Abstract
The role of Bordetella bronchiseptica in a natural outbreak of canine infectious respiratory disease was investigated both by culture and serological analysis. B. bronchiseptica was found in the lungs of a large proportion of clinically healthy dogs and in a greater proportion of dogs with respiratory disease. Using a lipopolysaccharide (LPS) antigen-based enzyme-linked immunosorbent assay, we analyzed the serological responses of a large number of dogs. Dogs with high antibody levels showed no protection from disease, and there was no correlation between the development of disease and rising antibody titer. Similarly, there was no difference in antibody levels in dogs with and without B. bronchiseptica in the lungs. Antibodies to LPS have no predictive value in determining which animals will contract respiratory disease, how severe the disease will be, or which dogs will have B. bronchiseptica colonizing the lungs.
Canine infectious respiratory disease (CIRD; also known as kennel cough) is an infection that affects dogs of all ages (2). The disease commonly occurs when large numbers of dogs are housed together in close confinement. The disease has high morbidity and is characterized by a dry hacking cough, anorexia, and depression and can lead to tracheobronchitis, pneumonia, and even death in severe cases. The disease has historically been regarded as a complex infection in which combined or sequential challenge with both viral (canine parainfluenza virus [CPIV] and canine adenovirus type 2 [CAV-2]) and bacterial agents produces a synergistic enhancement of the clinical symptoms (1, 20). The most common bacterial agent detected during the disease is Bordetella bronchiseptica (22, 29). Evidence that this organism has an etiological role in the disease was provided during early studies by Thompson et al. (28) and Bemis et al. (5, 6), who correlated the presence of B. bronchiseptica with respiratory disease and were able to reproduce experimental disease with B. bronchiseptica infection alone. Vaccination protection studies also support a role for B. bronchiseptica in respiratory disease (7, 23). However, Ueland (31) reported an outbreak of infectious tracheobronchitis during which B. bronchiseptica was not isolated, suggesting that other agents may be able to produce respiratory disease in dogs.
A major component of the cell membrane of gram-negative bacteria is lipopolysaccharide (LPS), and this molecule is highly toxic and immunogenic and plays an integral part in infection (32). Although the role of LPS in the pathogenesis of B. bronchiseptica infection is largely undefined (32), in mice LPS is required for both colonization of the respiratory tract and persistence via resistance to the adaptive immune system (16). To date, there is only one publication concerning the serum responses of dogs to B. bronchiseptica LPS (10). Dees et al. (10) found that the serum response to vaccination with an LPS mutant was reduced in comparison with the response to vaccination with the whole-cell bacterin and that antibodies to LPS are made during infection. However, the correlation between the presence of antibodies to LPS and protection from B. bronchiseptica infection was not examined. The purpose of the study was to evaluate whether levels of antibodies to B. bronchiseptica in the sera of dogs entering a large kennel could predict the susceptibilities of the dogs to natural infection with respiratory disease.
In collaboration with a well-established rehoming kennel with a long history of respiratory disease, we examined the serological responses of 424 dogs to B. bronchiseptica during the dogs' first 3 weeks at the center. It is during this period that approximately 90% of the animals contract the disease. An enzyme-linked immunosorbent assay (ELISA) was used to measure levels of canine antibodies to B. bronchiseptica LPS. High antibody levels, indicating past exposure to B. bronchiseptica, may be indicative of protective immunity, while low antibody levels may indicate predisposition to disease. Furthermore, the identification of a correlation between rising antibody levels, bacterial isolation, and clinical disease would support the view that B. bronchiseptica is a significant agent of the respiratory disease in this rehoming center.
MATERIALS AND METHODS
Dogs.
On day 1, the day of entry into the rehoming kennel, all dogs were routinely vaccinated with Kavak DA2PiP69 and Kavak L (Fort Dodge Animal Health), the former protecting against canine distemper virus (CDV), CAV-2, CPIV-2, and canine parvovirus, and the latter protecting against leptospirosis. Dogs were not vaccinated against B. bronchiseptica, but vaccination histories prior to entry into the rehoming center remain unknown. Dogs were housed in groups of three or less per kennel but shared runs and air space with other dogs. A total of 152 dogs were euthanized for welfare reasons, ranging from behavioral problems to signs of severe respiratory disease, at an average time period of 16 days after entry into the kennel. All dogs were necropsied within 4 h of euthanasia. Control sera were obtained from serum samples submitted to the Royal Veterinary College for routine evaluation from 18-month-old kennelled beagles which had never been vaccinated against B. bronchiseptica and had no history of respiratory disease.
Clinical evaluation.
On days 1, 7, and 21, the clinical condition of each dog was scored in relation to respiratory disease by using the following categories: 1, no respiratory symptoms; 2, mild cough (mild disease); 3, cough and nasal discharge (moderate disease); and 4, cough and nasal discharge with depression, inappetence, or signs of lower respiratory tract involvement (severe disease).
Serum samples.
Three milliliters of blood was taken on days 1 and 21 from each dog in a randomly chosen sample (approximately 10%) of dogs entering the kennel. Because many dogs were rehomed before day 21, the number of serum samples taken on day 1 was considerably greater than that taken on day 21. In total, 424 serum samples were taken on day 1 upon entry into the kennel, with 156 corresponding serum samples taken on day 21. Additional serum samples were obtained from 77 dogs just prior to euthanasia. All samples were transported in microtube 1.1-ml Z-gel tubes (Sarstedt) and centrifuged at 835 × g for 5 min. The sera were then separated and stored at −20°C.
Isolation of B. bronchiseptica.
The lungs of the 152 animals necropsied were subjected to lavage with 60 ml of Hanks balanced salts solution (Invitrogen, Paisley, United Kingdom). Recovered lavage fluid (0.05 ml) was plated onto blood agar and MacConkey agar and incubated aerobically at 37°C for 48 h. Typical oxidase-positive colonies were identified biochemically as B. bronchiseptica by using API 20NE (Biomerieux).
Antibody determination.
B. bronchiseptica LPS antigen was isolated from a lung lavage fluid bacterial isolate (B. bronchiseptica strain Bob30) from a dog within this kennel. This isolate was similar to other isolates from the kennel, as determined by restriction fragment length polymorphism and phenotypic analyses (data not shown). Briefly, 250 ml of stationary-phase B. bronchiseptica cells cultured at 37°C in brain heart infusion broth (Oxoid) were harvested by centrifugation at 9,300 × g for 20 min at 4°C, resuspended in 50 ml of Tris-NaCl (10 mM Tris-HCl, 150 mM NaCl [pH 7.5]), and then heated to 100°C for 1 h. Nonsoluble cellular matter was then harvested by centrifugation at 9,300 × g for 20 min at 4°C, and the LPS was extracted by mixing with an equal volume of water-saturated phenol at 68°C for 15 min with agitation. The aqueous phase was separated, dialyzed against three changes of deionized water, and stored in 1-ml aliquots at −20°C.
Each well of a 96-well plate (F96 MaxiSorp Nunc-Immuno plates) was coated with 100 μl of LPS antigen diluted 1:10 in NaCO3 buffer (95 mM Na2CO3, 50 mM NaHCO3 [pH 9.0]) at 37°C for 1 h at 180 rpm. The remaining antigen was then aspirated, and wells were blocked for 1 h at 37°C, 180 rpm, with 3% (wt/vol) skimmed milk powder in TNT (10 mM Tris-HCl, 150 mM NaCl [pH 8.0], 0.1% Tween). All wells were then washed in triplicate with TNT. One hundred microliters of each serum sample (diluted 1:1,000 in 3% milk-TNT) was then added to duplicate wells, and the plates were incubated at 37°C for 1 h at 180 rpm. Wells were then washed five times in TNT, and 100 μl of anti-canine immunoglobulin G (IgG)-horseradish peroxidase conjugate (Sigma, Poole, United Kingdom) diluted 1:5,000 in 3% milk-TNT was added for 1 h at 37°C and 180 rpm. Following five washes with TNT, 200 μl of substrate solution o-phenylenediamine dihydrochloride (Sigma) was added to each well, and the reaction developed for 30 min in the dark. The reaction was then stopped by the addition of 100 μl of 2 M H2SO4, and the absorbance at 490 nm was determined. Absorbance measurements were then converted into arbitrary antibody units by using the SOFT Max Pro version 2.6.1 computer program (Molecular Devices Corporation).
All paired sera (samples from the same dogs on days 1 and 21) were analyzed on the same microtiter plates, and duplicates of the negative control (no primary antibody) and positive control pool sera were added to each plate. The study included only naturally infected kennelled dogs and no experimentally infected dogs; thus, no positive control as such was available. In place of a known infected dog serum and to aid in determining assay accuracy and consistency, a pool of six sera which yielded high antibody levels on initial screening was included in duplicate on each plate, a procedure similar to the method of Trollfors et al. (30) except that pooled serum was used. Plate results were considered to be valid only when this high serum mix value was within two times the standard deviation (SD) of the mean of all sera included in the pool.
The test was further validated by the inclusion of a mix of sera from five 18-month-old kennelled nonvaccinated beagles with no history of respiratory illness. An equal volume of each dog serum was pooled, mixed, and diluted 1:1,000 in 3% milk-TNT. The serum mix was then aliquoted and stored at −20°C. Each beagle serum sample was also tested separately seven times in duplicate by the method described above, and a lower limit of 1,452 antibody units was determined by the mean of the individual samples plus three times the SD. Dogs were classed as negative if the antibody levels were at or below this cutoff point. All statistical analysis was performed with SPSS for Windows (SPSS Inc.) and GraphPad Prism 3.02 (GraphPad Software Inc.). The probability of a type I error (α) of 0.05 and the power to detect a clinically important difference (1 − β) of 0.8 were taken for all analyses.
RESULTS
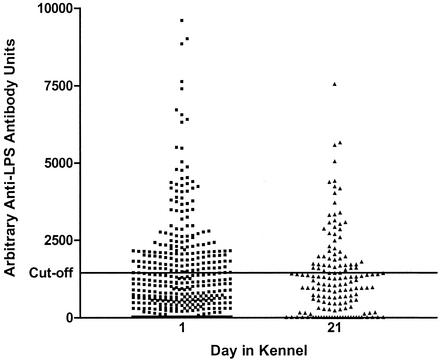
Serum samples from 424 dogs entering the rehoming center were analyzed for anti-B. bronchiseptica LPS antibodies. Of these animals, 421 (99.3%) had a clinical score of 1 (no respiratory disease) on day 1 and three (0.7%) had a clinical score of 2 (mild respiratory symptoms). On day 21, only 156 of the animals remained at the center and were reexamined and sampled. Thirty-seven (23.7%) showed a clinical score of 1 (including 19 dogs [12.2%] that had recovered from respiratory disease [convalescent dogs]), while 119 (76.3%) showed clinical scores ranging from 2 to 4, indicating that within 21 days 88.5% of the population had contracted respiratory disease. Dogs showed similar ranges of antibody levels at both time points (15 to 64,080 antibody units), with 156 (36.8%) and 53 (34.0%) dogs having positive titers (above 1,452 antibody units) on days 1 and 21, respectively. An independent sample t test was conducted to compare the arithmetic mean antibody levels for dogs on days 1 and 21. There was no significant difference in means for day 1 (mean, 1,578; SD, 3,514; n = 424) and day 21 (mean, 1,822; SD, 5,299; n = 156; t = −0.64; P = 0.522). The magnitude of differences in means (effect size) was very small (η2 [correlation ratio] = 0.0007). It was estimated that a sample size of 160 dogs would be required to detect a clinically important difference (of 1,452 antibody units) in the means (Fig. 1).
FIG. 1.
Antibody titers do not alter between days 1 and 21. LPS antibody levels of dogs on days 1 and 21 (day 1, n = 424; day 21, n = 156) in the kennel are shown. Any samples with levels above the cutoff point of 1,452 antibody units were deemed to be positive. For clarity, the y axis has been limited to 10,000 U.
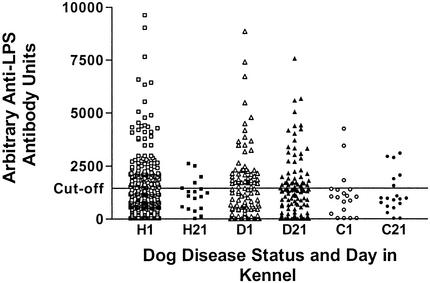
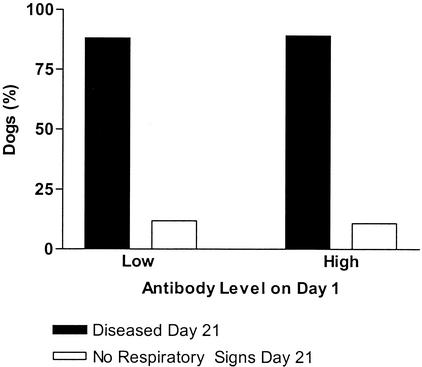
Subjects were divided by day and then into three groups according to clinical health status (healthy, diseased, and convalescent) on day 21. Examination of paired serum samples from days 1 and 21 from dogs categorized by clinical respiratory symptoms (Fig. 2) indicated that those animals remaining free of disease had arithmetic mean antibody levels above the negative cutoff point (1,452 antibody units) on day 1 only (day 1, 1,481 antibody units; day 21, 1,133 antibody units) but that convalescing animals had arithmetic mean antibody levels below the cutoff point on both day 1 and day 21 (1,113 and 1,208 antibody units, respectively). This was in contrast to those dogs which contracted the disease and showed positive arithmetic mean antibody levels on both day 1 and day 21 (1,886 and 2,025 antibody units, respectively). A one-way between-groups analysis of variance was conducted to explore the impact of clinical health status on the arithmetic mean antibody level. There was no significant difference in the means on day 1 [F (2,421) = 0.7; P = 0.482; n = 424] and day 21 [F (2,153) = 0.4; P = 0.696; n = 156]. The effect size was very small for both day 1 (η2 = 0.0035) and day 21 (η2 = 0.0047). An estimate of the number of dogs required to detect a clinically relevant difference (1,452 mean antibody units) gave sample sizes of 130 dogs (day 1) and 32 dogs (day 21). Dogs with low antibody levels (<1,452 antibody units) on day 1 were just as likely to develop respiratory disease as those dogs with high antibody levels (>1,452 antibody units) on day 1 (χ2 = 0.033 [Pearson's test]; P = 0.856; number of valid cases, 156) (Fig. 3). In order to detect a clinically relevant difference (5%) in disease status, it was estimated that a sample size of 12 dogs was required.
FIG. 2.
Antibody titers do not differ between healthy (H; day 1, n = 286; day 21, n = 18), diseased (D; n = 119), and convalescent (C; n = 19) dogs. LPS antibody levels in dogs on days 1 and 21, separated according to the disease statuses of the animals on day 21, are shown. Any samples with levels above the cutoff point of 1,452 antibody units were deemed to be positive. For clarity, the y axis has been limited to 10,000 U.
FIG. 3.
Percentage of dogs with either low (89 of 101 dogs) or high (49 of 55 dogs) antibody levels on day 1 which developed disease by day 21. High antibody titers offer no protection from disease.
To determine whether individual animals were seroconverting to B. bronchiseptica during infection, paired day 1 and day 21 serum samples were analyzed for rising titers. Of 156 dogs, 21 (13.5% of the total) showed an increase in titer of 500 U or more. The remainder showed a minor increase or decrease.
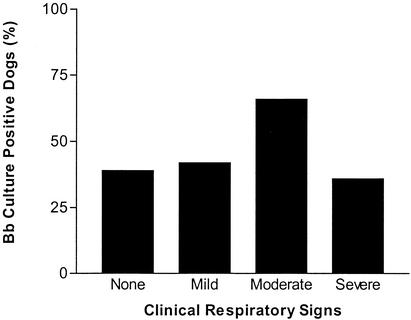
B. bronchiseptica was isolated from 47% of the lung washings obtained postmortem from the kennelled population (n = 152). However, the isolation rate from clinically healthy dogs was 39% (n = 54), which was considerably less than the percentage of dogs displaying any clinical respiratory symptoms (52%; n = 98) (Fig. 4), with the highest isolation rate seen in those dogs with moderate disease (66%). There was no significant difference in the numbers of healthy and diseased animals with B. bronchiseptica in the lungs (χ2 = 2.416; P = 0.12; number of valid cases, 152). In order to detect a clinically relevant difference (5%) in lung B. bronchiseptica colonization, it was estimated that a sample size of 32 dogs was required. However, a significant difference was found between the numbers of healthy animals and those with moderate disease that had B. bronchiseptica in the lungs (χ2 = 7.371; P = 0.007; number of valid cases, 101; estimated sample size required for detection of a clinically relevant difference of 5%, 26 dogs).
FIG. 4.
B. bronchiseptica isolation and disease. The percentages of dogs with lung cultures positive for B. bronchiseptica (Bb) are shown (no symptoms, n = 54; mild disease, n = 26; moderate disease, n = 47; severe disease, n = 25). B. bronchiseptica is isolated with higher frequency from animals with symptoms of moderate disease.
Serum samples were obtained from 77 dogs upon euthanasia and tested for antibodies to B. bronchiseptica LPS. While 68% of animals had raised antibody levels, there was no correlation between high titer and the isolation of B. bronchiseptica (52 and 48% of dogs with cultures positive and negative for B. bronchiseptica, respectively, had high antibody titers). In addition, dogs with high antibody titers were as likely to have B. bronchiseptica in the lungs as those with low antibody titers (χ2 = 0.42; P = 0.51; number of valid cases, 77). In order to detect a clinically relevant difference (5%) in lung B. bronchiseptica colonization, it was estimated that a sample size of 30 dogs was required. In this case, only 25 dogs that were available for postmortem analyses had antibody levels below the negative cutoff point on the day of entry into the kennel. The actual power of this test was calculated at 0.75. Dogs with low antibody titers at euthanasia showed a higher incidence of respiratory disease (68%) than the other dogs, but the incidence of disease was not significantly different from that in dogs with high antibody titers (54%; χ2 = 1.393; P = 0.238; number of valid cases, 77; estimated sample size required for detection of a clinically relevant difference of 5%, 22 dogs).
DISCUSSION
B. bronchiseptica has previously been associated with respiratory disease in dogs (28). This study relates to a natural outbreak of CIRD and confirms that B. bronchiseptica isolation increases with primary clinical respiratory symptoms and furthermore shows that B. bronchiseptica is found in a large proportion of dogs exhibiting no respiratory symptoms. Measurement of serum antibody levels indicates that there is no correlation between clinical respiratory symptoms and the levels of anti-B. bronchiseptica LPS antibodies.
The levels of anti-LPS antibodies varied considerably between individual animals, and no difference was found between diseased animals and those showing no respiratory symptoms. In 21 dogs, a clearly rising titer was detected over the 21-day period, indicating an LPS antibody response in these few animals. However, the fact that rising titers were found in only 13.5% of the population, even though 88.5% contracted the disease within 21 days, is surprising. In humans, infection with B. pertussis leads to a clear agglutinating antibody response, whether or not this response is related to protection (8, 30). However, Ashworth et al. (3) found no correlation between the clearance of Bordetella pertussis from the nasopharynx of rabbits and serum antibodies to LPS. In addition, Kono et al. (19) found that in B. bronchiseptica infections of pigs there was no correlation between the clearly developing antibody response and protection from colonization or disease. However, the same study also showed that 21 days may be insufficient for the development of serum antibodies to B. bronchiseptica in pigs, whereas in dogs serum IgG production occurs 5 days postinfection (33).
Even though full-length LPS has been shown to be essential for colonization by and survival (32) and virulence (16) of B. bronchiseptica in mice, it is possible that in dogs B. bronchiseptica LPS is not an immunodominant antigen. Recently, Mattoo et al. (21) have reported that fimbriae constitute a dominant antigen in the immune response to B. bronchiseptica in rats. Furthermore, using a whole-cell antigen-based ELISA, Dees et al. (10) found that 68% of the household dog population that they tested were seropositive for B. bronchiseptica, a percentage considerably higher than the level detected in the present study. Additionally, Ellis et al. (11) reported an increase in IgG and IgA in vaccinated dogs by using a whole-cell antigen-based ELISA. However, the use of a whole-cell antigen-based ELISA in this study would have provided limited information as to the role of LPS antibodies in susceptibility to disease.
Dogs with high antibody levels were just as likely to contract respiratory disease as those animals with low titers. This could indicate that in this natural outbreak of respiratory disease, even though B. bronchiseptica was present and found in increasing numbers in those dogs with respiratory disease, it was not the causative agent of disease or that antibodies to B. bronchiseptica LPS are nonprotective. In concordance with the results of this investigation, Trollfors et al. (30) observed that in humans anti-LPS serum IgG antibodies to B. pertussis and B. parapertussis are nonprotective and increases in anti-LPS antibodies are seen only in some patients. Furthermore, Thrusfield et al. (29) found no correlation between levels of antibodies to B. bronchiseptica antigen in serum and protection. In addition, a study with piglets (24) has shown that antibodies to B. bronchiseptica LPS have a limited role in protection, even though they are produced following vaccination. However, IgA titers correlated with the development of resistance to clinical infection (7) and several authors (11, 27, 33) found that local antibodies were protective against B. bronchiseptica. In fact, both Shade and Goodnow (27) and Ellis et al. (11) reported protection against B. bronchiseptica following intranasal vaccination. It may therefore be suggested that the role of mucosal immunity to B. bronchiseptica infection is more important than that of a serum response. Indeed, Yamamoto et al. (33) found that local antibodies (IgG and IgA) in dogs are produced and confer partial protection at the local site of injection of B. bronchiseptica, and therefore local antibodies may play an important role in natural infection. However, serum IgG is used to determine the efficacy of B. bronchiseptica vaccination in dogs (11, 12) and has also been shown to increase following natural and experimental infection of dogs (28), and this serum response should be further defined. Chodorowska (9) observed that IgM rather than IgG is the major antibody produced in response to B. pertussis LPS infections of children. Yet for B. bronchiseptica infections in dogs, Yamamoto et al. (33) observed that IgG was produced in serum 5 days postinfection, at the same time as and at a higher titer than IgM.
Traditionally, CIRD is referred to as a disease complex involving multiple infectious agents, such as B. bronchiseptica and CPIV or CAV-2. In addition, clinical symptoms other than coughing are not found in dogs experimentally infected with B. bronchiseptica (4, 18). It is likely that agents other than B. bronchiseptica were involved in this natural outbreak of respiratory disease. It may be asked whether CDV played a role in this outbreak of respiratory disease. However, all dogs are vaccinated against CDV on entry into the kennel, and reverse transcription-PCR analysis detected no CDV in 34 dogs tested (data not shown). Mycoplasma species have also been implicated in this disease syndrome (14, 25, 26), and certain mycoplasmas are known to have immunosuppressive activities during other infections (13). During the infection process within this rehoming center, B. bronchiseptica or other microbial agents may have immunosuppressive activities, inhibiting the production of anti-LPS antibodies. Indeed, Horiguchi et al. (17) reported that the dermonecrotizing toxin of B. bronchiseptica suppresses in vivo antibody responses in mice. Furthermore, Guzman et al. (15) noted that B. bronchiseptica enters mouse dendritic cells and postulated that residence within dendritic cells not only accounts for the chronic nature of Bordetella infection but could also lead to an altered immune response.
In conclusion, levels of antibodies to B. bronchiseptica LPS in serum were shown to have no predictive value for susceptibility to naturally occurring respiratory disease in dogs in this rehoming center and no correlation with the presence of B. bronchiseptica within the canine lung.
REFERENCES
- 1.Appel, M., and D. Bemis. 1977. Canine respiratory disease complex, p. 1287-1292. In R. Kirk (ed.), Current veterinary therapy, vol. 6. W. B. Saunders Co., New York, N.Y.
- 2.Appel, M., and L. N. Binn. 1987. Canine infectious bracheobronchitis short review: kennel cough, p. 209-211. In M. Appel (ed.), Virus infections of carnivores. Elsevier, New York, N.Y.
- 3.Ashworth, L. A., R. B. Fitzgeorge, L. L. Irons, C. P. Morgan, and A. Robinson. 1982. Rabbit nasopharyngeal colonisation by Bordetella pertussis: the effects of immunisation on clearance and on serum and nasal antibody levels. J. Hyg. 88:475-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bemis, D. A. 1992. Bordetella and Mycoplasma respiratory infection in dogs and cats. Vet. Clin. N. Am. Small Anim. Pract. 22:1173-1186. [DOI] [PubMed] [Google Scholar]
- 5.Bemis, D. A., L. E. Carmichael, and M. J. Appel. 1977. Naturally occurring respiratory disease in a kennel caused by Bordetella bronchiseptica. Cornell Vet. 67:282-293. [PubMed] [Google Scholar]
- 6.Bemis, D. A., H. A. Greison, and M. J. G. Appel. 1977. Pathogenesis of canine bordetellosis. J. Infect. Dis. 135:753-762. [DOI] [PubMed] [Google Scholar]
- 7.Bey, R. F., F. J. Shade, R. A. Goodnow, and R. C. Johnson. 1981. Intranasal vaccination of dogs with live avirulent Bordetella bronchiseptica: correlation of serum agglutination titre and the formation of secretory IgA with protection against experimentally induced infectious tracheobronchitis. Am. J. Vet. Res. 42:1130-1132. [PubMed] [Google Scholar]
- 8.Bordet, J., and O. Gengou. 1906. Le microbe de la coqueluche. Ann. Inst. Pasteur (Paris) 20:731-741. [Google Scholar]
- 9.Chodorowska, M. 1999. Humoral reaction to Bordetella pertussis antigens: pertussis toxin, filamentous hemagglutinin and lipopolysaccharide in children with clinical symptoms of whooping cough. II. Occurrence and level of B. pertussis antigens in children with suspected whooping cough. Med. Dosw. Mikrobiol. 51:269-280. (In Polish.) [PubMed]
- 10.Dees, C., M. W. Fountain, V. S. Panangala, L. J. Swango, and R. D. Schultz. 1982. An ELISA test to detect antibody to Bordetella bronchiseptica. Vet. Immunol. Immunopathol. 3:539-545. [DOI] [PubMed] [Google Scholar]
- 11.Ellis, J. A., D. M. Haines, K. H. West, J. H. Burr, A. Dayton, H. G. G. Townsend, E. W. Kanara, C. Konoby, A. Crichlow, K. Martin, and G. Headrick. 2001. Effect of vaccination on experimental infection with Bordetella bronchiseptica in dogs. J. Am. Vet. Med. Assoc. 218:367-375. [DOI] [PubMed] [Google Scholar]
- 12.Ellis, J. A., G. S. Krakowka, A. D. Dayton, and C. Konoby. 2002. Comparative efficacy of an injectable vaccine and an intranasal vaccine in stimulative Bordetella bronchiseptica reactive antibody responses in sero-positive dogs. J. Am. Vet. Med. Assoc. 220:43-48. [DOI] [PubMed] [Google Scholar]
- 13.Friedman, S. M., Y. Li, G. Zagon, J. R. Tunmang, G. R. Sun, and M. K. Crow. 1994. A potential role for microbial superantigens in autoimmune disease. IOM Lett. 3:688-689. [Google Scholar]
- 14.Greig, A. S. 1954. The significance of a pleuropneumonia-like organism in kennel cough. Can J. Comp. Med. 18:275-279. [PMC free article] [PubMed] [Google Scholar]
- 15.Guzman, C. A., M. Rohde, M. Bock, and K. N. Timmis. 1994. Invasion and intracellular survival of Bordetella bronchiseptica in mouse dendritic cells. Infect. Immun. 62:5528-5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harvill, E. T., A. Preston, P. A. Cotter, A. G. Allen, D. J. Maskell, and J. F. Miller. 2000. Multiple roles for Bordetella lipopolysaccharide molecules during respiratory tract infection. Infect. Immun. 68:6720-6728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horiguchi, Y., H. Matsuda, H. Koyama, T. Nakai, and K. Kume. 1992. Bordetella bronchiseptica dermonecrotizing toxin suppresses in vivo antibody responses in mice. FEMS Microbiol. Lett. 69:229-234. [DOI] [PubMed] [Google Scholar]
- 18.Keil, D. J., and B. Fenwick. 1998. The role of Bordetella bronchiseptica in infectious tracheobronchitis in dogs. J. Am. Vet. Med. Assoc. 212:200-207. [PubMed] [Google Scholar]
- 19.Kono, Y., S. Suzuki, T. Muika, K. Okazaki, E. Honda, and T. Yamashiro. 1993. Detection of specific systemic and local IgG and IgA antibodies of pigs after infection with Bordetella bronchiseptica by ELISA. J. Vet. Med. Sci. 56:249-253. [DOI] [PubMed] [Google Scholar]
- 20.Kontor, E. J., R. J. Wegrzn, and R. A. Goodnow. 1981. Canine infectious tracheobronchitis: effects of an intranasal live canine parainfluenza-Bordetella bronchiseptica vaccine on viral shedding and clinical tracheobronchitis (kennel cough). Am. J. Vet. Res. 42:1694-1698. [PubMed] [Google Scholar]
- 21.Mattoo, S., J. F. Miller, and P. A. Cotter. 2000. Role of Bordetella bronchiseptica fimbriae in tracheal colonization and development of a humoral immune response. Infect. Immun. 68:2024-2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCandlish, I. A. P., H. Thompson, and N. G. Wright. 1978. Vaccination against Bordetella bronchiseptica infection in dogs using a heat-killed bacterial vaccine. Res. Vet. Sci. 25:45-50. [PubMed] [Google Scholar]
- 23.McCandlish, I. A. P., H. Thompson, and N. G. Wright. 1978. Vaccination against canine bordetellosis: protection from contact challenge. Vet. Rec. 102:479-483. [DOI] [PubMed] [Google Scholar]
- 24.Novotny, P., M. Kobisch, K. Cownley, A. P. Chubb, and J. A. Montaraz. 1985. Evaluation of Bordetella bronchiseptica vaccines in specific-pathogen-free piglets with bacterial cell surface antigens in enzyme-linked immunosorbent assay. Infect. Immun. 50:190-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Randolph, J. F., N. S. Moise, J. M. Scarlett, S. J. Shin, J. T. Blue, and J. R. Corbett. 1993. Prevalence of mycoplasmal and ureaplasmal recovery from tracheobronchial lavages and of mycoplasmal recovery from pharyngeal swab specimens in cats with or without pulmonary disease. Am. J. Vet. Res. 54:897-900. [PubMed] [Google Scholar]
- 26.Rosendal, S. 1972. Mycoplasmas as a possible cause of enzootic pneumonia in dogs. Acta Vet. Scand. 13:137-139. [PubMed] [Google Scholar]
- 27.Shade, F. J., and R. A. Goodnow. 1979. Intranasal immunisation of dogs against Bordetella bronchiseptica-induced tracheobronchitis (kennel cough) with a modified live-Bordetella bronchiseptica vaccine. Am. J. Vet. Res. 40:1241-1243. [PubMed] [Google Scholar]
- 28.Thompson, H., I. A. P. McCandlish, and N. G. Wright. 1976. Experimental respiratory disease in dogs due to Bordetella bronchiseptica. Res. Vet. Sci. 20:16-23. [PubMed] [Google Scholar]
- 29.Thrusfield, M. V., C. G. G. Aitken, and R. H. Muirhead. 1991. A field investigation of kennel cough: incubation period and clinical signs. J. Small Anim. Pract. 32:215-220. [Google Scholar]
- 30.Trollfors, B., T. Lagergard, J. Taranger, E. Bergfors, R. Schneerson, and J. B. Robbins. 2001. Serum immunoglobulin G antibody responses to Bordetella pertussis lipooligosaccharide and B. parapertussis lipopolysaccharide in children with pertussis and parapertussis. Clin. Diagn. Lab. Immunol. 8:1015-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ueland, K. 1990. Serological, bacteriological and clinical observations on an outbreak of canine infectious tracheobronchitis in Norway. Vet. Rec. 126:481-483. [PubMed] [Google Scholar]
- 32.West, N. P., H. Jungnitz, J. T. Fitter, J. D. McArthur, C. A. Guzman, and M. J. Walker. 2000. Role of phosphoglucomutase of Bordetella bronchiseptica in lipopolysaccharide biosynthesis and virulence. Infect. Immun. 68:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamamoto, S., T. Shida, M. Honda, Y. Ashida, Y. Rikihisa, M. Okadura, S. Hayashi, M. Nomura, and Y. Isayame. 1994. Serum C-reactive protein and immune responses in dogs inoculated with Bordetella bronchiseptica (phase I cells). Vet. Res. Commun. 18:347-357. [DOI] [PubMed] [Google Scholar]