Abstract
Theiler's virus infection in the central nervous system (CNS) induces a demyelinating disease very similar to human multiple sclerosis. We have assessed cytokine gene activation upon Theiler's murine encephalomyelitis virus (TMEV) infection and potential mechanisms in order to delineate the early events in viral infection that lead to immune-mediated demyelinating disease. Infection of SJL/J primary astrocyte cultures induces selective proinflammatory cytokine genes (interleukin-12p40 [IL-12p40], IL-1, IL-6, tumor necrosis factor alpha, and beta interferon [IFN-β]) important in the innate immune response to infection. We find that TMEV-induced cytokine gene expression is mediated by the NF-κB pathway based on the early nuclear NF-κB translocation and suppression of cytokine activation in the presence of specific inhibitors of the NF-κB pathway. Further studies show this to be partly independent of dsRNA-dependent protein kinase (PKR) and IFN-α/β pathways. Altogether, these results demonstrate that infection of astrocytes and other CNS-resident cells by TMEV provides the early NF-κB-mediated signals that directly activate various proinflammatory cytokine genes involved in the initiation and amplification of inflammatory responses in the CNS known to be critical for the development of immune-mediated demyelination.
Theiler's virus induces an immune-mediated demyelinating disease similar to human multiple sclerosis. Studies have shown the importance of genetic factors like major histocompatibility complex (MHC), T-cell receptor, and gender in the susceptibility to the disease (21). Intracerebral infection of susceptible strains such as SJL/J mice results in an acute, polio-like phase of the gray matter that later develops into a chronic demyelination in the white matter manifested by severe hind limb paralysis and incontinence (25). Demyelination is characterized by mononuclear cell infiltration with the primary involvement of activated macrophages that correlates with virus-specific delayed-type hypersensitivity responses (7, 51). The Theiler's murine encephalomyelitis virus (TMEV)-specific T-cell responses to viral determinants are well characterized in this Th1-mediated disease (21). However, the early innate immune response of central nervous system (CNS)-resident cells that lead to the proinflammatory milieu critical in the recruitment and development of virus-specific Th1 responses, are largely unknown.
An innate immune response to viral and bacterial infection often results in the production of immune molecules, including cytokines, chemokines, MHC, and enzymes, etc., that act in concert to control the infectious agents (13). These very same molecules can however induce dysregulated inflammation that leads to target tissue destruction (9, 26). Recent studies suggest that toll-like receptors are involved in the induction of innate immune responses following exposure to bacterial and/or viral components (18, 34). In particular, TLR-3 (as well as double-stranded RNA [dsRNA]-dependent protein kinase [PKR]) is involved in activation of a variety of proinflammatory cellular genes through the NF-κB pathway upon recognition of dsRNA, a replication intermediate of TMEV (2, 55). NF-κB represents a family of dimeric transcription factors that play a central role in these inflammatory responses by regulation of gene expression and inhibition of apoptosis (11, 20). The sequestration of NF-κB by IκB in the cytoplasm and IκB phosphorylation leading to proteasomal degradation resulting in activation and translocation of NF-κB to the nucleus is essential in the transcription of many proinflammatory chemokines and cytokines such as IP-10, RANTES, monocyte chemotactic protein 1 (MCP-1), interleukin-10 (IL-10), tumor necrosis factor alpha (TNF-α), and IL-6, (3, 4).
Many investigators have extensively investigated the potential roles of cytokines in TMEV-induced, immune-mediated inflammatory demyelination (5, 6, 21, 44, 49). The accumulation of proinflammatory cytokines has been demonstrated in the CNS of TMEV-infected mice and precedes that of anti-inflammatory cytokines (IL-4 and IL-10) during the course of disease (37). Recent studies have also shown sustained upregulation of proinflammatory cytokines (gamma interferon [IFN-γ], IL-6, IL-12, and TNF-α) and transforming growth factor β, downregulating cytolytic responses in the CNS of SJL mice compared to resistant C57BL/6 mice. These results correlate well with susceptibility to demyelinating disease (6). However, the mechanisms that initiate and expand cytokine gene expression in the CNS inflammatory disease induced following TMEV infection are less clear. We and others have previously reported that TMEV is able to directly activate selective chemokine and cytokine genes in the CNS-resident glial cell populations (32, 36, 38, 46). However, the range of cytokines induced and the signal transduction mechanisms involved in this virus-mediated cytokine gene activation has not been previously explored.
In order to delineate the potential pathogenic role of the innate immune response in virus-induced CNS demyelination, we have examined the molecular mechanisms underlying early cytokine gene activation after TMEV infection of primary astrocyte cultures. Here we demonstrate the ability of TMEV to directly induce selective proinflammatory cytokines (IL-12, IL-1, IL-6, TNF-α, and IFN-β) in glial cells. The expression of cytokine genes was evident for some, but not for all, as early as 30 min postinfection, and the activation of NF-κB was demonstrated within 5 min of TMEV infection. Additionally, various NF-κB inhibitors, as well as an IκB super-repressor, were shown to inhibit TMEV-induced cytokine gene expression. These results clearly indicate that NF-κB activation is necessary in TMEV-induced cytokine gene activation. The PKR as well as IFN-α/β pathways do not appear to play a major role in the cytokine gene activation. Altogether, these results strongly support the crucial role of CNS resident cells in the initiation of inflammatory demyelination by production of various proinflammatory cytokines via an NF-κB-dependent innate immune response to TMEV infection.
MATERIALS AND METHODS
Animals.
Female SJL/J mice from Charles Rivers (Wilmington, Mass.) were housed in the animal facility at Northwestern University Medical School under specific guidelines set by the Animal Care Use Committee. IFN-α/β receptor knockout mice (IFN-α/BR-KO) were kindly provided by Herbert (Skip) Virgin (Washington University, St. Louis, Mo.), and control 129Ev/Sv mice purchased from Taconic (Germantown, N.Y.).
Chemicals and reagents.
Poly(I-C) (Calbiochem, La Jolla, Calif.) was used at 25 to 100 μg/ml in phosphate-buffered saline (PBS). Stock solutions (0.01 to 10 μM) of NF-κB inhibitors (caffeic ester phenyl ester [CAPE] and MG-132; Calbiochem) in 50% ethanol were further diluted with plain Dulbecco's modified Eagle medium (DMEM) for treatment 3 h prior to TMEV infection. The adenovirus IκB super-repressor was a kind gift from R. Balfour Sartor (17) and used at a multiplicity of infection (MOI) of 25 or 100 for 24 h prior to TMEV infection. The serine/threonine kinase inhibitor for PKR, 2-aminopurine (Sigma, St. Louis, Mo.) was used at 3 and 10 mM for 2 h prior to TMEV infection. Antibody to the p65 subunit of NF-κB was from Santa Cruz Biotechnology (Santa Cruz, Calif.). Secondary antibodies labeled with Alexa 594 and 4′,6′-diamidino-2-phenylindole (DAPI) for nuclear counterstain were used for immunohistochemistry (Molecular Probes, Eugene, Oreg.).
Glial cell cultures.
Primary astrocytes, oligodendrocytes, and microglial cells were derived from 0- to 3-day-old neonates by conventional methods using differential shaking (47). Single-cell suspensions from neonatal brains were seeded on poly-l-lysine-coated flasks (25 μg/ml), in DMEM supplemented with 2 mM l-glutamine, antibiotics (Gibco BRL, Grand Island, N.Y.), and 10% fetal calf serum (<10 U of endotoxin/ml; HyClone, Logan, Utah). After 8 days in culture at 37°C, flasks were placed in a shaker at 200 rpm overnight, and detached oligodendrocytes were seeded into new flasks. The remaining cells were cultured again for another 4 days at 37°C before shaking at 200 rpm for 1 h to harvest detached microglial cells. Four days later, cultures were again agitated overnight (250 rpm) to harvest adherent astrocytes. Cell preparations yielded at least 95% purity as confirmed by staining with cell-type specific antibodies (CNPase, oligodendrocyte marker; Mac-1, macrophage/microglial marker [Boehringer Mannheim, Indianapolis, Ind.]; and GFAP, astrocyte marker [Dako, Carpenteria, Calif.]).
Virus infection.
The BeAn strain of TMEV was expanded in vitro using permissive BHK cells and partially purified by ultracentrifugation as previously described (54). Viral titer was determined by plaque assay on BHK cells and subsequently used for infection of cells in vitro at various MOIs. Cells were washed and resuspended with infection media (DMEM with 0.1% bovine serum albumin) before addition of TMEV. Chemical inhibitors were added 2 to 3 h prior to TMEV infection. For specific NF-κB inhibition, the adenovirus coding for an IκB super-repressor was also used (17). Cells were infected with the adenoviruses in plain α-MEM medium (Gibco BRL) for 2 h at 37°C before the addition of serum-supplemented medium for 24 h prior to TMEV infection. This procedure resulted in >90% adenovirus-infected astrocytes as assessed by fluorescence-activated cell sorting (FACS) after infection with a green fluorescent protein (GFP)-expressing control adenovirus (Adv-Gfp).
RNase protection assay.
Glial cultures were seeded on six-well plates at a density of up to 5 × 105 cells per well and infected with TMEV BeAn (MOI = 0.1 to 100) for 0 to 24 h at 37°C. Total RNA was isolated using Trizol (Gibco BRL) as specified by the manufacturer's instructions. The RNA multiprobe sets (mck-2b and mck-3b; Pharmingen, San Diego, Calif.) were used throughout without any modifications to analyze cytokine gene expression by RNase protection assay (RPA) according to manufacturer's instructions. Briefly, 5 to 10 μg total RNA was hybridized at 56°C for 14 to 16 h. RNA samples were analyzed on a 5% denaturing polyacrylamide gel, exposed on film for 6 to 12 h or a phosphorscreen and subsequently analyzed using the Quantity One software (Bio-Rad, Richmond, Calif.).
Immunohistochemistry.
Astrocytes were cultured on four-well chamber slides (Nunc, Rochester, N.Y.) and infected with TMEV or treated with TNF-α (20 ng/ml) from 5 to 120 min before washing in PBS and fixation in 4% paraformaldehyde for 15 min at room temperature. Slides were then washed with 0.1% Triton X-100 in Tris-buffered saline (pH 7.5) and incubated with anti-NF-κB p65 primary antibodies (1:50 overnight at 4°C). After washing, slides were further incubated in secondary antibodies labeled with Alexa 594 (1:1,000; 75 min at room temperature), and then DAPI (0.5 μg/ml; 15 min at room temperature) for nuclear counterstain (Molecular Probes). Specific staining was subsequently visualized under a fluorescent microscope.
In situ hybridization.
The infectivity of TMEV to astrocytes was determined after 6 h of infection by in situ hybridization using a negative-strand digoxigenin-labeled VP4 probe. Control and virus-infected astrocyte monolayers were fixed with 4% paraformaldehyde and then hybridized with the VP4 probe for 2 h at 45°C in 50% formaldehyde-hybridization buffer (4× SSC [1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate], 1× Denhardt's solution, and salmon sperm DNA [40 μg/ml]). After three washings with 50% formamide in 1× SSC, cells were stained with antidigoxigenin Fab-alkaline phosphatase and nitroblue tetrazolium.
Electrophoretic mobility shift assay (EMSA).
Astrocytes (5 × 106) were harvested by scraping in cold PBS and then were pelleted. Nuclear extracts were prepared as previously described (31) by resuspension of the cell pellet in 1 ml of buffer A (20 mM HEPES, pH 7.9; 10 mM KCl; 1 mM MgCl2; 0.1 mM EDTA; 1 mM dithiothreitol [DTT]; 0.4 mM phenylmethylsulfonyl fluoride; 1 mM NaF; aprotinin, 10 μg/ml; leupeptin, 10 μg/ml; 1 mM Na3VO4) containing 0.1% Nonidet P-40 for 10 min at 0°C. The nuclei were then pelleted at 3,000 × g for 10 min and resuspended in 200 μl of high-salt buffer (10 mM HEPES, pH 7.9; 1 mM MgCl2; 10 mM KCl; 0.1 mM EDTA; 400 mM NaCl; 15% glycerol; 1 mM DTT; 0.4 mM phenylmethylsulfonyl fluoride; 1 mM NaF; aprotinin, 10 μg/ml; leupeptin, 10 μg/ml; 1 mM Na3VO4). The suspension was rocked gently for 30 min at 4°C followed by centrifugation at 12,000 × g for 10 min at 4°C. The protein concentration in the supernatant was determined by Bradford assay.
Double-stranded oligonucleotides containing the NF-κB consensus sequence (5′-AGT TGA GGG GAC TTT CCC AGG C-3′) from Santa Cruz Biotechnology were used for EMSA. The oligonucleotide was end-labeled with [γ-32P]ATP (NEN, Boston, Mass.) using T4 polynucleotide kinase. EMSA was performed in a total volume of 20 μl at 4°C. Typically, 10 μg of nuclear extracts was equilibrated for 15 min in binding buffer (10 mM Tris-HCl, pH 8.0; 75 mM KCl; 2.5 mM MgCl2,; 0.1 mM EDTA; 10% glycerol; 0.25 mM DTT) and 1 μg of poly(dI-dC) (Amersham Pharmacia Biotech, Piscataway, N.J.). The mixture was incubated with 32P-labeled oligonucteotide probe for 20 min on ice and resolved by electrophoresis on a 5% native polyacrylamide gel at 10 V/cm. For competition analysis, a 50-fold molar excess of unlabeled oligonucleotide was added to the nuclear extract 30 min prior to the addition of labeled probe.
RESULTS
TMEV is a potent inducer of proinflammatory cytokine expression in astrocytes.
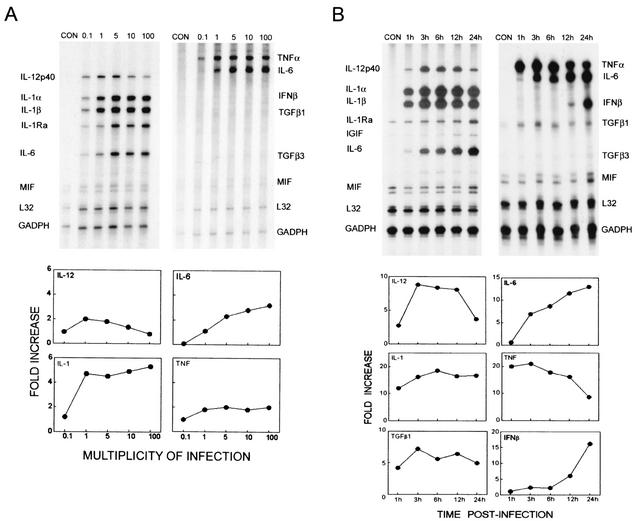
To delineate the early mechanisms of immune-mediated demyelination, we assessed the levels of various cytokine genes expressed in astrocytes after TMEV infection by using sensitive RPA. TMEV infection (6 h) at various MOIs results in the marked upregulation of proinflammatory cytokine message levels (IL-12p40, IL-1, IL-1Ra, IL-6, and TNF-α), but not IL-10 in primary astrocyte cultures (Fig. 1A). Significant cytokine gene expression was observed at an MOI as low as 0.1 and reached a peak at MOIs of 1 and 5. Interestingly, the level of IL-12p40 was reduced at MOIs of 10 and 100 compared to an MOI of 1, while the levels of other cytokine genes expressed remain at peak levels. In contrast, IL-1 and IL-6 continuously increased at higher MOIs. These results strongly suggest that the regulation of cytokine gene expression induced by TMEV infection may be somewhat different depending on the cytokine, perhaps influenced by the level of signals provided by viral infection. The levels of gene expression in control cultures incubated with the identical infection medium alone without virus were minimal, indicating that the activation signal is induced by viral infection. In addition, control cultures with BHK lysates or UV-inactivated TMEV induced minimal cytokine gene expression in these cultures (not shown), similar to our previous results for chemokine gene activation (36). Therefore, these results demonstrate the potent ability of TMEV to rapidly induce proinflammatory cytokines in astrocytes implying an important role in setting the proinflammatory milieu in the CNS that can lead to immune-mediated inflammatory disease.
FIG. 1.
TMEV-induced cytokine expression in SJL/J astrocytes. (A) Dose-dependent TMEV-induced cytokine expression was observed in SJL/J primary astrocytes. Astrocytes were infected with TMEV at different MOIs and incubated for 6 h. (B) Kinetics of TMEV-induced cytokine expression in astrocytes infected with an MOI of 10 at different time points. Note the robust Th1 proinflammatory cytokine expression (IL-12 p40, IL-1, IL-6, and TNF-α) and not Th2 cytokine (IL-10) as early as 1 h postinfection and at a low MOI (0.1). Cytokine expression was analyzed by RPA and represented as fold increases using the Bio-Rad Quantity One software.
To further determine the kinetics of cytokine gene expression, primary astrocytes were infected at the same MOI (MOI = 10) for different time periods ranging from 1 to 24 h (Fig. 1B). As early as 1 h postinfection, significantly elevated expression (5- to 20-fold) of proinflammatory cytokines was observed. However, the patterns of gene expression were significantly different from each other depending on the cytokines. For example, the expression of IL-12p40 reached a peak at 3 h after viral infection and maintained its level until a decrease at 24 h, whereas the level of IL-6 continuously increased. On the other hand, TNF-α reached a peak early (at 1 to 3 h) and then decreased steadily thereafter, while the significant expression of IFN-β message started rather late (12 h) and continuously increased (24 h). Although the mechanisms involved in the differences in expression kinetics of these cytokines are not clear, some of the late expression (e.g., continuous increases in IL-6 and IFN-β at the late time periods) may represent secondary induction and/or positive feedback by chemokines and cytokines induced early by viral infection.
Similar proinflammatory cytokine expression is induced in oligodendrocytes and microglia after TMEV infection.
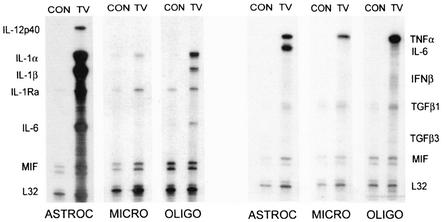
To determine whether other CNS glial cells also contribute to the overall proinflammatory environment in the CNS after TMEV infection, oligodendrocytes and microglial cells isolated from neonatal mice were also infected with TMEV in vitro (Fig. 2). Proinflammatory cytokines (IL-1, IL-1Ra, and TNF-α) similar to that induced in astrocytes were also expressed in these glial cell types. However, induction of IL-6 and IL-12p40 gene expression was most prominent in astrocyte cultures. The level of cytokine expression in microglia is generally much lower compared to that in oligodendrocytes and astrocytes, except for TNF-α. The level of mRNA expression corresponded well with the protein level assessed by proteome array (Pierce Endogen Searchlight Proteome Array Technology; data not shown). These results indicate that other glial cells like microglia and oligodendrocytes are capable of significantly expressing proinflammatory cytokines as a response to TMEV infection, although the level of cytokines induced may vary depending on the cell type.
FIG. 2.
Similar TMEV-induced cytokine expression in oligodendrocytes and microglia. Other glial cells like oligodendrocytes and microglia were also analyzed for their response to TMEV infection. Similar to astrocytes, enhanced levels of proinflammatory cytokine expression was demonstrated after 6 h postinfection at an MOI of 10 except for IL-6 and IL-12 p40. Cytokine expression was analyzed by RPA and represented as fold increases using the Bio-Rad Quantity One software.
Infection of TMEV results in the rapid activation of NF-κB.
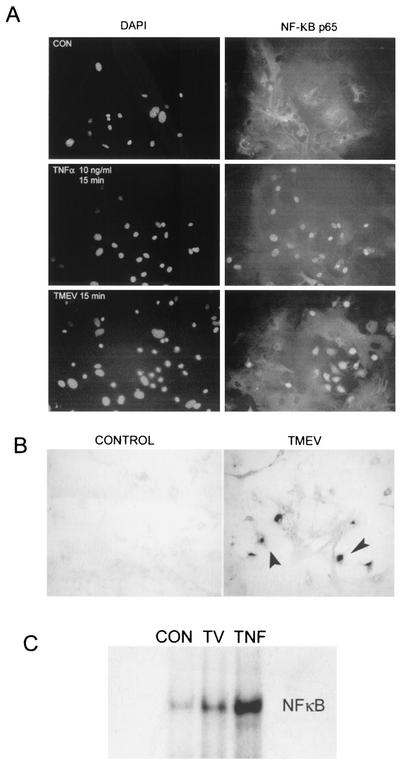
To identify the mechanism(s) involved in TMEV-induced cytokine expression in glial cells, we initially assessed the activation of NF-κB in astrocytes by immunohistochemistry and EMSA (Fig. 3). Staining with antibodies to the p65 subunit of NF-κB and counterstaining of the nuclei with DAPI, we observed rapid NF-κB nuclear translocation within 5 to 15 min after TMEV infection (MOI = 10) in approximately 25% of the cells (Fig. 3A). TNF-α treatment (20 ng/ml) was used as a positive control for NF-κB activation, and this treatment induced nearly 100% NF-κB translocation and/or activation within 5 to 15 min. The number of astrocytes that show nuclear translocation changed little even after 1 h postinfection, perhaps reflecting only a subpopulation of cells activated for such nuclear translocation. However, it is not yet clear whether only a fraction of the astrocyte population is infected by TMEV or activated. Further experiments assessing TMEV infected astrocytes by in situ hybridization suggested that not all astrocytes are infected with TMEV even at an MOI of 10 at 6 h postinfection (Fig. 3B), corresponding to the nuclear translocation of NF-κB. In fact, a similar percentage (∼30%) of astrocytes was positive for viral messages at 6 h after infection, suggesting that only these virus-infected cells are activated to express cytokine genes. The activation of NF-κB was also demonstrated by EMSA using nuclear extracts from astrocytes after 30 min of TMEV infection (Fig. 3C). Though some background binding of NF-κB to the 32P-labeled NF-κB specific oligonucleotides was observed in uninfected control astrocytes, significantly increased binding of the NF-κB probe was demonstrated in nuclear extracts of TMEV-infected astrocytes. Altogether, these results conclusively demonstrate the activation of NF-κB in astrocytes after TMEV infection.
FIG. 3.
Rapid nuclear translocation of NF-κB in astrocytes after TMEV infection. Nuclear translocation of NF-κB is apparent as early as 15 min after TMEV infection. TNF-α was used as a positive control. (A) NF-κB translocation was determined by immunohistochemistry using anti-p65 antibody for NF-κB and DAPI counterstaining for nuclei locations at 15 min postinfection with an MOI of 10. (B) Viral infectivity was assessed by in situ hybridization of TMEV-infected astrocytes (6 h) with digoxigenin-labeled antisense VP4 probe followed by development with alkaline-phosphatase-labeled antidigoxigenin. Arrowheads indicate the virus-infected cells. (C) Specific-binding ability of nuclear NF-κB was determined by EMSA at 30 min postinfection with an MOI of 10. Astrocytes were treated with TNF-α (10 ng/ml) for 30 min and used as a positive control. CON, control; TV, TMEV-infected; TNF, TNF-α treated.
NF-κB antagonists inhibit TMEV-induced cytokine gene expression.
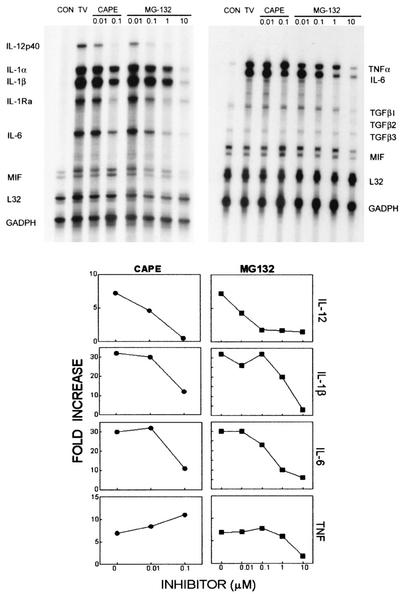
To correlate NF-κB activation and cytokine gene expression induced by TMEV, chemical inhibitors for the NF-κB pathway were used. CAPE, a specific inhibitor of NF-κB that acts by interfering in DNA binding (30), and MG132, a general proteasome inhibitor preventing the degradation of IκB (41), were added at different molar concentrations 2 h prior to TMEV infection. As shown in Fig. 4, the pretreatment of astrocytes with CAPE inhibited cytokine gene expression induced by TMEV infection in a dose-dependent manner. CAPE was found to be toxic at concentrations higher than 0.1 μM. MG132 was less toxic and showed nearly complete inhibition of the cytokine induction. Sensitivity to these inhibitors is somewhat different depending on the cytokines. For example, TNF-α was relatively more resistant to the inhibitors, although it was also inhibited with the treatment of the highest concentration of MG-132 (10 μM). Nevertheless, these results strongly suggest that NF-κB activation is required for selective cytokine gene expression in primary astrocyte cultures induced after TMEV infection.
FIG. 4.
TMEV-induced cytokine expression in astrocytes is inhibited by NF-κB inhibitors. Various concentrations (0.01 to 10 μM) of CAPE (NF-κB inhibitor) and MG-132 (proteasome inhibitor) were added 3 h prior to TMEV infection to determine its specific role in cytokine gene expression. Cytokine expression in control and experimental astrocyte cultures was analyzed at 6 h postinfection (MOI = 10) by RPA using two separate murine cytokine multiprobe sets (Pharmingen).
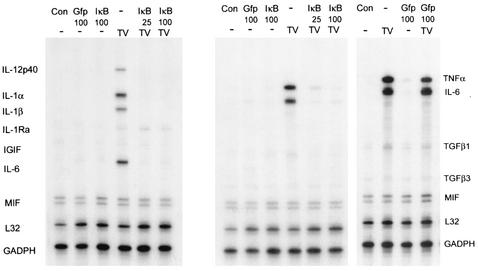
Infection with adenovirus expressing a dominant-negative IκB completely inhibits TMEV-induced cytokine gene expression.
Although nuclear translocation and significant inhibition by chemical inhibitors were observed indicating a role of NF-κB in TMEV-induced cytokine expression in astrocytes, specificity and efficiency may be a concern. Thus, we have utilized a replication-deficient adenovirus expressing a mutant form of the IκB protein (Adv-IκB) whose two phosphorylation sites required for NF-κB activation are mutated. As a control, we have generated a similar replication-deficient adenovirus expressing green fluorescent protein (Adv-Gfp) to assess infectivity and toxicity in primary astrocytes. By FACS analysis, close to 100% of primary astrocytes are infected by adenovirus without apparent toxicity thus suitable for studies with the adenovirus IκB super-repressor. Infection with either the Adv-Gfp or Adv-IκB was initiated 24 h prior to TMEV infection and then analyzed by RPA at the peak of cytokine response (6 h; Fig. 5). The IκB super-repressor (Adv-IκB at MOI of 25 and 100) completely inhibited cytokine gene expression induced by TMEV. This inhibition not only was complete but also covers the whole spectrum of cytokines inducible by TMEV. Neither Adv-Gfp nor Adv-IκB infection alone induced significant background cytokine gene expression. In addition, no inhibition of TMEV-induced cytokine expression was observed when infected with control Adv-Gfp prior to TMEV, indicating no apparent nonspecific alteration in TMEV-induced gene expression following infection with adenovirus. These data combined with the previous results (nuclear translocation and chemical inhibitors) unequivocally demonstrate the crucial role of NF-κB activation in TMEV-mediated cytokine gene expression in primary astrocytes.
FIG. 5.
IκB super-repressor inhibits TMEV-induced cytokine gene expression. The specific role of NF-κB in cytokine expression was further demonstrated by use of the adenovirus IκB super-repressor. The infection of primary astroctyes with control adenoviruses (Adv-Gfp) showed virtually 100% infectivity as determined by FACS (data not shown). Adenoviruses expressing an IκB mutant (Adv-IκB) (MOI = 25 and 100) or control expressing GFP (Adv-Gfp) (MOI = 100) were used to infect astrocytes 24 h prior to TMEV infection (TV). Infection of Adv-IκB or Adv-Gfp alone did not induce any background nor did Adv-Gfp inhibit TMEV-mediated cytokine expression.
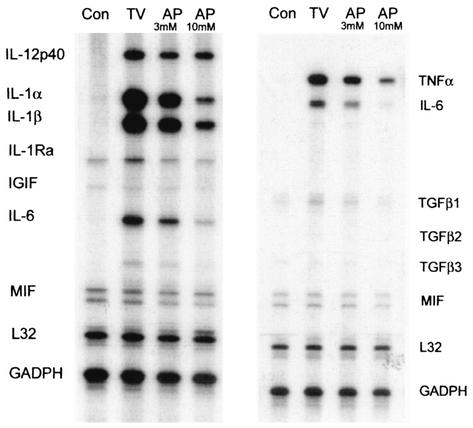
TMEV-induced cytokine expression is partly independent of PKR.
Many pathways can lead to NF-κB activation that result in the specific transcription of various genes including proinflammatory cytokines (3, 4, 14, 19, 45). To assess the upstream pathways leading to NF-κB activation, we initially investigated whether the PKR is involved, since it has been shown to activate NF-κB (16, 23) and dsRNA is generated as viral replication intermediates during TMEV replication (50). Pretreatment of astrocytes with 2-aminopurine, a serine threonine protein kinase inhibitor of PKR (15) was able to partially reduce the level of some of the TMEV-induced cytokines expressed but not all (Fig. 6). However, the degree of sensitivity to the inhibitor was different depending on the cytokines. Gene expression of IL-1, TNF-α, and IL-6 was more sensitive (3- to 10-fold) to 2-aminopurine at a concentration of 10 mM, whereas IL-12p40 expression was relatively resistant. Furthermore, TMEV-induced cytokine expression was not significantly compromised in a PKR-deficient fibroblast cell line (TIK [data not shown]) (1, 53), indicating that NF-κB activation induced by TMEV can be independent of the PKR pathway. Therefore, different activation pathways may be utilized for the induction of cytokines by TMEV infection, although some cytokines (e.g., TNF-α and IL-6) may be more PKR-dependent than others.
FIG. 6.
2-Aminopurine incompletely inhibits TMEV-induced cytokine expression. Two different concentrations (3 and 10 mM) of PKR inhibitor, 2-aminopurine were added 2 h prior to TMEV infection to initially assess the upstream pathways leading to cytokine expression. Cytokine expression at 6 h post-TMEV infection was analyzed by RPA. Con, control astrocyte cultures mock infected with a BHK lysate; TV, astrocytes infected with TMEV alone; AP, astrocytes pretreated with 2-aminopurine and then infected with TMEV.
IFN-α/β receptor-deficient astrocytes show similar activation of cytokine genes after TMEV infection
The IFN-α/β interferons are induced as an innate immune response to various infections, including viruses (13), and these can further induce a variety of other cytokines (TNF-α, IL-12, IL-1, and IL-6), chemokines, and immunoregulatory molecules (MHC, adhesion molecules, and NO, etc.) through NF-κB activation (3, 4, 14). In addition, this cytokine may also be directly involved in controlling viral replication (13). To determine the potential role of IFN-α/β in the induction of cytokines by TMEV infection, astrocytes from control and IFN-α/β receptor-deficient (IFN-α/βR-KO) mice were infected and the level of cytokine gene expression was examined (Fig. 7). As early as 1 h after TMEV infection, enhanced expression (2- to 20-fold) of the cytokine genes (IL-12p40, IL-1, TNF-α, and IL-6) was observed in IFN-α/βR-KO astrocytes that is similar to that in wild-type SJL astrocytes (Fig. 1B) as well as control 129 astrocytes (data not shown). The overall kinetics of the initial cytokine gene expression was similar to astrocytes with intact IFN-α/β receptor. The levels of IL-1 and IL-6 expression were similar to that in astrocyte cultures throughout the time points examined. However, the levels of IL-12p40 and TNF-α, critically important for inflammatory responses, are significantly lower in the IFN-α/βR-KO astrocytes during the late time periods (12 to 24 h). The low level of TNF-α in IFN-α/βR-KO mice does not appear to reflect the genetic differences with the SJL astrocytes. Therefore, this may reflect the fact that, in part, cytokine gene expression in late periods is secondarily induced by IFN-α/β; the lack of additional stimulation may result in a reduction in cytokine gene expression at late time points. In fact, IFN-β treatment was able to induce significant levels of TNF-α, IL-6, and tumor growth factor β-1 in astrocyte cultures derived from the wild-type mice, suggesting this possibility (data not shown). Nevertheless, these results show that the cytokines induced early by TMEV is not dependent on the IFN-α/β pathway though it may contribute to amplification and sustaining the immune response by continuous stimulation of other cytokines.
FIG. 7.
Enhanced TMEV-induced cytokine expression in IFN-α/β-deficient astrocytes. The role of IFN-α/β in TMEV-induced cytokine induction was examined by using primary astrocytes from IFN-α/βR-KO. Primary astrocytes were infected with TMEV (MOI = 10) for 1 to 24 h and then analyzed by RPA.
DISCUSSION
TMEV-induced demyelination is associated with the level of virus-specific inflammatory Th1 responses (7, 10, 51, 54). In addition, the different immune components specific for TMEV have been extensively studied in order to understand the pathogenic mechanisms involved in this apparently immune-mediated demyelinating disease (21). We and others have shown the ability of astrocytes to process and present viral antigens resulting in Fas-mediated lysis thereby compromising the BBB (12, 39). Many previous studies have also demonstrated the predominant expression of proinflammatory cytokines (IL-12, IL-1, IFN-γ, TNF-α, and IL-6) in the CNS of TMEV-infected animals both at the early (prior to T-cell infiltration) and late (clinical) stages of disease (5, 6, 21, 44, 49). The suppressive effects on demyelinating disease following administration of cytokine antagonists or expression of Th2 cytokine transgenes strongly suggest the importance of cytokines in promoting demyelination (21, 35). However, virtually nothing is known about the innate immune mechanisms involved in the initiation of inflammatory demyelination.
Our recent studies indicate a potential crucial role of glial cells (astrocytes) in the initiation of immune-mediated demyelination by orchestrating the migration of mononuclear cells into the CNS via selective production of chemokines, IP-10 and RANTES (36). Here, we demonstrate that a robust Th1-promoting, proinflammatory cytokine expression (IL-12, IL-1, and TNF-α) is directly induced as early as 1 h after TMEV infection in astrocytes and other glial cells as an early innate response (Fig. 1). Interestingly, a lack of Th2 cytokine induction (IL-10) was observed. In addition, the kinetics of cytokine gene induction appears to be different depending on the cytokines. IL-1 and TNF-α genes were induced fastest reaching near maximal levels at 1 h, IL-12 and IL-6, intermediate taking 3 to 6 h, and IFN-β, the slowest requiring 24 h for a significant induction. Similar cytokine responses are also observed with other glial cells like oligodendrocytes and microglias, and yet the patterns are different (Fig. 2). While the induction of rapidly expressed cytokines (IL-1 and TNF-α) is similar, intermediate (IL-12 and IL-6)- and late (IFN-β)-expressed cytokines are not readily detectable. In contrast, a previous study examining cytokines induced at 48 h after TMEV infection in microglia by utilizing RT-PCR showed significant upregulation of IL-1, IL-6, IL-12, and TNF-α as well as IFN-α/β genes (32). The difference between this study and ours may reflect the differences in the assay method and the duration of induction, as we have analyzed the induction by using quantitative RPA at early time points after TMEV infection. Thus, it is possible that microglia and perhaps oligodendrocytes may be slower in kinetics of cytokine gene induction and/or some of the cytokine gene induction in the late time period may represent gene expression secondarily induced by the rapid accumulation of early induced cytokines like IL-1 and TNF-α. In addition, we cannot rule out the possibility that TMEV may induce cytokine gene activation in oligodendrocytes and microglial cells via signal pathways different from that of astrocytes. These proinflammatory cytokines induced by viral infection are identical to the cytokines produced by inflammatory cells, also capable of inducing many of the same cytokines. Thus, it is most likely that further amplification and sustaining of the virus-induced initial inflammatory immune response will occur by the cytokines produced by resident as well as infiltrating immune cells during the ongoing pathogenic processes leading to viral demyelination.
The innate immune response following viral infections lead to the activation of various cytokine genes (IFN-α/β, TNF-α, IL-12, and IL-6) as well as other immune molecule (MHC and adhesion molecule) genes (13, 14). In particular, dsRNA is known to induce potent innate immune responses via TLR-3 and PKR leading to mitogen-activated protein kinase (MAPK) and NF-κB activation (2, 55). These cytokines ultimately act in concert to control viral replication either directly by inhibiting host cell protein synthesis, viral assembly, degrading viral transcripts, and direct antiviral properties or indirectly by cytokine-mediated activation of effector cells like NK, macrophages, and CD8+ T cells (13, 14). However, the production of these immune factors can also be associated with marked destruction of the target tissue especially in a situation of persistent viral infection. Viral persistence is a key in determining susceptibility to disease (27, 37, 40) by perhaps driving the inflammatory immune response in the CNS of susceptible mice. Astrocytes are most abundant in the CNS and play a pivotal role for maintaining the integrity of the blood brain barrier (8). In addition, TMEV infects this CNS-resident cell type more persistently in vivo and in vitro, compared to oligodendrocytes as well as microglia and macrophages (56). Thus, it is likely that their early response to viral infection may be critically important in shaping the proinflammatory milieu in the CNS. Altogether these results strongly suggest that the innate immunity of CNS-resident glial cells to TMEV infection is likely important in the development of demyelinating disease.
The mechanisms involved in the signal transduction leading to various cytokine gene expression following TMEV infection have not been previously reported. To determine the molecular mechanisms of TMEV-induced cytokine expression in astrocytes, we initially assessed the translocation and activation of NF-κB. The rapid activation of NF-κB following TMEV infection was observed (Fig. 3). In addition, the cytokine gene expression induced by TMEV was completely abolished by inhibitors specific for the NF-κB pathway, indicating that this pathway is critical for the expression of all of these cytokine genes (Fig. 4 and 5). This is not surprising as activation of NF-κB is a hallmark in many different viral infections (13, 14, 28). In addition, NF-κB binding sites are present in the promoter regions of numerous cytokines, chemokines, adhesion molecules, and other immune mediators (33), suggesting that this nuclear transcription factor plays an important role in the development of the inflammatory immune response. It is conceivable that the activation signal may be induced via viral receptor engagement, interaction with viral proteins or viral RNA production leading to NF-κB activation. However, it is most likely that viral infection is required for cytokine gene activation since a similar % of astrocytes showed NF-κB translocation and viral messages. In addition, UV-inactivated virus failed to induce vigorous cytokine gene activation as shown with chemokine gene activation (36). The cooperative action of other transcription factors cannot be ruled out as it is often known that maximal gene expression require the action of NF-κB with other transcription factors such as AP-1 (33). The activation of c-Fos, forming the transcription factor AP-1 in conjunction with c-Jun, was observed in TMEV-infected astrocytes (42). However, it is not clear whether this transcription factor is required for cytokine gene upregulation induced by TMEV infection. We could not detect significant levels of activation of MAPK assessed by phosphorylation of ERK1/2, p38 and c-Jun (data not shown). In addition, inhibitors for MAPKs (p38 and ERK1/2) failed to suppress the cytokine gene expression (data not shown). These results suggest that AP-1 may not be important in the cytokine gene activation by TMEV in mouse astrocytes. Nevertheless, cooperative actions between NF-κB and other transcription factors may be operational for optimal cytokine gene expression.
To begin to address upstream signals leading to NF-κB activation, we examined whether NF-κB activation is dependent on PKR as shown in some virus systems such as VSV (48). Activation of NF-κB by treatment with dsRNA or infection with certain viruses involves PKR through direct phosphorylation of IκBα (23). We observe that synthetic dsRNA, poly(I-C), induces cytokine gene expression similar to TMEV in astrocytes (data not shown). However, our results using a PKR inhibitor, 2-aminopurine (Fig. 6) and PKR-deficient cells (data not shown), indicate that TMEV-induced cytokine expression can be PKR-independent. Similar PKR-independent NF-κB activation has been documented in other viral systems (1, 53). The potential involvement of IFN-α/β, which is central to the innate immune response to infection, was assessed, since this cytokine produced after various infections is known to induce various proinflammatory cytokines and chemokines (22, 24). The cytokine response induced following TMEV infection is also IFN-α/β-independent, as IFN-α/βR-deficient astrocytes are capable of inducing cytokine gene expression (Fig. 7). This is consistent with the kinetics of the delayed IFN-α/β gene expression in normal astrocytes (Fig. 1). Thus, two major pathways important in innate immunity (PKR and IFN-α/β) do not seem to be necessary for TMEV-induced cytokine expression. However, IFN-α/β may play an important role in sustaining the levels of critical proinflammatory cytokines and further amplifying the initial inflammatory response, since the expression of such cytokine genes (e.g., IL-12p40 and TNF-α) is low in the absence of IFN-α/β stimulation (Fig. 7). Very recently, it has been reported that dsRNA and certain viral infection may activate NF-κB pathway via TLR-3 (2). It is not yet clear whether TMEV infection activates cytokine gene expression by NF-κB is primarily activated via TLRs. Nevertheless, our results indicate that NF-κB activation is necessary for cytokine gene expression induced directly by TMEV in astrocytes. Many recent studies have utilized NF-κB inhibitors in attempts to control various inflammatory diseases, including multiple sclerosis (29, 43, 52). Thus, the potential therapeutic effects of NF-κB antagonists on inflammatory disease may also include minimizing the initial innate cytokine and chemokine responses and prevention of further propagation of the inflammatory response following microbial infections.
Acknowledgments
We thank John Bell (Ottawa Regional Cancer Center Research Laboratories) and Skip Virgin (Washington University) for their kind gifts of PKR-deficient cells and IFN-αβ-receptor-deficient mice, respectively, as well as R. B. Sartor for the adenovirus IκB super-repressor. We also thank Mike Lyman and Jinjong Myoung for the generation of the control adenoviruses expressing GFP.
This work was supported by the U.S. Public Health Service (grants NS 28752 and NS 33008) and the National Multiple Sclerosis Society (grant RG3126-A4).
REFERENCES
- 1.Abraham, N., D. F. Stojdl, P. I. Duncan, N. Methot, T. Ishii, M. Dube, B. C. Vanderhyden, H. L. Atkins, D. A. Gray, M. W. McBurney, A. E. Koromilas, E. G. Brown, N. Sonenberg, and J. C. Bell. 1999. Characterization of transgenic mice with targeted disruption of the catalytic domain of the double-stranded RNA-dependent protein kinase, PKR. J. Biol. Chem. 274:5953-5962. [DOI] [PubMed] [Google Scholar]
- 2.Alexopoulou, L., A. C. Holt, R. Medzhitov, and R. A. Flavell. 2001. Recognition of double-stranded RNA and activation of NF-κB by Toll-like receptor 3. Nature 413:732-738. [DOI] [PubMed] [Google Scholar]
- 3.Baeuerle, P. A., and V. R. Baichwal. 1997. NF-κB as a frequent target for immunosuppressive and anti-inflammatory molecules. Adv. Immunol. 65:111-137. [PubMed] [Google Scholar]
- 4.Barnes, P. J., and M. Karin. 1997. Nuclear factor-kappaB: a pivotal transcription factor in chronic inflammatory diseases. N. Engl. J. Med. 336:1066-1071. [DOI] [PubMed] [Google Scholar]
- 5.Begolka, W. S., C. L. Vanderlugt, S. M. Rahbe, and S. D. Miller. 1998. Differential expression of inflammatory cytokines parallels progression of central nervous system pathology in two clinically distinct models of multiple sclerosis. J. Immunol. 161:4437-4446. [PubMed] [Google Scholar]
- 6.Chang, J. R., E. Zaczynska, C. D. Katsetos, C. D. Platsoucas, and E. L. Oleszak. 2000. Differential expression of TGF-β, IL-2, and other cytokines in the CNS of Theiler's murine encephalomyelitis virus-infected susceptible and resistant strains of mice. Virology 278:346-360. [DOI] [PubMed] [Google Scholar]
- 7.Clatch, R. J., H. L. Lipton, and S. D. Miller. 1986. Characterization of Theiler's murine encephalomyelitis virus (TMEV)-specific delayed-type hypersensitivity responses in TMEV-induced demyelinating disease: correlation with clinical signs. J. Immunol. 136:920-927. [PubMed] [Google Scholar]
- 8.Dong, Y., and E. N. Benveniste. 2001. Immune function of astrocytes. Glia 36:180-190. [DOI] [PubMed] [Google Scholar]
- 9.Fontana, A., D. Constam, K. Frei, U. Koedel, W. Pfister, and M. Weller. 1996. Cytokines and defense against CNS infection, p. 188-219. In R. M. Ramschoff and E. Benveniste (ed.), Cytokines and the CNS. CRC Press, Boca Raton, Fla.
- 10.Gerety, S. J., M. K. Rundell, M. C. Dal Canto, and S. D. Miller. 1994. Class II-restricted T cell responses in Theiler's murine encephalomyelitis virus-induced demyelinating disease. VI. Potentiation of demyelination with and characterization of an immunopathologic CD4+ T cell line specific for an immunodominant VP2 epitope. J. Immunol. 152:919-929. [PubMed] [Google Scholar]
- 11.Ghosh, S., M. J. May, and E. B. Kopp. 1998. NF-κB and Rel proteins: evolutionarily conserved mediators of immune responses. Annu. Rev. Immunol. 16:225-260. [DOI] [PubMed] [Google Scholar]
- 12.Girvin, A. M., K. B. Gordon, C. J. Welsh, N. A. Clipstone, and S. D. Miller. 2002. Differential abilities of central nervous system resident endothelial cells and astrocytes to serve as inducible antigen-presenting cells. Blood 99:3692-3701. [DOI] [PubMed] [Google Scholar]
- 13.Guidotti, L. G., and F. V. Chisari. 2001. Noncytolytic control of viral infections by the innate and adaptive immune response. Annu. Rev. Immunol. 19:65-91. [DOI] [PubMed] [Google Scholar]
- 14.Hatada, E. N., D. Krappmann, and C. Scheidereit. 2000. NF-κB and the innate immune response. Curr. Opin. Immunol. 12:52-58. [DOI] [PubMed] [Google Scholar]
- 15.Hu, Y., and T. W. Conway. 1993. 2-Aminopurine inhibits the double-stranded RNA-dependent protein kinase both in vitro and in vivo. J. Interferon Res. 13:323-328. [DOI] [PubMed] [Google Scholar]
- 16.Iordanov, M. S., J. Wong, J. C. Bell, and B. E. Magun. 2001. Activation of NF-κB by double-stranded RNA (dsRNA) in the absence of protein kinase R and RNase L demonstrates the existence of two separate dsRNA-triggered antiviral programs. Mol. Cell. Biol. 21:61-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jobin, C., A. Panja, C. Hellerbrand, Y. Iimuro, J. Didonato, D. A. Brenner, and R. B. Sartor. 1998. Inhibition of proinflammatory molecule production by adenovirus-mediated expression of a nuclear factor κB super-repressor in human intestinal epithelial cells. J. Immunol. 160:410-418. [PubMed] [Google Scholar]
- 18.Kaisho, T., and S. Akira. 2000. Critical roles of Toll-like receptors in host defense. Crit. Rev. Immunol. 20:393-405. [PubMed] [Google Scholar]
- 19.Karin, M., and Y. Ben-Neriah. 2000. Phosphorylation meets ubiquitination: the control of NF-κB activity. Annu. Rev. Immunol. 18:621-663. [DOI] [PubMed] [Google Scholar]
- 20.Karin, M., and A. Lin. 2002. NF-κB at the crossroads of life and death. Nat. Immunol. 3:221-227. [DOI] [PubMed] [Google Scholar]
- 21.Kim, B. S., M. A. Lyman, B. S. Kang, H. K. Kang, H. G. Lee, M. Mohindru, and J. P. Palma. 2001. Pathogenesis of virus-induced immune-mediated demyelination. Immunol. Res. 24:121-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim, M. O., Q. Si, J. N. Zhou, R. G. Pestell, C. F. Brosnan, J. Locker, and S. C. Lee. 2002. Interferon-beta activates multiple signaling cascades in primary human microglia. J. Neurochem. 81:1361-1371. [DOI] [PubMed] [Google Scholar]
- 23.Kumar, A., J. Haque, J. Lacoste, J. Hiscott, and B. R. Williams. 1994. Double-stranded RNA-dependent protein kinase activates transcription factor NF-κB by phosphorylating IκB. Proc. Natl. Acad. Sci. USA 91:6288-6292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Le Bon, A., and D. F. Tough. 2002. Links between innate and adaptive immunity via type I interferon. Curr. Opin. Immunol. 14:432-436. [DOI] [PubMed] [Google Scholar]
- 25.Lipton, H. L. 1975. Theiler's virus infection in mice: an unusual biphasic disease process leading to demyelination. Infect. Immun. 11:1147-1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ludewig, B., T. Junt, H. Hengartner, and R. M. Zinkernagel. 2001. Dendritic cells in autoimmune diseases. Curr. Opin. Immunol. 13:657-662. [DOI] [PubMed] [Google Scholar]
- 27.McAllister, A., F. Tangy, C. Aubert, and M. Brahic. 1990. Genetic mapping of the ability of Theiler's virus to persist and demyelinate. J. Virol. 64:4252-4257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mogensen, T. H., and S. R. Paludan. 2001. Molecular pathways in virus-induced cytokine production. Microbiol. Mol. Biol. Rev. 65:131-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muijsers, R. B., and K. L. Goa. 2002. Balsalazide: a review of its therapeutic use in mild-to-moderate ulcerative colitis. Drugs 62:1689-1705. [DOI] [PubMed] [Google Scholar]
- 30.Natarajan, K., S. Singh, T. R. Burke, Jr., D. Grunberger, and B. B. Aggarwal. 1996. Caffeic acid phenethyl ester is a potent and specific inhibitor of activation of nuclear transcription factor NF-κB. Proc. Natl. Acad. Sci. USA 93:9090-9095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nguyen, V. T., W. S. Walker, and E. N. Benveniste. 1998. Post-transcriptional inhibition of CD40 gene expression in microglia by transforming growth factor-beta. Eur. J. Immunol. 28:2537-2548. [DOI] [PubMed] [Google Scholar]
- 32.Olson, J. K., A. M. Girvin, and S. D. Miller. 2001. Direct activation of innate and antigen-presenting functions of microglia following infection with Theiler's virus. J. Virol. 75:9780-9789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ondrey, F. G., G. Dong, J. Sunwoo, Z. Chen, J. S. Wolf, C. V. Crowl-Bancroft, N. Mukaida, and C. Van Waes. 1999. Constitutive activation of transcription factors NF-κB, AP-1, and NF-IL6 in human head and neck squamous cell carcinoma cell lines that express pro-inflammatory and pro-angiogenic cytokines. Mol. Carcinog. 26:119-129. [DOI] [PubMed] [Google Scholar]
- 34.O'Neill, L. A. 2002. Toll-like receptor signal transduction and the tailoring of innate immunity: a role for Mal? Trends Immunol. 23:296-300. [DOI] [PubMed] [Google Scholar]
- 35.Owens, T., H. Wekerle, and J. Antel. 2001. Genetic models for CNS inflammation. Nat. Med. 7:161-166. [DOI] [PubMed] [Google Scholar]
- 36.Palma, J. P., and B. S. Kim. 2001. Induction of selected chemokines in glial cells infected with Theiler's virus. J. Neuroimmunol. 117:166-170. [DOI] [PubMed] [Google Scholar]
- 37.Palma, J. P., S. H. Park, and B. S. Kim. 1996. Treatment with lipopolysaccharide enhances the pathogenicity of a low-pathogenic variant of Theiler's murine encephalomyelitis virus. J. Neurosci. Res. 45:776-785. [DOI] [PubMed] [Google Scholar]
- 38.Palma, J. P., R. L. Yauch, H. K. Kang, H. G. Lee, and B. S. Kim. 2002. Preferential induction of IL-10 in APC correlates with a switch from Th1 to Th2 response following infection with a low pathogenic variant of Theiler's virus. J. Immunol 168:4221-42230. [DOI] [PubMed] [Google Scholar]
- 39.Palma, J. P., R. L. Yauch, S. Lang, and B. S. Kim. 1999. Potential role of CD4+ T cell-mediated apoptosis of activated astrocytes in Theiler's virus-induced demyelination. J. Immunol. 162:6543-6551. [PubMed] [Google Scholar]
- 40.Pullen, L. C., S. H. Park, S. D. Miller, M. C. Dal Canto, and B. S. Kim. 1995. Treatment with bacterial LPS renders genetically resistant C57BL/6 mice susceptible to Theiler's virus-induced demyelinating disease. J. Immunol. 155:4497-4503. [PubMed] [Google Scholar]
- 41.Rock, K. L., C. Gramm, L. Rothstein, K. Clark, R. Stein, L. Dick, D. Hwang, and A. L. Goldberg. 1994. Inhibitors of the proteasome block the degradation of most cell proteins and the generation of peptides presented on MHC class I molecules. Cell 78:761-771. [DOI] [PubMed] [Google Scholar]
- 42.Rubio, N., and B. Martin-Clemente. 1999. Theiler's murine encephalomyelitis virus infection induces early expression of c-fos in astrocytes. Virology 258:21-29. [DOI] [PubMed] [Google Scholar]
- 43.Salvarani, C., P. Macchioni, I. Olivieri, A. Marchesoni, M. Cutolo, G. Ferraccioli, F. Cantini, F. Salaffi, A. Padula, C. Lovino, L. Dovigo, G. Bordin, C. Davoli, G. Pasero, and O. D. Alberighi. 2001. A comparison of cyclosporine, sulfasalazine, and symptomatic therapy in the treatment of psoriatic arthritis. J. Rheumatol. 28:2274-2282. [PubMed] [Google Scholar]
- 44.Sato, S., S. L. Reiner, M. A. Jensen, and R. P. Roos. 1997. Central nervous system cytokine mRNA expression following Theiler's murine encephalomyelitis virus infection. J. Neuroimmunol. 76:213-223. [DOI] [PubMed] [Google Scholar]
- 45.Siebenlist, U., G. Franzoso, and K. Brown. 1994. Structure, regulation and function of NFκB. Annu. Rev. Cell Biol. 10:405-455. [DOI] [PubMed] [Google Scholar]
- 46.Sierra, A., and N. Rubio. 1993. Theiler's murine encephalomyelitis virus induces tumour necrosis factor-alpha in murine astrocyte cell cultures. Immunology 78:399-404. [PMC free article] [PubMed] [Google Scholar]
- 47.Skias, D. D., D. K. Kim, A. T. Reder, J. P. Antel, D. W. Lancki, and F. W. Fitch. 1987. Susceptibility of astrocytes to class I MHC antigen-specific cytotoxicity. J. Immunol. 138:3254-3258. [PubMed] [Google Scholar]
- 48.Stojdl, D. F., N. Abraham, S. Knowles, R. Marius, A. Brasey, B. D. Lichty, E. G. Brown, N. Sonenberg, and J. C. Bell. 2000. The murine double-stranded RNA-dependent protein kinase PKR is required for resistance to vesicular stomatitis virus. J. Virol. 74:9580-9585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Theil, D. J., I. Tsunoda, J. E. Libbey, T. J. Derfuss, and R. S. Fujinami. 2000. Alterations in cytokine but not chemokine mRNA expression during three distinct Theiler's virus infections. J. Neuroimmunol. 104:22-30. [DOI] [PubMed] [Google Scholar]
- 50.Vilcek, J., and G. C. Sen. 1996. Interferons and other cytokines, p. 375-399. In D. M. Knipe, B. N. Fields, and P. M. Howley (ed.), Virology. Lippincott-Raven, Philadelphia, Pa.
- 51.Welsh, C. J., P. Tonks, A. A. Nash, and W. F. Blakemore. 1987. The effect of L3T4 T cell depletion on the pathogenesis of Theiler's murine encephalo-myelitis virus infection in CBA mice. J. Gen. Virol. 68:1659-1667. [DOI] [PubMed] [Google Scholar]
- 52.Wiendl, H., and R. Hohlfeld. 2002. Therapeutic approaches in multiple sclerosis: lessons from failed and interrupted treatment trials. BioDrugs 16:183-200. [DOI] [PubMed] [Google Scholar]
- 53.Yang, Y. L., L. F. Reis, J. Pavlovic, A. Aguzzi, R. Schafer, A. Kumar, B. R. Williams, M. Aguet, and C. Weissmann. 1995. Deficient signaling in mice devoid of double-stranded RNA-dependent protein kinase. EMBO J. 14:6095-6106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yauch, R. Y., J. P. Palma, H. Yahikozawa, C.-S. Koh, and B. S. Kim. 1998. Role of individual T cell epitopes of Theiler's virus in the pathogenesis of demyelination correlates with the ability to induce a Th1 response. J. Virol. 72:6169-6174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zamanian-Daryoush, M., T. H. Mogensen, J. A. DiDonato, and B. R. Williams. 2000. NF-κB activation by double-stranded-RNA-activated protein kinase (PKR) is mediated through NF-κB-inducing kinase and IκB kinase. Mol. Cell Biol. 20:1278-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zheng, L., M. A. Calenoff, and M. C. Dal Canto. 2001. Astrocytes, not microglia, are the main cells responsible for viral persistence in Theiler's murine encephalomyelitis virus infection leading to demyelination. J. Neuroimmunol. 118:256-267. [DOI] [PubMed] [Google Scholar]