Abstract
The development of reliable assay systems that can measure lymphocyte activation in vitro has been a major goal of immunodiagnostics. Traditionally, tritiated thymidine incorporation has been used to monitor T-cell activation. Other methods include enzyme-linked immunosorbent assay (ELISA), enzyme-linked immunospot assay, and colorimetric assays. We have established a lymphocyte activation assay that utilizes fluorescein isothiocyanate (FITC)-streptavidin bound to recombinant biotinylated human interleukin-2 (IL-2). Utilizing recombinant DNA technology, a unique monobiotinylated human IL-2 has been created and isolated using the Promega PinPoint vector system. ELISA has been used to demonstrate streptavidin binding and recognition by a human IL-2-specific antibody. IL-2 function has been demonstrated using a murine IL-2-dependent T-cell line, CTLL-2, responsive to human IL-2. Recombinant biotinylated human IL-2 conjugated to streptavidin-FITC or streptavidin-horseradish peroxidase has been used to monitor T-cell activation in the presence of antigen as well as mitogen. The sensitivity and convenience of this method make this lymphocyte activation assay an attractive alternative to tritiated thymidine incorporation as a method for monitoring T-cell activation. In addition, the availability of a recombinant biotinylated human IL-2 will permit the production of a uniform product suitable for diagnostic and clinical application.
Interleukin-2 (IL-2) is the principal cytokine responsible for progression of T lymphocytes from the G1 to the S phase of the cell cycle. It is produced by CD4 T cells and to a lesser degree by CD8 T cells (9). On activation, T cells express high levels of the high-affinity form of the IL-2 receptor (IL-2R), to which IL-2 binds (12). IL-2, biotinylated by chemical means, has been used to detect activated T cells (3), isolate IL-2R (5), monitor IL-2R expression (3, 21, 24), and study IL-2R internalization (15). Studies also show that targeting IL-2 to tumor cells facilitates tumor elimination, presumably via enhanced T-cell activation (8, 10, 11, 14, 26). Therefore, the availability of a recombinant biotinylated human IL-2 (rbhIL-2) could not only provide a tool for monitoring T-cell activation but also facilitate the development of a therapeutic tool which utilizes single-chain variable-region antibody fragment-IL-2 fusion proteins to target IL-2 to tumors (8, 10, 11, 14) as well as antigen-presenting cells (APC) (23). In addition, large-scale production and purification of a uniformly biotinylated IL-2 product, required for diagnostic and clinical applications, would be facilitated. However, no genetically engineered biotinylated IL-2 is available at present.
The availability of the Promega PinPoint Xa protein purification system now makes the construction of rbhIL-2 possible. This system takes advantage of the fact that Escherichia coli produces a naturally biotinylated protein that is essential for fatty acid synthesis and growth of this organism (2, 18). The Promega PinPoint Xa vector contains a biotinylation tag similar to that present in the endogenous protein and is designed for the production and purification of fusion proteins, which uniformly contain a single biotin. In chemical biotinylation, the number of biotins per protein molecule is variable. Also, in the Promega PinPoint vector system, the biotinylation tag can be easily removed by enzymatic cleavage should one wish to remove the biotinylation stub once the cytokine is purified. Furthermore, biotinylated fusion proteins produced in this fashion can be affinity purified using the SoftLink soft-release avidin resin, which, importantly, permits elution of the fusion protein under nondenaturing conditions.
In this paper the generation of an rbhIL-2 fusion protein is described, as well as its use to monitor human T-cell activation in vitro. This biotinylated fusion protein binds both streptavidin (SA) and the IL-2-specific antibody (Ab) and induces the proliferation of a murine IL-2-dependent T-cell line, CTLL-2. Furthermore, by utilizing fluorescein isothiocyanate (FITC)- or horseradish peroxidase (HRP)-labeled SA in combination with the rbhIL-2, antigen- and mitogen-induced T-lymphocyte activation can be monitored via flow cytometry or chemiluminescence assay, respectively.
MATERIALS AND METHODS
Generation of the rbhIL-2 construct.
E. coli containing human IL-2 cDNA inserted into a cloning vector was obtained from the American Type Culture Collection (ATCC, Manassas, Va.). The cloning vector containing the IL-2 insert, PTCGF-11, was isolated by an alkaline lysis plasmid preparation and cleaned by the Qiagen method (Qiagen, Inc., Valencia, Calif.). PCR was used to create unique HindIII and KpnI restriction enzyme sites (Promega Corp., Madison, Wis.) at the 5′ and 3′ ends of the IL-2 insert, respectively. The PCR product was digested with HindIII and KpnI and cleaned by the Qiagen method. The PinPoint Xa3 vector (Promega) was digested with HindIII and KpnI, and the released human IL-2 DNA was inserted into the PinPoint Xa3 expression vector. E. coli JM109 was then transformed with the PinPoint Xa3 vector containing the IL-2 DNA insert or the same vector lacking the IL-2 DNA insert. The product produced by the latter construct served as a negative control in many of the assays described here. 2YT (Becton Dickinson & Co., San Jose, Calif.) plates containing 100 μg of ampicillin (Sigma Chemical Co., St. Louis, Mo.) per ml were used to select for transformants. Transformed E. coli JM109 was then cultured at 37°C with agitation on a Gyrotory shaker (New Brunswick Scientific Co., Inc., Edison, N.J.) in 2YT plates containing 100 μg of ampicillin per ml and 8 μM biotin (Sigma). After 2 h, the culture was induced to produce rbhIL-2 protein with 100 μM isopropyl-β-d-thiogalactopyranoside (IPTG) (Sigma). After 4 h, the transformed E. coli JM109 cells were isolated from bulk culture and resuspended in lysis buffer containing 50 mM Tris (Fisher Biotech, Fair Lawn, N.J.), 50 mM NaCl (Sigma), and 0.02% sodium azide (Sigma), with one Complete mini protease inhibitor cocktail tablet (Roche, Indianapolis, Ind.).
Purification of the rbhIL-2 protein.
The transformed E. coli JM109 cells were mechanically lysed with a French press (American Instrument Co., Inc., Silver Spring, Md.), and the rbhIL-2 was isolated on a SoftLink Avidin column as described by the manufacturer (Promega). The eluate containing the rbhIL-2 was concentrated using a Speed Vac concentrator (Savant, Hicksville, N.Y.) and dialyzed against phosphate-buffered saline (PBS) using a Slide-A-Lyzer cassette (15-kDa exclusion; Pierce Chemical Co., Rockford, Ill.). Purity was verified using Western blotting, and the protein concentration was determined using the biocinchoninic acid assay as specified by the manufacturer (Pierce).
SA binding and IL-2-specific Ab recognition of rbhIL-2.
To illustrate SA binding by rbhIL-2 and its recognition by an IL-2-specific Ab, an enzyme-linked immunosorbent assay (ELISA) was performed. A high-binding 96-well ELISA plate (Corning Inc., Corning, N.Y.) was coated with 10 μg of SA (Pierce) per ml in PBS and incubated at 4°C overnight. After the wells were washed three times with PBS containing 2 mg of bovine serum albumin (BSA) per ml and 0.02% sodium azide (PBS-BSA), they were blocked with PBS-BSA at room temperature for 1 h. The blocking solution was discarded, rbhIL-2 was added to appropriate wells, and the wells were incubated at room temperature for 90 min. The wells were then washed three times with PBS-BSA, and goat anti-human IL-2 (R&D Systems, Minneapolis, Minn.) was added for 4 h at room temperature. After the wells were washed three times with PBS-BSA, rabbit anti-goat IgG-alkaline phosphatase (AP) (Vector Laboratories, Inc., Burlingame, Calif.) was added at a 1:500 dilution in PBS-BSA, and the wells were incubated at room temperature for 90 min. The wells were then washed three times with PBS-BSA, and 1 mg of AP substrate (Sigma) per ml diluted in ELISA buffer (0.05 Na2CO3, 10 mM MgCl [pH 9.8]) was added. The samples were incubated at room temperature for 1 h, and absorbance was read on a microplate reader (Molecular Devices, Sunnyvale, Calif.) at 405 nm.
Stimulation of an IL-2-dependent T-cell line by using rbhIL-2.
To demonstrate rbhIL-2 function, the IL-2-dependent murine T-cell line CTLL-2 (ATCC), which responds to recombinant human IL-2 (rhIL-2), was utilized. The CTLL-2 cells were maintained at 37°C and 5% CO2 for 2 days in 25-cm2 flasks (Becton Dickinson) containing RPMI 1640 (GibcoBRL), 10% fetal bovine serum (Sigma), 10 mM HEPES (Sigma), 2 mM sodium pyruvate (Sigma), 2 mM glutamine (Sigma), 2.5 g of glucose (Sigma) per liter, and 1 U of rhIL-2 (R&D Systems) per ml. The cells were then washed three times with medium and resuspended, and 50 μl of cells/well was plated in a 96-well plate at 5 × 103 cells/well. The plates were then incubated at 37°C and 5% CO2 for 2 days in the presence of rbhIL-2 or rhIL-2 at different concentrations. Subsequently, the CTLL-2 cells were pulsed with 1 μCi of tritiated thymidine ([3H]TdR) (ICN, Aurora, Ohio) 24 h before being harvested. The cells were harvested using a Harvester 96 (Tomtec, Hamden, Conn.), and [3H]TdR incorporation was measured using a 1450 microbeta liquid scintillation counter (EG&G Wallac, Gaithersburg, Md.).
rbhIL-2 binding to activated T cells.
To demonstrate binding of rbhIL-2 to antigen- and mitogen-activated T cells, human peripheral blood mononuclear cells (PBMC) were isolated from peripheral blood as previously described (7). With regard to the use of human subjects, this research has complied with all relevant federal guidelines and institutional policies. PBMC were resuspended in AIM V (Gibco-BRL) at 2.5 × 106 cells/ml. Then 50-μl volumes of cells were added to individual wells of a 96-well plate containing 5.0 μg of phytohemagglutinin mitogen (PHA) (Sigma) per ml. The plates were then incubated at 37°C and 5% CO2 for 3 days. One day prior to completion of this incubation, 60 μg of rbhIL-2 per ml was combined in equal volume with 120 μg of SA-FITC (Vector) per ml in PBS-BSA and incubated at 4°C overnight on a Vari-Mix rocker. Following the 3-day incubation of cells, stimulated PBMC were washed three times in AIM V and resuspended at 12.5 × 106 cells/ml in AIM V. Then 50-μl volumes of cells were added to wells of a 96-well plate, followed by 50 μl of rbhIL-2-SA-FITC conjugate, and the plates were incubated at 4°C for 2 h with rocking. Biotin-labeled anti-human CD25 (IL-2R) monoclonal Ab (MAb) (Becton Dickinson) bound to SA-FITC was used as a positive control for T-cell activation (IL-2R upregulation) (1). SA-FITC was used as a negative control for rbhIL-2 binding. The cells were then washed three times with PBS-BSA and resuspended in 2% methanol-free formalin in PBS (Eastman Kodak, Rochester, N.Y.). PBMC were analyzed by flow cytometry, and mean fluorescence intensity (MFI) was determined using Cell Quest (Becton Dickinson).
Evaluation of lymphocyte activation by using rbhIL-2-SA-FITC conjugates and flow cytometry.
PBMC were resuspended in AIM V with different concentration of tetanus toxoid antigen (TT) (Accurate Chemical & Scientific Corp., Westbury, N.Y.) or PHA and incubated in a 96-well plate as described above. rbhIL-2-SA-FITC conjugate was then combined with stimulated PBMC as described above and incubated at 4°C for 1 h with rocking. The cells were then washed, fixed, and analyzed by flow cytometry.
Cell specificity of rbhIL-2 binding.
To demonstrate that the majority of lymphocytes detected in this assay are activated T cells, a lymphocyte activation assay was performed using PBMC incubated for 1 day in AIM V as described above with 1.0 μg of PHA per ml. In this case, however, equal volumes of rbhIL-2 (15 μg/ml) and SA-FITC (60 μg/ml) were combined and incubated at 4°C overnight on a Vari-Mix rocker to obtain the rbhIL-2-SA-FITC conjugate. To identify T and B lymphocytes, mouse immunoglobulin G1 (IgG1) anti-human CD3 peridinin chlorophyll protein (PerCP) (Becton Dickinson) and mouse IgG1 anti-human CD19 phycoerythrin (PE) (Becton Dickinson) were also added to the cells in combination with rbhIL-2-SA-FITC. Mouse IgG1 PE (Becton Dickinson) and mouse IgG1 PerCP (Becton Dickinson) were used as negative controls. The cells were washed, fixed, and analyzed using flow cytometry.
Evaluation of lymphocyte activation using rbhIL-2-SA-HRP and chemiluminescence.
PBMC were resuspended in AIM V at 2.5 × 106 cells/ml with different concentrations of TT or PHA and incubated for 1 day as described above. Equal volumes of rbhIL-2 (15 μg/ml) and SA-HRP (Pierce) (10 μg/ml) were combined and incubated at 4°C overnight on a Vari-Mix rocker. The rbhIL-2-SA-HRP conjugate was combined with stimulated PBMC and incubated at room temperature for 1 h with rocking, as was done with the rbhIL-2-FITC conjugate. The chemiluminescence assay was performed as described by the manufacturer (Boehringer Mannheim, Indianapolis, Ind.). The cells were washed three times with PBS and resuspended in 100 μl of chemiluminescence working solution. Chemiluminescence was measured using a 1450 microbeta liquid scintillation counter.
Comparison of the rbhIL-2-SA-HRP assay to lymphocyte activation measured by [3H]TdR incorporation.
PBMC were added to plates followed by different concentrations of PHA or TT and incubated for 3 days as described above for the chemiluminescence analysis. To monitor T-cell activation using [3H]TdR incorporation, PBMC were pulsed with 1 μCi of [3H]TdR overnight before being harvested. The cells were harvested, and [3H]TdR incorporation was measured as described above when using CTLL-2 cells to detect rbhIL-2-induced T-cell proliferation.
RESULTS
Production of rbhIL-2.
By utilizing recombinant DNA technology, rbhIL-2 was created and isolated using the Promega PinPoint Xa3 vector system, as described in Materials and Methods. Western blot analysis with SA-HRP revealed a banding pattern of 28.9 kDa, consistent with the presence of an rbhIL-2 protein (data not shown).
SA binding and Ab recognition of rbhIL-2 by ELISA.
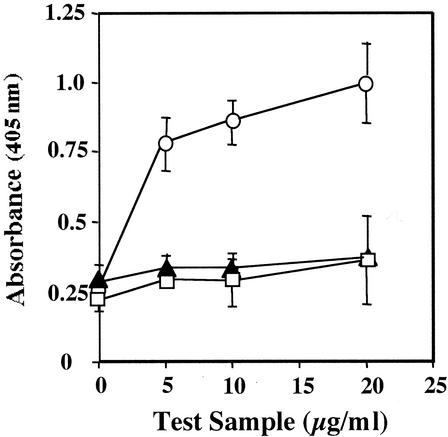
ELISA, utilizing SA-coated wells and goat anti-human IL-2, was used to demonstrate the binding of rbhIL-2 to SA and its recognition by Ab specific for human IL-2. When added to SA-coated wells, the rbhIL-2 bound to SA and was detected by the addition of goat anti-human IL-2 followed by rabbit anti-goat Ig-AP. When the PinPoint vector control or nonbiotinylated rhIL-2 was used in place of rbhIL-2, binding of goat anti-human IL-2 was not detected (Fig. 1).
FIG. 1.
rbhIL-2 detection by ELISA. PinPoint vector control (open squares), nonbiotinylated rhIL-2 (solid triangles), and rbhIL-2 (open circles) were incubated in wells coated with SA, and goat anti-human IL-2 and rabbit anti-goat IgG-AP were added. Samples were developed and read as described in Materials and Methods. Data represent the mean and standard deviation for triplicate samples.
rbhIL-2 stimulates the proliferation of an IL-2-dependent T-cell line.
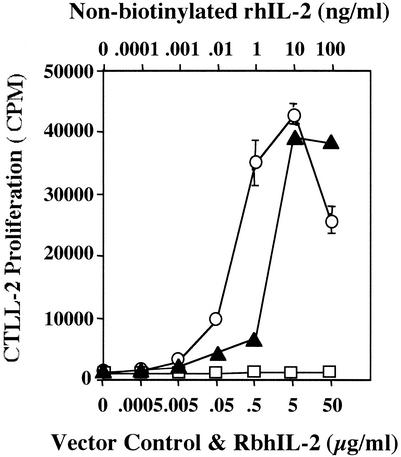
To illustrate the function of rbhIL-2, the murine (human IL-2-responsive) T-cell line CTLL-2 was used. rbhIL-2 induced CTLL-2 proliferation in a dose-responsive manner, as did nonbiotinylated rhIL-2. However, rhIL-2 was 20- to 30-fold more active than was rbhIL-2. The PinPoint vector control, which did not contain the rbhIL-2 protein, did not induce CTLL-2 proliferation (Fig. 2).
FIG. 2.
CTLL-2 proliferation in response to rbhIL-2. PinPoint vector control (open squares), nonbiotinylated rhIL-2 (solid triangles), and rbhIL-2 (open circles) were used to stimulate CTLL-2 cells as described in Materials and Methods. Data represent the mean and standard deviation for triplicate samples.
rbhIL-2 binds to PHA-activated T cells.
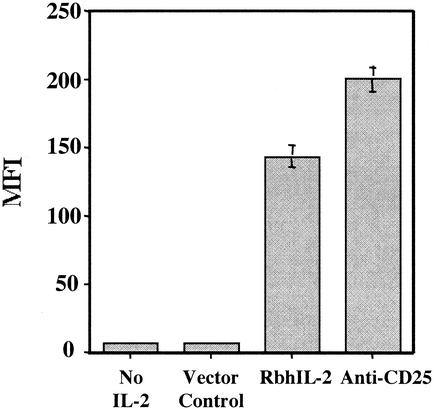
PHA-stimulated human PBMC were incubated with rbhIL-2-SA-FITC to demonstrate the binding of rbhIL-2 to activated T lymphocytes. rbhIL-2 did bind to PHA-activated PBMC, although at a level 25% lower than that of anti-CD25-FITC. Despite this, rbhIL-2-SA-FITC binding (MFI = 145) was significantly higher than that of SA-FITC or the PinPoint vector control (MFI = 10) (Fig. 3).
FIG. 3.
rbhIL-2 binds to PHA-activated lymphocytes. PBMC were stimulated in the presence of PHA. rbhIL-2 or anti-CD25 combined with SA-FITC was then added, and the fluorescence (MFI) was measured by flow cytometry as described in Materials and Methods. Data represent the mean and standard deviation for triplicate samples.
Detection of lymphocyte activation by using rbhIL-2-SA-FITC conjugates.
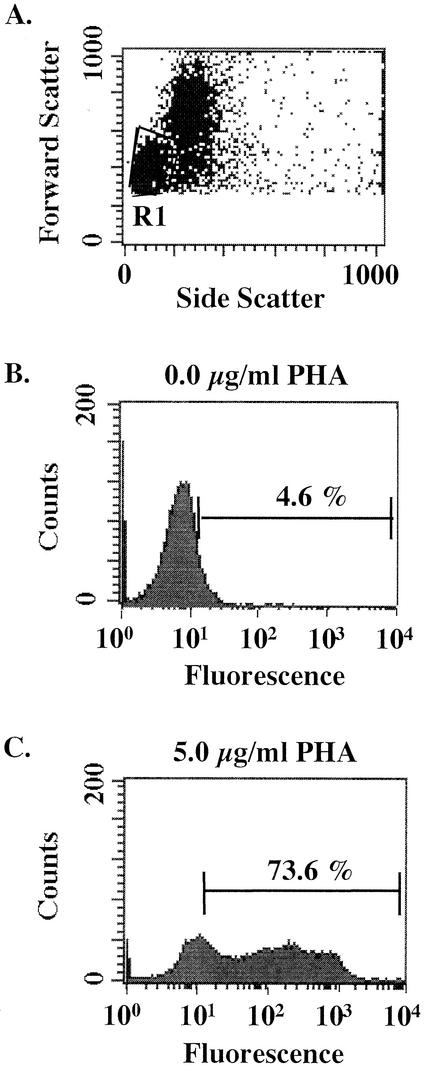
To illustrate that rbhIL-2-SA-FITC conjugates can be used to distinguish activated from nonactivated lymphocytes, PBMC incubated in the presence or absence of PHA were incubated with rbhIL-2-SA-FITC. Approximately 74% of the cells bound rbhIL-2-SA-FITC following stimulation with PHA, whereas approximately 5% of the cells bound rbhIL-2-SA-FITC in the absence of PHA stimulation (Fig. 4).
FIG. 4.
rbhIL-2 binding is specific for activated lymphocytes. PBMC were incubated in the presence or absence of PHA and subsequently labeled with rbhIL-2-SA-FITC conjugates as described in Materials and Methods. (A) Dot plot indicating the lymphocyte population gated on for analysis of rbhIL-2-SA-FITC binding. (B) Histogram representing gated lymphocytes and rbhIL-2-SA-FITC binding after incubation in the absence of PHA. (C) Histogram representing gated lymphocytes and rbhIL-2-SA-FITC binding after incubation in the presence of PHA.
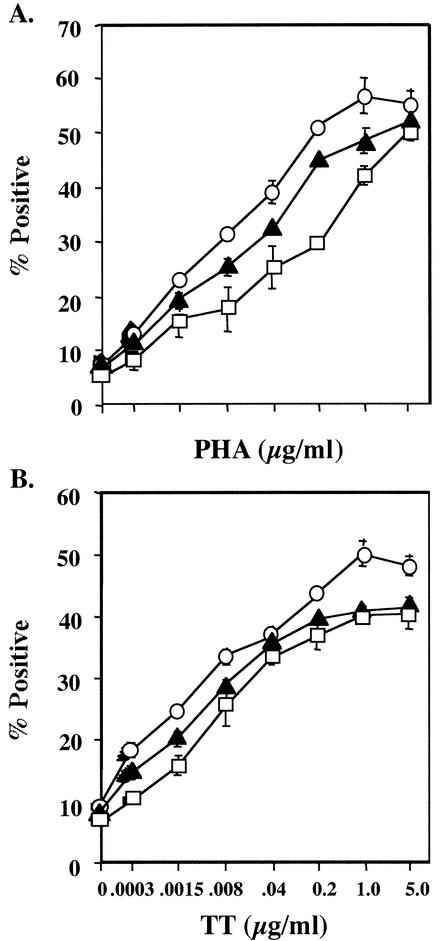
The incubation time required for the successful detection of lymphocyte activation and the ability to detect differences in the level of cell stimulation were also evaluated. Cell activation could be detected over a wide range of PHA and TT concentrations following a single day of incubation. There was little apparent advantage to increasing the incubation time to 2 or 3 days (Fig. 5).
FIG. 5.
rbhIL-2-SA-FITC binds to PHA- and TT-activated lymphocytes after 1 day of incubation over a wide range of PHA and TT concentrations. PBMC were incubated with different concentrations of PHA or TT for 1 day (open squares), 2 days (solid triangles), or 3 days (open circles) and were then labeled with rbhIL-2-SA-FITC, as described in the text. Data represent the mean and standard deviation for triplicate samples.
T-cell specificity of rbhIL-2 binding.
B cells are known to express IL-2R (4). To demonstrate that the majority of lymphocytes detected with rbhIL-2-SA-FITC conjugate are in fact T cells, PBMC incubated with 1.0 μg of PHA or TT per ml for 1 day were then stained with anti-CD3-PerCP, anti-CD19-PE, and rbhIL-2-SA-FITC. Approximately 21% of the cells stimulated with PHA were positive for both rbhIL-2-SA-FITC and anti-CD3-PerCP, whereas only 2.4% were positive for both rbhIL-2-SA-FITC and anti-CD19-PE. When stimulated with TT, approximately 4.1% of the cells were positive for both rbhIL-2-SA-FITC and anti-CD3-PerCP whereas only 0.5% were positive for both rbhIL-2-SA-FITC and anti-CD19-PE (Table 1).
TABLE 1.
Specificity of rbhIL-2-SA-FITC conjugate bindinga
| Stimulant | Ab specificity | % of cells positive forb:
|
|
|---|---|---|---|
| rbhIL-2-SA-FITC | SA-FITC | ||
| PHA | CD3 (T) | 20.97 ± 1.75 | 0.06 ± 0.02 |
| CD19 (B) | 2.39 ± 0.14 | 0.05 ± 0.00 | |
| TT | CD3 (T) | 4.09 ± 0.17 | 0.00 ± 0.00 |
| CD19 (B) | 0.48 ± 0.20 | 0.00 ± 0.00 | |
The analysis represents the examination of the lymphocyte population only, as determined by forward and side scatter.
Data represent the percentage of cells positive for binding of rbhIL-2-SA-FITC or SA-FITC and Ab against the indicated markers.
T-lymphocyte activation monitored using rbhIL-2-SA-HRP conjugates and chemiluminescence.
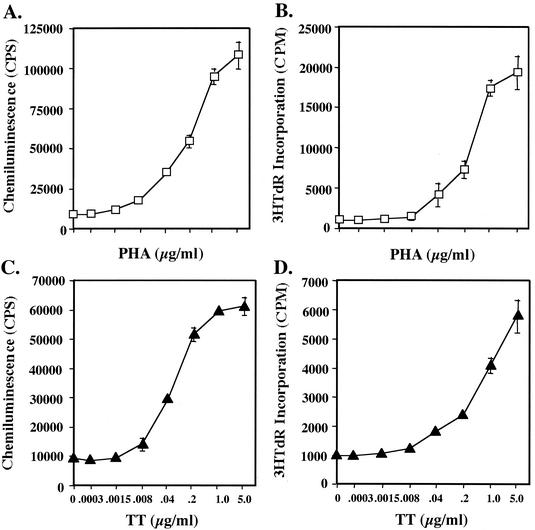
To illustrate the versatility with which different detection mechanisms can be applied when using rbhIL-2, the above strategy was applied in combination with rbhIL-2-SA-HRP and chemiluminescence detection methods. Similar to the results obtained when using rbhIL-2-SA-FITC conjugate and flow cytometry, as the concentration of PHA or TT increased, chemiluminescence (rbhIL-2-SAHRP binding) also increased in a dose-responsive manner (Fig. 6).
FIG. 6.
Analysis of T-cell activation by using rbhIL-2 and chemiluminescence versus [3H]TdR incorporation. PBMC were incubated in the presence of PHA (open squares) (A and B) or TT (solid triangles) (C and D) for 1 day for chemiluminescence analysis (A and C), and 3 days for [3H]TdR incorporation (B and D). Incubations and assays were carried out as described in Materials and Methods. Data represent the mean and standard deviation for triplicate samples.
Comparison of the chemiluminescence (rbhIL-2-SA-HRP) assay to T-lymphocyte activation measured by [3H]TdR incorporation.
To compare the ability of rbhIL-2-SA-HRP conjugate and chemiluminescence to detect T-cell activation with the ability of [3H]TdR incorporation to do so, TT- or PHA-stimulated PBMC were used as described above, except that T-cell activation was monitored via [3H]TdR incorporation. For PHA stimulation, titration curves using [3H]TdR incorporation were similar to those generated via the chemiluminescence assay (Fig. 6A and B). For TT stimulation and the chemiluminescence assay, however, a 50% maximal response was detected at 0.04 μg of TT per ml whereas the same value was approximately 0.4 μg/ml when using [3H]TdR incorporation (Fig. 6C and D). It should be noted, however, that in the latter case, a maximal (saturable) response to TT was not actually obtained. Thus, although we have traditionally observed saturation of the TT response at between 5 and 10 μg of TT per ml when using [3H]TdR incorporation, the actual dose of TT required to achieve a 50% maximal response may be higher.
DISCUSSION
The availability of highly purified recombinant cytokines has facilitated advances in immunological research and the development of cytokine-based immunotherapies. Several prokaryotic and eukaryotic systems have been used for the production of recombinant cytokines. Alternative approaches to cytokine production using retroviral, adenoviral, and direct DNA gene transfer also have met with success. Genetic manipulation of autologous or allogeneic cells transduced to secrete cytokines also was highly effective in several murine models (19, 25). No genetically engineered biotinylated cytokines are commercially available at present.
The PinPoint vector system allows the production of unique, monobiotinylated proteins which can be affinity purified using SoftLink soft-release avidin resin. In addition, the biotinylation tag can be easily removed enzymatically to produce nonbiotinylated product, should this be required. Therefore, we attempted to use this system to generate a monobiotinylated human IL-2. One potential caveat was the presence of an endogenously biotinylated protein normally present in E. coli, which may coisolate with rbhIL-2. While we did not observe significant levels of this protein in our rbhIL-2 preparation, potential solutions to this problem include dialysis of the rbhIL-2 preparation by using a size exclusion filter through which the endogenous protein can pass but rbhIL-2 cannot. Alternatively, molecular genetic techniques could be used to tag the endogenously biotinylated protein and facilitate its removal from rbhIL-2 preparations. Another caveat, which we did observe, was reduced activity of rbhIL-2 compared to nonbiotinylated rhIL-2. One possible explanation for this observation could be steric interference, or structural alterations, due to the presence of the biotinylation stub.
Having produced a genetically engineered rbhIL-2, we determined whether it could be used to monitor T-cell activation. Traditionally, T-cell activation has been monitored by [3H]TdR incorporation, a process that is closely related to underlying changes in the T-cell number. Other methods to monitor in vitro T-lymphocyte activation include detection of T-cell cytokines such as IL-2 and gamma interferon by ELISA (16), measurement of CD8 T-cell activation by granzyme B enzyme-linked immunospot assay (17), use of the IL-2-dependent cell line CTLL-2, MTT reduction (22), and detection of the lymphoid activation marker CD69 by flow cytometry (20). Additional assays include light absorbency to measure lymphocyte proliferation (6) and a combination of intracellular cytokine expression, cytokine secretion, and cytokine receptor expression (1). However, most of these assays require substantial time and the use of radioactive isotopes and/or are expensive to run.
We provide an alternative method to monitor T-cell activation by using FITC-SA or HRP-SA conjugated to rbhIL-2. This assay can be completed in 1 day and does not require the use of radioactive isotopes. This method is comparable in sensitivity to the traditional [3H]TdR incorporation assay when stimulating cells with PHA and is up to 10-fold more sensitive when analyzing TT-stimulated cells by chemiluminescence techniques (Fig. 6). In addition, the time required to complete the assay is one-quarter to one-sixth of that required to measure [3H]TdR incorporation. It remains to be determined whether the use of a biotinylated anti-CD25 (IL-2Rα) MAb would be an equally effective tool for monitoring T-cell activation by the described strategy. However, rbhIL-2 would be expected to bind only IL-2R capable of binding IL-2 itself (3, 21). IL2R binding by rbhIL-2 is indicated by the fact that purified rbhIL-2 stimulates the proliferation of an IL-2-dependent T-cell line whereas similarly purified material from bacteria transfected with a biotinylation vector lacking the IL-2 insert does not (Fig. 2). In addition, rbhIL-2 binds primarily to T cells (Table 1), the binding correlates with CD25 expression (Fig. 3), and it increases with the level of T-cell activation, as measured by [3H]TdR incorporation (Fig. 6). In addition, bhIL-2 would not be expected to bind to Fc receptors, a potential complication when using intact anti-CD25 MAb. Furthermore, therapeutic use of anti-CD25 MAb would probably be problematical, since host Ab would be generated to the variable regions and any mouse components that are present. This is important since rbhIL-2 has the potential for application in vaccines and immunotherapeutics (8, 10, 11, 13). For example, our laboratory has recently developed a targeting strategy which has the ability to target biotinylated proteins to APC (23). We are currently exploring its use in similarly targeting cytokines to APC in order to influence the degree and direction of the immune response. Furthermore, by changing the specificity of the targeting component, it may also be possible to target biotinylated cytokines to tumors, a strategy previously shown to be effective for the elimination of cancer cells (8, 10, 11, 14).
In summary, an rbhIL-2 has been generated which has potential application not only in immunodiagnostics but in clinical immunotherapeutics as well.
Acknowledgments
This work was supported by Public Health Service grants AI46968, AI40442, and AI35327 from the National Institutes of Health.
We thank the members of the Flow Cytometry Core Facility at Albany Medical College for their assistance and for the use of the equipment and facilities.
REFERENCES
- 1.Collins, D. P., B. J. Luebering, and D. M. Shaut. 1998. T-lymphocyte functionality assessed by analysis of cytokine receptor expression, intracellular cytokine expression, and femtomolar detection of cytokine secretion by quantitative flow cytometry. Cytometry 33:249-255. [DOI] [PubMed] [Google Scholar]
- 2.Cronan, J. E., Jr. 1990. Biotinylation of proteins in vivo, a post-translational modification to label, purify, and study proteins. J. Biol. Chem. 265:10327-10333. [PubMed] [Google Scholar]
- 3.De Jong, M. O., H. Rozemuller, J. G. Bauman, and J. W. Visser. 1995. Biotinylation of interleukin-2 (IL-2) for flow cytometric analysis of IL-2 receptor expression. Comparison of different methods. J. Immunol. Methods 184:101-112. [DOI] [PubMed] [Google Scholar]
- 4.de Totero, D., P. Francia di Celle, A. Cignetti, and R. Foa. 1995. The IL-2 receptor complex: expression and function on normal and leukemic B cells. Leukemia 9:1425-1431. [PubMed] [Google Scholar]
- 5.Foxwell, B. M., D. Taylor, B. Greiner, M. J. Mihatsch, V. Olivieri, and B. Ryffel. 1988. Biotinylated recombinant interleukin-2. A tool for research on the interleukin-2 receptor. J. Immunol. Methods 113:221-229. [DOI] [PubMed] [Google Scholar]
- 6.Gao, Z. H., W. A. Briggs, N. R. Rose, and J. F. Burdick. 1998. Use of a simple light absorbance assay to measure lymphocyte proliferation. J. Immunoassay 19:129-143. [DOI] [PubMed] [Google Scholar]
- 7.Gosselin, E. J., K. Wardwell, D. R. Gosselin, N. Alter, J. L. Fisher, and P. M. Guyre. 1992. Enhanced antigen presentation using human Fc gamma receptor (monocyte/macrophage)-specific immunogens. J. Immunol. 149:3477-3481. [PubMed] [Google Scholar]
- 8.Holden, S. A., Y. Lan, A. M. Pardo, J. S. Wesolowski, and S. D. Gillies. 2001. Augmentation of antitumor activity of an antibody-interleukin 2 immunocytokine with chemotherapeutic agents. Clin. Cancer Res. 7:2862-2869. [PubMed] [Google Scholar]
- 9.Jadus, M. R. 1999. Clinical uses of cytokines and their receptors, p. 311-330. In J. H. Nichols (ed.), Therapeutic drug monitoring and toxicology, vol. 20. American Association of Clinical Chemistry, Inc., Washington, D.C.
- 10.Lode, H. N., and R. A. Reisfeld. 2000. Targeted cytokines for cancer immunotherapy. Immunol. Res. 21:279-288. [DOI] [PubMed] [Google Scholar]
- 11.Matsumoto, H., S. Liao, F. Arakawa, A. Ueno, H. Abe, A. Awasthi, and M. Kuroki. 2002. Targeting of interleukin-2 to human MK-1-expressing carcinoma by fusion with a single-chain Fv of anti-MK-1 antibody. Anticancer Res. 22:2001-2007. [PubMed] [Google Scholar]
- 12.Nelson, B. H., and D. M. Willerford. 1998. Biology of the interleukin-2 receptor. Adv. Immunol. 70:1-77. [DOI] [PubMed] [Google Scholar]
- 13.Nichols, J., F. Foss, T. M. Kuzel, C. F. LeMaistre, L. Platanias, M. J. Ratain, A. Rook, M. Saleh, and G. Schwartz. 1997. Interleukin-2 fusion protein: an investigational therapy for interleukin-2 receptor expressing malignancies. Eur. J. Cancer Suppl. 1:S34-S36. [DOI] [PubMed] [Google Scholar]
- 14.Penichet, M. L., and S. L. Morrison. 2001. Antibody-cytokine fusion proteins for the therapy of cancer. J. Immunol. Methods 248:91-101. [DOI] [PubMed] [Google Scholar]
- 15.Peters, D. K., and D. H. Norback. 1990. Binding and internalization of biotinylated interleukin-2 in human lymphocytes. Blood 76:97-104. [PubMed] [Google Scholar]
- 16.Petrovsky, N., and L. C. Harrison. 1995. Cytokine-based human whole blood assay for the detection of antigen-reactive T cells. J. Immunol. Methods 186:37-46. [DOI] [PubMed] [Google Scholar]
- 17.Rininsland, F. H., T. Helms, R. J. Asaad, B. O. Boehm, and M. Tary-Lehmann. 2000. Granzyme B ELISPOT assay for ex vivo measurements of T cell immunity. J. Immunol. Methods 240:143-155. [DOI] [PubMed] [Google Scholar]
- 18.Samols, D., C. G. Thornton, V. L. Murtif, G. K. Kumar, F. C. Haase, and H. G. Wood. 1988. Evolutionary conservation among biotin enzymes. J. Biol. Chem. 263:6461-6464. [PubMed] [Google Scholar]
- 19.Schoenhaut, D. S., A. O. Chua, A. G. Wolitzky, P. M. Quinn, C. M. Dwyer, W. McComas, P. C. Familletti, M. K. Gately, and U. Gubler. 1992. Cloning and expression of murine IL-12. J. Immunol. 148:3433-3450. [PubMed] [Google Scholar]
- 20.Simms, P. E., and T. M. Ellis. 1996. Utility of flow cytometric detection of CD69 expression as a rapid method for determining poly- and oligoclonal lymphocyte activation. Clin. Diagn. Lab. Immunol. 3:301-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taki, S., T. Shimamura, M. Abe, T. Shirai, and Y. Takahara. 1989. Biotinylation of human interleukin-2 for flow cytometry analysis of interleukin-2 receptors. J. Immunol. Methods 122:33-41. [DOI] [PubMed] [Google Scholar]
- 22.VanBuskirk, A. M., P. W. Adams, and C. G. Orosz. 1995. Nonradioactive alternative to clinical mixed lymphocyte reaction. Hum. Immunol. 43:38-44. [DOI] [PubMed] [Google Scholar]
- 23.Walsh, M. C., J. A. Banas, S. P. Mudzinski, M. T. Preissler, R. F. Graziano, and E. J. Gosselin. 2003. A two component modular approach for enhancing T cell activation utilizing a unique anti-FcγR1-streptavidin construct and microspheres coated with biotinylated antigen. Biomol. Eng. 20:21-33. [DOI] [PubMed] [Google Scholar]
- 24.Wang, H., and S. K. Beckner. 1992. A colorimetric method for detection of specific ligand binding. Anal. Biochem. 204:59-64. [DOI] [PubMed] [Google Scholar]
- 25.Yoshimoto, T., K. Kojima, T. Funakoshi, Y. Endo, T. Fujita, and H. Nariuchi. 1996. Molecular cloning and characterization of murine IL-12 genes. J. Immunol. 156:1082-1088. [PubMed] [Google Scholar]
- 26.Zhang, G., W. Li, L. Holle, N. Chen, and W. Y. Chen. 2002. A novel design of targeted endocrine and cytokine therapy for breast cancer. Clin. Cancer Res. 8:1196-1205. [PubMed] [Google Scholar]