Abstract
Rhodococcus equi causes severe pyogranulomatous pneumonia in foals and in immunocompromised humans. Replication of virulent isolates within macrophages correlates with the presence of a large plasmid which encodes a family of seven virulence-associated proteins (VapA and VapC to VapH), whose functions are unknown. Although cell-mediated immunity is thought to be crucial in eliminating R. equi infection, antibody partially protects foals. The antibody response to both VapA and VapC was similar in six adult horses and six naturally exposed but healthy foals, as well as in eight foals with R. equi pneumonia. The immunoglobulin G (IgG) subisotype response of pneumonic foals to Vap proteins was significantly IgGb biased and also had a trend toward higher IgGT association compared to the isotype association of antibody in adult horses and healthy exposed foals. This suggests that in horses, IgGb and IgGT are Th2 isotypes and IgGa is a Th1 isotype. Furthermore, it suggests that foals which develop R. equi pneumonia have a Th2-biased, ineffective immune response whereas foals which become immune develop a Th1-biased immune response. Pneumonic foals had significantly more antibody to VapD and VapE than did healthy exposed foals. This may indicate a difference in the expression of these two Vap proteins during persistent infection. Alternatively, in pneumonic foals the deviation of the immune response toward VapD and VapE may reflect a bias unfavorable to R. equi resistance. These data indicate possible age-related differences in the equine immune response affecting Th1-Th2 bias as well as antibody specificity bias, which together favor the susceptibility of foals to R. equi pneumonia.
Rhodococcus equi causes pyogranulomatous bronchopneumonia in foals younger than 4 months and induces significant economic losses on endemically infected horse-breeding farms (29). This gram-positive, facultatively intracellular bacterium is an opportunistic pathogen in immunocompromised humans, such as those infected with human immunodeficiency virus (2, 9, 13). Foal-virulent R. equi strains possess an 81-kb plasmid and express VapA, a plasmid-encoded, surface-expressed lipoprotein (38, 40-42). VapA is a member of a family of seven Vap proteins (VapA and VapC to VapH), which have homology in their C-terminal halves and are encoded within a 27-kb pathogenicity island on the large plasmid (37). The function of these proteins is not known.
Protection of foals against R. equi appears to rely on cooperation and interdependency between the antibody-mediated and cell-mediated immune response. Antibody appears to contribute to protection since the period of maximum susceptibility coincides with declining levels of maternally derived antibody (29). In addition, antibody opsonizes R. equi for uptake and killing by macrophages and neutrophils in vitro (45, 47, 48) and may facilitate the killing of R. equi in vivo. Vap-specific antibodies protect immunosuppressed mice since purified immunoglobulin G (IgG) from APTX (a VapA-enriched antigen)-vaccinated horses protected against intraperitoneal challenge with R. equi whereas nonimmune equine IgG failed to protect (10).
Pulmonary clearance of virulent R. equi in mice requires functional T lymphocytes (3, 46). Both CD4+ and CD8+ T cells apparently contribute to protection (26, 33), and CD4+ cells are necessary for complete pulmonary clearance of R. equi in mice (16). Mice in which a Th2 cytokine response was induced by administration of monoclonal antibodies against gamma interferon (IFN-γ) prior to experimental infection with virulent R. equi failed to clear the bacteria and developed pulmonary granulomas (17). In contrast, immunocompetent BALB/c mice developed a Th1 cytokine response and cleared the infection (17). Adoptive transfer of R. equi-specific Th1 or Th2 cell lines in mice supported the conclusion that a Th2 response is detrimental whereas a Th1 response is beneficial in clearance of R. equi (18). Virulent R. equi can modulate the cytokine response in foals, down-regulating IFN-γ mRNA expression in CD4+ T cells and up-regulating lung interleukin-10 expression (12). These cytokines may influence the Th1-Th2 balance of the immune response in foals.
Although antibody provides partial protection against R. equi pneumonia (14), the role of the Ig isotype has not been described. Prescott et al. determined that the antibody response of young foals to the APTX antigen with aluminium hydroxide adjuvant induced a more IgGb- and IgGT-biased subisotype response than did natural infection, which induced an IgGa-dominant response (30). Vaccination with this antigen and adjuvant exacerbated disease in the foals after natural challenge with R. equi (30). An aluminium hydroxide-based influenza vaccine also induced an IgGT-biased response in horses, with some evidence of an IgGc response. These vaccinated horses were not resistant to infection even though they had an anamnestic IgGT response (25). In contrast, natural infection with influenza virus induced virus-specific IgGa and IgGb (25).
In mice and humans, the antibody isotype reflects the Th1-Th2 bias of the immune response. A similar bias may occur in horses. The IgG subisotype profile of the antibody response associated with protective immunity to R. equi infection has not been described. The study reported here addresses the hypothesis that resistance or susceptibility to R. equi pneumonia in foals is associated with distinct IgG subisotype-related antibody responses to the seven virulence-related Vap proteins and that in pneumonic foals the profile reflects a Th2-biased response whereas in healthy foals and adults the profile reflects a Th1-biased response.
MATERIALS AND METHODS
Experimental design.
Serum was collected biweekly from clinically normal pony foals (n = 6) kept on pasture at a University of Guelph research farm that had a history of R. equi infections in foals. The serum sample in which the peak antibody response to VapA was observed over the first 6 months of a foal's life was used to determine the isotype profile of the anti-Vap response compared to sera obtained from a group of clinically normal, unrelated adult horses (n = 6) at the same farm and from a third group of foals with clinical R. equi pneumonia (n = 8). The clinical case samples were obtained from client animals at the University of Florida and the Ontario Veterinary College. The horses were categorized according to defined resistance or susceptibility status to provide a framework in which to make correlations based on specificity and isotype relatedness of serum anti-Vap antibody. The three groups represent (i) a clinically normal, healthy R. equi-exposed foal group; (ii) a clinically normal and healthy adult group (and since adult horses do not become sick with R. equi pneumonia, presumably an immune group); and (iii) a susceptible group of foals that had developed R. equi pneumonia and were therefore assumed to be nonimmune. The use of the horses for this research complied with all relevant national guidelines and University of Guelph Animal Care Committee policies.
Vap protein purification.
Vap proteins were obtained as glutathione S-transferase fusion proteins. Each vap gene was amplified from the R. equi strain 103+ plasmid by PCR using primers described in Table 1. The primers contained 5′ extensions encoding restriction enzyme recognition sites for BamHI or EcoRI. Amplified products were digested with BamHI and EcoRI, and the digestion products were ligated to a similarly digested plasmid vector (pGEX-2T; Amersham Pharmacia Biotech, Baie d'Urfé, Quebec, Canada). The resulting plasmids were transformed into Escherichia coli XL1Blue (Stratagene, La Jolla, Calif.). These plasmids allowed in vitro production of recombinant Vap proteins fused to glutathione S-transferase. Fusion proteins were purified, using glutathione-Sepharose 4B beads (Amersham Pharmacia Biotech), and Vap proteins were cleaved from glutathione S-transferase using thrombin (Amersham Pharmacia Biotech). Protein production was assessed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, Western blot analysis, and protein assay (Pierce, Rockford, Ill.).
TABLE 1.
Primers used for PCR of each of the seven members of the Vap family
| Vap | Primer 1 | Primer 2 |
|---|---|---|
| VapA | 5′-GCCGGATCCACTAATGCGACCGTTCTT-3′ | 5′-CATGAATTCCTAGGCGTTGTGCCA-3′ |
| VapC | 5′-GCCGGATCCGCCAATGTAGTCGCTCCGTC-3′ | 5′-CATGAATTCGCGAGCGTTTACCTTCCGAC-3′ |
| VapD | 5′-GCCGGATCCACGGCGAGAAGACGAAAGTGT-3′ | 5′-CATGAATTCTTGTTCCTCACGCAGCCCAC-3′ |
| VapE | 5′-GCCGGATCCATAGCATTGGTTCTAATCGCA-3′ | 5′-CATGAATTCTACTGCACTGCAGCCGGACT-3′ |
| VapF | 5′-CACGGATCCAACAGATGTTGTGGCGATTGC-3′ | 5′-CCTGAATTCTTCGTCCGCTTCTGCCAATC-3′ |
| VapG | 5′-GCGAATTCCAGAAGAGGTACGTGGCATTG-3′ | 5′-CTGGGATCCATGGTATCCACTACAGCAGC-3′ |
| VapH | 5′-CATGGATCCGTGTACCAACGGCTGAAGCTC-3′ | 5′-CCTGAATTCCACGTGATTCTTCGCTTGGAC-3′ |
Although cloning and protein production were successful for each Vap protein, purification using the glutathione-Sepharose 4B beads was not always achieved because of the inability of the fusion protein to bind to the beads. In these cases, a protein agglutination protocol was employed to concentrate the Vap protein. Briefly, a 100-ml overnight culture, which had reached an optical density at 600 nm (OD600) of 0.6, was stimulated with 5 mM isopropyl-β-d-1-thiogalactopyranoside (IPTG) for 3 h. The culture was then centrifuged at 1,500 × g for 15 min at 4°C, and the supernatant was decanted. The pellet was resuspended in 4 ml of 25% sucrose-50 mM Tris (pH 8.0) and frozen at −70°C. The suspension was then thawed quickly in a 37°C water bath, and 1 ml of a lysozyme solution (10 mg of lysozyme [Sigma-Aldrich, Oakville, Ontario, Canada] per ml of 250 mM Tris [pH 8.0] was added. This mixture was incubated on ice for 15 min, and 30 ml of a 2× RIPA-TET detergent mixture at a 5:4 ratio (2 × RIPA is 20 mMTris-HCl [pH 8.0], 300 mM NaCl and 2% sodium deoxycholate; TET is 100 mM Tris-HCl[pH 8.0], 50 mM EDTA [pH 8.0], and 2% Triton X-100) was added. The mixture was left on ice for a further 5 min and then sonicated on ice three times for 30 s. The sonicate was then centrifuged at 15,000 × g for 15 min at 4°C, and the supernatant was decanted. The pellet was resuspended in 500 μl of sterile water and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, Western blot analysis, and protein assay.
Serological testing.
Equine sera were absorbed for E. coli-specific antibodies because Vap proteins produced by agglutination contained trace amounts of E. coli antigens. Briefly, E. coli XL1Blue cells were grown on tryptic soy agar plates at 37°C for 24 h. The bacteria were washed from the plates with 5 ml of phosphate-buffered saline, using a glass rod. The OD of the bacterial suspension was measured and adjusted with phosphate-buffered saline to an OD600 of 2.0. The suspension was subjected to three rounds of 3 min of sonication and freeze-thawing and cultured to confirm killing. The suspension was then added in a 1:1 ratio to each serum sample and incubated at 4°C for 18 h with gentle rocking (Lab-line 3-D bidirectional rotator; Fisher Scientific, Nepean, Ontario, Canada). The sera were then centrifuged (14,000 × g for 30 min) to remove debris.
Titers of serum antibodies to each of the Vap proteins were determined by enzyme-linked immunosorbent assay (ELISA). The natural log of the dilution which produced an OD double that of the negative control was considered the titer of that sample. Each sample was run in duplicate, and the average titer of the group (adults, clinically ill foals, and clinically normal foals) was used in a comparison of the total antibody response (expressed as a ratio over the total antibody response to VapA) and the IgG subisotype response to each of the Vap proteins. A ratio was used for the total antibody comparison to control for possible variation associated with differences in antibody avidity. It was not used for the comparison of isotype response because this would have removed most of the data. Ninety-six-well polystyrene plates (Immunolon 1; Dynatech Laboratories Inc., Chantilly, Va.) were coated overnight at 4°C with each Vap protein at 2 μg/ml in carbonate-bicarbonate coating buffer (1.59 g of Na2CO3 per liter, 2.93 g of NaHCO3 per liter [pH 9.6]), washed three times using 0.05% PBS-Tween 20, and blocked for 1 h at room temperature using 0.05% Tween 20 and 0.5% gelatin in PBS (PBS-TG). To measure antibody associated with all Ig isotypes, peroxidase-conjugated goat anti-horse Ig (Jackson ImmunoResearch Laboratories, Inc., West Grove, Pa.) was used at 1:10,000 in PBS-TG and incubated for 1 h at 37°C. Substrate was then applied (ABTS; Roche Diagnostics, Mannheim, Germany), the plate was incubated for 1 h in the dark at room temperature, and the OD was read at 405/630 nm (BioTek EL311; Biotek Instruments, Fisher Scientific). To determine isotype-specific response, peroxidase-conjugated anti-IgGa, anti-IgGb, anti-IgGc, and anti-IgGT antibodies (Serotec Inc., Oxford, England) were used at 1:100,000 for anti-IgGa, anti-IgGb, and anti-IgGc and 1:10,000 for anti-IgGT. Positive and negative controls were obtained from a known R. equi-exposed mare and a foal immediately following birth, respectively.
Analysis.
Two-tailed Student's t tests and LSD random permutation tests (analysis of variance) in SAS (SAS/STAT user's guide version 6, 1990; SAS Institute Inc., Cary, N.C.) were used to analyze the data and to determine the significance of differences between groups (P ≤ 0.05). The first test was used to test for significant differences in the actual titers of each group, and the second test was used to test for significant differences between the groups for each antibody subisotype against each Vap protein in terms of there being detectable antibody or no antibody.
RESULTS
Antibody response to each of the Vap proteins.
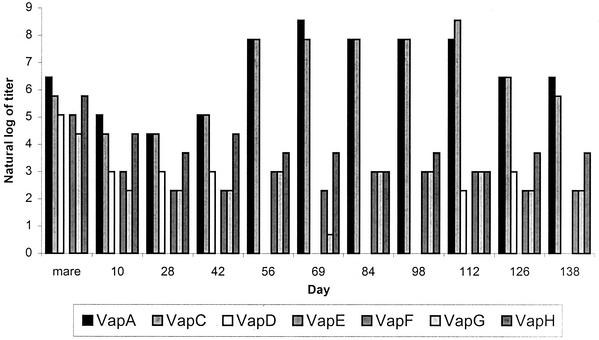
Antibody to VapA and VapC increased following natural exposure to R. equi (Fig. 1). In contrast, antibody to VapF, VapG, and VapH did not change throughout the 6-month observation period and antibody to VapE was not detected. The level of anti-VapD antibody rose slightly in some foals a few weeks after the peak of the response to VapA and VapC. Figure 1 data from one foal are representative of the pattern of the response observed in six foals kept at pasture, which remained apparently healthy, although the response pattern shifted along the x axis between foals, so that the day on which the anti-VapA response peaked differed between foals. For this reason, data for foals were not combined.
FIG. 1.
Titer of serum antibody to each of the seven R. equi Vap proteins as measured in sera from a clinically normal foal over a 5-month period (the x axis indicates the days postparturition). Titers were determined by ELISA and expressed as natural logs. Mare serum was obtained on the day of parturition. This pattern was typical of the six foals maintained at pasture which remained apparently healthy over the course of the study.
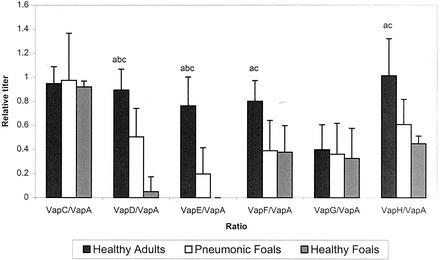
There was a marked antibody response to VapA and VapC by all groups in the study. The antibody response relative to anti-VapA was significantly lower in both foal groups than in the immune adult horses for anti-VapD, anti-VapE, anti-VapF, and anti-VapH (Fig. 2). The pneumonic foals had significantly more antibody to VapD and VapE than did the normal foals, which as a group had no antibody response to VapE and only a low response to VapD by one foal.
FIG. 2.
Mean antibody response of the adult horse group, the pneumonic foal group, and the clinically normal foal group to each of the six Vap proteins (VapC to VapH) expressed as a ratio to the VapA titer. Titers of antibody to each protein were determined by ELISA. Significant differences (P ≤ 0.05 by Student's t test) between the adult and pneumonic groups are indicated by a, those between the pneumonic and clinically normal foal groups are indicated by b, and those between the adult and clinically normal group are indicated by c.
Subisotype of the IgG response.
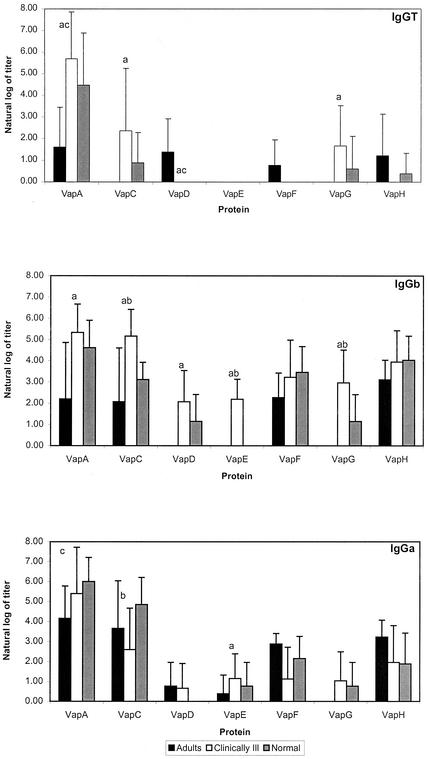
The antibody response to each of the proteins by the three groups of animals (pneumonic foals, clinically normal foals, and immune adults) was further studied by investigating the subisotype (IgGT, IgGb, IgGc, and IgGa) of IgG and the differences were analyzed by Student's t test. With the exception of three pneumonic and two clinically normal foals responding to VapA, an IgGc subisotype response was not detected to any of the Vap proteins. The IgGT response of pneumonic foals was significantly greater than that of the immune adult group to VapA, VapC, and VapG (Fig. 3). The adults produced an IgGa- versus an IgGT- or IgGb-biased response against VapA, VapC, VapF, and VapH (Fig. 3). Although the pneumonic foals also produced IgGa against VapA and VapC, this group had an IgGb-dominant response to all the Vap family members (Fig. 3). The IgGb response of ill foals was significantly greater (P ≤ 0.05 by Student's t test) than that of immune adults for VapA, VapC, VapD, VapE, and VapG and significantly greater than the normal foal group for VapC, VapE, and VapG (Fig. 3).
FIG. 3.
IgGT, IgGa, and IgGb responses to each of the seven Vap proteins (VapA and VapC to VapH) by adult horses, clinically normal foals, and pneumonic foals. Significant differences (P ≤ 0.05 by Student's t test) between the adult and pneumonic group are indicated by a, those between the pneumonic and clinically normal foal groups are indicated by b, and those between the adult and clinically normal group are indicated by c.
The mean IgGa-to-IgGb and IgGa-to-IgGT ratios against all of the Vap family members were consistently higher in the adults than in the pneumonic foals (Table 2) and were usually higher in adults than in healthy foals. When the immune adult group was compared to the pneumonic-foal group, the IgGa-to-IgGb ratio was as much as three times higher and the ratio difference was significant for VapA, VapC, and VapF. The IgGa-to-IgGb ratios in the healthy foals tended to also be higher than in the pneumonic foals, as did the IgGa-to-IgGT ratio. When the overall IgGb response to all the Vap proteins was compared between the horse groups by analysis of variance, the IgGb response was significantly (P ≤ 0.05) greater in the pneumonic-foal group than in both the clinically normal group and the adult group.
TABLE 2.
IgGa-to-IgGb and IgGa-to-IgGT ratios for mean IgGa-, IgGb-, and IgGT-associated antibody to each of the R. equi Vap proteins for each horse group
| Group and ratio | Value of ratio for:
|
||||||
|---|---|---|---|---|---|---|---|
| VapA | VapC | VapD | VapE | VapF | VapG | VapH | |
| IgGa/IgGb | |||||||
| Healthy adults | 1.89 ± 0.61 | 1.76 ± 0.94 | —a | — | 1.27 ± 0.45 | — | 1.04 ± 0.91 |
| Pneumonic foals | 1.01 ± 1.75 | 0.50 ± 1.66 | 0.37 ± 0.85 | 0.53 ± 1.31 | 0.35 ± 0.91 | 0.35 ± 0.94 | 0.50 ± 1.24 |
| Healthy foals | 1.30 ± 0.94 | 1.56 ± 0.94 | 0.00 ± 0.00 | — | 0.62 ± 0.91 | 0.67 ± 0.94 | 0.47 ± 1.36 |
| Significanceb | ac | ab | — | — | a | b | |
| IgGa/IgGT | |||||||
| Healthy adults | 2.57 ± 0.88 | — | 0.56 ± 0.77 | — | 3.75 ± 0.44 | — | 2.62 ± 0.44 |
| Pneumonic foals | 0.95 ± 1.07 | 1.10 ± 0.72 | — | — | — | 0.62 ± 0.78 | — |
| Healthy foals | 1.35 ± 0.50 | 5.49 ± 0.98 | — | — | — | 1.25 ± 0.79 | 4.90 ± 1.64 |
| Significanceb | ac | b | |||||
Dashes indicate an inability to calculate the ratio due to the absence of a response and hence a zero denominator.
Significant differences (P ≤ 0.05 by Student's t test) between the adult and pneumonic foal group are indicated by a, those between the pneumonic and healthy foal groups are indicated by b, and those between the adult and healthy foal group are indicated by c.
DISCUSSION
Antibody function differs by immunoglobulin subisotype (7, 22). The Ig subisotype bias of the response to infectious organisms reflects a finely regulated multifactorial system that steers the response to provide an environment appropriate for control of the particular organism (15). In effect, the isotype profile of the immune response reflects the overall type of response (Th1 or Th2) induced by the pathogen or vaccine (32). This division of the CD4+ T helper cell population into two complementary groups has been extensively investigated by using humans and mice (32). The relationship of the bias of the response (Th1/Th2) to the degree of resistance to specific pathogens or to the response to vaccination has become an important area of research since it may be possible to increase resistance to disease by steering the immune response (11).
Assuming that the Th1 bias is protective in equine infections by the intracellular pathogen R. equi as it is in mice, the results reported here support the conclusion that IgGT reflects a Th2-like response since a significantly greater IgGT response to R. equi antigens VapA, VapC and VapG was observed in foals that had developed R. equi pneumonia than in the immune adult group. However, this difference was not noted between the two foal groups. The significantly greater overall amount of IgGb anti-R. equi antibody in the sera of pneumonic foals compared to adult and healthy foals suggests that the ratio of IgGa to IgGb is important in determining resistance, since the amounts of IgGa were similar in clinically normal foals, pneumonic foals, and adults (Fig. 3). These results suggest that a higher IgGa-to-IgGb anti-R. equi ratio (Table 2) reflects a Th1-biased immune response and greater protection from R. equi infection. This is an important finding, since a fundamental question regarding R. equi pneumonia in foals is why foals are particularly susceptible to disease since this infection is almost unique to foals (as well as occurring in immunodeficient individuals of other species). The present results support the hypothesis that foals develop R. equi pneumonia because an inappropriate Th2-dominant response to infection develops whereas foals and adults which develop a Th1 response become immune (and remain so as adults). It has been suggested that the neonatal period in general is marked by a high susceptibility to infections and that although neonatal T cells are immunocompetent, their differentiation is biased toward a Th2 profile under neutral conditions (19). Work with rodents and humans indicates that this susceptibility may be a result of a combination of factors including a greater costimulatory requirement of neonatal T cells than of adult T cells (1), differences in antigen handling by neonatal B cells and low major histocompatibility complex-peptide density which favors priming of Th2-type CD4 cells (23), the lack of anatomical structures (germinal centers) required for lymphoid cell maturation (8, 24), and an immature phenotype of neonatal B cells in comparison to adult B cells (21). In addition to these possible mechanisms which may or may not operate to produce a relative Th2 bias in foals, the R. equi virulence plasmid may drive a Th2-biased immune response. For example, Giguère et al. (12) reported that foals with severe pneumonia caused by virulent R. equi strains differed significantly from foals infected with avirulent R. equi strains in that the former had reduced amounts of IFN-γ mRNA in bronchial lymph node CD4+ T cells as well as enhanced quantities of interleukin-10 mRNA in the lungs. Since the presence of the virulence plasmid carrying R. equi Vap genes was the only difference between the virulent and avirulent R. equi strains used in the experimental infections, this suggests that an important function of the virulence plasmid involves driving an ineffective Th2 immune response so that foals develop pneumonic disease rather than clearing infection. One factor determining whether foals become immune (Th1 response) or develop disease (Th2 response) in response to virulent R. equi may be the dose of virulent bacteria initiating the infection. By analogy, the dose of Mycobacterium tuberculosis BCG has been shown experimentally to determine whether a Th1 or a Th2 response developed, with relatively low doses leading to an almost exclusively cell-mediated, Th1 response (28). It may also be predicted that, as in mycobacterial infections, individuals vary in their ability to mount Th1 and Th2 immune responses (36, 44).
Aluminum hydroxide induces a Th2-biased response in mice, humans, and other species, which is reflected in the Ig subisotype of the antibodies produced (5, 6, 43). It was previously demonstrated that vaccination of foals with APTX antigen in aluminum hydroxide was associated with exacerbation of R. equi pneumonia and induced a greater IgGb and IgGT response than did natural infection, which induced an IgGa-biased response (30). Serum antibody responses following vaccination of horses with an aluminum hydroxide-based influenza vaccine which failed to produce protection were restricted to IgGT, whereas protective immunity produced by natural infection resulted in an IgGa and IgGb serum antibody response. An IgGa isotype bias was also observed in horses naturally infected with Streptococcus equi (35), in contrast to vaccinated animals, which produced only small amounts of serum IgGa. Horses infected with intestinal nematodes such as Anoplacephala perfoliata and Strongylus vulgaris produced a strong IgGT response (27, 31). These studies and the data presented here suggest that in horses the IgGT isotype reflects a Th2-like response and the IgGa isotype reflects a Th1-like response. The role of IgGb (the predominant isotype in serum, comprising 77% of total serum Ig) (34) is still unclear. However, the present data indicate that they reflect susceptibility in foals to R. equi pneumonia and therefore that they reflect a Th2 bias in the immune response. Interestingly, Lopez et al. (20) recently observed a greater than fourfold increase in VapA-specific IgGa and IgGb antibody levels following intrabronchial challenge of adult horses with live R. equi whereas the IgGT antibody levels increased only 2.6-fold. IgGa and IgGb opsonize microbes and fix complement, whereas IgGT not only does not fix complement but also may inhibit complement fixation by IgGa and IgGb (4, 22). A recent study of foals naturally exposed to R. equi infection also identified the dominance of IgGa and IgGb in these foals but did not relate this to the development of pneumonia in any of the foals (39). The course of the antibody response to each of the seven members of the R. equi Vap protein family in clinically normal foals suggests either that there is a differential pattern of antigen expression and/or that VapA and VapC are more immunogenic than the other Vap proteins, as well as possibly being cross-reactive. VapE appears not to be expressed early in infection or has low immunogenicity, since antibody to this protein was undetectable or present only in small amounts in all six pneumonic foals. The relative antibody response to each of the Vap proteins suggests that horses eventually develop antibodies to VapE. Anti-VapE antibody was detected in the pneumonic foal group, whereas the clinically normal foals had no response to this protein. The antibody response to VapD was also significantly greater in the pneumonic foals than in the clinically normal group. It may be possible to use a lack of antibody response to VapE in parallel with an antibody response to VapA to identify pneumonic foals or foals at risk of disease.
In conclusion, pneumonic foals had significantly more antibody to VapD and VapE than did healthy exposed foals, which may indicate a difference in the expression of these two proteins during persistent infection. Alternatively, it may reflect a skewing of the immune response toward VapD and VapE, which may influence anti-R. equi resistance. In addition, this study suggests that in horses the Ig isotypes IgGT and IgGb are Th2 related and that antibodies associated with them are probably ineffective in clearance of R. equi and allow for colonization and disease in foals. The IgGa isotype, in contrast, reflects a Th1 response in foals which do not develop pneumonia. The relative IgGa-to-IgGb and IgGa-to-IgGT ratios appear to influence the outcome of infection with R. equi (Table 2). The results of the comparison of immune adults and healthy foals to pneumonic foals suggest that the higher the IgGa-to-IgGb and IgGa-to-IgGT ratios, the better the animals are protected against R. equi. This study also suggests a possible age-related difference in the equine immune response affecting the Th1-Th2 bias, which, together with the antibody specificity bias toward VapD and VapE, may favor a susceptibility of foals to R. equi pneumonia.
Acknowledgments
This work was supported by the Grayson-Jockey Club Equine Research Fund, the Natural Sciences and Engineering Research Council of Canada, and the Ontario Ministry of Agriculture and Food.
We thank Steeve Giguère for pneumonic foal sera, Vivian Nicholson for technical assistance, and William Matthes-Sears for assistance with statistical analysis.
REFERENCES
- 1.Adkins, B. 1999. T-cell function in newborn mice and humans. Immunol. Today 20:330-335. [DOI] [PubMed] [Google Scholar]
- 2.Arlotti, M., G. Boboli, G.L. Moscatelli, G. Magnati, R. Masterati, V. Borghi, M. Andreoni, M. Libanore, L. Bonazzi, A. Piscina, and R. Ciammarughi. 1996. Rhodococcus equi infection in HIV-positive subjects: a retrospective analysis of 24 cases. Scand. J. Infect. Dis. 28:463-467. [DOI] [PubMed] [Google Scholar]
- 3.Balson, G. A., J. A. Yager, and B. A. Croy. 1992. SCID/beige mice in the study of immunity to Rhodococcus equi, p. 49-53. In P. D. Rossdale and J. F. Wade (ed.), Equine infectious diseases, 6th ed. R&W Publications, Newmarket, United Kingdom.
- 4.Banks, K. L., and T. C. McGuire. 1975. Surface receptors on neutrophils and monocytes from immunodeficient and normal horses. Immunology 28:581-588. [PMC free article] [PubMed] [Google Scholar]
- 5.Bomford, R. 1998. Will adjuvants be needed for vaccines of the future? Dev. Biol. Stand. 92:13-17. [PubMed] [Google Scholar]
- 6.Brewer, J. M., M. Conacher, C. A. Hunter, M. Mohrs, F. Brombacher, and J. Alexander. 1999. Aluminium hydroxide adjuvant initiates strong antigen-specific responses in the absence of IL-4 or IL-13-mediated signaling. J. Immunol. 163:6448-6454. [PubMed] [Google Scholar]
- 7.Burton, D. R., and J. M. Woof. 1992. Human antibody effector function. Adv. Immunol. 51:1-84. [DOI] [PubMed] [Google Scholar]
- 8.Dijkstra, C., and E. Dopp. 1983. Ontogenetic development of T- and B-lymphocytes and non-lymphoid cells in the white pulp of the rat spleen. Cell Tissue Res. 229:351-363. [DOI] [PubMed] [Google Scholar]
- 9.Donisi, A., M. G. Suardi, S. Casari, M. Longo, G. P. Cadeo, and G. Carosi. 1996. Rhodococcus equi infection in HIV-infected patients. AIDS 10:359-362. [DOI] [PubMed] [Google Scholar]
- 10.Fernandez, A. S., J. F. Prescott, and V. M. Nicholson. 1997. Protective effect against Rhodococcus equi infection in mice of IgG purified from horses vaccinated with virulence associated protein (VapA)-enriched antigen. Vet. Microbiol. 56:187-192. [DOI] [PubMed] [Google Scholar]
- 11.Furesz, S. E., B. N. Wilkie, B. A. Mallard, S. Rosendal, and J. I. MacInnes. 1998. Anti-haemolysin IgG1 to IgG2 ratios correlate with haemolysin neutralization titers and lung lesion scores in Actinobacillus pleuropneumonia infected pigs. Vaccine 16:1971-1975. [DOI] [PubMed] [Google Scholar]
- 12.Giguère, S., B. N. Wilkie, and J. F. Prescott. 1999. Modulation of cytokine response of pneumonic folas by virulent Rhodococcus equi. Infect. Immun. 67:5041-5047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harvey, R. L., and J. C. Sunstrum. 1991. Rhodococcus equi infections in patients with and without human immunodeficiency virus infection. Rev. Infect. Dis. 13:139-145. [DOI] [PubMed] [Google Scholar]
- 14.Hooper-McGrevy, K. E., S. Giguere, B. N. Wilkie, and J. F. Prescott. 2000. Evaluation of equine immunoglobulin specific for Rhodococcus equi virulence-associated proteins A and C for use in protecting foals against Rhodococcus equi-induced pneumonia. Am. J. Vet. Res. 62:1307-1313. [DOI] [PubMed] [Google Scholar]
- 15.Jefferis, R., and D. S. Kumararatne. 1990. Selective IgG deficiency: quantification and clinical relevance. Clin. Exp. Immunol. 81:357-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kanaly, S. T., S. A. Hines, and G. H. Palmer. 1993. Failure of pulmonary clearance of Rhodococcus equi infection in CD4+ T-lymphocyte-deficient transgenic mice. Infect. Immun. 61:4929-4932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kanaly, S. T., S. A. Hines, and G. H. Palmer. 1995. Cytokine modulation alters pulmonary clearance of Rhodococcus equi and development of granulomatous pneumonia. Infect. Immun. 63:3037-3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kanaly, S. T., S. A. Hines, and G. H. Palmer. 1996. Transfer of CD4+ Th1 cell line to nude mice effects clearance of Rhodococcus equi from the lung. Infect. Immun. 64:1126-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kovarik, J., and C.-A. Siegrist. 1998. Immunity in early life. Immunol. Today 19:150-152. [DOI] [PubMed] [Google Scholar]
- 20.Lopez, A. M., M. T. Hines, G. H. Palmer, D. C. Alperin, and S. A. Hines. 2002. Identification of pulmonary T-lymphocyte and serum antibody isotype responses associated with protection against Rhodococcus equi. Clin. Diagn. Lab. Immunol. 9:1270-1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marshall-Clark, S., D. Reen, L. Tasker, and J. Hassan. 2000. Neonatal immunity: how well has it grown up? Immunol. Today 21:35-41. [DOI] [PubMed] [Google Scholar]
- 22.McGuire, T. C., T. B. Crawford, and J. B. Henson. 1972. The isolation, characterization and functional properties of equine immunoglobulin classes and subclasses, p. 364-381. In J. T. Bryans and H. Gerber (ed.), Proceedings of the 3rd International Conference on Equine Infectious Diseases. S. Karger, Basel, Switzerland.
- 23.Murry, J. 1998. How the MHC selects Th1/Th2 immunity. Immunol. Today 19:157-163. [DOI] [PubMed] [Google Scholar]
- 24.Namikawa, R., T. Mizuno, H. Matsuoka, H. Fukumi, R. Ueda, and G. Itoh. 1983. Ontogenic development of T and B cells and non-lymphoid cells in the white pulp of human spleen. Immunology 57:61-69. [PMC free article] [PubMed] [Google Scholar]
- 25.Nelson, K. M., B. R. Schram, M. W. McGregor, A. S. Sheoran, C. W. Olsen, and D. P. Lunn. 1998. Local and systemic isotype-specific antibody responses to equine influenza virus infection versus conventional vaccination. Vaccine 16:1306-1313. [DOI] [PubMed] [Google Scholar]
- 26.Nordmann, P., E. Ronco, and C. Nauciel. 1992. Role of T-lymphocyte subsets in Rhodococcus equi infection. Infect. Immun. 60:2748-2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patton, S., R. E. Mock, J. H. Drudge, and D. Morgan. 1978. Increase in immunoglobulin T concentration in ponies as a response to experimental infection with the nematode Strongylus vulgaris. Am. J. Vet. Res. 39:19-24. [PubMed] [Google Scholar]
- 28.Power, C. A., G. Wei, and P. A. Bretscher. 1998. Mycobacterial dose defines the Th1/Th2 nature of the immune response independently of whether immunization is administered by the intravenous, subcutaneous, or intrademal route. Infect. Immun. 66:5743-5750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prescott, J. F. 1991. Rhodococcus equi: an animal and human pathogen. Clin. Microbiol. Rev. 4:20-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prescott, J. F., V. M. Nicholson, M. C. Patterson, M. C. Zandona Meleiro, A. Caterino de Araujo, J. A. Yager, and M. A. Holmes. 1997. Use of Rhodococcus equi virulence-associated protein for immunization of foals against Rhodococcus equi pneumonia. Am. J. Vet. Res. 58:356-359. [PubMed] [Google Scholar]
- 31.Proudman, C. J., and A. J. Trees. 1996. Correlation of antigen specific IgG and IgGT responses with Anoplocephala perfoliata infection intensity in the horse. Parasite Immunol. 18:499-506. [DOI] [PubMed] [Google Scholar]
- 32.Romangnani, S. 1997. The Th1/Th2 paradigm. Immunol. Today 18:263-266. [DOI] [PubMed] [Google Scholar]
- 33.Ross, T. L., G. A. Balson, J. S. Miners, G. D. Smith, P. E. Shewen, J. F. Prescott, and J. A. Yager. 1997. Role of CD4+, CD8+ and double negative T-cells in the protection of SCID/beige mice against respiratory challenge with Rhodococcus equi. Can. J. Vet. Res. 60:186-192. [PMC free article] [PubMed] [Google Scholar]
- 34.Sheoran, A. S., and M. A. Holmes. 1996. Separation of equine IgG subclasses (IgGa, IgGb and IgGT) using their differential binding characteristics for staphylococcus protein A and streptococcal protein G. Vet. Immunol. Immunopathol. 55:33-43. [DOI] [PubMed] [Google Scholar]
- 35.Sheoran, A. S., B. T. Sponseller, M. A. Holmes, and J. F. Timoney. 1997. Serum and mucosal antibody isotype responses to M-like protein (SeM) of Streptococcus equi in convalescent and vaccinated horses. Vet. Immunol. Immunopathol. 59:239-251. [DOI] [PubMed] [Google Scholar]
- 36.Surcel, H. M., M. Troye-Bloomberg, S. Paulie, G. Anderson, C. Moreno, G. Pasvol, and J. Ivanyi. 1994. Th1/Th2 profiles in tuberculosis, based on the proliferation and cytokine response of blood lymphocytes to mycobacterial antigens. Immunology 81:171-176. [PMC free article] [PubMed] [Google Scholar]
- 37.Takai, S., S. Hines, T. Sekizaki, V. M. Nicholson, D. A. Alperin, M. Osaki, D. Takamatsu, M. Nakamura, K. Suzuki, N. Ogino, T. Kakuda, H. Dan, and J. F. Prescott. 2000. DNA sequence and comparison of virulence plasmids from Rhodococcus equi ATCC 33701 and 103. Infect. Immun. 68:6840-6847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takai, S., K. Koike, S. Ohbushi, C. Izumi, and S. Tsubaki. 1991. Identification of 15- to 17-kilodalton antigens associated with virulent Rhodococcus equi. J. Clin. Microbiol. 29:439-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takai, S., I. Nakata, N. Fujui, Y. Kimura, Y. Sasaki, T. Kakuda, S. Tsubaki, T. Kondo, and T. Sugiura. 2002. Isotype-specific antibody responses to Rhodococcus equi in foals on a horse-breeding farm with a persistent incidence of R. equi infection. J. Equine Sci. 13:63-70. [Google Scholar]
- 40.Takai, S., T. Sekizaki, T. Ohbushi, C. Izumi, T. Sungawara, Y. Watanabe, and S. Tsubaki. 1991. Association between a large plasmid and 15- to 17-kilodalton antigens in virulent Rhodococcus equi. Infect. Immun. 59:4056-4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tan, C., J. F. Prescott, M. C. Patterson, and V. Nicholson. 1995. Molecular characterization of a lipid-modified virulence-associated protein of Rhodococcus equi and its potential in protective immunity. Can. J. Vet. Res. 59:51-59. [PMC free article] [PubMed] [Google Scholar]
- 42.Tkachuk-Saad, O., and J. F. Prescott. 1991. Rhodococcus equi plasmid: isolation and partial characterization. J. Clin. Microbiol. 29:2696-2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Victoratos, P., M. Yiangou, N. Avramidis, and L. Hadjipetrou. 1997. Regulation of cytokine gene expression by adjuvants in vivo. Clin. Exp. Immunol. 109:569-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wilsher, M. L., C. Hagan, R. Prestidge, A. U. Wells, and G. Murison. 1999. Human in vitro responses to Mycobacterium tuberculosis. 79:371-377. [DOI] [PubMed]
- 45.Yager, J. A., C. K. Duder, J. F. Prescott, and M. C. Zink. 1987. The interaction of Rhodococcus equi and foal neutrophils in vitro. Vet. Microbiol. 14:287-294. [DOI] [PubMed] [Google Scholar]
- 46.Yager, J. A., C. A. Prescott, D. P. Kramer, H. Honnah, G. A. Balson, and B. A. Croy. 1991. The effect of experimental infection with Rhodococcus equi on immunodeficient mice. Vet. Microbiol. 28:363-376. [DOI] [PubMed] [Google Scholar]
- 47.Zink, M. C., J. A. Johnson, J. F. Prescott, and P. J. Pascoe. 1982. The interaction of Corynebacterium equi and equine alveolar macrophages in vitro. J. Reprod. Fertil. Suppl. 32:491-496. [PubMed] [Google Scholar]
- 48.Zink, M. C., J. A. Yager, J. F. Prescott, and M. A. Fernando. 1987. Electron microscopic investigation of intracellular events after ingestion of Rhodococcus equi by foal alveolar macrophages. Vet. Microbiol. 14:295-305. [DOI] [PubMed] [Google Scholar]