dahlgren, and A. Karlsson. 1999. Identification of CD66a and CD66b as the major galectin-3 receptor candidates in human neutrophils. J. Immunol. 163:5592-5598. [PubMed] [Google Scholar]
- 6.Hansel, T. T., I. J. M. De Vries, T. Iff, S. Rihs, M. Wandzilak, S. Betz, K. Blaser, and C. Walker. 1991. An improved immunomagnetic procedure for the isolation of highly purified human blood eosinophils. J. Immunol. Methods 145:105-110. [DOI] [PubMed] [Google Scholar]
- 7.Kuijpers, T. W., A. T. Tool, C. E. van der Schoot, L. A. Ginsel, J. J. Onderwater, D. Roos, and A. J. Verhoeven. 1991. Membrane surface antigen expression on neutrophils: a reappraisal of the use of surface markers for neutrophil activation. Blood 78:1105-1111. [PubMed] [Google Scholar]
- 8.Lehninger, A. L. 1977. The molecular components of cells, p. 268-271. In A. L. Lehninger (ed.), Biochemistry. Worth Publishers, New York, N.Y.
- 9.Lowy, F. D. 1998. Staphylococcus aureus infections. N. Engl. J. Med. 339:520-532. [DOI] [PubMed] [Google Scholar]
- 10.Lund-Johansen, F., J. Olweus, F. W. Symington, A. Arli, J. S. Thompson, R. Vilella, K. Skubitz, and V. Horejsi. 1993. Activation of human monocytes and granulocytes by monoclonal antibodies to glycosylphosphatidylinositol-anchored antigens. Eur. J. Immunol. 23:2782-2791. [DOI] [PubMed] [Google Scholar]
- 11.Matsumoto, K., J. Appiah-Pippim, R. P. Schleimer, C. A. Bickel, L. A. Beck, and B. S. Bochner. 1998. CD44 and CD69 represent different types of cell-surface activation markers for human eosinophils. Am. J. Respir. Cell Mol. Biol. 18:860-866. [DOI] [PubMed] [Google Scholar]
- 12.Mattsson, E., L. Verhage, J. Rollof, A. Fleer, J. Verhoef, and H. van Dijk. 1993. Peptidoglycan and teichoic acid from Staphylococcus epidermidis stimulate human monocytes to release tumour necrosis factor-alpha, interleukin-1 beta and interleukin-6. FEMS Immunol. Med. Microbiol. 7:281-287. [DOI] [PubMed] [Google Scholar]
- 13.Mattsson, E., H. Herwald, L. Björck, and A. Egesten. 2002. Peptidoglycan from Staphylococcus aureus induces tissue factor expression and procoagulant activity in human monocytes. Infect. Immun. 70:3033-3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Plotz, S. G., A. Lentschat, H. Behrendt, W. Plotz, L. Hamann, J. Ring, E. T. Rietschel, H. D. Flad, and A. J. Ulmer. 2001. The interaction of human peripheral blood eosinophils with bacterial lipopolysaccharide is CD14 dependent. Blood 97:235-241. [DOI] [PubMed] [Google Scholar]
- 15.Sabroe, I., E. C. Jones, L. R. Usher, M. K. Whyte, and S. K. Dower. 2002. Toll-like receptor (TLR)2 and TLR4 in human peripheral blood granulocytes: a critical role for monocytes in leukocyte lipopolysaccharide responses. J. Immunol. 168:4701-4710. [DOI] [PubMed] [Google Scholar]
- 16.Schwandner, R., R. Dziarski, H. Wesche, M. Rothe, and C. Kirschning. 1999. Peptidoglycan- and lipoteichoic acid-induced cell activation is mediated by toll-like receptor 2. J. Biol. Chem. 274:17406-17409. [DOI] [PubMed] [Google Scholar]
- 17.Skubitz, K. M., M. Kuroki, P. Jantscheff, A. P. Skubitz, and F. Grunert. 1999. CD66b. J. Biol. Regul. Homeost. Agents 13:242-243. [PubMed] [Google Scholar]
- 18.Wedi, B., D. Wieczorek, T. Stunkel, K. Breuer, and A. Kapp. 2002. Staphylococcal exotoxins exert proinflammatory effects through inhibition of eosinophil apoptosis, increased surface antigen expression (CD11b, CD45, CD54, and CD69), and enhanced cytokine-activated oxidative burst, thereby triggering allergic inflammatory reactions. J. Allergy Clin. Immunol. 109:477-484. [DOI] [PubMed] [Google Scholar]
- 19.Weller, P. F. 1997. Human eosinophils. J. Allergy Clin. Immunol. 100:283-287. [DOI] [PubMed] [Google Scholar]