Abstract
Bacteroides forsythus is one of the important periodontopathic bacteria, and this microorganism is known to have an S-layer outside the outer membrane. The S-layer-like antigens were recently isolated from B. forsythus, and they were found to be 270- and 230-kDa proteins in the envelope fraction. In this study, these proteins were confirmed to be specific to B. forsythus by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and they were clearly recognized by sera from patients with adult and early-onset periodontitis in Western immmunoblot analysis. We compared the immunoglobulin G (IgG) responses against the purified S-layer-like antigen by enzyme-linked immunosorbent assay. IgG responses against this antigen were low in healthy control subjects, but they were significantly higher in subjects with adult and early-onset periodontitis. Together with the fact that the IgG responses against the crude extract of B. forsythus did not rise significantly in patients with periodontitis, S-layer-like proteins are considered to be specific antigens of B. forsythus and may play an important role in the progression of periodontitis.
Bacteroides forsythus is a gram-negative, fusiform, and fastidious anaerobe which was first reported by Tanner et al. (28). Recent evidence supports the idea that this organism is one of the important periodontopathic bacteria (32). This organism is often isolated from active periodontitis sites (5), and it is also reported that the presence of B. forsythus is a risk factor for periodontal disease (7, 8, 29). B. forsythus is frequently isolated with Porphyromonas gingivalis, which is one of the important periodontopathic bacteria (4, 16), and these two bacteria are known to have a synergistic effect in animal models (27, 34).
Because of the fastidious nature and difficulty in culture of this bacterium, pathogenicity of B. forsythus is not completely known. Only a few virulent factors have been identified, and these include trypsin-like protease (28), sialidase (11), apoptosis-inducing activity (1), prtH protease (22), and a cell surface-associated BspA protein (24). B. forsythus is also known to have a unique surface structure. As first reported by Tanner et al. (28), B. forsythus has an S-layer outside the outer membrane. The functions and pathogenicity of S-layers are not fully understood, but they are known to have crystalline protein or glycoprotein structures (25) and are considered to be related to molecular sieving, adhesion, and phage receptors (2). Virulence of S-layers is reported for some bacteria. S-layer of Aeromonas salmonicida is related to furnculosis in fish (12), and S-layer of Campylobacter fetus is considered to have an antiphagocytic function (31). Among periodontopathic bacteria, Campylobacter rectus (15) is also known to have an S-layer. The S-layer of C. rectus is considered to induce proinflammatory cytokines in patients with periodontitis (30). The functions of an S-layer of B. forsythus are as yet unknown, but Kerosuo suggests that S-layers may contribute to the rigidity of the cell wall (13). Recently, Higuchi et al. reported that 270- and 230-kDa proteins in the envelope fraction of B. forsythus are constituents of the S-layer of this bacterium (10).
On the other hand, patients with periodontitis are known to have high antibody titers against periodontopathic bacteria (6, 17). The humoral immune response has been extensively studied with P. gingivalis. Patients with severe periodontitis are known to have higher immunoglobulin G (IgG) titers against lipopolysaccharide (LPS) (23), fimbriae (20, 35), and gingipains (19), which are major pathogenic factors of this bacterium. In this way, higher antibody titers are in part correlated with the pathogenicity of bacteria. Humoral immune response against B. forsythus is not so extensively studied as that of P. gingivalis. Califano et al. could not detect higher antibody titers against a crude extract of B. forsythus in patients with periodontitis (3). On the other hand, Persson et al. (21) reported higher antibody titers against B. forsythus for patients with periodontitis compared to those of patients with gingivitis. Haffajee et al. grouped periodontitis subjects according to their elevated serum antibody levels to specific subgingival species (9). However, there is no report as of yet which has examined the immune reactions against purified antigens of B. forsythus in patients with periodontitis. In this study, we confirmed that S-layer-like proteins are specific antigens of B. forsythus and that they were found to be recognized by sera from patients with early-onset periodontitis (EOP) and adult periodontitis (AP).
MATERIALS AND METHODS
Human subjects.
Nine patients with EOP (8 males, 1 female; mean age, 27.7), 27 patients with AP (12 males, 15 females; mean age, 51.8), and 11 periodontally healthy controls (HC) (6 males, 5 females; mean age, 24.9) participated in this study. The patients were diagnosed as having EOP or AP according to the classification reported by Califano et al. (3). All subject participants were otherwise healthy and had not undergone periodontal or antibiotic therapy for at least 3 months prior to this study. We obtained informed consents from all participants according to the Helsinki Declaration, and blood samples were taken from them. The blood was allowed to clot and was centrifuged at 750 × g for 10 min, and the sera were separated, aliquoted, and stored at −80°C until use.
Bacterial strains and growth conditions.
B. forsythus ATCC 43037 was maintained on CDC-anaerobic blood agar (BBL Microbiology Systems, Cockeysville, Md.) under anaerobic conditions (85% N2, 10% H2, 5% CO2). B. forsythus was grown in large scale in brain heart infusion broth (Difco Laboratories, Detroit, Mich.) containing 0.5% yeast extract, 5 μg of hemin per ml, 0.5 μg of menadione per ml, 0.001% N-acetylmuramic acid (Sigma Chemical Co., St. Louis, Mo.), and 5% fetal bovine serum (Gibco-BRL, Grand Island, N.Y.). Other bacteria, including P. gingivalis ATCC 33277, Bacteroides fragilis ATCC 25285, Fusobacterium nucleatum ATCC 25586, and Prevotella intermedia ATCC 25611, were grown in brain heart infusion broth containing 0.5% yeast extract, 5 μg of hemin per ml, and 0.5 μg of menadione per ml under anaerobic conditions (33). Escherichia coli DH5α was grown in Luria-Bertani broth (Gibco-BRL).
SDS-PAGE and Western immunoblot.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed with a 1-mm-thick 9% running gel according to the method described by Laemmli (14). For Western immunoblot analysis, proteins separated by SDS-PAGE were transferred to nitrocellulose membranes in 25 mM Tris-glycine buffer. The unoccupied sites on the membranes were blocked with phosphate-buffered saline (PBS) containing 5% dry skim milk powder (Difco). The membrane was then treated with the sera from HC and patients with EOP or AP (1:1,000). Preabsorption of sera was performed according to the method of Nardin et al. (18). Sera were incubated with formalin-killed bacterial cell mix which contained P. gingivalis, P. intermedia, B. fragilis, F. nucleatum, and E. coli at 4°C overnight, and bound antibodies on bacterial cells were removed by centrifugation. The membranes were washed and incubated with goat anti-human IgG conjugated with alkaline phosphatase (1:2,000; Bio-Rad). The membranes were washed, and the bound antibodies were visualized by using 5-bromo-4-chloro-3-indolyl-1-phosphate and nitroblue tetrazolium (Bio-Rad).
Purification of S-layer from B. forsythus.
S-layer-like proteins were purified from sonicated extract of B. forsythus ATCC 43037 according to the methods of Higuchi et al. (10). In brief, the washed cells were sonicated on ice in the presence of protease inhibitors (0.25 mM N-α-p-tosyl-l-lysine chloromethyl ketone, 0.2 mM phenylmethylsulfonyl fluoride, and 0.1 mM leupeptin; Sigma). The cell extracts were obtained by removing the unbroken cells by centrifugation, and the envelope fraction was separated from the soluble fraction by ultracentrifugation at 143,000 × g for 60 min. The envelope fractions were subjected to SDS-PAGE, and the 270- and 230-kDa bands were excised and collected. Each protein was eluted from the gel slices by using an electroelutor (Model 422; Bio-Rad).
ELISA.
Enzyme-linked immunosorbent assay (ELISA) was performed according to the modified methods of O'Brien-Simpson et al. (19). Ninety-six-well microtiter plates were coated with the crude or purified antigens of B. forsythus at 4°C overnight. After removing the unbound antigens, the uncoated wells were blocked with PBS containing 3% bovine serum albumin (blocking buffer) at room temperature for 1 h. After being washed, serially diluted subject sera in the blocking buffer were added and incubated at 37°C for 3 h. After being washed, goat anti-human IgG conjugated with alkaline-phosphatase (1:1,000 dilution; Bio-Rad) was added and incubated for 1.5 h at 37°C. After being washed, p-nitrophenylphosphate (Bio-Rad) was added to the wells and the optical density (OD) at 405 nm of the developed color was measured by using a microplate reader. The assays were performed with triplicate wells for each individual serum, and the median data were used for analysis.
RESULTS
Protein profiles of bacterial strains.
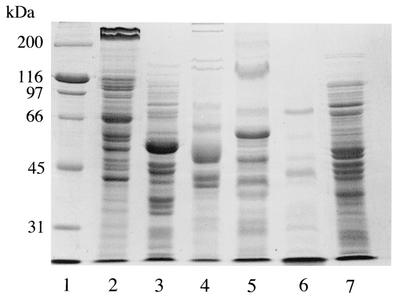
We first examined the protein profiles of sonicated extracts of six bacterial strains by SDS-PAGE (Fig. 1). Cells of B. forsythus, P. gingivalis, P. intermedia, F. nucleatum, B. fragilis, and E. coli were disrupted by a sonicator, and the extracts were subjected to SDS-PAGE. B. forsythus had two unique high-molecular-size proteins, and they were considered to be the 270- and 230-kDa S-layer-like proteins reported by Higuchi et al. (10).
FIG. 1.
SDS-PAGE of sonicated extracts of B. forsythus and other gram-negative bacteria on a 9% gel. Lanes: 1, molecular size standard; 2, B. forsythus ATCC 43037; 3, B. fragilis ATCC 25285; 4, F. nucleatum ATCC 25586; 5, P. intermedia ATCC 25611; 6, P. gingivalis ATCC 33277; 7, E. coli DH5α. B. forsythus exhibited the characteristic two high-molecular-size bands of 270 and 230 kDa.
Antibody reaction against cell extract of B. forsythus.
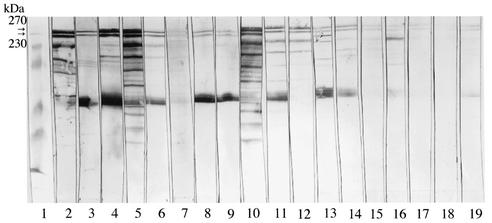
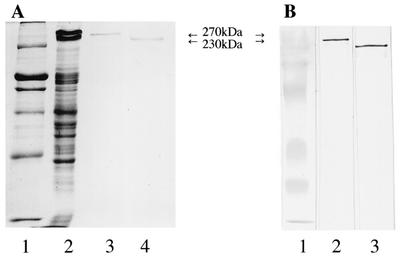
Next we examined the antibody reaction against sonicated extracts of B. forsythus by the absorbed sera from HC and patients with EOP and AP by Western immunoblot (Fig. 2). Six serum samples for the EOP group (a total of 9 patients), nine serum samples for the AP group (a total of 27 patients), and three serum samples for the HC group (a total of 11 donors) were chosen for this experiment, because preliminary experiments showed that these sera had higher antibody titers against sonicated extracts of B. forsythus among each group (data not shown). There is a possibility that some proteins may cross-react with the antibody against other bacteria, so the sera were first absorbed with other bacterial cells that are used in Fig. 1. After SDS-PAGE, the transferred membranes were reacted with absorbed sera from HC or patients with EOP or AP. All sera from patients with EOP and AP recognized proteins of B. forsythus, and they clearly reacted with 270- and 230-kDa proteins, although they also strongly recognized the protein with a molecular size of about 50 kDa. Sera from two HC also weakly reacted with 270- and 230-kDa proteins. We could not see these two bands with other subjects from HC (data not shown). We isolated S-layer-like antigens from the crude extracts of B. forsythus and examined the purity of these antigens by SDS-PAGE and Western immunoblot. Both 270- and 230-kDa antigens were purified (Fig. 3A), and the two proteins were equally recognized by serum from one of the patients with EOP (Fig. 3B). All sera from patients with EOP and AP also recognized the purified S-layer-like antigens, and the representative data with 270-kDa antigens are shown in Fig. 4. It was suggested that 270- and 230-kDa antigens were antigenically similar, so we used the 270-kDa antigen for further studies.
FIG. 2.
Western immunoblot analysis. Sonicated extract of B. forsythus was separated on 9% gels and was transferred to nitrocellulose membranes. Membranes were excised, and each strip was probed with preabsorbed sera from human subjects (1:1,000) followed by goat anti-human IgG conjugated with alkaline phosphatase (1:2,000). Lanes: 1, prestained molecular size marker; 2 to 7, sera from patients with EOP; 8 to 16, sera from patients with AP; 17 to 19, sera from HC. All sera from patients and two sera from HC recognized 270- and 230-kDa proteins (arrows).
FIG. 3.
(A) SDS-PAGE of S-layer-like antigens of B. forsythus on 9% gels. Lanes: 1, molecular size standard; 2, sonicated extracts of B. forsythus; 3, purified 270-kDa protein; 4, purified 230-kDa protein. (B) Western immunoblot analysis of S-layer-like antigens of B. forsythus. Purified antigens were loaded on 9% gels and were transferred to nitrocellulose membranes. Membranes were probed with nonabsorbed serum from a patient with EOP (1:2,000) followed by goat anti-human IgG conjugated with alkaline phosphatase (1:2,000). Lanes: 1, prestained molecular size marker; 2, purified 270-kDa protein; 3, purified 230-kDa protein.
FIG. 4.
Western immunoblot analysis with the purified 270-kDa antigen. The purified 270-kDa antigen was transferred to nitrocellulose membrane after SDS-PAGE. Membranes were excised, and each strip was probed with nonabsorbed sera from human subjects (1:2,000) followed by goat anti-human IgG conjugated with alkaline phosphatase (1:2,000). Lanes: 1 to 3, sera from patients with EOP; 4 to 9, sera from patients with AP; 10 to 12, sera from HC.
IgG responses against S-layer-like antigens.
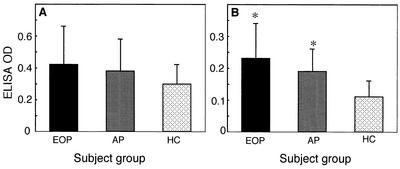
We first examined the IgG responses of sera from HC and patients with periodontitis against the sonicated extract from B. forsythus (Fig. 5A). We measured the ELISA OD with sera (1:500 dilution) according to the methods by O'Brien-Simpson et al. (19). Sera from subjects of each group exhibited IgG responses, but there was no significant difference among these subject groups. On the other hand, when the purified 270-kDa S-layer-like protein was used as an antigen, sera (1:500 dilution) from patients with EOP and AP exhibited significantly higher IgG responses compared to those from the sera from HC (Fig. 5B).
FIG. 5.
(A) IgG responses against sonicated extract from B. forsythus. Microtiter wells coated with sonicated extract were blocked with PBS containing bovine serum albumin and then were incubated with 1:500-diluted nonabsorbed sera from human subjects. The microtiter wells were then incubated with goat anti-human IgG conjugated with alkaline phosphatase (1:1,000) and color was developed with p-nitrophenylphosphate. (B) IgG responses against purified 270-kDa S-layer-like antigen. The procedures were same as those described for panel A. EOP, sera from patients with EOP (n = 9); AP, sera from patients with AP (n = 27); HC, sera from HC (n = 11). An asterisk means significantly different from HC (P < 0.005).
To further confirm the difference among these subject groups, we examined the IgG responses of serially diluted sera against the S-layer-like protein. When the sera were diluted to 1:1,000, all ELISA OD for the HC group were below the cutoff point (OD of 0.15). At this dilution, the numbers of positive samples over the cutoff points were 4 out of 9 and 5 out of 27 for the EOP and AP groups, respectively. Both are significantly larger than the number of positives for the HC group (P < 0.01 and P < 0.05, respectively), but because the overall IgG responses were low, we did not present the titers, which were calculated from the final dilution at the positive cutoff points. The data of ELISA are representative of three independent experiments.
DISCUSSION
The present study revealed that S-layer-like proteins of B. forsythus were specific antigens of this bacterium. As shown by SDS-PAGE (Fig. 1), these proteins were found to be unique among the six bacterial strains used in this study. The result of the Western immunoblot (Fig. 2 and 4) showed that the S-layer-like proteins were clearly recognized by sera from patients with EOP and AP, although the intensities of the bands varied by subject. Lower-molecular-size proteins were recognized by some sera, but 270- and 230-kDa proteins were recognized by all sera from patients with periodontitis. Sera from two HC subjects, which exhibited relatively high antibody titers among the HC group, also recognized these proteins weakly. These two HC subjects may harbor B. forsythus in their periodontal sulci or they may have a history of prior infection by this organism, because 270- and 230-kDa proteins are considered to be specific to B. forsythus. Further analysis is necessary to examine other possibilities, for example, cross-reaction with host proteins such as heat shock proteins.
Higuchi et al. (10) reported that S-layer-like proteins of B. forsythus had a strong antigenicity in rabbits, and our results supported the finding of antigenicity in human subjects as well. Two-hundred seventy- and 230-kDa proteins showed similar antigenicity, and this was also confirmed with the purified antigens (Fig. 3B). Higuchi et al. also reported that these two proteins are considered to be encoded by the same gene, and the difference in size may come from the difference in processing (10).
The IgG responses against S-layer-like antigens measured by ELISA were higher for patients with EOP and AP than for HC donors, although we could not obtain such difference when we used the crude extract of B. forsythus as an antigen. Our results indicated that S-layer-like antigens of B. forsythus were recognized by sera of patients with periodontitis and that the difference of antibody responses between patients and healthy donors were statistically significant, but the overall responses were very low compared to those against other bacterial antigens, such as P. gingivalis (data not shown). Due to the low IgG responses against B. forsythus, we did not show the exact titers of the antibodies. We need to compare the titers among subject groups by more sensitive methods in the future. Califano et al. also reported the lower response against the sonicated extract of B. forsythus compared to that of P. gingivalis in patients with periodontitis (3). It may be because of the low penetrating activity of B. forsythus into periodontal tissues or the low antigenicity of this organism. They also reported that there was not a significant difference between patients with periodontitis and healthy donors. Our data, which were performed with the sonicated extract antigen, supported their results. One of the reasons that there was not a significant difference among these patient groups may be the presence of common antigen among oral and nonoral bacteria. Healthy donors may have antibodies against such antigens, and we may have failed to count the specific immune response to B. forsythus. Further studies will be necessary to identify the true reason by using greater numbers of patients.
In any event, the S-layer-like proteins were specifically recognized by patients with periodontitis, so the S-layer of B. forsythus may have potential as a pathogenic factor as reported for S-layers of other bacteria (12, 30, 31). We cannot exclude the possibility that the S-layer-like proteins used in this experiment were contaminated with LPS, but we did not detect the characteristic ladder pattern of LPS by Western immunoblot, so the content of LPS is negligible in this experiment. We are now trying to isolate ultrapure S-layer-like antigens devoid of LPS for further bioassay.
Periodontitis is now considered to be the typical biofilm infection, and a mixed infection of several periodontopathic bacteria is the cause of this chronic disease (26). Takemoto et al. (27) reported that coinfection of B. forsythus and P. gingivalis induced larger abscess formation in rabbits compared to that of monoinfection with each bacterium. This synergism was previously confirmed by using a mouse model, and it was found that this synergism is in part related to the gingipains of P. gingivalis (34), but the virulence factors in B. forsythus could not be found in that experiment. Our results in this study revealed that S-layer of B. forsythus is a specific antigen of this bacterium. S-layer of B. forsythus may play an important role in biofilm formation in periodontal tissues. We are now constructing the isogenic mutant strain of B. forsythus devoid of S-layer-like antigens. Thus, we will be better able to study the function and virulence of S-layer of B. forsythus, and we will be able to access the mechanism of synergism between B. forsythus and P. gingivalis.
Acknowledgments
We appreciate Toru Eguchi of Sun Star Co. Ltd. and Mamiko Yoshimura of Nagasaki University for providing us with some of the bacteria used in this experiment. We also thank Fuminobu Yoshimura of Aichi Gakuin University for his helpful advice in performing this experiment.
This work was supported in part by Grants-in-Aid from Scientific Research 13672189 from the Ministry of Education, Sports, and Culture of Japan.
REFERENCES
- 1.Arakawa, S., T. Nakajima, H. Ishikura, S. Ichinose, I. Ishikawa, and N. Tsuchida. 2000. Novel apoptosis-inducing activity in Bacteroides forsythus: a comparative study with three serotypes of Actinobacillus actinomycetemcomitans. Infect. Immun. 68:4611-4615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baumeister, W., and H. Engelhardt. 1987. Three-dimensional structure of bacterial surface layers, p. 121-126. In J. R. Harris and R. W. Horne (ed.). Electron microscopy of proteins; membranous structures, vol. 6. Academic Press, London, United Kingdom.
- 3.Califano, J. V., J. C. Gunsolley, H. A. Schenkein, and J. G. Tew. 1997. A comparison of IgG antibody reactive with Bacteroides forsythus and Porphyromonas gingivalis in adult and early-onset periodontitis. J. Periodontol. 68:734-738. [DOI] [PubMed] [Google Scholar]
- 4.Darveau, R. P., A. Tanner, and R. C. Page. 1997. The microbial challenge in periodontitis. Periodontol. 2000 14:12-32. [DOI] [PubMed] [Google Scholar]
- 5.Gersdorf, H., A. Meissner, K. Peltz, G. Krekeler, and U. B. Gobel. 1993. Identification of Bacteroides forsythus in subgingival plaque from patients with advanced periodontitis. J. Clin. Microbiol. 31:941-946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gmür, R., K. Hrodek, U. P. Saxer, and B. Guggenheim. 1986. Double-blind analysis of the relation between adult periodontitis and systemic host response to suspected periodontal pathogens. Infect. Immun. 52:768-776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grossi, S. G., R. J. Genco, E. E. Machtei, A. W. Ho, G. Koch, R. G. Dunford, J. J. Zambon, and E. Hausmann. 1995. Assessment of risk for periodontal disease. II. Risk indicators for alveolar bone loss. J. Periodontol. 66:23-29. [DOI] [PubMed] [Google Scholar]
- 8.Grossi, S. G., J. J. Zambon, A. W. Ho., G. Koch, R. G. Dunford, E. E. Machtei, O. M. Norderyd, and R. J. Genco. 1994. Assessment of risk for periodontal disease. I. Risk indicators for attachment loss. J. Periodontol. 65:260-267. [DOI] [PubMed] [Google Scholar]
- 9.Haffajee, A. D., S. S. Socransky, M. A. Taubman, J. Sioson, and D. J. Smith. 1995. Patterns of antibody response in subjects with periodontitis. Oral Microbiol. Immunol. 10:129-137. [DOI] [PubMed] [Google Scholar]
- 10.Higuchi, N., Y. Murakami, K. Moriguchi, N. Ohno, H. Nakamura, and F. Yoshimura. 2000. Localization of major, high molecular weight proteins in Bacteroides forsythus. Microbiol. Immunol. 44:777-780. [DOI] [PubMed] [Google Scholar]
- 11.Holt, S. C., and T. E. Barman. 1991. Factors in virulence expression and their role in periodontal disease pathogens. Crit. Rev. Oral Biol. Med. 2:177-281. [DOI] [PubMed] [Google Scholar]
- 12.Ishiguro, E. E., W. W. Kay, T. Ainsworth, J. B. Chamberlain, R. A. Austen, J. T. Buckley, and T. J. Trust. 1981. Loss of virulence during culture of Aeromonas salmonicida at high temperature. J. Bacteriol. 148:333-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kerosuo, E. 1988. Ultrastructure of the cell envelope of Bacteroides forsythus strain ATCC 43037. Oral Microbiol. Immunol. 3:134-137. [DOI] [PubMed] [Google Scholar]
- 14.Laemmli, U. K. 1976. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 15.Lai, C. H., M. A. Listgarten, A. C. R. Tanner, and S. S. Socransky. 1981. Ultrastructures of Bacteroides gracilis, Campylobacter concisus, Wolinella recta, and Eikenella corrodens from humans with periodontal disease. Int. J. Syst. Bacteriol. 31:465-475. [Google Scholar]
- 16.Lotufo, R. F., J. Flynn, C. Chen, and J. Slots. 1994. Molecular detection of Bacteroides forsythus in human periodontitis. Oral Microbiol. Immunol. 9:154-160. [DOI] [PubMed] [Google Scholar]
- 17.Naito, Y., K. Okuda, I. Takazoe, H. Watanabe, and I. Ishikawa. 1985. The relationship between serum IgG levels to subgingival gram-negative bacteria and degree of periodontal destruction. J. Dent. Res. 64:1306-1310. [DOI] [PubMed] [Google Scholar]
- 18.Nardin, A. M., H. T. Sojar, S. G. Grossi, L. A. Christernsson, and R. J. Genco. 1991. Humoral immunity of older adults with periodontal disease to Porphyromonas gingivalis. Infect. Immun. 59:4363-4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O'Brien-Simpson, N. M., C. L. Black, P. S. Bhogal, S. M. Cleal, N. Slakeski, T. J. Higgins, and E. C. Reynolds. 2000. Serum immunogloblin G (IgG) and IgG subclass responses to the RgpA-Kgp proteinase-adhesin complex of Porphyromonas gingivalis in adult periodontitis. Infect. Immun. 68:2704-2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ogawa, T., Y. Kusumoto, S. Hamada, J. R. McGhee, and H. Kiyono. 1990. Bacteroides gingivalis-specific serum IgG and IgA subclass antibodies in periodontal diseases. Clin. Exp. Immunol. 82:318-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Persson, G. R., B. Schlegel-Bregenzer, W. O. Chung, L. Houston, T. Oswald, and M. C. Roberts. 2000. Serum antibody titers to Bacteroides forsythus in elderly subjects with gingivitis or periodontitis. J. Clin. Periodontol. 27:839-845. [DOI] [PubMed] [Google Scholar]
- 22.Saito, T., K. Ishihara, T. Kato, and K. Okuda. 1997. Cloning, expression, and sequencing of a protease gene from Bacteroides forsythus ATCC 43037 in Escherichia coli. Infect. Immun. 65:4888-4891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schenk, K., and T. E. Michaelsen. 1987. IgG subclass distribution of serum antibodies against lipopolysaccharide from Bacteroides gingivalis in periodontal health and disease. Acta Pathol. Microbiol. Scand. 95:41-46. [DOI] [PubMed] [Google Scholar]
- 24.Sharma, A., H. T. Sojar, I. Glurich, K. Honma, H. K. Kuramitsu, and R. J. Genco. 1998. Cloning, expression, and sequencing of a cell surface antigen containing leucine-rich repeat motif from Bacteroides forsythus ATCC 43037. Infect. Immun. 66:5703-5710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sleytr, U. B., and P. Messner. 1983. Crystalline surface layers on bacteria. Annu. Rev. Microbiol. 37:311-339. [DOI] [PubMed] [Google Scholar]
- 26.Socransky, S. S., and A. D. Haffajee. 2002. Dental biofilms: difficult therapeutic targets. Periodontol. 2000. 28:12-55. [DOI] [PubMed] [Google Scholar]
- 27.Takemoto, T., H. Kurihara, and G. Dahlen. 1997. Characterization of Bacteroides forsythus isolates. J. Clin. Microbiol. 35:1378-1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tanner, A. C. R., M. A. Listgarten, J. Ebersole, and M. N. Strezempko. 1986. Bacteroides forsythus sp. nov., a slow-growing, fusiform Bacteroides sp. from the human oral cavity. Int. J. Syst. Bacteriol. 36:213-221. [Google Scholar]
- 29.Tran, S. D., J. D. Rudney, B. S. Sparks, and J. S. Hodges. 2001. Persistent presence of Bacteroides forsythus as a risk factor for attachment loss in a population with low prevalence and severity of adult periodontitis. J. Periodontol. 72:1-10. [DOI] [PubMed] [Google Scholar]
- 30.Wang, B., E. Kraig, and D. Kolodrubetz. 2000. Use of defined mutants to assess the role of the Campylobacter rectus S-layer in bacterium-epithelial cell interactions. Infect. Immun. 68:1465-1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Winter, A. J., E. C. McCoy, S. S. Fullmer, K. Burda, and P. J. Bier. 1978. Microcapsule of Campylobacter fetus: chemical and physical characterization. Infect. Immun. 22:963-971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ximenez-Fyvie, L. A., A. D. Haffajee, and S. S. Socransky. 2000. Comparison of the microbiota of supra- and subgingival plaque in health and periodontitis. J. Clin. Periodontol. 27:648-657. [DOI] [PubMed] [Google Scholar]
- 33.Yoneda, M., K. Maeda, and M. Aono. 1990. Suppression of bactericidal activity of human polymorphonuclear leukocytes by Bacteroides gingivalis. Infect. Immun. 58:406-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yoneda, M., T. Hirofuji, H. Anan, A. Matsumoto, T. Hamachi, K. Nakayama, and K. Maeda. 2001. Mixed infection of Porphyromonas gingivalis and Bacteroides forsythus in a murine abscess model: involvement of gingipains in a synergistic effect. J. Periodotal Res. 36:237-243. [DOI] [PubMed] [Google Scholar]
- 35.Yoshimura, F., T. Sugano, M. Kawanami, H. Kato, and T. Suzuki. 1987. Detection of specific antibodies against fimbriae and membrane proteins from the oral anaerobe Bacteroides gingivalis in patients with periodontal disease. Microbiol. Immunol. 31:935-941. [DOI] [PubMed] [Google Scholar]