Abstract
Otitis media with effusion (OME) is one of the most common ear diseases. Bacterial endotoxins and several inflammatory cytokines appear to be involved in the pathogenesis of OME in children; however, little is known of the immunological aspects of the onset of OME in adults. We sought to determine the presence of macrophage migration inhibitory factor (MIF) as well as interleukin 1β (IL-1β), tumor necrosis factor alpha (TNF-α), RANTES (regulated upon activation, normal T-cell expressed and presumably secreted), and endotoxin in middle ear effusions (MEEs) from adult patients with OME. In addition, the levels of MIF in MEEs from adults and children were compared. MEE was obtained from 95 adults and 11 children. The levels of MIF, IL-1β, TNF-α, and RANTES were determined by enzyme-linked immunosorbent assay, and the concentrations of endotoxin and total protein were determined by the Endospec assay and bicinchoninic acid assay, respectively. MIF was detected in 97.9% of the MEEs from adults, while endotoxin, IL-1β, TNF-α, and RANTES were detected in 96.8, 12.6, 5.3, and 43.9%, respectively. In addition, the level of MIF was significantly higher than those of endotoxin, IL-1β, and TNF-α. A positive correlation between the levels of MIF and endotoxin was observed. MIF and endotoxin were detected in 81.8 and 72.7%, respectively, of the MEEs from the children. The level of MIF was significantly higher in the children, and conversely that of endotoxin was significantly higher in the adults. These results suggest that the interaction between MIF and endotoxin may promote fluid collection in the middle ear, particularly in adults.
Otitis media with effusion (OME) is one of the most common middle ear diseases and leads to an intractable hearing loss in both children and adults (12, 14). In children, many studies have demonstrated the involvement of immune reactions and/or eustachian tube dysfunction induced by bacterial infections in the middle ear and/or nasopharynx in the pathogenesis of OME (4, 10, 13, 14, 16, 30, 31). To date, several inflammatory cytokines such as interleukin 1 (IL-1), IL-2, IL-6, IL-8, and tumor necrosis factor alpha (TNF-α) are observed in middle ear effusions (MEEs) (13, 30, 31). In addition, bacterial DNAs and components such as endotoxins are also detected in MEEs, whereas bacterial culture from MEEs is often negative (4, 10, 16).
On the other hand, little is known of the immunological aspects of the onset of OME in adults (12, 17, 22, 28). Recently, proinflammatory cytokines such as IL-1β and TNF-α were detected in some cases of MEEs from adult OME, although the presence of these cytokines in adults was less frequent than in children (22). RANTES (regulated upon activation, normal T-cell expressed and presumably secreted) may contribute to the pathogenesis of OME in children (27). Furthermore, the majority of MEEs from adult OME are PCR positive for bacterial genomic sequences, whereas most MEEs are culture negative for bacteria (17). In addition, one recent study revealed that sinusitis is also a common causal factor for adult onset OME (12). Endotoxin-producing pathogens such as Haemophilus influenzae and Moraxella catarrhalis can be detected in adult patients with sinusitis (23), thus suggesting that the immune responses triggered by bacterial endotoxins may be involved in the pathogenesis of the onset of OME in adults.
A 1966 report identified macrophage migration inhibitory factor (MIF) as a factor that inhibits the migration of macrophages in vitro and as the first cytokine secreted from activated T cells (9). Recently, MIF has been found to be secreted from not only T cells but also various cells such as monocytes/macrophages, eosinophils, keratinocytes, epithelial cells, and corticotropic cells of the anterior lobe of hypophysis in response to infection and stress (7, 19, 25). Among these, endotoxins from gram-negative bacteria have been shown to be powerful inducers of MIF secretion by macrophages, and MIF induces the release of TNF-α from macrophages through autocrine and paracrine mechanisms (7). MIF is currently believed to play a pivotal role in regulating inflammatory responses triggered by bacterial endotoxins since mice deficient in the MIF gene are resistant to septic shock (5). In addition, MIF functions as a counterbalance of the anti-inflammatory and immunosuppressive effects of glucocorticoids (6).
Although one very early report from 1976 has demonstrated the existence of MIF in MEE (3), the role of MIF in OME, particularly in adults, has not been determined over the past 2 decades. In the present study, we investigated the existence of MIF as well as IL-1β, TNF-α, RANTES, and endotoxin in MEEs from adult patients with OME. In addition, the levels of MIF in adult and pediatric patients with OME were compared. Our results demonstrate that MIF is present in most MEEs from adult OME and may play an important role in the pathogenesis of OME after the host's exposure to endotoxins in the middle ear.
MATERIALS AND METHODS
Patients and samples.
MEEs were collected from 95 adult subjects (47 males and 48 females, aged 35 to 92 years [mean, 64.9 years]) and 11 children (4 males and 7 females, aged 6 months to 13 years [mean, 4.9 years]) after myringotomy or insertion of a ventilation tube. All patients had obvious effusion, and the effusion could be detected through the eardrum. Prior to collection of MEEs, 8 of the 95 adult subjects and 6 of the 11 children had had an episode of acute otitis media (AOM) involving earache, fever, and/or otorrhea within the previous month. No patients took antibiotics or immunosuppressive drugs, including corticosteroids, within the 2 weeks prior to MEE collection. In addition, no neoplasms, including nasopharyngeal cancer, or immune deficiency was detected in any patient. Nineteen patients had allergic rhinitis.
The collected MEEs were transferred to 0.65-ml microcentrifuge tubes (Corning) and then were classified into two groups (mucoid or serous type) based on whether or not they flowed on inversion. Of the 95 adult subjects, 30 had MEEs of the mucoid type and 65 had MEEs of the serous type. On the other hand, five children had mucoid-type MEEs, while the remaining six had serous-type MEEs. The collected MEEs were stored at −80°C until analysis. All MEEs were analyzed within 6 months after collection. Myringotomy and ventilation tube insertion were performed after informed consent was obtained from the patient or guardian. Since the samples used were surplus material following surgery, additional consent from patients and clearance by an ethics committee were not required.
Measurement of MIF, IL-1β, TNF-α, RANTES, endotoxin, and total protein in MEEs.
Samples were spun in a centrifuge at 2,236 × g for 10 min to separate cellular components. After centrifugation, the concentrations of MIF, IL-1β, TNF-α, RANTES, endotoxin, and total protein in MEEs were determined. Levels of IL-1β and TNF-α in MEEs were measured with Opt enzyme immunoassay sets (Pharmingen) according to the manufacturer's instructions. The detection limit of these assays was 10 pg/ml for both IL-1β and TNF-α. The levels of RANTES in the 66 adult subjects were measured with Quantikine (R&D Systems Inc.) according to the manufacturer's instructions. The level of MIF was determined by sandwich enzyme-linked immunosorbent assay as described previously (21). Briefly, Maxisorp 96-well plates (Nunc Laboratories Ltd.) were coated with 50 μl of mouse anti-human MIF monoclonal antibody (R&D Systems Inc.) at 2.0 mg/ml in 0.05 M carbonate buffer, pH 9.6, and incubated overnight at 4°C. After the plate was washed with phosphate-buffered saline (PBS) containing 0.05% Tween 20 (PBS-Tween 20), each well was blocked with PBS containing 10% fetal bovine serum (FBS) and allowed to stand at 37°C for 2 h. The plate was washed again with PBS-Tween 20, 50 μl of either the sample diluted 1:20 in PBS or purified recombinant human MIF (R&D Systems Inc.) was added to two wells, and the plate was then allowed to stand at 37°C for 2 h. Standard curves were created with recombinant human MIF (0 to 8,000 pg/ml), and the sensitivity of detection was 10 pg/ml. After the plate was washed four times with PBS-Tween 20, 80 μl of 1-mg/ml biotinylated anti-human MIF (R&D Systems Inc.) diluted in PBS-10% FBS was added to each well, and then plate was allowed to stand at 37°C for 2 h. After the plate was washed, 80 μl of ExtrAvidin-peroxidase (Sigma Chemical Co., St. Louis, Mo.) diluted 1:1,000 with PBS-10% FBS was added to each well, and the plate was allowed to stand at 37°C for 1 h. After the plate was washed, 80 μl of tetramethylbenzidine substrate (Kirkegaard and Perry) was added to each well, and the plate was allowed to stand at room temperature for 15 min. The reaction was stopped with 25 μl of 5% phosphoric acid, and the absorbance was determined at 450 nm with a microplate reader (Molecular Devices).
The concentration of endotoxin in MEEs was determined with an Endospec assay kit (Seikagaku Kogyo Corporation, Tokyo, Japan) according to the manufacturer's instructions. The detection limit for the assay was 4 pg/ml. The concentration of total protein in the MEEs was determined by bicinchoninic acid assay according to the manufacturer's instructions (Pierce, Rockford, Ill.). All samples were determined in duplicate, and the measured values were then averaged. The concentrations of MIF, IL-1β, TNF-α, and endotoxin were divided by the concentration of total protein (milligrams of total protein per milliliter) for standardization.
Statistical analysis.
Data represent the means ± standard deviations. Statistical analysis was performed by using the nonparametrical Mann-Whitney U test, χ2 test, and Spearman's rank correlation. Significant difference was established at a P level of <0.05.
RESULTS
Levels of MIF, endotoxin, IL-1β, TNF-α, and RANTES in MEEs from adult subjects.
MIF was detected in the MEEs of 93 of the 95 adult subjects (97.9%), with concentrations ranging from 3.7 to 83,255.7 pg/mg of total protein (mgTP) (25th percentile, 78.3 pg/mgTP; 50th percentile, 174.7 pg/mgTP; 75th percentile, 412.7 pg/mgTP), while endotoxin, IL-1β, and TNF-α were detected in the MEEs from 92 (96.8%; range, 2.5 to 3,435.7 pg/mgTP), 12 (12.6%; range, 0.6 to 1,473.2 pg/mgTP), and 5 (5.3%; range, 2.7 to 554.6 pg/mgTP) of the 95 adult subjects, respectively (Table 1). MIF was present in significantly higher concentrations than endotoxin (P < 0.001), IL-1β (P < 0.001), and TNF-α (P < 0.001). In addition, endotoxin was present in significantly higher concentrations than IL-1β (P < 0.001) and TNF-α (P < 0.001). In contrast, no significant difference between the concentrations of IL-1β and TNF-α was observed (P = 0.090).
TABLE 1.
Incidence of endotoxin, cytokines (MIF, IL-1β, and TNF-α), and RANTES in MEEs in adults
| Protein | No. of positive samples/total no. of samples | % Positive samples |
|---|---|---|
| MIF | 93/95 | 97.9 |
| IL-1β | 12/95 | 12.6 |
| TNF-α | 5/95 | 5.3 |
| RANTES | 29/66 | 43.9 |
| Endotoxin | 92/95 | 96.8 |
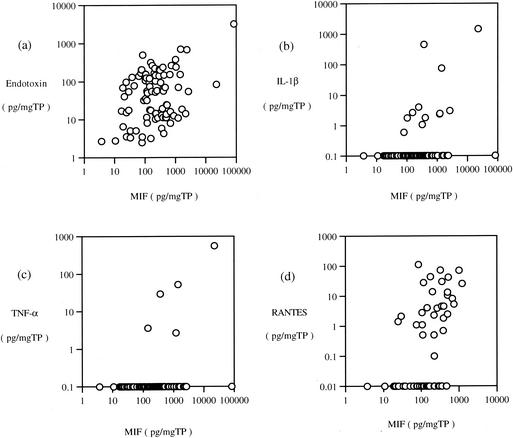
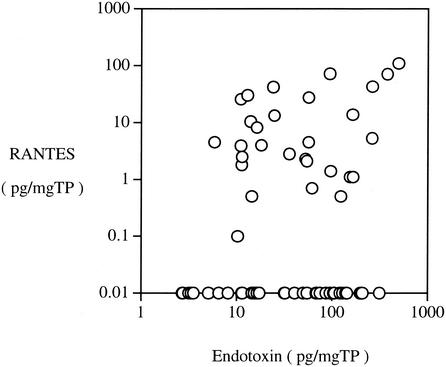
Significantly a positive correlation between the levels of MIF and endotoxin in adult MEEs was observed (Fig. 2a; P < 0.001). In addition, the level of MIF showed significant correlations with the levels of IL-1β (Fig. 2b; P = 0.008) and TNF-α (Fig. 2c; P = 0.022). A significantly positive correlation between the levels of endotoxin and IL-1β was observed (P = 0.038). However, no significant correlation between the level of endotoxin and that of TNF-α was obtained (P = 0.887). On the other hand, a highly significant strong correlation between the levels of IL-1β and TNF-α was obtained (P < 0.001). The RANTES level in adult MEEs showed significant correlation with the levels of MIF (Fig. 2d; P < 0.001) and endotoxin (Fig. 3; P < 0.001).
FIG. 2.
Correlation between the levels of MIF and endotoxin (a), IL-1β (b), TNF-α (c), and RANTES (d) in MEEs in adult cases. Concentrations of MIF, endotoxin, IL-1β, and TNF-α were divided by the concentration of total protein of each sample for standardization. The level of MIF showed significant correlation with the levels of endotoxin, IL-1β, TNF-α, and RANTES.
FIG. 3.
Significant positive correlation between the levels of RANTES and endotoxin in MEEs in the 66 adult cases.
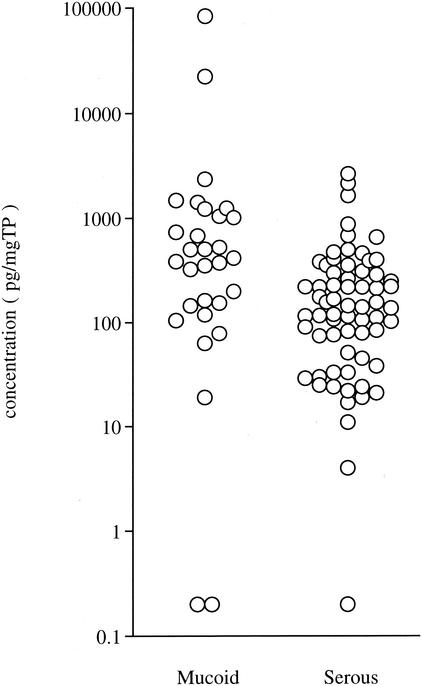
The level of MIF in mucoid MEEs (mean, 4,040.6 ± 15,492.3 pg/mgTP) was significantly higher than that in serous effusions (mean, 279.4 ± 458.9 pg/mgTP; P = 0.002) (Fig. 4). The levels of endotoxin (mean, 357.6 ± 822.6 pg/mgTP) and TNF-α (mean, 21.3 ± 101.3 pg/mgTP) were also significantly higher in mucoid MEEs than in serous MEEs (mean for endotoxin, 69.1 ± 85.3 pg/mgTP [P = 0.027]; mean for TNF-α, 0.1 ± 0.5 pg/mgTP [P = 0.016]) (Table 2). The level of IL-1β in mucoid MEEs (mean, 67.0 ± 278.5 pg/mgTP) was high, but no significant difference from that in serous effusions (mean, 0.2 ± 0.8 pg/mgTP [P = 0.120]) was observed.
FIG. 4.
The concentrations of MIF in mucoid and serous MEEs in the 95 adult cases.
TABLE 2.
Concentrations of endotoxin and cytokines in mucoid and serous MEEs
| Type | Patient | No. of cases | Mean concn (pg/mgTP) ± SD of:
|
|||
|---|---|---|---|---|---|---|
| MIF | IL-1β | TNF-α | Endotoxin | |||
| Mucoid | Adult | 30 | 4,040.6 ± 15,492.3 | 67.0 ± 278.5 | 21.3 ± 101.3 | 357.6 ± 822.6 |
| Child | 5 | 9,078.0 ± 18,209.3 | 35.6 ± 79.6 | 0.0 ± 0.0 | 41.8 ± 74.4 | |
| Serous | Adult | 65 | 279.4 ± 458.9 | 0.2 ± 0.8 | 0.1 ± 0.5 | 69.1 ± 85.3 |
| Child | 6 | 692.3 ± 593.2 | 5.7 ± 14.1 | 0.0 ± 0.0 | 52.4 ± 113.4 | |
In addition, patients who experienced AOM within 1 month prior to the collection of MEEs showed significantly higher levels of IL-1β (mean, 250.9 ± 518.2 pg/mgTP) and TNF-α (mean, 79.5 ± 192.9 pg/mgTP) than patients with no history of AOM during the same period (mean for IL-1β, 0.2 ± 0.7 pg/mgTP [P < 0.001]; mean for TNF-α, 0.1 ± 0.5 pg/mgTP [P < 0.001]) (Table 3). On the other hand, the levels of MIF and endotoxin for the patients with (MIF, 3,095.3 ± 7,807.4 pg/mgTP; endotoxin, 44.0 ± 51.2 pg/mgTP) or without (MIF, 1,317.4 ± 8,901.5 pg/mgTP [P = 0.688]; endotoxin, 170.9 ± 501.9 pg/mgTP [P = 0.321]) a history of AOM were similar.
TABLE 3.
Concentrations of endotoxin and cytokines in MEEs from patients with or without AOM prior to OME
| Prior AOM | Patient | No. of cases | Mean concn (pg/mgTP) ± SD of:
|
|||
|---|---|---|---|---|---|---|
| MIF | IL-1β | TNF-α | Endotoxin | |||
| Present | Adult | 8 | 3,095.3 ± 7,807.4 | 250.9 ± 518.2 | 79.5 ± 192.9 | 44.0 ± 51.2 |
| Child | 6 | 7,745.1 ± 16,616.5 | 35.4 ± 71.2 | 0.0 ± 0.0 | 78.5 ± 121.5 | |
| Absent | Adult | 87 | 1,317.4 ± 8,901.5 | 0.2 ± 0.7 | 0.1 ± 0.5 | 170.9 ± 501.9 |
| Child | 5 | 614.6 ± 403.2 | 0.0 ± 0.0 | 0.0 ± 0.0 | 10.5 ± 8.2 | |
Comparison of MIF level in MEEs from children and adult patients with OME.
MIF, endotoxin, and IL-1β were detected in the MEEs of 9 (81.8%; range, 520.7 to 41,646.3 pg/mgTP), 8 (72.7%; range, 2.8 to 283.4 pg/mgTP), and 2 (18.2%; range, 34.6 to 177.9 pg/mgTP), respectively, of the 11 children. However, TNF-α was not detected in the MEEs of these children. The incidence of IL-1β in MEEs was significantly lower than that of MIF (P = 0.003 by χ2 test) or endotoxin (P = 0.010).
The mean levels of MIF, endotoxin, and IL-1β in the children's MEEs were 4,504.0 ± 12,328.3, 47.6 ± 93.1, and 19.3 ± 53.6 pg/mgTP, respectively. MIF was present at a significantly higher concentration than either endotoxin (P = 0.006) or IL-1β (P < 0.001). In addition, endotoxin was present at a significantly higher concentration than IL-1β (P = 0.045). However, no significant correlation between the level of endotoxin and that of MIF was obtained (r = 0.461, P = 0.158).
A comparison of the levels of MIF, endotoxin, and IL-1β in MEEs from adult (n = 95) and pediatric (n = 11) patients revealed that the level of MIF was significantly higher in the children (P = 0.013) and that, conversely, the level of endotoxin was significantly higher in the adults (P = 0.022). The concentrations of IL-1β in MEEs from adults and children were not significantly different (P = 0.526).
DISCUSSION
In the present study, we demonstrated that MIF is frequently found in MEEs. In particular, this is the first study to detect MIF in MEEs from not only children but also most (97.9%) of the adult OME patients examined. These findings suggest that MIF may play a key role in the onset and/or exacerbation of adult OME. Despite the fact that adult OME is not an uncommon disease (12), the pathogenesis of the onset of adult OME remains unclear. Nasopharyngeal carcinoma, sinusitis, bacteria, and production of proinflammatory cytokines are all thought to be involved in the onset of this disease (12, 13, 17, 22, 26, 28). In the present study, we excluded patients with neoplasms, including nasopharyngeal carcinoma, since most of those patients received radiotherapy and/or chemotherapy, which, in addition to the tumor burden itself, may have affected host immunity (15).
Several cytokines such as IL-1β, TNF-α, and IL-8 have been found in adult MEEs (13, 22). Ondrey et al. reported that IL-1β and TNF-α were present in 12.0 and 8.0%, respectively, of MEEs from adult OME (22). Although we did not examine the level of IL-8 in MEEs, the present study detected IL-1β and TNF-α in 12.6 and 5.3%, respectively, of MEEs from adults. This result is consistent with the report of Ondrey et al. demonstrating IL-1β and TNF-α in 12.0 and 8.0%, respectively, of MEEs from adult OME (22). The low rates of these two cytokines in adult MEEs suggest that the onset or prognosis or both of adult OME are not strictly associated with IL-1β and TNF-α. Schousboe et al. reported that RANTES was present in 94 (82%) of 114 effusions from children, with a median concentration of 79.7 pg/mgTP (27). The level of RANTES in adult subjects is lower (17.5 pg/mgTP).
Another fascinating finding is that endotoxin was frequently (96.8%) detected in MEEs from our adult patients with OME. Endotoxins are lipopolysaccharide complexes found on the outer surfaces of most gram-negative bacteria such as Haemophilus influenzae and Moraxella catarrhalis (10). In pediatric OME, it is well known that endotoxin-positive MEE is detected at high rates. DeMaria et al., Iino et al., and Dingman et al. reported that 80.0, 69.0, and 76.4%, respectively, of MEEs from children exhibit endotoxin activity (10, 11, 16). Our present result that 72.7% of pediatric MEEs contain endotoxins is consistent with those reports.
We did not attempt to culture bacteria from the MEEs, as bacteria in MEEs are notoriously difficult to culture (4, 11, 16). Beswick et al. demonstrated that many bacterial species were detected in MEEs by PCR, whereas no bacterial growth was detected by culture-based approaches for 10 of their 12 patients (4). In addition, endotoxin has been shown to be present in MEEs negative for bacteria by both culture and Gram stain (10). Thus, the high prevalence of endotoxin-positive MEE may reflect the presence of gram-negative bacteria in the middle ear.
The levels of MIF and endotoxin in MEEs are positively and significantly correlated (P < 0.001). Significant correlation between the levels of endotoxin and IL-1β is observed (P = 0.005). On the other hand, the amount of endotoxin present is not correlated with the levels of TNF-α (P = 0.887). In pediatric MEEs, there are strong correlations between the concentrations of endotoxin and TNF-α and between the concentrations of endotoxin and IL-1β (29). These results strongly suggest that the interaction between endotoxin and MIF rather than that between endotoxin and TNF-α may be involved in the pathogenesis of OME in adults. In addition, a very significant strong correlation between the level of IL-1β and that of TNF-α (P < 0.001) in adult MEEs was observed; this is consistent with the report by Ondrey et al. demonstrating that the amount of TNF-α present is strongly correlated with the levels of IL-1β in pediatric MEEs (22). The levels of RANTES and endotoxin in MEEs are positively and significantly correlated (P < 0.001). This result is consistent with the report of Schousboe et al. demonstrating RANTES in MEEs from children (27). In addition, the levels of RANTES and MIF in MEEs are positively and significantly correlated (P < 0.001).
MIF promotes inflammatory responses, including release of proinflammatory mediators such as TNF-α, by macrophages and activating T cells (7). After stimulation of endotoxin, MIF is rapidly released from various cells such as macrophages and pituitary cells and promotes innate immune responses through activation of NF-κB and Toll-like receptor 4 (2, 7, 24). In fact, mice deficient in the MIF gene are resistant to endotoxic shock, and MIF is absent from the serum of MIF gene-deficient mice (5). Mice are protected from endotoxic shock when MIF activity is neutralized by anti-MIF antibodies (8). Furthermore, MIF is produced rapidly following infection with Escherichia coli, and mortality and TNF-α production decrease in mice upon treatment with anti-MIF antibodies (8). In addition, septic shock due to E. coli-induced peritonitis is exacerbated in mice inoculated with recombinant MIF (8). Thus, the present results suggest that responses against endotoxin in the middle ear lead to MIF production and subsequently induce MEEs, particularly in adults.
In the present study, adult patients who had a recent history of AOM and those with no history of AOM showed similar levels of MIF and endotoxin in MEEs, suggesting that active infection with gram-negative bacteria is not involved in the production of MIF but that the presence of the bacteria and subsequent release of endotoxin may be key factors in the induction of MIF production in the middle ear. On the other hand, adult patients showed significantly higher levels of IL-1β and TNF-α when they had a recent history of AOM. This result further suggests that active infection promotes IL-1β and TNF-α production in the middle ear. In fact, upper airway infection is known to increase the level of TNF-α in MEEs (20). In addition, Barzilai et al. reported that the level of IL-1β in MEEs was significantly higher in culture-positive patients than in culture-negative children (1).
The level of MIF, as well as those of endotoxin and TNF-α, in MEEs was significantly higher in mucoid-type than in serous-type MEEs. TNF-α plays an important role in the development of mucous otitis media by promoting Muc2, the mucin gene, expressed in the middle ear epithelium (18). As described above, production of MIF is stimulated by TNF-α, and subsequently MIF induces the release of TNF-α from macrophages through autocrine and paracrine mechanisms (7). Thus, the interaction between MIF and TNF-α may cause the MEEs to be mucous in nature.
Although we examined limited samples, MIF was detected in 81.8% of MEEs from pediatric OME, suggesting that MIF may also play an important role in the pathogenesis of pediatric OME.
In conclusion, we demonstrated that MIF and endotoxin are frequently detected in adult MEEs. Importantly, a strong correlation between the presence of MIF and endotoxin was obtained, and both were detected even in those who had no recent history of AOM. In addition, MIF was also frequently detected in pediatric MEEs. These results suggest that the interaction between MIF and endotoxin may promote fluid collection in the middle ear, particularly in adults. However, whether the level of MIF is a result of the endotoxin-induced MEEs or is actually part of the cause is unclear. Endotoxin induces the elaboration of MIF, which can induce TNF-α. The latter is not detected in many MEEs by enzyme-linked immunosorbent assay. In the future, when appropriate anti-MIF treatments are possible in humans, it might be possible to diminish the MEEs from OME. These results provide a basis for future therapeutic approaches in the management of OME by regulating MIF activation triggered by endotoxins.
RANTES was detected in the MEEs from 29 (43.9%; range, 0.1 to 109.9 pg/mgTP) of the 66 adult subjects. The incidence of MIF-positive MEEs was significantly greater than that of IL-1β- (P < 0.001 by χ2 test) and TNF-α-positive (P < 0.001) samples but not that of endotoxin-positive samples (P = 0.650). The incidence of endotoxin-positive MEEs was also significantly greater than that of IL-1β- (P < 0.001 by χ2 test) and TNF-α-positive (P < 0.001) MEEs. In contrast, no significant difference between the detection rates for IL-1β and TNF-α was observed (P = 0.075).
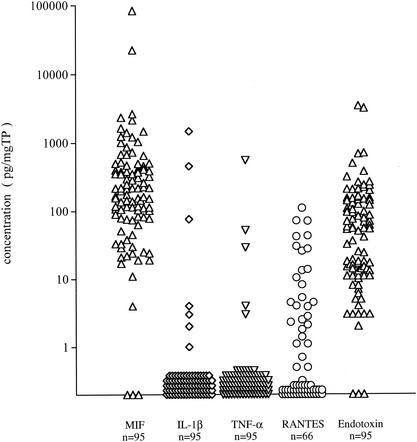
The mean levels of MIF, endotoxin, IL-1β, TNF-α, and RANTES in MEEs from adult OME were 1,467.1 ± 8,790.8, 160.2 ± 481.6, 21.3 ± 157.8, 6.8 ± 57.1, and 17.5 ± 26.8 pg/mgTP, respectively (Fig. 1).
FIG. 1.
Concentration of cytokines, RANTES, and endotoxin in MEEs in adult cases. Concentrations of MIF, IL-1β, TNF-α, RANTES, and endotoxin were divided by the concentration of total protein of each sample for standardization. MIF showed a significantly higher concentration than IL-1β (P < 0.001), TNF-α (P < 0.001), RANTES (P < 0.001), and endotoxin (P < 0.001) (nonparametrical Mann-Whitney U test).
Acknowledgments
We thank Yuko Okano for editorial assistance.
This work was supported by grants from the Ministry of Education, Culture, Sports, Science and Technology, Japan (14704043).
REFERENCES
- 1.Barzilai, A., E. Leibovitz, J. H. Laver, L. Piglansky, S. Raiz, M. R. Abboud, D. M. Fliss, A. Leiberman, and R. Dagan. 1999. Dynamics of interleukin-1 production in middle ear fluid during acute otitis media treated with antibiotics. Infection 27:173-176. [DOI] [PubMed] [Google Scholar]
- 2.Bernhagen, J., T. Calandra, R. A. Mitchell, S. B. Martin, K. J. Tracey, W. Voelter, K. R. Manogue, A. Cerami, and R. Bucala. 1993. MIF is a pituitary-derived cytokine that potentiates lethal endotoxaemia. Nature 365:756-759. [DOI] [PubMed] [Google Scholar]
- 3.Bernstein, J. M. 1976. Biological mediators of inflammation in middle ear effusions. Ann. Otol. Rhinol. Laryngol. 85:90-96. [DOI] [PubMed] [Google Scholar]
- 4.Beswick, A. J., B. Lawley, A. P. Fraise, A. L. Pahor, and N. L. Brown. 1999. Detection of Alloiococcus otitis in mixed bacterial populations from middle-ear effusions of patients with otitis media. Lancet 354:386-389. [DOI] [PubMed] [Google Scholar]
- 5.Bozza, M., A. R. Satoskar, G. Lin, B. Lu, A. A. Humbles, C. Gerard, and J. R. David. 1999. Targeted disruption of migration inhibitory factor gene reveals its critical role in sepsis. J. Exp. Med. 189:341-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calandra, T., J. Bernhagen, C. N. Metz, L. A. Spiegel, M. Bacher, T. Donnelly, A. Cerami, and R. Bucala. 1995. MIF as a glucocorticoid-induced modulator of cytokine production. Nature 377:68-71. [DOI] [PubMed] [Google Scholar]
- 7.Calandra, T., J. Bernhagen, R. A. Mitchell, and R. Bucala. 1994. The macrophage is an important and previously unrecognized source of macrophage migration inhibitory factor. J. Exp. Med. 179:1895-1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calandra, T., B. Echtenacher, D. LeRoy, J. Pugin, C. N. Metz, L. Hültner, D. Heumann, D. Männel, R. Bucala, and M. P. Glauser. 2000. Protection from septic shock by neutralization of macrophage migration inhibitory factor. Nat. Med. 6:164-170. [DOI] [PubMed] [Google Scholar]
- 9.David, J. R. 1966. Delayed hypersensitivity in vitro: its mediation by cell-free substances formed by lymphoid cell-antigen interaction. Proc. Natl. Acad. Sci. USA 56:72-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeMaria, T. F., R. B. Prior, B. R. Briggs, D. J. Lim, and H. G. Birck. 1984. Endotoxin in middle-ear effusions from patients with chronic otitis media with effusion. J. Clin. Microbiol. 20:15-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dingman, J. R., M. G. Rayner, S. Mishra, Y. Zhang, M. D. Ehrlich, J. C. Post, and G. D. Ehrlich. 1998. Correlation between presence of viable bacteria and presence of endotoxin in middle-ear effusions. J. Clin. Microbiol. 36:3417-3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Finkelstein, Y., D. Ophir, Y. P. Talmi, A. Shabtai, M. Strauss, and Y. Zohar. 1994. Adult-onset otitis media with effusion. Arch. Otolaryngol. Head Neck Surg. 120:517-527. [DOI] [PubMed] [Google Scholar]
- 13.Hotomi, M., T. Samukawa, and N. Yamanaka. 1994. Interleukin-8 in otitis media with effusion. Acta Oto-Laryngol. 114:406-409. [DOI] [PubMed] [Google Scholar]
- 14.Howie, V. M., J. H. Ploussard, and J. Sloyer. 1975. The “otitis-prone” condition. Am. J. Dis. Child. 129:676-678. [DOI] [PubMed] [Google Scholar]
- 15.Hsu, M. M., K. R. Wang, T. C. Lynn, T. Hsieh, S. C. Huang, and S. M. Tu. 1980. Immunologic reactivity in patients with nasopharyngeal carcinoma. Otolaryngol. Head Neck Surg. 88:384-390. [DOI] [PubMed] [Google Scholar]
- 16.Iino, Y., Y. Kaneko, and T. Takasaka. 1985. Endotoxin in middle ear effusion with lumulus assay. Acta Oto-Laryngol. 100:42-50. [DOI] [PubMed] [Google Scholar]
- 17.Liederman, E. M., J. C. Post, J. J. Aul, D. A. Sirko, G. J. White, C. A. Buchman, and G. D. Ehrlick. 1998. Analysis of adult otitis media: polymerase chain reaction versus culture for bacteria and viruses. Ann. Otol. Rhinol. Laryngol. 107:10-16. [DOI] [PubMed] [Google Scholar]
- 18.Lin, J., A. Haruta, H. Kawano, S. B. Ho, G. L. Adams, S. K. Juhn, and Y. Kim. 2000. Induction of mucin gene expression in middle ear of rats by tumor necrosis factor-alpha: potential cause for mucoid otitis media. J. Infect. Dis. 182:882-887. [DOI] [PubMed] [Google Scholar]
- 19.Maaser, C., L. Eckmann, G. Paesold, H. S. Kim, and M. F. Kagnoff. 2002. Ubiquitous production of macrophage migration inhibitory factor by human gastric and intestinal epithelium. Gastroenterology 122:667-680. [DOI] [PubMed] [Google Scholar]
- 20.Nell, M. J., and J. J. Grote. 1999. Endotoxin and tumor necrosis factor-alpha in middle ear effusions in relation to upper airway infection. Laryngoscope 109:1815-1819. [DOI] [PubMed] [Google Scholar]
- 21.Okano, M., A. R. Satoskar, K. Nishizaki, and D. A. Harn. Jr. 2001. Lacto-N-fucopentaose III found on Schistosoma mansoni egg antigens functions as adjuvant for proteins by inducing Th2-type response. J. Immunol. 167:442-450. [DOI] [PubMed] [Google Scholar]
- 22.Ondrey, F. G., S. K. Juhn, and G. L. Adams. 1998. Early-response cytokine expression in adult middle ear effusions. Otoryngol. Head Neck Surg. 119:342-345. [DOI] [PubMed] [Google Scholar]
- 23.Ramadan, H. H. 1995. What is the bacteriology of chronic sinusitis in adults? Am. J. Otolaryngol. 16:303-306. [DOI] [PubMed] [Google Scholar]
- 24.Roger, T., J. David, M. P. Glauser, and T. Calandra. 2001. MIF regulates innate immune responses through modulation of Toll-like receptor 4. Nature 414:920-924. [DOI] [PubMed] [Google Scholar]
- 25.Rossi, A. G., C. Haslett, N. Hirani, A. P. Greening, I. Rahman, C. N. Metz, R. Bucala, and S. C. Donnelly. 1998. Human circulating eosinophils secrete macrophage migration inhibitory factor (MIF): potential role in asthma. J. Clin. Investig. 15:2869-2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sade, J., and C. Fuchs. 1996. Secretory otitis media in adults. II. The role of mastoid pneumatization as a risk factor. Ann. Otol. Rhinol. Laryngol. 105:643-647. [DOI] [PubMed] [Google Scholar]
- 27.Schousboe, L. P., L. M. Rasmussen, and T. Ovesen. 2001. RANTES in otitis media with effusion: presence, role and correlation with cytokines and microbiology. APMIS 109:441-446. [DOI] [PubMed] [Google Scholar]
- 28.Schuknecht, H. F., G. M. Zaytoun, and C. N. Moon, Jr. 1982. Adult-onset fluid in the tympanomastoid compartment. Diagnosis and management. Arch. Otolaryngol. 108:759-765. [DOI] [PubMed] [Google Scholar]
- 29.Willet, D. N., R. P. Rezaee, J. M. Billy, M. B. Tighe, and T. F. DeMaria. 1998. Relationship of endotoxin to tumor necrosis-alpha and interleukin-1 beta in children with otitis media with effusion. Ann. Otol. Rhinol. Laryngol. 107:28-33. [DOI] [PubMed] [Google Scholar]
- 30.Yellon, R. F., G. Leonard, P. T. Marucha, R. Craven, R. J. Carpenter, W. B. Lehmann, J. A. Burleson, and D. L. Kreutzer. 1991. Characterization of cytokines present in middle ear effusions. Laryngoscope 101:165-169. [DOI] [PubMed] [Google Scholar]
- 31.Yellon, R. F., G. Leonard, P. Marucha, J. Sidman, R. Carpenter, J. Burleson, J. Carlson, and D. Kreutzer. 1992. Demonstration of interleukin 6 in middle ear effusions. Arch. Otolaryngol. Head Neck Surg. 118:745-748. [DOI] [PubMed] [Google Scholar]