Abstract
The accumulation of cellular transcripts from cells infected with herpes simplex virus 1 (HSV-1) as measured with the aid of Affymetrix microchips has been reported elsewhere. Among these transcripts were genes that respond to stress and that could have a noxious effect on viral replication. We have selected the stress-inducible cellular gene encoding the immediate-early response protein IEX-1 to verify and determine the significance of the accumulation of these transcripts in infected cells. We report that we verified the increase in accumulation of IEX-1 transcripts after infection by Northern analyses and real-time PCR. These transcripts reach peak levels between 3 and 7 h after infection and decrease thereafter. However, IEX-1 protein was detected in cells 1 h after infection but not at later intervals. Studies designed to elucidate the failure of IEX-1 protein to be synthesized revealed the following points. (i) IEX-1 RNA transported to the cytoplasm after 1 h of infection consisted of at least two populations, a partially degraded population and a population consisting of unspliced IEX-1 RNA. Neither of these RNAs could translate the authentic IEX-1 protein. (ii) The partially degraded IEX-1 RNA was not detected in the cytoplasm of cells infected with a mutant virus lacking the UL41 gene encoding the virion host shutoff protein (vhs). Although degradation of RNA mediated by vhs was reported to be 5′ to 3′, the partially degraded IEX-1 RNA lacked the 3′ sequences rather than the 5′ sequences. (iii) The unspliced pre-RNA form containing the IEX-1 intron sequences was detected in the cytoplasm of cell infected with wild-type virus but not in those infected with a mutant lacking the α27 gene encoding the infected cell protein No. 27. (iv) Overexpression of IEX-1 protein by transduction of the gene prior to infection with 1 PFU of HSV-1 per cell had no effect on the accumulation of late genes and virus yield. We conclude that the failure of IEX-1 to express its protein reflects the numerous mechanisms by which the virus thwarts the cells from expressing its genes after infection.
An earlier article reported on the results of Affymetrix microchip assays of the accumulation of cellular transcripts after herpes simplex virus 1 (HSV-1) infection of quiescent human foreskin fibroblasts (29). These analyses showed that transcripts of approximately 2% of the human genes represented in the microchips accumulated in amounts significantly above the levels present in uninfected cells. The patterns of accumulation varied. While a few transcripts were elevated throughout the 12-h interval of study, others exhibited defined temporal patterns that appeared at least superficially to be related to the function of the gene products. The results of microchip assays must be verified with respect to actual changes in the accumulated amounts and tested for significance of the upregulation. The focus of this report is on the apparent increase in the accumulation of transcripts encoding the stress-inducible immediate-early response gene IEX-1.
IEX-1, also named p22/PRG1, Dif-2, or gly96 (its mouse homologue), is a stress-inducible gene. Its transcription can be rapidly activated by irradiation; growth factors, such as epidermal growth factor; viral infection; inflammatory cytokines, such as tumor necrosis factor alpha and interleukin-1β; lipopolysaccharide; and steroid hormones, such as 1α,25-dihydroxyvitamin D3 (6, 15, 16). The IEX-1 promoter contains several consensus sequences for transcription factors, including NF-κB/rel, p53, c-Myc, Sp1, p300, and Sox, which are conserved in human, rat, and mouse (18, 23). IEX-1 has been identified as an NF-κB/rel target gene (23). It has been recently reported that NF-κB/rel-mediated activation of IEX-1 expression is enhanced by p53 and is strongly inhibited by c-Myc (12).
The IEX-1 gene encodes a protein of 156 amino acids that undergoes additional posttranslational modification by glycosylation to yield a product with an apparent Mr of 27,000 to 29,000 (15). The protein sequence of IEX-1 contains a nuclear localization signal sequence and an endoplasmic reticulum, membrane-associated domain (4, 15). The protein is translocated from the nucleus to the cytosol or vice versa depending on cellular events or stimuli (8, 14, 16). IEX-1 has been shown to inhibit cell proliferation in some cells but to accelerate cell cycle progression in others (11, 16, 27). It was also reported to promote apoptosis in 293 cells, HeLa cells, and keratinocytes when the cells are subjected to stress stimuli (2, 11, 25). In contrast to these in vitro studies, it has been reported recently that IEX-1 exerts in vivo an antiapoptotic effect on activation-induced cell death of T lymphocytes (32). IEX-1 is also a substrate for extracellular signal-regulated kinases (ERKs) with a dual role in ERK signaling by acting as an ERK downstream effector mediating survival and as a regulator of ERK activation (10).
We have selected IEX-1 for initial analyses with the expectation that it may be a cell-activated stress response to infection of HSV-1. The question we posed is whether IEX-1 is indeed activated in infected cells and whether it assists or has a negative effect on viral replication. We report that the IEX-1 gene is activated after infection but that the protein expression does not correlate with the time course of mRNA accumulation. In the course of these studies we have discovered that the interdiction to IEX-1 gene expression is multifaceted and involves novel, hitherto undescribed mechanisms.
MATERIALS AND METHODS
Cells and viruses.
Telomerase-transformed primary human foreskin fibroblasts (HFF) (3), a kind gift of Thomas E. Shenk (Princeton University), were cultured in Dulbecco's modified Eagle's minimal essential medium supplemented with 10% fetal calf serum. SK-N-SH and HeLa cells obtained from the American Type Culture Collection were propagated in Dulbecco's modified Eagle's minimal essential medium supplemented with 5% newborn calf serum. HSV-1(F) is the prototype HSV-1 strain used by our laboratory (7). The ΔUL54 mutant virus (vBSΔ27) and complementing cells (Vero 2.2) (28) were a kind gift of Saul Silverstein (Columbia University). The ΔUL41 mutant virus, R2621, was reported elsewhere (20).
Immunoblots.
Cells were collected, rinsed once with cold phosphate-buffered saline (PBS), and lysed in radioimmunoprecipitation assay buffer (PBS containing 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate, 1 mM sodium orthovanadate, 5 mM EDTA, and protease inhibitor mixture [Complete protease mixture; Roche Diagnostics]). Protein concentration was determined, and 50 to 100 μg of proteins was separated on a 12.5% denaturing polyacrylamide gel and electrically transferred to a nitrocellulose membrane at 300 mA (constant) for 4 h in Tris-glycine-methanol buffer at 4°C. The membranes were blocked for 2 h with 5% nonfat dry milk in PBS and were reacted with the appropriate primary antibody overnight at 4°C, rinsed, and then exposed to secondary antibody at room temperature for 1 h. The antibodies were diluted in PBS containing 1% bovine serum albumin and 0.05% Tween 20. Secondary antibodies were alkaline phosphatase (AP)-conjugated goat anti-mouse diluted 1:3,000 and goat anti-rabbit diluted 1:3,000 (Bio-Rad, Hercules, Calif.), AP-conjugated rabbit anti-goat diluted 1:2,000, and peroxidase-conjugated rabbit anti-goat diluted 1:1,000 (Sigma; St. Louis, Mo.). All rinses were done in PBS containing 0.05% Tween 20. To develop AP-conjugated secondary antibodies, the immunoblots were reacted with AP buffer (100 mM Tris [pH 9.5], 100 mM NaCl, 5 mM MgCl2) followed by AP buffer containing 5-bromo-4-chloro-3-indolyl phosphate and nitroblue tetrazolium. To develop peroxidase-conjugated secondary antibodies, the immunoblots were reacted with ECL Western blotting detection reagents according to the manufacturer's instructions (Amersham-Pharmacia). For IEX-1 protein detection, a goat anti-IEX-1 polyclonal antibody available from Santa Cruz Biotechnology (Santa Cruz, Calif.) was used. The HSV-1 proteins were detected with the anti-US11 monoclonal antibody (21) and the polyclonal anti-UL38 antibody reported elsewhere (30).
Isolation of total and cytoplasmic RNA.
Total RNA was extracted with the aid of TRIZOL Reagent (Life Technologies, Rockville, Md.) according to the manufacturer's instructions. DNase treatment (Life Technologies), phenol-chloroform extraction, and ethanol precipitation (Fisher Scientific, Houston, Tex.) were carried out to remove possible DNA contamination. Cytoplasmic RNA was isolated with the aid of an RNeasy mini kit according to the protocol suggested by the manufacturer (Qiagen, Valencia, Calif.).
Real-time PCR.
Real-time PCR was carried out as previously described (29). The following primers were used: rt-IEX-1 forward, 5′-CCGTCCTCGAGCCCTTTAA-3′, and reverse, 5′-TGCTGAGGTCCAGAGCGTAGT-3′; 5′ IEX-1 forward, 5′-ACCCTCACTTGGCCTTACAC-3′, and reverse, 5′-AGCTGCGAGAGTGACACATG-3′; 3′ IEX-1 forward, 5′-ACTGCGGCAAAGTAGGAGAA-3′, and reverse, 5′-CGCCGAAGTCTCACACAGTA-3′.
Northern blot analyses.
For Northern blot analyses, 15 μg of total RNA or 8 μg of cytoplasmic RNA was loaded onto denaturing formaldehyde gel and was probed with random hexanucleotide-primed 32P-labeled specific probe after transfer onto a nylon membrane. For IEX-1 mRNA detection, the fragment containing the entire IEX-1 coding sequence, amplified by reverse transcription-PCR (RT-PCR) of total RNA purified from HSV-1-infected HFF (see below), was used as probe. Prehybridization and hybridization were performed with the ULTRAhyb buffer (Ambion, Austin, Tex.) supplemented with 200 μg of denatured salmon sperm DNA (Stratagene, La Jolla, Calif.) per ml. The membrane was prehybridized for 2 h at 42°C and then was prehybridized overnight after the addition of the 32P-labeled probe. The membrane was rinsed as suggested by the ULTRAhyb's manufacturer and was exposed to film for signal detection.
5′ RLM-RACE.
5′ RNA ligase-mediated rapid amplification of cDNA ends (RLM-RACE) was carried out on cytoplasmic RNA with the aid of a FirstChoice RLM-RACE kit (Ambion) according to the manufacturer's instructions. Briefly, 2.5 μg of cytoplasmic RNA was first treated with calf intestinal phosphatase (CIP) in a total volume of 20 μl to remove free 5′ phosphates from molecules, such as rRNA, tRNA, and contaminating genomic DNA. The CIP-treated RNA was then treated with tobacco acid pyrophosphatase to remove the cap structure from full-length RNA in a total volume of 10 μl. Five microliters of CIP- and/or tobacco acid pyrophosphatase-treated RNA population was ligated to a 45-base RNA adapter oligonucleotide (5′ RACE adapter) by using 5 U of T4 RNA ligase in a total reaction volume of 10 μl. Two microliters of ligated RNA were then reverse transcribed with random decamers and Moloney murine leukemia virus reverse transcriptase in a total reaction volume of 20 μl for 1 h at 42°C. One microliter of the RT mixture was directly subjected to PCR amplification by using the 5′ RACE outer primer specific for the 5′ RACE adapter and provided by the kit along with IEX-1-specific outer primer (5′-ATCTGGCAGAAGACGATGGT-3′) complementary to the region located 300 bases downstream of the IEX-1 start codon. PCR was carried out with AmpliTaq DNA polymerase (Applied Biosystems, Foster City, Calif.) under the following conditions: 3 min at 94°C; 45 s at 94°C, 45 s at 55°C, 45 s at 72°C for 35 cycles; 7 min at 72°C. Two microliters of the amplified PCR mixture was subjected to a second round of amplification by using 5′ IEX-1-specific primer (5′-CGGTCCTGAGATCTTCACCT-3′) located 100 bases downstream of the start codon along with an IEX-1-specific inner primer (5′-TGGTGAGCAGCAGAAAGAGA-3′) partially overlapping the IEX-1-specific outer primer. The same PCR conditions used in the first amplification were applied to the second one. Ten microliters of each sample was run in a 2% agarose gel containing 0.5 μg of ethidium bromide per ml, and the PCR products were visualized with the aid of a UV transilluminator.
Cloning of PCR products in pGEM-T vector.
Gel-purified PCR products were cloned in pGEM-T easy vector (Promega, Madison, Wis.) according to the manufacturer's instructions. Resulting clones were subsequently sequenced.
Construction of plasmids for baculovirus production.
All the recombinant baculoviruses were constructed with the aid of the shuttle vector pRB5850 (MTS1) derived from pAcSG2 baculovirus transfer vector (PharMingen). pRB5850 was generated as described elsewhere (26). IEX-1 coding sequence was amplified by RT-PCR as follows. One microgram of total RNA purified from HSV-1-infected HFF RNA collected at 7 h after infection was reverse transcribed to yield single-stranded cDNA by using 25 U of avian myeloblastosis virus reverse transcriptase (Promega) in a total reaction volume of 30 μl. The RT products were primed with a specific primer complementary to the IEX-1 stop codon containing a NotI restriction site for cloning in pRB5850 (IEX-1-reverse, 5′-GGGAGTGCGCGGCCG CACAGTTAGAAGG-3′). A pool of nucleotides consisted of 1 mM concentrations (each) of dGTP, dATP, dTTP, and dCTP, and 40 U of RNasin (Promega) was added to the reaction mixture. The mixture containing only the RNA template along with the specific reverse primer was first heated at 70°C for 10 min and then chilled on wet ice, and after the addition of the other components it was incubated at 42°C for 45 min, shifted at 52°C for 45 min to alter mRNA secondary structure, and then heat inactivated at 95°C for 5 min. Total RT mixture was subjected to PCR amplification in a final volume of 50 μl in presence of 2.5 U of Pfu polymerase (Stratagene) and 1× Pfu polymerase reaction buffer provided by the manufacturer. The reverse primer used in the RT reaction was added again to the PCR mixture along with the forward primer stretching the IEX-1 start codon and containing an EcoRI restriction site for cloning in pRB5850 (IEX-1-forward, 5′-GCTGAATTCACCATGTGTCACTCTCGCA-3′). PCR was carried out under the following conditions: 4 min at 94°C; 1 min at 94°C, 1 min at 50°C, 1 min 30 s at 72°C for 30 cycles; 10 min at 72°C. The generated fragment was ligated into pRB5850 after cleavage by EcoRI and NotI restriction enzymes and was subsequently sequenced. IEX-1-pRB5850 was used as template to generate by PCR the IEX-1 coding sequence in antisense orientation to the cytomegalovirus promoter. The sequence of the primers used to generate IEX-1-antisense were: anti-IEX-1 forward, 5′-ACACTCGCGCGGCCGCCCATGTGTCAC-3′, containing a NotI restriction site; anti-IEX-1 reverse, 5′-CGGGAATTCACAGTTAGAAGGCGG-3′, containing an EcoRI restriction site. All of the recombinant baculoviruses were generated by cotransfection of Sf9 insect cells with the IEX-1-pRB5850 or IEX-1-antisense-pRB5850 transfer plasmids along with BaculoGold DNA (Pharmingen) according to the manufacturer's instructions. The recombinant baculoviruses were amplified, and their titers were determined in Sf9 cells. The expression of the genes cloned in baculovirus was tested in HEp-2, HeLa, and rabbit skin cells by Northern blot and/or Western blot analysis.
RESULTS
Confirmation of increased accumulation of IEX-1 mRNA by real-time PCR.
Studies based on microchip analyses indicated a significant increase in the accumulation of IEX-1 RNA in HFF after infection with HSV-1 (29). To verify the results obtained with microchip assays we performed a real-time PCR analysis on total RNA extracted from HFF harvested at 1, 3, 7, or 12 h after infection with HSV-1(F). The RNA was reverse transcribed by using random hexamers and was normalized with respect to reverse-transcribed 18S rRNA. The amount of IEX-1 RNA from cells harvested 1 h after mock infection was used as a calibrator. The results were that IEX-1 mRNA levels were increased by 1 h, reached peak levels between 3 and 7 h, and decreased by 12 h after infection (Fig. 1A). To determine whether the increased accumulation of IEX-1 transcripts was cell type dependent, we also carried out similar tests on RNA extracted from infected HeLa cells. As shown in Fig. 1B, the time course of accumulation of IEX-1 transcripts in infected HeLa cells was similar to that observed in infected HFF. We conclude from these studies that the accumulation of IEX-1 mRNA was upregulated in wild-type virus-infected cells. The increased accumulation of RNA was not cell type dependent.
FIG. 1.
Real-time PCR analysis of IEX-1 transcripts accumulating in HSV-1-infected cells. Total RNA was extracted at the indicated hours after infection from HSV-1(F)-infected HFF (A) or HeLa (B) cells and was analyzed by real-time PCR. The amount of total RNA was normalized with respect to reverse-transcribed 18S rRNA. The amount of IEX-1 RNA accumulating in infected cells was calculated as the fold change compared to that from cells harvested 1 h after mock infection.
Detection of IEX-1 protein in HSV-1-infected cells.
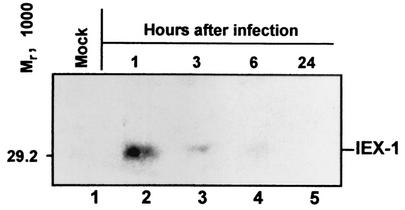
To verify whether the accumulation of IEX-1 mRNA in HSV-1-infected cells resulted in concurrent accumulation of the protein product, HeLa cells harvested at 1, 3, 6, or 24 h after exposure to 10 PFU of HSV-1(F) per cell were solubilized, electrophoretically separated on a 12.5% denaturing polyacrylamide gel, electrically transferred to a nitrocellulose sheet, and reacted with a specific anti-IEX-1 antibody. As shown in Fig. 2, IEX-1 protein was present in cells harvested at 1 h after infection (lane 2) but was significantly reduced in amount in cells harvested at 3 h (lane 3), becoming barely detectable in the 6-h sample (lane 4) and completely disappearing at later times after infection (lane 5). The absence of IEX-1 protein in mock-infected cells suggested that the amount detected at 1 h after infection represents a response to viral infection. The decrease in the amount of IEX-1 protein observed 1 h after infection did not correlate with the pattern of accumulation of IEX-1 RNA (Fig. 1B). This discrepancy could be explained by (i) failure of the RNA to be exported from the nucleus to the cytoplasm, (ii) improper processing of the mRNA, (iii) inhibition of translation of cellular RNA, or (iv) rapid degradation of the protein. The objective of the experiments detailed below was to analyze the state and integrity of the IEX-1 RNA accumulating in infected cells.
FIG. 2.
Accumulation of IEX-1 protein in HeLa cells after HSV-1 infection. HeLa cells were infected with 10 PFU of HSV-1(F)/cell and were collected at 1, 3, 6, and 24 h after infection (lanes 2 to 5). Mock-infected cells were treated and collected in parallel (lane 1). The cells were lysed as described in Materials and Methods, and protein extracts were electrophoretically separated on a 12.5% denaturing polyacrylamide gel, electrically transferred to a nitrocellulose sheet, and reacted with a specific antibody directed to IEX-1 protein.
Multiple forms of IEX-1 RNA accumulate in the cytoplasm of HSV-1-infected cells.
To test whether the lack of IEX-1 protein product could be related to the absence of IEX-1 mRNA in the cytoplasm of infected cells, HeLa cells were infected with 10 PFU of HSV-1(F) per cell, and total and cytoplasmic RNAs were purified at 1, 2, 3, and 5 h after infection. Mock-infected cells were treated in parallel, and total and cytoplasmic RNAs were collected at 1 and 5 h after incubation at 37°C. The accumulation of IEX-1 mRNA was determined by Northern blot assays, and the results are shown in Fig. 3. An increase in the intensity of the signal for IEX-1 RNA was observed, as expected, in all the RNA samples purified from HSV-1-infected cells (Fig. 3A, lanes 3 to 5, and 3B, lanes 3 to 6). The presence of IEX-1 RNA in the cytoplasmic fraction indicated no defects in IEX-1 RNA transportation. A positive signal observed for IEX-1 RNA in lysates of cells harvested at 1 h after mock infection could be the result of a cellular stress response to the exposure of the cells to low temperatures during intervals of virus absorption. To avoid this problem, all the subsequent experiments were carried out entirely at 37°C.
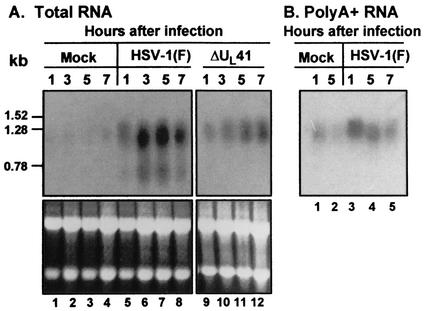
FIG. 3.
Total and cytoplasmic IEX-1 RNA accumulations in HeLa cells infected with HSV-1(F). HeLa cells were mock infected or were infected with 10 PFU of HSV-1(F) per cell. (A) Total RNA was extracted from cells harvested from cells at the indicated times after mock infection (lanes 1 and 2) or exposure to HSV-1(F) (lanes 3 to 5). Fifteen micrograms of total RNA was loaded onto a denaturing formaldehyde gel and was probed with a 32P-labeled fragment containing the entire coding sequence of IEX-1. (B) Cytoplasmic RNA was purified from cells harvested at the indicated times after mock infection (lanes 1 and 2) or infection with HSV-1(F) (lanes 3 to 6). Eight micrograms of total RNA was loaded onto a denaturing formaldehyde gel and was probed with a 32P-labeled fragment containing the entire coding sequence of IEX-1. The position of 18S rRNA is indicated. The ethidium bromide staining of total and cytoplasmic RNA samples is shown as loading controls. The labels A, B, and C indicate the different forms of IEX-1 RNA.
An unexpected observation apparent in Fig. 3 was that the size of the IEX-1 RNA became progressively smaller starting at 2 h after infection. The expected size for IEX-1 RNA (band A) is approximately 1.3 kb. The sizes of the other two bands appearing in HSV-1-infected cells were approximately 1.1 to 1.2 kb (band B) and 0.75 kb (band C), respectively. It is well established that virion host shutoff (vhs) protein accelerates the turnover of viral and cellular RNA after HSV-1 infection (9). To test if vhs was responsible for the shortening of IEX-1 mRNA in HSV-1-infected cells, replicate cultures of HeLa cells were mock infected or were exposed to 10 PFU per cell of HSV-1(F) or the ΔUL41 mutant virus lacking the vhs protein. Total RNA was purified at 1, 3, 5, or 7 h after infection and was analyzed as described above. The results (Fig. 4) show that the smaller IEX-1 RNA forms were present in lysates of cells infected with the wild-type virus but not in the lysates of cells infected with the ΔUL41 mutant virus (Fig. 4A, lanes 9 to 12).
FIG. 4.
Total and poly(A)+ IEX-1 RNA accumulation in HeLa cells after wild-type or ΔUL41 mutant HSV-1 infection. HeLa cells were mock infected or were infected with 10 PFU of HSV-1(F)/cell or with ΔUL41 mutant virus (A). Total RNA was extracted at the indicated hours after infection from mock-infected (lanes 1 to 4) or HSV-1(F)-infected cells (lanes 5 to 8) or ΔUL41-infected cells (lanes 9 to 12). (B) Poly(A)+ RNA was purified from total RNA extracted from mock-infected (lanes 1 and 2) or HSV-1(F)-infected cells (lanes 3 to 5). Total RNA and Poly(A)+ RNA were electrophoretically separated on denaturing formaldehyde gel and were probed with a 32P-labeled fragment containing the entire coding sequence of IEX-1. The position of some bands of a 0.16- to 1.77-kb RNA ladder (Life Technologies) is indicated. The ethidium bromide staining of total RNA samples is shown as a loading control.
An additional series of experiments was carried out to determine whether the rapidly migrating forms of IEX-1 RNA were polyadenylated. In this series of experiments poly(A)+ RNA was extracted from total RNA purified from HSV-1(F)-infected HeLa cells. The results showed that the smallest form of IEX-1 RNA (band C) could not be detected in the poly(A)+ RNA isolated from HSV-1(F)-infected HeLa cells (Fig. 4B lanes 3 to 5), indicating that this form did not contain a poly(A) tail.
We make the following conclusions from these series of observations. (i) IEX-1 RNA is transported into the cytoplasm of infected cells. (ii) A fraction of this RNA appears to be degraded. The degradation appears to be linked to the presence of a functional vhs product inasmuch as the rapidly migrating form of IEX-1 RNA was absent from cells infected with the ΔUL41 mutant. (iii) A large portion of the rapidly migrating RNA lacks the poly(A) tail, since it was absent in the poly(A)+ fraction. (iv) Finally, the presence of degraded IEX-1 RNA could partially account for the absence of IEX-1 protein in infected cells.
Analysis of the 5′ end of IEX-1 transcript.
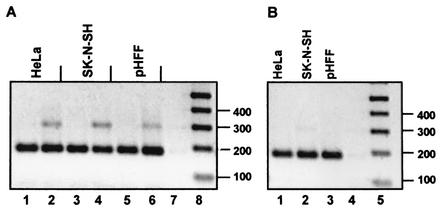
It has been reported that HSV-1 vhs protein induces degradation of sequences at the 5′ end of the mRNA more rapidly than those at the 3′ end of the transcript (13). To analyze the 5′ end of the IEX-1 RNA forms, HFF, HeLa, and SK-N-SH cells were harvested 5 h after exposure to 10 PFU of HSV-1(F) per cell. Cytoplasmic RNA was isolated and subjected to the 5′ RLM-RACE. The 5′ RLM-RACE is designed to amplify cDNA only from full-length, capped mRNA. The results are shown in Fig. 5A. A specific band of the expected size (185 bp) was observed in both mock (lanes 1, 3, and 5) and HSV-1(F)-infected cells (lanes 2, 4, and 6), indicating that in HSV-1-infected cells the 5′ end of IEX-1 RNA was intact and capped. Consistent with the results obtained in the preceding section, this observation indicates that the IEX-1 RNA was degraded at its 3′ terminus.
FIG. 5.
5′ RLM-RACE. HFF, HeLa, and SK-N-SH cell cultures were mock infected or were infected with 10 PFU of HSV-1(F) or ΔUL54 mutant virus per cell. Cytoplasmic RNA was extracted from cells harvested at 5 h after infection, and 2 μg was subjected to the 5′ RLM-RACE procedure described in Materials and Methods. (A) Nested PCR with 5′ IEX-specific primer and IEX inner primer (see Materials and Methods) on RACE-treated RNA purified from mock-infected (lanes 1, 3, and 5) or HSV-1(F)-infected HeLa cells, SK-N-SH cells, and HFF (lanes 2, 4, and 6), respectively. (B) Nested PCR with 5′ IEX-specific primer and IEX inner primer on RACE-treated RNA purified from ΔUL54-infected HeLa cells, SK-N-SH cells, and HFF (lanes 1, 2, and 3, respectively). Lane 7 in panel A and lane 4 in panel B show no template PCR control. Lane 8 in panel A and lane 5 in panel B show 1 kb plus ladder.
An unexpected finding was the presence of an extra band approximately 100 bp longer than the expected PCR product (Fig. 5, lanes 2, 4, and 6). Since the primers used for the PCR were located in the two different IEX-1 exons and the size of the additional sequence was similar to the size of IEX-1 intron (112 bp), the additional amplification product observed in HSV-1-infected cells could contain the IEX-1 intron. This would suggest the presence of unspliced IEX-1 RNA in the cytoplasm of infected cells.
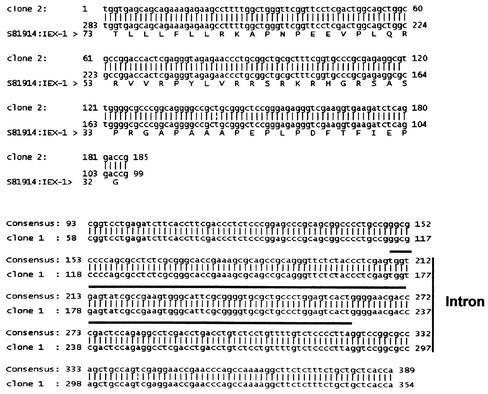
In an earlier article it was reported that ICP27 induces cytoplasmic accumulation of unspliced α-globin pre-mRNA in infected HeLa cells (5). To test whether ICP27 was also responsible for the presence of unspliced IEX-1 RNA in the cytoplasm of HSV-1-infected cells, HeLa cells, SK-N-SH cells, and HFF were infected with ΔUL54 (ICP27-lacking) mutant virus. Cytoplasmic RNA was isolated at 5 h after infection and was subjected to the same 5′ RLM-RACE procedure. As shown in Fig. 5B (lanes 1 to 3), only the expected IEX-1 mRNA amplification product was observed in the ΔUL54-infected cells. To verify that the RNA accumulating in the cytoplasm of infected cells contained the IEX-1 intron, the PCR products were purified from the gel, cloned in pGEM-T-Easy vector, and sequenced. As shown in Fig. 6, bottom panel, the longer PCR fragment aligned perfectly with the genomic sequence of IEX-1. We conclude from these studies that a fraction of the IEX-1 mRNA transported into the cytoplasm of cells infected with wild-type virus was unspliced. The transport of unspliced RNA was linked to the function of ICP27 and could further affect the synthesis of IEX-1 protein. If the unspliced IEX-1 were translated it would yield a protein product that shares only the amino-terminal portion with the authentic IEX-1 protein.
FIG. 6.
Comparison of the sequence of PCR product derived from 5′ RLM-RACE with the published IEX-1 sequence. The upper panel shows the comparison between the sequence of a representative clone (clone 2) obtained after cloning of the shorter PCR product derived from 5′ RLM-RACE (see Fig. 5A) with the published IEX-1 mRNA sequence. The lower panel shows the comparison between the sequence of a representative clone (clone 1) obtained after cloning of the longer PCR product derived from 5′ RLM-RACE (see Fig. 5A) with the genomic sequence of IEX-1. The solid line above the sequence identifies the 112-bp intron sequence.
A fraction of IEX-1 RNA accumulating early in infection lacks the 3′ sequences.
The preceding sections showed the presence of IEX-1 RNAs containing intact 5′ termini and partially degraded RNAs. To obtain a more thorough characterization of the various IEX-1 RNA populations, a real-time PCR analysis was set up with three different sets of primers scattered along the IEX-1 RNA sequence. The location of the primers is shown in Fig. 7A, and they measure the 5′-end region (5′ IEX-1 primers), the coding domain (rt-IEX-1 primers), and the 3′ noncoding domain (3′ IEX-1 primers). Replicate cultures of HeLa cells were mock infected or were exposed to 10 or 20 PFU of HSV-1(F) per cell. The cells were harvested at 1, 3, 7, or 12 h after infection or mock infection. The extracted total RNA was reverse transcribed by random hexamers and was normalized with respect to reverse-transcribed 18S rRNA. The amount of IEX-1 RNA from cells harvested 1 h after mock infection was used as a calibrator for each pair of primers. The results (Fig. 7B) were as follows.
FIG. 7.
Degradation of IEX-1 RNA after HSV-1 infection. (A) Location along the IEX-1 RNA sequence of the three sets of primers used for real-time PCR. The heavy line represents the coding domain. (B) The extracted total RNA was reverse transcribed by using random hexamers and was normalized with respect to reverse-transcribed 18S rRNA. The amount of IEX-1 RNA from cells harvested 1 h after mock infection was used as a calibrator for each pair of primers.
(i) As predicted by the experiments described above, the assays with the three primer sets verified the increase in the total amount of IEX-1 RNA after infection. IEX-1 RNA reached peak levels at 3 h after infection and declined thereafter, regardless of the multiplicity of infection. The IEX-1 RNA present in mock-infected cells decreased gradually to approximately half its initial concentration by 12 h.
(ii) At 1 h after infection, the amounts of RNA detected by the three primer sets were approximately equal. With time after infection, the amounts detected by the three primer sets diverged in decreasing order depending on the distance of the primer sets from the 5′ terminus of the RNA. Approximately 40% of the IEX-1 RNA extracted at 3 h after infection with 10 PFU of wild-type virus per cell lacked the 3′ sequences measured by the 3′ IEX-1 primers. Similar results were obtained for cells infected with 20 PFU/cell. In contrast, the quantities of IEX-1 RNA detected with the three primer sets in extracts of mock-infected cells were similar. We conclude from these studies that a significant fraction of the RNA accumulating after the first hour of infection with wild-type virus was truncated at the 3′ end.
Effects of IEX-1 overexpression on HSV-1-infected cells.
IEX-1 overexpression is associated with an increase in the rate of apoptosis when cells are stressed by UV irradiation, DNA damaging agents, camptothecin, or serum deprivation (25). The rate of apoptosis does not increase in cells not subjected to stress. In cells exposed to epidermal growth factor, serum, or other growth-promoting factors, IEX-1 serves to enhance the growth rate of cells (16, 24). The expression of the IEX-1 gene is under control of NF-κB (23), and the induction of IEX-1 transcription after HSV-1 infection could be explained by the persistent NF-κB nuclear translocation (17) observed in HSV-1-infected cells as a result of the activation of the IκB kinase and degradation of IκBα early in infection (1). The results presented above indicate that HSV-1 is able to overcome the possible effects of IEX-1 protein overexpression by blocking the translation of IEX-1 RNA.
Two series of experiments were done to determine the effect of overexpression of IEX-1 protein prior to infection on the outcome of viral replication in these cells. As described in Materials and Methods, we constructed baculoviruses containing IEX-1 open reading frames in the sense or antisense orientation driven by the cytomegalovirus immediate-early promoter. In the first series, SK-N-SH cells were mock infected or were infected with 10 PFU of wild-type, IEX-1-expressing-, or antisense-IEX-1-expressing baculoviruses. The efficacy of antisense-IEX-1 in blocking the expression of IEX-1 was tested by coinfection of the IEX-1- and antisense-IEX-1-recombinant baculoviruses (data not shown). After 16 h of incubation in the presence of 5 mM sodium butyrate, the baculovirus-infected cells were exposed to 1 PFU of HSV-1(F)/cell. The cells were harvested at 6 and 12 h after exposure to HSV-1(F), solubilized, subjected to electrophoresis in denaturing polyacrylamide gels, and reacted with antibodies against IEX-1 and late HSV-1 proteins. The synthesis of the HSV-1 proteins selected for these studies requires robust expression of α proteins as well as the synthesis of viral DNA. As shown in Fig. 8, the accumulation of US11 and UL38 proteins was similar in cells exposed to wild-type or recombinant baculoviruses (Fig. 8A, lanes 2 to 5 and 7 to 10), suggesting that the exogenous IEX-1 does not affect HSV-1 protein expression.
FIG. 8.
Effect of IEX-1 overexpression on HSV-1 replication. (A) SK-N-SH cells were mock infected (lanes 1, 2, 6, and 7) or were infected with 10 PFU of wild-type baculovirus (Bac-WT; lanes 3 and 8), IEX-1 baculovirus (Bac-IEX-1; lanes 4 and 9), or anti-IEX-1 baculovirus (Bac-anti-IEX-1; lanes 5 and 10) per cell, respectively. After 16 h of incubation the cells were exposed to 1 PFU of HSV-1(F)/cell (lanes 2 to 5 and 7 to 10). Cells were collected at 6 (lanes 1 to 5) and 12 h (lanes 6 to 10) after exposure to HSV-1(F). Protein extracts were electrophoretically separated on a 12.5% denaturing polyacrylamide gel, electrically transferred to a nitrocellulose sheet, and reacted with the indicated antibodies as described in Materials and Methods. (B) IEX-1 protein detection. Protein extracts from SK-N-SH cells infected with IEX-1 baculovirus and exposed to HSV-1 for 6 (lane 1) or 12 h (lane 2) or only exposed to HSV-1 for 6 h (lane 3) were electrophoretically separated on a 12.5% denaturing polyacrylamide gel, electrically transferred to a nitrocellulose sheet, and reacted with a goat anti-IEX-1 polyclonal antibody. inf, infection.
In the second series of experiments, replicate cultures of HeLa cells were mock infected or were exposed to 15 PFU of wild-type or recombinant baculoviruses per cell. After 16 h of incubation, the cells were exposed to 1 PFU of HSV-1(F) virus per cell. The cells were harvested at 24 h after HSV-1(F) infection, and the progeny virus was titered in Vero cells. The yields were 1.1 × 107 HSV-1(F) PFU for cells mock infected with baculoviruses, 1.5 × 107 PFU for cells infected with baculoviruses expressing IEX-1, and 1.0 × 107 PFU for cells exposed to wild-type baculovirus.
We conclude that overexpression of IEX-1 prior to HSV-1 infection had no effect on viral replication of HSV-1.
DISCUSSION
The salient features of the results presented in this report are as follows.
(i) The initial observation of increasing accumulation of IEX-1 RNA upon HSV-1 infection has been verified and confirmed by Northern analyses and real-time PCR. However, analyses of the HSV-1-infected cells for the presence of IEX-1 protein yielded unexpected results. Although the IEX-1 transcript reached peak levels between 3 and 7 h after infection, the IEX-1 protein decreased in amount after the first hour after infection.
(ii) Attempts to resolve this discrepancy revealed that the IEX-1 RNA accumulating in the cytoplasm of HSV-1-infected cells consisted of three populations. The first population, present primarily at 1 h after infection, consisted of apparently full-length, normally spliced RNA. The second population represented partially degraded forms of IEX-1 RNA. Lastly, a fraction of the IEX-1 RNA contained the intron sequence and appeared to be the unspliced precursor RNA.
(iii) Preliminary results based on 5′ RLM-RACE analyses indicated that the truncated forms of IEX-1 RNA accumulating in HSV-1-infected cells contained an intact 5′ terminus, suggesting that the 3′ terminus was truncated. This was also supported by the lack of polyadenylation of truncated RNA forms. A detailed analysis based on real-time PCR with three sets of primers specific for the 5′ domain, the coding domain, and the 3′ noncoding domain verified this conclusion. After the first hour of HSV-1 infection, a significant fraction of the total IEX-1 RNA lacked the 3′ sequences as measured by the 3′ IEX-1 primers.
One significant observation made in the course of these studies is that the appearance of the 3′-truncated RNA is related to the function of vhs protein inasmuch as the partially degraded RNA forms were not present in cells infected with the ΔUL41 mutant virus. Earlier studies done on viral thymidine kinase (13) have reported that the vhs protein mediates the degradation of mRNA starting from the 5′ terminus. Our data are not consistent with these studies. One hypothesis that could explain the discrepancy in our results and those reported earlier is that the site of initial degradation is dependent on the secondary structure of the RNA. In addition, earlier reports have shown that RNA degraded by vhs disappears very rapidly, in stark contrast to the IEX-1 RNA, whose truncated forms persisted in the cytoplasm for several hours. We expect that many more mRNA will have to be analyzed to determine the site initially targeted by the vhs protein.
(iv) Unspliced pre-RNA containing the IEX-1 intron sequences were detected in the cytoplasm of wild-type-infected but not in the ΔUL54 mutant virus-infected cells. A similar observation was reported by Cheung et al. (5) on the transport of α-globin precursor RNA into the cytosol of infected cells. Our observations and those of Cheung et al. (5) suggest that in addition to inhibiting splicing, ICP27 also disables the uninfected cell restriction to transport of unspliced RNA into the cytoplasm. In this respect ICP27 resembles the function of the human immunodeficiency virus 1 rev protein (19).
It is noteworthy that an early study reported the synthesis and accumulation of a variant of IEX-1 in cells exposed to phorbol myristate acetate or tumor necrosis factor alpha (31). This long form of IEX-1 (IEX-1L) has been reported to act as an apoptosis inhibitor in NF-κB-mediated survival in Jurkat cells (31), but its existence has been questioned (22). The reported IEX-1L sequence differs from that of the genomic IEX-1 by the one insertion and two deletions that resulted in the translation of a protein with an in-frame insertion of 37 amino acids compared to that of IEX-1 (22). The sequence of the unspliced mRNA recovered from the cytoplasm of HSV-1-infected cells aligns unambiguously with the IEX-1 genomic sequence. Attempts to clone the IEX-1L cDNA by using appropriately designed primers failed (data not shown).
(v) Overexpression of exogenous IEX-1 protein delivered to the cells by the baculovirus system prior to HSV-1 infection had no effect on viral gene accumulation and virus yield, even at low multiplicities of infection. The significance if this observation is unclear. Either IEX-1 is not able to block viral replication or HSV-1 can block the function of preexisting IEX-1 protein.
To sum up, the pattern of IEX-1 RNA accumulation in infected cells suggests that the gene was activated immediately after infection and that at that time the transcripts were capable of being translated into the authentic IEX-1 protein. The rate of transcription of the RNA was most likely higher than the rate of degradation of the RNA mediated by the vhs protein, a phenomenon similar to that of synthesis and degradation of viral mRNA early in infection. With time the transcription abated, the rate of degradation equaled or exceeded the rate of transcription, and moreover a fraction of the late transcripts was unspliced and hence unable to direct the synthesis of authentic RNA. The rapid turnover of the protein coupled with the degradation of the RNA rendered the IEX-1 response to infection moot.
This report centered on one of a large number of genes whose RNA accumulated in HSV-1(F)-infected cells at levels significantly above those present in mock-infected cells. Our expectation was that the accumulation of IEX-1 RNA reflected a host-induced response to infection designed to interdict or at least diminish the ability of the virus to replicate in the infected cells. The results presented here are of interest for several respects. The virus employed at least three different pathways to block the expression of the IEX-1 gene. The RNA accumulating in the cytoplasm of infected cells after the first hour consists of partially 3′-end-degraded forms and in part of unspliced RNA form. None of these RNAs could direct the synthesis of authentic IEX-1 protein. In addition, the expression of IEX-1 protein was transient, and the protein soon disappeared from the infected cell.
HSV-1-infected cells accumulated significant amounts of the transcripts of other proapoptotic genes. It remains to be determined whether HSV-1 handles other cellular gene products expressed after infection in the same fashion as IEX-1. The results presented in this report indicate that not all that glitters in the profiles of HSV RNA is in fact expressed and alters the physiology of the infected cell.
Acknowledgments
These studies were aided by grants from the National Cancer Institute (CA78766, CA71933, CA83939, CA87661, and CA88860) of the U.S. Public Health Service. A.E. is a recipient of a fellowship from the Association pour la Recherche sur le Cancer (ARC France).
We thank Beatrice Fineschi and the Biological Sciences Collegiate Divisions for making available the real-time PCR machine.
REFERENCES
- 1.Amici, C., G. Belardo, A. Rossi, and M. G. Santoro. 2001. Activation of I kappa b kinase by herpes simplex virus type 1. A novel target for anti-herpetic therapy. J. Biol. Chem. 276:28759-28766. [DOI] [PubMed] [Google Scholar]
- 2.Arlt, A., O. Grobe, A. Sieke, M. L. Kruse, U. R. Folsch, W. E. Schmidt, and H. Schafer. 2001. Expression of the NF-kappa B target gene IEX-1 (p22/PRG1) does not prevent cell death but instead triggers apoptosis in HeLa cells. Oncogene 20:69-76. [DOI] [PubMed] [Google Scholar]
- 3.Bresnahan, W. A., G. E. Hultman, and T. Shenk. 2000. Replication of wild-type and mutant human cytomegalovirus in life-extended human diploid fibroblasts. J. Virol. 74:10816-10818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Charles, C. H., J. K. Yoon, J. S. Simske, and L. F. Lau. 1993. Genomic structure, cDNA sequence, and expression of gly96, a growth factor-inducible immediate-early gene encoding a short-lived glycosylated protein. Oncogene 8:797-801. [PubMed] [Google Scholar]
- 5.Cheung, P., K. S. Ellison, R. Verity, and J. R. Smiley. 2000. Herpes simplex virus ICP27 induces cytoplasmic accumulation of unspliced polyadenylated α-globin pre-mRNA in infected HeLa cells. J. Virol. 74:2913-2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Domachowske, J. B., C. A. Bonville, A. J. Mortelliti, C. B. Colella, U. Kim, and H. F. Rosenberg. 2000. Respiratory syncytial virus infection induces expression of the anti-apoptosis gene IEX-1L in human respiratory epithelial cells. J. Infect. Dis. 181:824-830. [DOI] [PubMed] [Google Scholar]
- 7.Ejercito, P. M., E. D. Kieff, and B. Roizman. 1968. Characterization of herpes simplex virus strains differing in their effects on social behavior of infected cells. J. Gen. Virol. 2:357-364. [DOI] [PubMed] [Google Scholar]
- 8.Feldmann, K. A., M. R. Pittelkow, P. C. Roche, R. Kumar, and J. P. Grande. 2001. Expression of an immediate early gene, IEX-1, in human tissues. Histochem. Cell Biol. 115:489-497. [DOI] [PubMed] [Google Scholar]
- 9.Fenwick, M. L., and M. M. McMenamin. 1984. Early virion-associated suppression of cellular protein synthesis by herpes simplex virus is accompanied by inactivation of mRNA. J. Gen. Virol. 65:1225-1228. [DOI] [PubMed] [Google Scholar]
- 10.Garcia, J., Y. Ye, V. Arranz, C. Letourneux, G. Pezeron, and F. Porteu. 2002. IEX-1: a new ERK substrate involved in both ERK survival activity and ERK activation. EMBO J. 21:5151-5163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grobe, O., A. Arlt, H. Ungefroren, G. Krupp, U. R. Folsch, W. E. Schmidt, and H. Schafer. 2001. Functional disruption of IEX-1 expression by concatemeric hammerhead ribozymes alters growth properties of 293 cells. FEBS Lett. 494:196-200. [DOI] [PubMed] [Google Scholar]
- 12.Huang, Y. H., J. Y. Wu, Y. Zhang, and M. X. Wu. 2002. Synergistic and opposing regulation of the stress-responsive gene IEX-1 by p53, c-Myc, and multiple NF-kappaB/rel complexes. Oncogene 21:6819-6828. [DOI] [PubMed] [Google Scholar]
- 13.Karr, B. M., and G. S. Read. 1999. The virion host shutoff function of herpes simplex virus degrades the 5′ end of a target mRNA before the 3′ end. Virology 264:195-204. [DOI] [PubMed] [Google Scholar]
- 14.Kobayashi, T., M. R. Pittelkow, G. M. Warner, K. A. Squillace, and R. Kumar. 1998. Regulation of a novel immediate early response gene, IEX-1, in keratinocytes by 1α,25-dihydroxyvitamin D3. Biochem. Biophys. Res. Commun. 251:868-873. [DOI] [PubMed] [Google Scholar]
- 15.Kondratyev, A. D., K. N. Chung, and M. O. Jung. 1996. Identification and characterization of a radiation-inducible glycosylated human early-response gene. Cancer Res. 56:1498-1502. [PubMed] [Google Scholar]
- 16.Kumar, R., T. Kobayashi, G. M. Warner, Y. Wu, J. L. Salisbury, W. Lingle, and M. R. Pittelkow. 1998. A novel immediate early response gene, IEX-1, is induced by ultraviolet radiation in human keratinocytes. Biochem. Biophys. Res. Commun. 253:336-341. [DOI] [PubMed] [Google Scholar]
- 17.Patel, A., J. Hanson, T. I. McLean, J. Olgiate, M. Hilton, W. E. Miller, and S. L. Bachenheimer. 1998. Herpes simplex type 1 induction of persistent NF-kappa B nuclear translocation increases the efficiency of virus replication. Virology 247:212-222. [DOI] [PubMed] [Google Scholar]
- 18.Pietzsch, A., C. Buchler, and G. Schmitz. 1998. Genomic organization, promoter cloning, and chromosomal localization of the Dif-2 gene. Biochem. Biophys. Res. Commun. 245:651-657. [DOI] [PubMed] [Google Scholar]
- 19.Pollard, V. W., and M. H. Malim. 1998. The HIV-1 rev protein. Annu. Rev. Microbiol. 52:491-532. [DOI] [PubMed] [Google Scholar]
- 20.Poon, A. P., and B. Roizman. 1997. Differentiation of the shutoff of protein synthesis by virion host shutoff and mutant gamma (1)34.5 genes of herpes simplex virus 1. Virology 229:98-105. [DOI] [PubMed] [Google Scholar]
- 21.Roller, R. J., and B. Roizman. 1992. The herpes simplex virus 1 RNA binding protein US11 is a virion component and associates with ribosomal 60S subunits. J. Virol. 66:3624-3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schafer, H., A. Arlt, A. Trauzold, A. Hunermann-Jansen, and W. E. Schmidt. 1999. The putative apoptosis inhibitor IEX-1L is a mutant nonspliced variant of p22(PRG1/IEX-1) and is not expressed in vivo. Biochem. Biophys. Res. Commun. 262:139-145. [DOI] [PubMed] [Google Scholar]
- 23.Schafer, H., J. Diebel, A. Arlt, A. Trauzold, and W. E. Schmidt. 1998. The promoter of human p22/PACAP response gene 1 (PRG1) contains functional binding sites for the p53 tumor suppressor and for NFκB. FEBS Lett. 436:139-143. [DOI] [PubMed] [Google Scholar]
- 24.Schafer, H., P. Lettau, A. Trauzold, M. Banasch, and W. E. Schmidt. 1999. Human PACAP response gene 1 (p22/PRG1): proliferation-associated expression in pancreatic carcinoma cells. Pancreas 18:378-384. [DOI] [PubMed] [Google Scholar]
- 25.Schilling, D., M. R. Pittelkow, and R. Kumar. 2001. IEX-1, an immediate early gene, increases the rate of apoptosis in keratinocytes. Oncogene 20:7992-7997. [DOI] [PubMed] [Google Scholar]
- 26.Sciortino, M. T., B. Taddeo, A. P. Poon, A. Mastino, and B. Roizman. 2002. Of the three tegument proteins that package mRNA in herpes simplex virions, one (VP22) transports the mRNA to uninfected cells for expression prior to viral infection. Proc. Natl. Acad. Sci. USA 99:8318-8323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Segev, D. L., T. U. Ha, T. T. Tran, M. Kenneally, P. Harkin, M. Jung, D. T. MacLaughlin, P. K. Donahoe, and S. Maheswaran. 2000. Mullerian inhibiting substance inhibits breast cancer cell growth through an NFkappa B-mediated pathway. J. Biol. Chem. 275:28371-28379. [DOI] [PubMed] [Google Scholar]
- 28.Soliman, T. M., R. M. Sandri-Goldin, and S. J. Silverstein. 1997. Shuttling of the herpes simplex virus type 1 regulatory protein ICP27 between the nucleus and the cytoplasm mediates the expression of late proteins. J. Virol. 71:9188-9197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taddeo, B., A. Esclatine, and B. Roizman. 2002. The patterns of accumulation of cellular RNAs in cells infected with a wild-type and a mutant herpes simplex virus 1 lacking the virion host shutoff gene. Proc. Natl. Acad. Sci. USA 99:17031-17036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ward, P. L., W. O. Ogle, and B. Roizman. 1996. Assemblons: nuclear structure defined by aggregation of immature capsids and some tegument proteins of herpes simplex virus 1. J. Virol. 70:4623-4631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu, M. X., Z. Ao, K. V. Prasad, R. Wu, and S. F. Schlossman. 1998. IEX-1L, an apoptosis inhibitor involved in NF-κB-mediated cell survival. Science 281:998-1001. [DOI] [PubMed] [Google Scholar]
- 32.Zhang, Y., S. F. Schlossman, R. A. Edwards, C. N. Ou, J. Gu, and M. X. Wu. 2002. Impaired apoptosis, extended duration of immune responses, and a lupus-like autoimmune disease in IEX-1-transgenic mice. Proc. Natl. Acad. Sci. USA 99:878-883. [DOI] [PMC free article] [PubMed] [Google Scholar]