Abstract
MD-2 has been reported to be required for the translocation of the Toll-like receptor 4 (TLR4) to the cell surface. However, the mechanism by which MD-2 promotes TLR4 translocation is unknown. We identified the presence of two forms of TLR4 with different molecular masses (approximately 110 and 130 kDa) when TLR4 was expressed together with MD-2. Expressing TLR4 alone produced only the 110-kDa form. Using a membrane-impermeable biotinylation reagent, we found that only the 130-kDa form of TLR4 was expressed on the cell surface. When a cellular extract prepared from cells expressing TLR4 and MD-2 was treated with N-glycosidase, the two forms of TLR4 converged into a single band whose size was smaller than the 110-kDa form of TLR4. Mutation of TLR4 at Asn526 or Asn575 resulted in the disappearance of the 130-kDa form and prevented TLR4 from being expressed on the cell surface without affecting the ability of TLR4 to associate with MD-2. These results indicate that TLR4 is able to undergo multiple glycosylations without MD-2 but that the specific glycosylation essential for cell surface expression requires the presence of MD-2.
Toll-like receptors (TLRs) are mammalian homologues of the Drosophila Toll protein which plays critical roles in the establishment of dorsoventral polarity and antifungal response in adult flies (9). The TLR family is involved in the innate immune system in mammals. Among TLRs, TLR4 is responsible for lipopolysaccharide (LPS)-induced signaling (5, 14, 15). TLR4 associates with MD-2 and CD14 on the cell surface, forming a receptor complex for LPS. TLR4 recognizes LPS and transmits the LPS signal into the cytoplasm. Although MD-2 is known to be essential for LPS-induced signaling via TLR4 (16), the precise role of MD-2 in LPS signaling has been ambiguous. Recently, several groups have investigated the precise role of MD-2 in LPS signaling and demonstrated that MD-2 is involved in the recognition of LPS molecules (1, 10, 11, 19) and in the membrane surface translocation of TLR4 (6, 12). However, the mechanism through which MD-2 promotes the translocation of TLR4 to the membrane surface remains unknown.
In this study, we demonstrate that MD-2 is required for the specific glycosylation of TLR4, which is essential for the cell surface expression of TLR4.
MATERIALS AND METHODS
Cells and reagents.
The human embryonic kidney 293 cell line (obtained from the Human Science Research Resource Bank, Tokyo, Japan) was grown in Dulbecco's modified Eagle's medium (Gibco BRL, Rockville, Md.) supplemented with 10% (vol/vol) heat-inactivated fetal calf serum (Gibco BRL), penicillin (100 U/ml), and streptomycin (100 μg/ml). The rabbit antiserum (no. 1060) against amino acids 11 to 21 of equine infectious anemia virus (EIAV) Tat protein (amino acid sequence, ADRRIPGTAEE) (18) was a kind gift of Nancy Rice (National Cancer Institute Frederick Cancer Research and Development Center). The goat anti-human TLR4 antibody was purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, Calif.). Sulfosuccinimidyl-6-(biotinamido)-6-hexanamido hexanoate (sulfo-NHS-LC-LC-biotin) and immobilized streptavidin agarose were from Pierce (Rockford, Ill.). PNGase F was purchased from New England BioLabs (Beverly, Mass.). Unless otherwise noted, all other chemicals were from Wako Pure Chemical Industries, Ltd. (Osaka, Japan).
Plasmid construction and site-directed mutagenesis.
The coding regions of human MD-2 and TLR4 were amplified by reverse transcription-PCR from total RNA prepared from THP-1 cells and human spleen total RNA (OriGene Technologies, Inc., Rockville, Md.), respectively. cDNAs encoding EIAV-tagged human TLR4 and human MD-2 were cloned into the pEIAV vector as previously described (13). Point mutations at potential TLR4 glycosylation sites (Asn497, Asn526, and Asn575) were created by PCR; in each mutation, asparagine (TLR4 N497Q, TLR4 N526Q, or TLR4 N575Q) was replaced with glutamine. The sequences of the primers used for the PCR were as follows: 5′-CAG AGC TGA GAC AGT TGA CCT TCC TGG ACC TCT-3′ and 5′-TGA AGA TAT CTG GAA GGA AGT TTT CCT GGA-3′ for TLR4 N497Q, 5′-AGT CTT CAG GTA CTA CAG ATG AGC CAC AAC AAC-3′ and 5′-GGA GAG TGA GTT AAA TGC TGT TGG AGA C-3′ for TLR4 N526Q, and 5′-AGT CTA GCT TTC TTA CAG CTT ACT CAG AAT GAC-3′ and 5′-ACT TGG AAA ATG CTG TAG TTC CTG TTT TTT GG-3′ for TLR4 N575Q. All of the mutations were confirmed by automated DNA sequencing with a Dye Terminator cycle sequencing kit (Amersham Pharmacia Biotech Inc., Piscataway, N.J.) and an ABI Prism 310 genetic analyzer (Perkin-Elmer, Applied Biosystems, Foster City, Calif.). A plasmid for hexahistidine-tagged MD-2 (His-tagged MD-2) was constructed by inserting a coding sequence of hexahistidine into the EIAV-TLR4 plasmid described above at the position just downstream of the EIAV tag epitope sequence.
Glycosidase treatment of cellular extracts.
After plating 293 cells in 6-cm-diameter dishes, the cells were transfected with expression plasmids for TLR4 and MD-2 (5 μg each) by the calcium phosphate precipitation method. Twenty-four hours later, the cells were lysed on ice for 10 min by using a lysis buffer (10 mM HEPES-KOH, 5 mM EDTA, 0.5% Nonidet P-40, and 10 mM KCl [pH 7.9]) containing a protease inhibitor mix (Boehringer Mannheim GmbH, Mannheim, Germany). Following centrifugation at 1,000 × g for 5 min, the protein concentrations of supernatants were measured by a protein assay kit (Bio-Rad, Hercules, Calif.). The supernatants containing equal amounts of protein were treated with PNGase F, as previously described (13), and then subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). TLR4 and MD-2 were detected by using Western blot analysis and the rabbit anti-EIAV antiserum. The signals were visualized with an enhanced chemiluminescence system (Amersham).
Treatment of cellular extracts with calf intestinal alkaline phosphatase.
Cellular extracts were prepared as described above and were measured by a protein assay kit. The cellular extracts containing equal amounts of protein were diluted with phosphate-buffered saline (PBS) to a final volume of 20 μl. Twenty units of calf intestinal alkaline phosphatase (Takara Bio, Inc., Shiga, Japan) were added to the cellular extract. The extract was incubated at 37°C for 1 h. The extract was then used in a Western blotting analysis, and TLR4 and MD-2 were detected by using the rabbit anti-EIAV antiserum as described above.
Biotinylation of cell surface protein.
After plating 293 cells in 6-cm-diameter dishes, the cells were transfected with TLR4 and MD-2 expression plasmids (5 μg each) by the calcium phosphate precipitation method. Twenty-four hours later, the cells were washed with PBS twice and treated with 2 ml of ice-cold 0.5 mg of sulfo-NHS-LC-LC-biotin/ml, a membrane-impermeable biotinylation reagent, as previously described (13). Cellular extracts were prepared with 200 μl of a lysis buffer, as described above. The cellular extracts containing 30 μg of protein were diluted with PBS containing 0.5% Nonidet P-40 to a final volume of 500 μl and then incubated with immobilized streptavidin agarose at 4°C for 1 h. After washing three times with PBS containing 0.5% Nonidet P-40, the agarose was boiled in an SDS-PAGE sample buffer. The resulting supernatant was subjected to SDS-PAGE and Western blot analysis. TLR4 and MD-2 were detected by the rabbit anti-EIAV antiserum as described above.
Tunicamycin treatment.
After plating in 6-cm-diameter dishes, 293 cells were transfected with TLR4 and MD-2 expression plasmids (5 μg each). At 8 h after transfection, the medium was replaced with normal culture medium containing the indicated concentration of tunicamycin (Wako), and the cells were incubated at 37°C for 16 h. Cellular extracts were prepared as described above. Cellular extracts containing 5 μg of protein were subjected to SDS-PAGE and Western blot analysis. TLR4 and MD-2 were detected by the rabbit anti-EIAV antiserum as described above.
Precipitation of hexahistidine-tagged MD-2.
293 cells were transfected with the plasmids for TLR4 mutant and His-tagged MD-2 (5 μg each). After 24 h, cellular extracts were prepared with 200 μl of binding buffer (PBS containing 0.5% Nonidet P-40, 500 mM NaCl, 10 mM imidazole, and protease inhibitor mix). The cellular extracts containing 50 μg of protein were diluted with a binding buffer to a final volume of 1,00 μl and then incubated with Ni-nitrilotriacetic acid (NTA) His-bound resins (Novagen, Inc., Madison, Wis.) at 4°C for 1 h while being rocked. After washing three times with a washing buffer (PBS containing 500 mM NaCl, 20 mM imidazole), the resins were boiled in an SDS-PAGE sample buffer. The resulting supernatant was subjected to SDS-PAGE and Western blot analysis. TLR4 and MD-2 were detected with the rabbit anti-EIAV antiserum as described above.
Immunoprecipitation of TLR4.
Since the anti-EIAV antiserum does not work for immunoprecipitation, an anti-TLR4 antibody was used. Cellular extracts were prepared as described above. The cellular extracts containing equal amounts of protein were diluted with PBS containing 0.5% Nonidet P-40 to a final volume of 500 μl. Following the addition of anti-human TLR4 antibody and protein G-Sepharose (Pierce), the diluted cellular extracts were then incubated for 1 h at 4°C while being rocked. After washing with PBS containing 0.5% Nonidet P-40, the Sepharose beads were boiled in an SDS-PAGE sample buffer. The resulting supernatant was subjected to SDS-PAGE and Western blot analysis. TLR4 and MD-2 were detected with the rabbit anti-EIAV antiserum as described above.
Electrophoresis and Western blotting.
Discontinuous SDS-10% polyacrylamide gel (acrylamide/bisacrylamide ratio, 29:1) was prepared in a AE-6400 electrophoresis cell (ATTO Corp., Tokyo, Japan) according to the method of Laemmli (8). Following the addition of the reducing Laemmli's sample buffer, samples were boiled for 5 min and then electrophoresed. Electrophoretically separated samples were transferred to a polyvinylidene difluoride membrane (Immobilon-P; Millipore Corp., Bedford, Mass.) with a semidry blot electrophoretic transfer system (EB-150; Toyo Roshi Kaisha Ltd., Tokyo, Japan). After blocking with 5% nonfat dry milk dissolved in TBST (10 mM Tris, 100 mM NaCl, 0.2% Tween 20 [pH 7.5]), the membrane was probed with the indicated antibody and washed with TBST three times. The membrane was probed with a peroxidase-labeled second antibody and then washed with TBST three times. The signals were visualized by using an enhanced chemiluminescence system (Amersham).
RESULTS
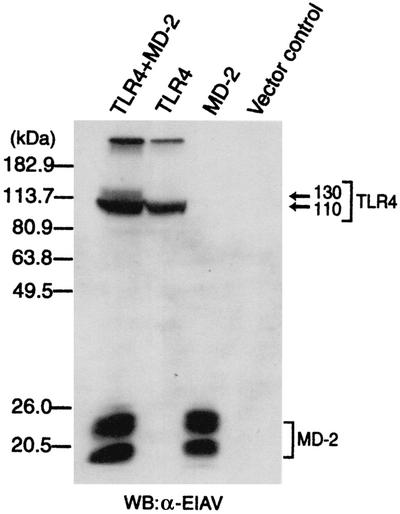
cDNAs encoding TLR4 and MD-2 were transiently transfected in human embryonic kidney 293 cells, and cellular extracts were prepared. Western blotting analysis for TLR4 revealed the presence of at least two forms of TLR4 with different molecular masses (110 and 130 kDa) (Fig. 1). In contrast, an analysis of cellular extracts prepared from cells expressing TLR4 alone produced only the 110-kDa form of TLR4. An analysis of cellular extracts prepared from cells transfected with MD-2 or vector alone did not produce any of the TLR4-specific protein bands, indicating that the bands observed in the above analysis had originated from TLR4. These observations indicate that TLR4 is modified in some way when MD-2 is coexpressed and that this modification leads to the appearance of the 130-kDa form of TLR4.
FIG. 1.
Expression of TLR4 with MD-2 results in the appearance of two forms of TLR4 with different molecular masses. After plating 293 cells in 6-cm-diameter dishes, the cells were transfected with a control vector or the expression plasmid(s) indicated (5 μg each). After 24 h, cellular extracts were prepared from the cells. The cell extracts (5 μg each) were analyzed for TLR4 and MD-2 by Western blotting (WB) with the rabbit anti-EIAV (α-EIAV) antiserum.
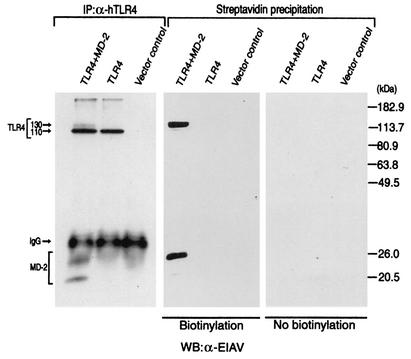
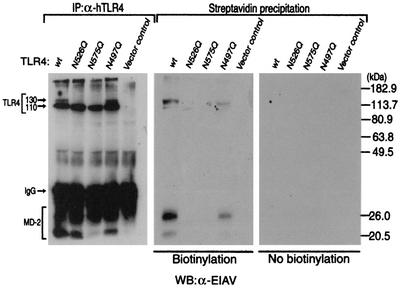
Since MD-2 has been reported to be required for the translocation of TLR4 to the cell surface (12), we next examined the membrane surface expression of TLR4 with or without coexpression of MD-2. TLR4 was expressed with or without MD-2 in 293 cells, and the membrane surface proteins were labeled with a membrane-impermeable biotinylation reagent (3, 17). Both the 130- and 110-kDa forms of TLR4 were detected in the anti-TLR4 immunoprecipitate obtained from cells expressing TLR4 and MD-2, whereas only the 130-kDa form of TLR4 was detected in the streptavidin precipitate obtained from cells expressing TLR4 and MD-2. When TLR4 was expressed alone, no signal of TLR4 was detected in the streptavidin precipitate and only the 110-kDa form of TLR4 was detected in the anti-TLR4 immunoprecipitate (Fig. 2). These results indicate that only the 130-kDa form of TLR4, which only appears in the presence of MD-2, is expressed on the membrane surface.
FIG. 2.
Only the 130-kDa form of TLR4 is expressed on the cell surface. 293 cells were transfected with a control vector or the expression plasmid(s) indicated (5 μg each). After 24 h, cell surface proteins were either untreated (right panel) or treated (center panel) with a membrane-impermeable biotinylating reagent, sulfo-NHS-LC-LC-biotin. Cellular extracts were prepared from the cells and divided into two portions (30 μg each). Biotinylated proteins were collected from one portion of the extracts with immobilized streptavidin. Another portion of the extracts was subjected to immunoprecipitation (IP) with a goat anti-human TLR4 (α-hTLR4) immunoglobulin G (IgG) (left panel). The precipitates were boiled in an SDS-PAGE sample buffer for 5 min, and the supernatants were analyzed for TLR4 and MD-2 by Western blotting (WB) with the rabbit anti-EIAV (α-EIAV) antiserum.
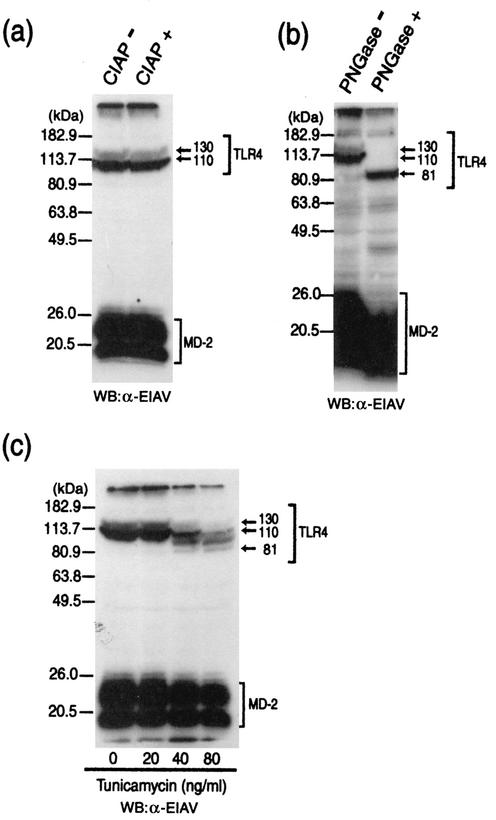
To identify what modification is involved in the production of the 130-kDa form of TLR4, the following experiments were performed. TLR4 and MD-2 were transiently expressed in 293 cells, and the cellular extracts were treated with calf intestinal alkaline phosphatase. This treatment did not affect the mobility of TLR4 (Fig. 3a), indicating that phosphorylation is not involved in the production of the 130-kDa form of TLR4. Since TLR4 has been reported to possess nine potential N-linked glycosylation sites (7), we next examined the involvement of N-linked glycosylation. Cellular extracts prepared from 293 cells transiently expressing TLR4 and MD-2 were treated with the N-glycosidase, PNGase F, and TLR4 was detected by Western blotting analysis. The PNGase F treatment produced a single form of TLR4, whose mass is approximately 80 kDa (Fig. 3b). Treatment with tunicamycin, which prevents the addition of N-linked glycans to polypeptides, also reduced the size of TLR4 in a similar way to the PNGase F treatment in Western blotting analysis (Fig. 3c). These results indicate that TLR4 undergoes N-linked glycosylation at multiple positions and that the 130-kDa form was produced by additional glycosylations. Furthermore, the presence of MD-2 appears to be necessary for the additional glycosylations to occur.
FIG. 3.
TLR4 is additionally glycosylated in the presence of MD-2. (a and b) After plating 293 cells in 6-cm-diameter dishes, the cells were transfected with plasmids (5 μg each) for TLR4 and MD-2. After 24 h, the prepared cell extracts (15 μg protein) were either untreated (−) or treated (+) with calf intestinal alkaline phosphatase (CIAP) (a) and PNGase F (b). The extracts were then analyzed for TLR4 and MD-2 by Western blotting (WB) with the rabbit anti-EIAV (α-EIAV) antiserum. (c) After plating in 6-cm-diameter dishes, 293 cells were transfected with TLR4 and MD-2 expression plasmids (5 μg each). At 8 h after transfection, the medium was replaced with normal culture medium containing the indicated concentration of tunicamycin and the cells were incubated at 37°C for 16 h. The cellular extracts were prepared from the cells, and the extracts (5 μg protein) were analyzed for TLR4 and MD-2 by Western blotting with the rabbit anti-EIAV antiserum.
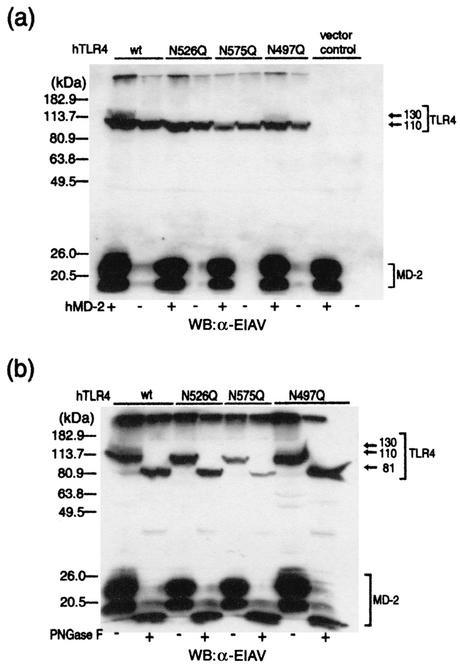
Since the glycosylation of TLR4 at Asn526 and Asn575 has been demonstrated to be important for the translocation of TLR4 to the membrane surface (7), we generated TLR4 mutants in which Asn526 or Asn575 was replaced with glutamine (named TLR4 N526Q and TLR4 N575Q) and examined for the appearance of the 130-kDa form upon coexpression of MD-2. Western blot analysis revealed that the 130-kDa form of TLR4 was not detected in 293 cells expressing these mutants even when MD-2 was coexpressed (Fig. 4a). On the other hand, the mutation of Asn497, whose glycosylation has been reported to be unrelated to its cell surface expression (7), did not affect the appearance of the 130-kDa form. Thus, glycosylation at Asn526 and/or Asn575 is likely to be involved in the appearance of the 130-kDa form of TLR4. PNGase F treatment further reduced the size of these mutants, indicating that these mutants are still glycosylated at positions other than mutated sites (Fig. 4b). Biotinylation studies revealed that both of TLR4 N526Q and TLR4 N575Q mutants were not expressed on the membrane surface even when MD-2 was coexpressed, although these mutants were expressed in cytosol (Fig. 5). On the other hand, N497Q was expressed on the membrane surface.
FIG. 4.
Asn526 and Asn575 are glycosylated in the presence of MD-2. (a) After plating 293 cells in 6-cm-diameter dishes, they were transfected with plasmids (5 μg each) for the TLR4 mutant with or without MD-2. After 24 h, cellular extracts were prepared from the cells and the extracts (10 μg protein) were analyzed for TLR4 and MD-2 by Western blotting (WB) with a rabbit anti-EIAV (α-EIAV) antiserum. wt, wild type; hTLR4, human TLR4. (b) After plating 293 cells in 6-cm-diameter dishes, they were transfected with plasmids (5 μg each) for the TLR4 mutant and MD-2. After 24 h, the prepared cellular extracts (10 μg protein) were either untreated or treated with PNGase F. The extracts were then analyzed for TLR4 and MD-2 by Western blotting with the rabbit anti-EIAV antiserum.
FIG. 5.
Asn526 and Asn575 are critical for the membrane translocation of TLR4. The 293 cells were transfected with plasmids for the TLR4 mutant and MD-2 (5 μg each). After 24 h, the cell surface proteins were either untreated (right panel) or treated with a membrane-impermeable biotinylating reagent, sulfo-NHS-LC-LC-biotin (center panel). Cellular extracts were prepared from the cells and divided into two portions (30 μg of protein each). The biotinylated proteins were collected from one portion of the extracts with immobilized streptavidin. Another portion of the extracts was subjected to immunoprecipitation (IP) with anti-human TLR4 (α-hTLR4) immunoglobulin G (IgG) (left panel). The precipitates were boiled in an SDS-PAGE sample buffer for 5 min, and the supernatants were analyzed for TLR4 and MD-2 by Western blotting (WB) with the rabbit anti-EIAV (α-EIAV) antiserum. wt, wild type.
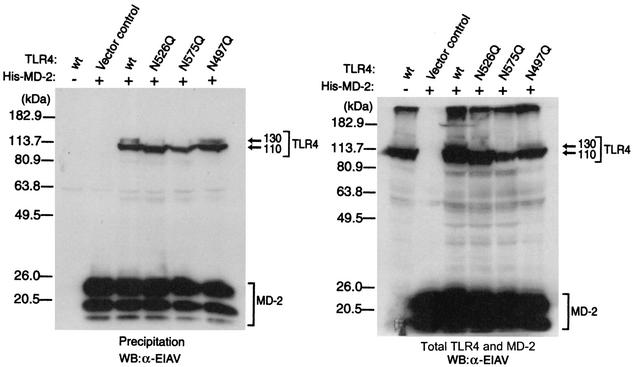
Precipitation assays were carried out to determine whether the loss of glycosylation at Asn526 and/or Asn575 of TLR4 disrupts the association between TLR4 and MD-2 (Fig. 6). His-tagged MD-2 and/or TLR4 was transiently expressed in 293 cells, and cellular extracts were prepared. Then His-tagged MD-2 was precipitated with Ni-NTA His-bound resin from the extracts, and TLR4 was detected by Western blotting analysis. When TLR4 and MD-2 were expressed alone, no signals of TLR4 were detected in the precipitate. On the other hand, TLR4 was detected in all of precipitates prepared from cells coexpressed with MD-2 proteins, indicating that these TLR4 mutants are still capable of associating with MD-2. Taken together, these results indicate that the presence of MD-2 promotes the modification of TLR4, which enables TLR4 to be translocated to the membrane surface, and that glycosylation at Asn526 and/or Asn575 is likely to be involved in this modification.
FIG. 6.
TLR4 mutants still associate with MD-2. 293 cells were transfected with the plasmids for TLR4 mutant and His-tagged MD-2. After 24 h, cell extracts were prepared from the cells and a portion of the extracts (50 μg of protein) was subjected to precipitation with Ni-NTA His-bound resin. Both the precipitates (left panel) and the cell extracts set aside (right panel) were analyzed for TLR4 and MD-2 by Western blotting (WB) with the rabbit anti-EIAV (α-EIAV) antiserum. wt, wild type.
DISCUSSION
In the present study, we found that the coexpression of MD-2 with TLR4 resulted in the production of the 130-kDa form of TLR4, which coincided with the form found on the cell membrane surface, and that mutation of TLR4 at Asn526 or Asn575 prevented the appearance of the 130-kDa form of TLR4. TLR4 has previously been reported to undergo glycosylation at several Asn residues, among which Asn526 and Asn575 have been demonstrated to be important for its cell surface expression (7). We also found, in this study, that the 110-kDa form of TLR4 was partially glycosylated at residues other than Asn526 and Asn575 and that this form was unable to translocate to the membrane surface. All of these results strongly suggest that MD-2 is required for the glycosylation of TLR4 at Asn526 and Asn575, which is critical for the translocation of TLR4 to the membrane surface.
We also demonstrated that TLR4 N526Q and TLR4 N575Q are still capable of associating with MD-2. da Silva et al. (7) also reported that mutations of these residues did not affect the ability of TLR4 to associate with MD-2, although they did not show the data. These mutants did not translocate to the membrane surface, even when MD-2 was coexpressed. These findings suggest that the binding of TLR4 to MD-2 is not sufficient for the translocation of TLR4 to the membrane surface and that the glycosylation of Asn526 and/or Asn575 is necessary for the translocation.
It has been reported (4) that the level of membrane surface expression of TLR4 is extremely low. Even in our transient expression system, in which higher expression can usually be achieved, only a small portion of TLR4 was expressed on the membrane surface. There may be a regulatory mechanism that limits the membrane trafficking of TLR4.
Akashi et al. (2) reported that a conformational change may occur in TLR4 molecules upon association with MD-2. Therefore, the association of MD-2 with TLR4 may lead to a conformational change that exposes TLR4 to a glycosylation enzyme.
Acknowledgments
This work was supported in part by grants from the Japan Health Sciences Foundation and the Ministry of the Environment.
REFERENCES
- 1.Akashi, S., Y. Nagai, H. Ogata, M. Oikawa, K. Fukase, S. Kusumoto, K. Kawasaki, M. Nishijima, S. Hayashi, M. Kimoto, and K. Miyake. 2001. Human MD-2 confers on mouse Toll-like receptor 4 species-specific lipopolysaccharide recognition. Int. Immunol. 13:1595-1599. [DOI] [PubMed] [Google Scholar]
- 2.Akashi, S., R. Shimazu, H. Ogata, Y. Nagai, K. Takeda, M. Kimoto, and K. Miyake. 2000. Cutting edge: cell surface expression and lipopolysaccharide signaling via the toll-like receptor 4-MD-2 complex on mouse peritoneal macrophages. J. Immunol. 164:3471-3475. [DOI] [PubMed] [Google Scholar]
- 3.Altin, J. G., and E. B. Pagler. 1995. A one-step procedure for biotinylation and chemical cross-linking of lymphocyte surface and intracellular membrane-associated molecules. Anal. Biochem. 224:382-389. [DOI] [PubMed] [Google Scholar]
- 4.Beutler, B. 2000. Tlr4: central component of the sole mammalian LPS sensor. Curr. Opin. Immunol. 12:20-26. [DOI] [PubMed] [Google Scholar]
- 5.Chow, J. C., D. W. Young, D. T. Golenbock, W. J. Christ, and F. Gusovsky. 1999. Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J. Biol. Chem. 274:10689-10692. [DOI] [PubMed] [Google Scholar]
- 6.da Silva, C. J., K. Soldau, U. Christen, P. S. Tobias, and R. J. Ulevitch. 2001. Lipopolysaccharide is in close proximity to each of the proteins in its membrane receptor complex: transfer from CD14 to TLR4 and MD-2. J. Biol. Chem. 276:21129-21135. [DOI] [PubMed] [Google Scholar]
- 7.da Silva, C. J., and R. J. Ulevitch. 2002. MD-2 and TLR4 N-linked glycosylations are important for a functional lipopolysaccharide receptor. J. Biol. Chem. 277:1845-1854. [DOI] [PubMed] [Google Scholar]
- 8.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 9.Lemaitre, B., E. Nicolas, L. Michaut, J. M. Reichhart, and J. A. Hoffmann. 1996. The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell 86:973-983. [DOI] [PubMed] [Google Scholar]
- 10.Mancek, M., P. Pristovsek, and R. Jerala. 2002. Identification of LPS-binding peptide fragment of MD-2, a toll-receptor accessory protein. Biochem. Biophys. Res. Commun. 292:880-885. [DOI] [PubMed] [Google Scholar]
- 11.Muroi, M., T. Ohnishi, and K. Tanamoto. 2002. MD-2, a novel accessory molecule, is involved in species-specific actions of Salmonella lipid A. Infect. Immun. 70:3546-3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nagai, Y., S. Akashi, M. Nagafuku, M. Ogata, Y. Iwakura, S. Akira, T. Kitamura, A. Kosugi, M. Kimoto, and K. Miyake. 2002. Essential role of MD-2 in LPS responsiveness and TLR4 distribution. Nat. Immunol. 3:667-672. [DOI] [PubMed] [Google Scholar]
- 13.Ohnishi, T., M. Muroi, and K. Tanamoto. 2001. N-linked glycosylations at Asn(26) and Asn(114) of human MD-2 are required for toll-like receptor 4-mediated activation of NF-kappaB by lipopolysaccharide. J. Immunol. 167:3354-3359. [DOI] [PubMed] [Google Scholar]
- 14.Poltorak, A., X. He, I. Smirnova, M. Y. Liu, C. V. Huffel, X. Du, D. Birdwell, E. Alejos, M. Silva, C. Galanos, M. Freudenberg, P. Ricciardi-Castagnoli, B. Layton, and B. Beutler. 1998. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282:2085-2088. [DOI] [PubMed] [Google Scholar]
- 15.Qureshi, S. T., L. Lariviere, G. Leveque, S. Clermont, K. J. Moore, P. Gros, and D. Malo. 1999. Endotoxin-tolerant mice have mutations in Toll-like receptor 4 (Tlr4). J. Exp. Med. 189:615-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shimazu, R., S. Akashi, H. Ogata, Y. Nagai, K. Fukudome, K. Miyake, and M. Kimoto. 1999. MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J. Exp. Med. 189:1777-1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Staros, J. V. 1982. N-hydroxysulfosuccinimide active esters: bis(N-hydroxysulfosuccinimide) esters of two dicarboxylic acids are hydrophilic, membrane-impermeant, protein cross-linkers. Biochemistry 21:3950-3955. [DOI] [PubMed] [Google Scholar]
- 18.Stephens, R. M., D. Derse, and N. R. Rice. 1990. Cloning and characterization of cDNAs encoding equine infectious anemia virus tat and putative Rev proteins. J. Virol. 64:3716-3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Viriyakosol, S., P. S. Tobias, R. L. Kitchens, and T. N. Kirkland. 2001. MD-2 binds to bacterial lipopolysaccharide. J. Biol. Chem. 276:38044-38051. [DOI] [PubMed] [Google Scholar]