Abstract
Control of the worldwide AIDS pandemic may require not only preventive but also therapeutic immunization strategies. To meet this challenge, the next generation of human immunodeficiency virus type 1 (HIV-1) vaccines must stimulate broad and durable cellular immune responses to multiple HIV antigens. Results of both natural history studies and virus challenge studies with macaques indicate that responses to both Gag and Pol antigens are important for the control of viremia. Previously, we reported increased Rev-independent expression and improved immunogenicity of DNA vaccines encoding sequence-modified Gag derived from the HIV-1SF2 strain (J. zur Megede, M. C. Chen, B. Doe, M. Schaefer, C. E. Greer, M. Selby, G. R. Otten, and S. W. Barnett, J. Virol. 74: 2628-2635, 2000). Here we describe results of expression and immunogenicity studies conducted with novel sequence-modified HIV-1SF2 GagPol and Pol vaccine antigens. These Pol antigens contain deletions in the integrase coding region and were mutated in the reverse transcriptase (RT) coding region to remove potentially deleterious enzymatic activities. The resulting Pol sequences were used alone or in combination with sequence-modified Gag. In the latter, the natural translational frameshift between the Gag and Pol coding sequences was either retained or removed. Smaller, in-frame fusion gene cassettes expressing Gag plus RT or protease plus RT also were evaluated. Expression of Gag and Pol from GagPol fusion gene cassettes appeared to be reduced when the HIV protease was active. Therefore, additional constructs were evaluated in which mutations were introduced to attenuate or inactivate the protease activity. Nevertheless, when these constructs were delivered to mice as DNA vaccines, similar levels of CD8+ T-cell responses to Gag and Pol epitopes were observed regardless of the level of protease activity. Overall, the cellular immune responses against Gag induced in mice immunized with multigenic gagpol plasmids were similar to those observed in mice immunized with the plasmid encoding Gag alone. Furthermore, all of the sequence-modified pol and gagpol plasmids expressed high levels of Pol-specific antigens in a Rev-independent fashion and were able to induce potent Pol-specific T- and B-cell responses in mice. These results support the inclusion of a gagpol in-frame fusion gene in future HIV vaccine approaches.
The AIDS pandemic caused by human immunodeficiency virus type 1 (HIV-1) is believed to have cost 3.1 million lives in the year 2002 alone, with over 42 million people believed to be infected worldwide (http://www.unaids.org/worldaidsday/2002/press/Epiupdate.html). At present, 20 years after the discovery of HIV/AIDS, no effective HIV vaccine has been identified and few candidates have advanced beyond early-phase clinical trials (20). While effective drug therapy is available in developed parts of the world, it is financially out of reach for most of the world's population of infected individuals. It is thus widely believed that an efficacious prophylactic vaccine is critical for the control of the global spread of HIV/AIDS. Furthermore, therapeutic vaccine approaches in combination with drug therapy, which allow patients to be off drugs for extended periods of time, also hold great promise for those already infected (18, 45).
While the primary focus for first-generation HIV vaccines was the induction of neutralizing antibodies using HIV envelope (Env)-based approaches, more recently, the focus has extended to the induction of CD8+ cytotoxic T-lymphocyte (CTL) responses against conserved internal viral antigens such as Gag and Pol (17). This shift was a result of studies of natural infections, long-term nonprogressors, and exposed uninfected individuals that have, in multiple studies, demonstrated an inverse correlation between the potency and breadth of CD4+ and CD8+ T-cell responses and HIV disease progression (7, 8, 14, 29, 31, 33, 44, 46). Moreover, vaccine approaches specifically designed to induce strong cellular immunity recently have shown promising results in nonhuman primate vaccine challenge models (2, 5, 49). In these studies, the induction of strong CD8+ T-cell responses against Gag in vaccinated macaques appeared to result in decreased viremia, morbidity, and mortality when animals were subsequently challenged with pathogenic simian/human immunodeficiency viruses. Nevertheless, this strategy of using gene-based vaccines alone to induce CD8+ T-cell responses does not appear to protect monkeys from infection and the challenge virus was able to eventually escape immune control, resulting in increased viremia and its sequelae (4, 24).
Interestingly, the use of prime-boost immunization strategies, including those that use Env antigens as the protein in several of these CTL-based vaccines, has repeatedly been shown to improve the degree of protection observed (23, 36, 37, 42, 43). Whether this is due to the priming of protective B- or T-cell responses has not been elucidated in these studies. In addition to the use of prime-boost strategies, the use of multiple genes in the vaccine to increase the number of potential T-cell epitopes has also improved the outcome after a virus challenge over that achieved with a single- or double-gene vaccine (2, 30). Therefore, the overall goal of our program has been to achieve the greatest breadth of cellular immunity directed to multiple HIV antigens in combination with broad neutralizing antibody responses, an approach that may be more successful at blocking infection than has been previously observed.
The goal of the present study was to evaluate the expression and immunogenicity of novel vaccine antigens based on portions of the HIV-1 Pol polyprotein administered alone or in combination with Gag. Pol is a conserved protein of HIV-1, and cross-clade CTL responses against Pol epitopes have been detected in both HIV-infected and exposed but uninfected individuals (6, 7, 47). The inclusion of the pol gene in the form of the gagpol precursor in earlier vaccine trials with humans and nonhuman primates was most likely suboptimal with regard to inefficient expression of the Pol antigen. The expression levels of the Pol protein generally are low during natural infection because of the frameshift required for translation of pol coding sequences. This mode of Pol expression results in an up to 95% reduction in Pol protein compared to Gag (27, 53). To increase Pol expression, the frameshift between gag and pol can be removed, resulting in equimolar or nearly equimolar expression of Gag and Pol whereas the secretion of virus-like particles (VLP) is impaired (28, 40). To evaluate the potential antigenic competition between Gag and Pol if they are encoded in one expression cassette, various expression cassettes were designed and tested with the antigens encoded on single or multigenic expression plasmids. Another consideration was the possible cytotoxic effect of the active viral protease and possible effects of the active protease on Gag and Pol antigen expression levels. Therefore, mutations known to attenuate or inactivate HIV-1 protease (32) were introduced. Additional safety features introduced into the pol expression cassette included the removal of integrase and the mutation of the reverse transcriptase (RT) to remove these potentially deleterious enzymatic activities.
Plasmid DNA vaccines encoding these sequence-modified gag, pol, and gagpol genes were evaluated for expression in vitro after transient transfection of 293 cells and subsequently in dose titration immunogenicity studies performed with mice. Overall, the cellular immune responses against Gag induced by the various multigenic Gag- and Pol-expressing plasmids were similar to those induced by the plasmid encoding Gag alone. All of the sequence-modified pol and gagpol plasmids expressed high levels of Pol-specific antigens in a Rev-independent fashion and were able to induce potent Pol-specific T-cell responses in mice. Moreover, removal of the frameshift between gag and pol resulted in increased expression of Pol and increased RT-specific immune responses, as expected. Lastly, while the activity of protease appeared to have an inhibitory effect on the expression of Gag and Pol antigens in vitro, the immunogenicities of constructs encoding active protease did not appear to be reduced in mice. The CD8+ T-cell responses against Gag- and RT-specific epitopes, as measured by flow cytometric analysis of gamma interferon (IFN-γ)-producing cells, were comparable for all constructs regardless of the level of protease activity. These results support the inclusion of a sequence-modified in-frame gagpol fusion cassette in future HIV vaccine approaches.
MATERIALS AND METHODS
Plasmid DNA cassettes.
A panel of expression cassettes based on the amino acid sequences of HIV-1SF2 subtype B Gag and Pol antigens was designed with sequence modifications as described previously (22, 56). All gene cassettes were cloned into eukaryotic expression vector pCMVKm2, which contained the cytomegalovirus immediate-early enhancer-promoter and the bGH terminator (Chiron Corporation, Emeryville, Calif.) (12). To further enhance the translation efficiency of all expression cassettes, an optimal “Kozak” consensus sequence (GCCACC) for initiation of translation was inserted (34). gag-only plasmid pCMVKm2.GagMod.SF2 (GenBank accession no. AF201927) and gagprotease cassettes GP1 and GP2 (pCMVKm2.GagProt.SF2; GenBank accession no. AF202464 and AF202465) have been described previously (56).
The entire integrase coding sequence in pol was deleted for safety reasons, and the catalytic triad and primer grip regions of the RT coding sequences were deleted to inactivate these enzymatic activities (39, 41). The construct gagFSpol was based on the GP2 cassette but was extended for pol up to the RNase H coding sequences. For the gagpol and gag-complete-pol (gagCpol) cassettes, the frameshift region was removed by insertion of an extra T nucleotide at the p1 “slippery sequence” (TTTTTTA) in order to express the gag and pol genes in frame. The pol region included p1p6Pol coding sequences up to RNase H for gagpol. To include p1p6Gag and for optimal processing of Gag and Pol by the protease, the p2p7p1p6 fragment was added to get gagCpol (see Fig. 1). The constructs gagRT and gagprotInaRT expressed fusion proteins of p55Gag and either p66RT or p10protease plus p66RT. Furthermore, gene cassettes for the expression of p66RT alone and proteaseRT and p2p7Gag plus p1p6Pol (p2pol) were also included. When indicated, the protease in some constructs was either attenuated (Att) or inactivated (Ina) by the introduction of specific point mutations (32) with the QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, Calif.).
FIG. 1.
Overview of HIV-1 gag and pol expression cassettes. All sequences are based on the HIV-1SF2 isolate (GenBank accession no. K02007) and were optimized for human codon usage. The coding sequence for RT was mutated for all affected constructs to yield a nonfunctional protein. The various versions of constructs with mutations to eliminate the frameshift (FS) and protease (Prot) activity are shown.
Testing for in vitro expression.
Human kidney 293 cells (no. 45504; American Type Tissue Collection, Manassas, Va.) were plated 1 day prior to transfection at a density of 5 × 105 cells per 35-mm-diameter well (Corning, Acton, Mass.) and transfected with endotoxin-free purified plasmid DNA (Qiagen, Valencia, Calif.). For the transfections, 2 μg of each plasmid DNA was mixed with Mirus TransIT-LT1 Polyamine transfection reagent (PanVera, Madison, Wis.). The cells were incubated with 2 ml of 10% Iscove's modified Dulbecco's medium (Invitrogen, Carlsbad, Calif.) per well for 48 and 72 h, and the supernatants and lysates were then harvested for further analysis. Quantitation of p24Gag protein in cell supernatants and lysates was performed with the Coulter p24 Antigen Capture enzyme-linked immunosorbent assay (ELISA; Coulter Corporation, Miami, Fla.). The Western blot assay for Gag and Pol expression analysis was done by using 4 to 12% Bis-Tris sodium dodecyl sulfate-polyacrylamide gels (Invitrogen) and then transfer onto 0.2-μm-pore-size nitrocellulose (Invitrogen). Prestained full-range rainbow marker (Amersham, Piscataway, N.J.) and recombinant HIV-1 p24Gag, p55Gag (Chiron), and p66RT (Protein Sciences, Meriden, Conn.) proteins were used as the size standard and positive controls, respectively. For detection of Gag proteins by immunostaining, membranes were incubated with HIV-1-positive human serum at a dilution of 1:400. For Pol proteins, an anti-p66RT monoclonal antibody (MAb; 1:200; Fitzgerald, Concord, Mass.) and pooled mouse serum (1:400) against p66RT (Chiron) were used. Secondary antibodies (1:20,000) were anti-human or anti-mouse immunoglobulin G (IgG) conjugated to horseradish peroxidase (Pierce, Rockford, Ill.). Detection was performed by using the enhanced chemiluminescence substrate (Amersham). The predicted molecular weights of the various expression cassettes tested were calculated from the predicted amino acid sequences by using MacVector software (Oxford Molecular Ltd.).
Immunization of mice.
To evaluate the relative potencies of the immune responses induced by the different constructs, female CB6F1 or C3H/HeN mice, 6 to 8 weeks old, were immunized bilaterally in the tibialis anterior muscles with 100-μl volumes of endotoxin-free plasmid DNA in isotonic saline (50 μl per site). The DNA concentrations of the test plasmids were adjusted to provide equal molar quantities of Gag or Pol at a given DNA dose. Furthermore, all DNA of <10 μg were adjusted to 10 μg by using noncoding vector pCMVKm2 as carrier DNA to avoid possible negative effects on immune potency that have been observed at low DNA doses (G. R. Otten, unpublished results). Table 1 contains a summary of the mouse studies performed and the immunization regimens used.
TABLE 1.
Overview of mouse studies
| Expt no. | Fig. no. | Mouse strain | Vaccinesa | Immunization (day[s]) | rVV challenge (day) | Blood collection (days) |
|---|---|---|---|---|---|---|
| 1 | 4 | CB6F1 | a, b, c | 0 | 28 | 0, 28, 33 |
| 2 | 5A, 6B | CB6F1 | a, d, f, g, h | 0 | 28 | 0, 28, 33 |
| 3 | 5B | CB6F1 | a, d, f, g, h | 0, 28 | None | 0, 28, 42 |
| 4 | 6A | CB6F1 | a, b, c | 0, 28 | None | 0, 28, 42 |
| 5 | 7A | C3H/HeN | e, f, g, i, k | 0 | 28 | 0, 28, 33 |
| 6 | 7C | C3H/HeN | e, f, g, i, k | 0, 28 | None | 0, 28, 42 |
| 7 | 7B | C3H/HeN | d, f, g, h, l | 0 | 28 | 0, 28, 33 |
| 8 | 7D | C3H/HeN | d, f, g, h, l | 0, 28 | None | 0, 28, 42 |
Vaccines: a, gag; b, GP1; c, GP2; d, gagFSpol; e, gagRT; f, gagprotInaRT; g, gagCpolIna; h, gagCpol; i, RT; k, protInaRT; l, p2polIna.
Measurements of antibody responses to p24Gag.
Plates (96 wells; Corning) were coated with 100 μl of recombinant HIV-1SF2 p24Gag antigen (Chiron) at a concentration of 2 μg/ml in 50 mM borate buffer, pH 9. Sera were diluted 1:25 and then serially diluted threefold in dilution buffer containing 1% casein as a blocking reagent. Pooled anti-p24Gag antibody-positive mouse sera served as both positive controls and assay standards. All sera were incubated for 1 h at 37°C, washed, and incubated with a 1:20,000 dilution of goat anti-mouse IgG plus IgM peroxidase conjugate (Pierce) for 1 h at 37°C. After washing of the plates, the tetramethylbenzidine substrate (Pierce) was added to each well and the reaction was stopped after 30 min by addition of 1 M H3PO4. The plates were read on an ELISA reader (312e; Bio-Tek Instruments, Inc., Winooski, Vt.) at 450 nm with a reference wavelength of 600 nm. The calculated titers are the reciprocal of the dilution of serum at a cutoff optical density of 0.4.
Challenge of immunized mice with recombinant vaccinia viruses (rVVs) expressing Gag or Pol.
Challenge of gag DNA-primed mice with rVV expressing HIV-1SF2 GagPol (with frameshift) (B. Doe and C. Walker, Letter, AIDS 10:793-794, 1996) can enhance humoral and cellular immune responses to Gag compared to those observed after DNA immunization alone (Otten, unpublished). Thus, the rVVgagpol challenge model can provide a useful means by which to obtain quantitative measurements of antigen-specific CD8+ T-cell function (Otten, unpublished). Mice were challenged 28 days postimmunization with an intraperitoneal injection of 107 PFU of rVV. Spleens were removed 5 days later, and spleen cells were isolated for further evaluation in an intracellular cytokine-staining (ICS) assay (described below). An rVV expressing HIV-1SF2 Pol was constructed to allow application of this challenge model for the measurement of Pol-specific T-cell responses. Because of the frameshift in gagpol, the expression of Pol was insufficient if rVVgagpol was used. The complete codon-optimized pol sequence, with the exception of integrase, was used. Protease and RT were left functional. The gene was cloned into the shuttle vector pSC11 (11) via XmaC1 and HindIII sites, and rVV expressing Pol was generated as described for rVVgagpol.
ICS for Gag- and Pol-specific IFN-γ-producing CD8+ lymphocytes.
Stimulation and staining of isolated spleen cells were done as described previously (56). Briefly, spleens were harvested 2 weeks post second DNA immunization or 5 days post rVV challenge and single-cell suspensions were prepared. Nucleated spleen cells (106) were cultured in duplicate at 37°C in the presence or absence of 10 μg of p7g peptide per ml (Doe and Walker, letter) for Gag or by using the RT39-47SF2 peptide TEMEKGEKI (35) for the stimulation of Pol-specific CD8+ cells. Unstimulated cells plus spleen cells from naive mice were used as background and negative controls. The background values were generally very low, between 0.01 and 0.1% of IFN-γ-secreting CD8+ cells. After 5 h, cells were washed, incubated with anti-CD16/32 (Pharmingen, San Diego, Calif.) to block Fcγ receptors, fixed in 1% (wt/vol) paraformaldehyde, and stored overnight at 4°C. On the following day, cells were stained with fluorescein isothiocyanate-conjugated CD8 MAb (Pharmingen), washed, treated with 0.5% (wt/vol) saponin (Sigma), and then incubated with phycoerythrin-conjugated mouse IFN-γ MAb (Pharmingen) in the presence of 0.1% (wt/vol) saponin. Cells were then washed and analyzed on a FACScalibur flow cytometer (Becton Dickinson Immunocytometry Systems, San Jose, Calif.).
Statistical analysis of Gag- and Pol-specific IFN-γ-producing CD8+ T-cell responses.
For analysis of the relative CD8+ T-cell responses in the mouse immunogenicity studies, a regression analysis was performed. Each regression analysis began with a single regression model incorporating indicator variables to allow for individual intercepts and slopes specifically for each treatment. The model is Yi = β0 + β0iδi + β1x + β1iδIx + ε, where i = 1…no. of treatments. Here Yi is the log10 background-corrected percentage of cells showing a positive CD8 IFN-γ response for peptide treatment group i and HIV DNA vaccine dose level x. The intercept for each treatment is the overall intercept, β0, plus an additional term, β0i, for treatment i. The slope for each treatment is β1x plus an additional term, β1iδix. The δi values are indicator variables that equal 1 for treatment i and are 0 otherwise. The model was iteratively reduced by removing first nonsignificant slope terms, those with P > 0.05, and then nonsignificant intercept terms, those with P > 0.05, in the reduced-slope model. The result was a final regression model with only the significant slope and intercept terms, those with P < 0.05. This model-building process was repeated for each of seven experiments, corresponding to Fig. 4, 5A and B, and 7A to D. Scatter plots for each figure including the significant regression model equations for each treatment were plotted by using SPlus 2000.
FIG. 4.
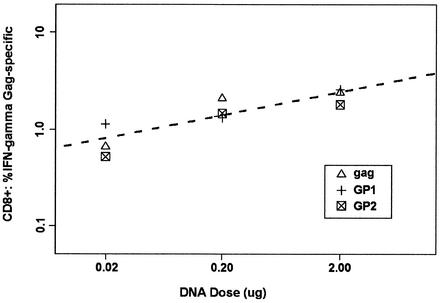
Quantitative analysis of Gag-specific, IFN-γ-secreting CD8+ T cells. CB6F1 mice were immunized twice at weeks 0 and 4 with titrated doses of codon-optimized HIV-1SF2 gag, GP1, and GP2 plasmid DNAs. Spleens were removed 2 weeks after the second immunization, and the pooled spleen cells were stimulated in duplicate for 5 h with the p7g peptide. On the following day, cells were stained for CD8+ and intracellular IFN-γ and analyzed by flow cytometry. Data were analyzed by a regression model (see Materials and Methods for details).
FIG. 5.
HIV-1SF2 Gag-specific CD8+ responses of CB6F1 mice immunized with titrated DNA doses of gag or gag-plus-pol cassettes. Groups of mice were either immunized once and challenged with rVVgagpol 4 weeks later (A) or received two immunizations with DNA at weeks 0 and 4 (B). Spleens were harvested 5 days post vaccinia virus challenge or 2 weeks post second immunization, respectively. Pooled splenocytes were stimulated with the Gag-specific peptide p7g for 5 h. Cells were stained for CD8+ and intracellular IFN-γ on the next day and analyzed by flow cytometry. Data were analyzed by a regression model (see Materials and Methods for details).
FIG. 7.
Frequencies of HIV-1SF2 RT-specific CD8+ T-cell responses of C3H/HeN mice immunized with titrated DNA doses of RT, protInaRT, p2polIna, or gag-plus-pol cassettes. Results for mice immunized once and challenged with rVVpol 4 weeks later are shown in panels A and B. Another set of animals received two DNA immunizations at weeks 0 and 4 (C and D). Spleen harvesting and staining for flow cytometry were performed as described in the legend to Fig. 5. Data were analyzed by a regression model (see Materials and Methods for details).
RESULTS
Construction of novel gag- and pol-derived expression cassettes.
Previously, we reported on the construction and characterization of a sequence-modified Gag plasmid that was found in several studies to be a potent inducer of Gag-specific immune responses (38, 56). In the present work, we sought to broaden the spectrum of viral epitopes represented in our DNA vaccine approach (without introducing a reduction of Gag-specific immune responses) through the addition of Pol coding sequences. For this purpose, we designed and evaluated several novel gag and pol expression cassettes. A summary of the sequence-modified gene cassettes evaluated here is shown in Fig. 1. The constructs GagMod (gag), GP1, and GP2 were described and characterized previously but are included for comparison (56). The gene cassette gagFSpol was based on GP2 with an extension of Pol including the p66RT coding region but without the integrase coding sequences. The integrase was excluded from all of the constructs described here to avoid possible integration of vaccine sequences into the host genome. To improve Pol expression, the frameshift region between the gag and pol genes was mutated by single-base insertion to create gagpol with both the Gag and Pol coding sequences in the same open reading frame. Creation of this construct resulted in the loss of p1p6Gag because of the mutation introduced to remove the frameshift. Because the p6 portion of Gag was shown to be important for the efficient release of Gag VLP (19), the cassette gagCpol was designed to include a repeat of p2p7p1p6 to restore p1p6Gag expression. Moreover, the p2p7Gag repeat was introduced to improve the secretion and autoprocessing of gagCpol by the protease (1, 57). Also, to enhance possible processing requirements for efficient expression, a pol cassette was designed to include p2p7gag (p2pol and p2pol). Because of concerns regarding potential cytotoxic properties of the functional viral protease (32) that could affect both antigen expression and immunogenicity, the protease gene was either attenuated (Att) or rendered inactive (Ina) in the designated constructs (Fig. 1). Fusion cassettes expressing Gag plus RT (gagRT) and Gag plus protease plus RT (gagprotInaRT) were also constructed and compared to gagCpol.
In vitro characterization of expression cassettes.
To evaluate the expression patterns of the various Gag- and Pol-containing constructs, 293 cells were transiently transfected and supernatants and cell lysates were analyzed by p24Gag antigen capture ELISA and immunoblotting. Because the p24Gag antigen capture ELISA preferentially recognizes processed forms of Gag (48, 56), comparative expression analyses were problematic to perform for all constructs. However, comparison of very similar constructs allowed us to test for differences in Gag expression.
Figure 2 illustrates the relative Gag expression levels. The cassette gagFSpol was designed to extend the Pol region and at the same time maintain the natural processing and frameshift translation of the expressed GagPol precursor polyprotein. In cell lysates, the expression level of Gag from this construct was about the same as that of Gag expressed by GP2 (Fig. 2B) but about fourfold less p24Gag was detected in the culture supernatant compared to that of GP2 (Fig. 2A). In the gagpol and gagCpol constructs, the frameshift sequences were altered so that Gag and Pol could be expressed by the same reading frame in order to increase the expression of Pol without affecting Gag expression. In alternative versions of these constructs, the protease gene was either mutated to produce attenuated (gagpolAtt, gagCpolAtt) or inactivated (gagpolIna, gagCpolIna) protease. As shown in Fig. 2C, no differences in p24Gag levels were observed in culture supernatants when similar versions of gagpol and gagCpol were compared. The same results were also obtained with the cell lysates (data not shown). Thus, the additional insertion of the p2p7p1p6 fragment appeared to have no influence on p24Gag expression levels as measured here.
FIG. 2.
Quantitative comparison of HIV-1 Gag expression by p24Gag antigen capture ELISA of supernatants (sup) and lysates (lys) of 293 cells 48 h posttransfection with gagpol constructs. Supernatants (A) and lysates (B) of GP2 versus gagFSpol are shown. Both cassettes express functional protease with an intact frameshift. In the experiment whose results are shown in panel C, supernatants were analyzed from different versions of in-frame gagpol versus gagCpol with functional, nonfunctional, or attenuated protease. The gagCpol cassettes included an additional p2p7p1p6gag sequence.
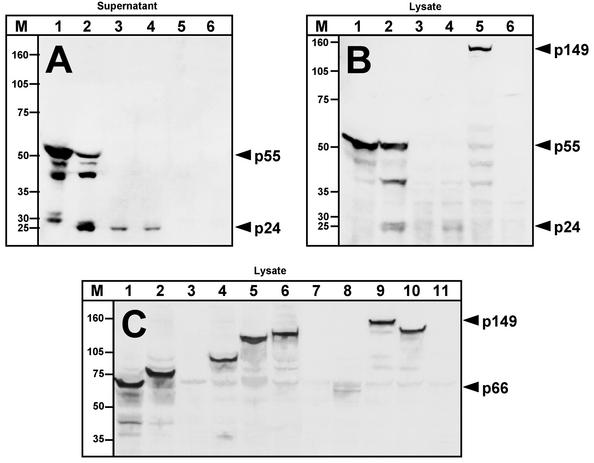
Western blot analysis was performed with all of the expression cassettes described in Fig. 1 by using Gag-specific, HIV-positive human antisera (Fig. 3A and B). Clear differences were observed between plasmids expressing processed and unprocessed forms of the Gag and GagPol polyproteins. The highest level of Gag-specific reactivity appeared to be found in supernatants (Fig. 3A) and lysates (Fig. 3B) of cultures of cells transfected with gag, followed by GP2 and gagFSpol (data not shown). GP2 and gagFSpol process the Gag polyprotein by using a protease that is underexpressed with the natural frameshift intact, and the bands observed included unprocessed p55Gag and processed forms of Gag. As would be expected in the absence of protease, very little or no processed p24Gag was seen in lysates of cells expressing Gag alone; nevertheless, the small amount of processing observed in the supernatants of these cells was likely due to the presence of nonspecific cellular protease activity. In transfections with two of the constructs expressing Gag and Pol in the same reading frame, gagCpol and gagCpolAtt, the band corresponding to p55Gag was not detectable in the cell supernatants or lysates and reduced amounts of p24Gag were seen in supernatants and lysates (Fig. 3A and B). For gagCpolIna with the nonfunctional protease, no Gag-specific bands were detected in cell supernatants (Fig. 3A) and a high-molecular-mass band corresponding to the unprocessed GagCPol polyprotein (149 kDa) was observed to migrate as expected in the cell lysate (Fig. 3B). Additional bands expressed from gagCpolIna included small amounts of p55Gag and p41Gag, but no p24Gag could be detected. Accordingly, when cells transfected with gagCpolIna, gagCpol, and gag were examined by electron microscopy, very few VLP were detected for gagCpolIna and no particles were detected for gagCpol, indicating impaired secretion of VLP compared to that achieved with gag (data not shown). The cassettes gagRT (121 kDa) and gagprotInaRT (131 kDa) showed levels of Gag comparable to those observed for gagCpolIna (data not shown).
FIG. 3.
Immunoblots of synthetic HIV-1 gag and pol expression cassettes. 293 cells were transfected, and supernatants and lysates were collected 48 h posttransfection, subjected to 4 to 12% Bis-Tris sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and blotted onto nitrocellulose membranes. Immunostaining was performed with either human HIV-1 patient serum (A and B) or pooled anti-p66RT mouse serum (C). For detection of Gag expression, supernatants (A) and lysates (B) were used. Lanes: 1, gag; 2, GP2; 3, gagCpol; 4, gagCpolAtt; 5, gagCpolIna; 6, mock transfection. For detection of Pol products, only cell lysates were analyzed (C). Lanes: 1, RT; 2, protInaRT; 3, p2pol; 4, p2polIna; 5, gagRT; 6, gagprotInaRT; 7, gagCpol; 8, gagCpolAtt; 9, gagCpolIna; 10, gagpolIna; 11, mock transfection. The values on the left are molecular sizes (M) in kilodaltons.
The expression of Pol in cell lysates from transfected 293 cells was also analyzed by Western blotting with RT-specific antisera (Fig. 3C). In general, both the single-gene cassettes in the absence of Gag (RT, proteaseRT, and p2polIna) and the gagpol fusion cassettes (gagRT, gagprotInaRT, gagCpolIna, and gagpolIna) appeared to be expressed well as long as the protease gene was absent or nonfunctional. The RT (66 kDa) and protInaRT (75 kDa) cassettes appeared to be expressed at the highest levels, followed by the p2polIna (93 kDa), gagRT (121 kDa), and gagprotInaRT (131 kDa) cassettes, followed by the gagCpolIna (149 kDa) and gagpolIna (132 kDa) cassettes. The latter two constructs exhibited high-molecular-weight bands of the expected relative mobilities (and slightly faster, respectively) of similar intensities indicative of comparable levels of expression. In constructs expressing the functional and attenuated HIV protease, p2pol, gagCpol, and gagCpolAtt, reduced expression of RT-specific bands was observed compared to the levels expressed by the p2polIna and gagCpolIna constructs. In summary, the addition of gag sequences to pol appeared to have very little influence on Pol-specific expression levels and vice versa but the addition of a functional protease gene resulted in reduced expression of Gag- and RT-specific bands.
Design of mouse immunogenicity studies.
The relative immunogenicities of the DNA plasmids encoding the various gene cassettes were evaluated in mice that were intramuscularly immunized with doses of plasmid DNA ranging from 0.002 to 20 μg (Table 1 contains a summary of the studies performed). This afforded a determination of the dose dependency for each plasmid. In each experiment, groups of 4 to 10 mice were immunized per dose of a given plasmid. One set of mice was immunized twice, at weeks 0 and 4, with spleen removal and analysis at week 6, and another set was immunized once with DNA and then challenged after 4 weeks with rVV expressing GagPol or Pol. Spleens were removed 5 days later, and cells were harvested for ICS to measure Gag- and Pol-specific IFN-γ-producing CD8+ lymphocytes. Because boosting with rVV enhanced specific immune responses to these antigens, T-cell responses could be evaluated after a single DNA prime even at the lowest DNA dose.
CD8+ T-cell responses to Gag.
Gag-specific CD8+ T-cell responses were analyzed by intracellular IFN-γ staining of CD8+ spleen cells that had been stimulated with Gag peptide p7g, an H-2Kd-restricted epitope (Doe and Walker, letter). In the first study (Fig. 4), addition of functional protease to Gag with a frameshift in constructs GP1 and GP2 was tested. The CD8+ T-cell responses after two DNA immunizations were indistinguishable for all three plasmids. Thus, from these results, protease-mediated cleavage of Gag apparently did not affect the processing and presentation of Gag in vivo. At the lowest plasmid dose (0.02 μg), Gag-specific CD8+ T cells were only 30 to 50% below maximum. Therefore, for the next studies, the lowest DNA dose was reduced further to 0.002 μg. Furthermore, new constructs were included and compared to gag. The potency of all of the plasmids tested with regard to the induction of Gag-specific CD8+ T cells was indistinguishable after a single DNA immunization followed by an rVVgagpol challenge or after two DNA immunizations (Fig. 5A and B).
Addition of pol sequences to gag in the DNA vaccine constructs evaluated here did not affect the induction of Gag-specific immune responses. Moreover, despite apparent differences between gagCpol and gagCpolIna in Gag expression as measured in vitro (Fig. 3), the induction of Gag-specific CD8+ T-cell responses was not affected by functional protease.
Antibody responses to Gag.
The measurement of Gag-specific antibody responses revealed a different pattern of responses for the various constructs compared to that observed for the cellular responses. In the first experiment, a comparison was drawn between gag and GP1 and GP2 (Fig. 6A) to look for possible effects of the functional protease on the immunogenicity of p55Gag when protease is expressed with the natural frameshift. The p55Gag antibody responses at 2 weeks post second DNA immunization demonstrated the overall weakest responses with GP1 and better responses with GP2. The gag DNA appeared to be more immunogenic, especially at the lower DNA doses, but GP2 was more comparable to gag at the highest DNA dose (20 μg). For the next experiment (Fig. 6B), antibody responses were analyzed 5 days after a vaccinia virus challenge. Additional cassettes expressing Gag and Pol in frame (gagprotInaRT, gagCpol, and gagCpolIna) were evaluated. In comparison to the previously described analysis (Fig. 6A), the differences between constructs were much more apparent. Two patterns of antibody induction emerged. The gag and gagFSpol cassettes induced strong humoral immune responses post vaccinia virus challenge, while the gagprotInaRT, gagCpol, and gagCpolIna cassettes were much less potent for the induction of antibody titers. The observed antibody responses appear to correlate with the relative amounts of secreted Gag proteins observed in the in vitro analysis (Fig. 3). The constructs that secreted the highest levels of Gag (gag and gagFSpol) primed for the most potent antibody responses, while those that expressed high-molecular-weight polyproteins in the cell lysates (gagprotInaRT and gagCpolIna) or overprocessed Gag (gagCpol) induced the poorest antibody responses.
FIG. 6.
Antibody (Ab) titers specific for HIV-1SF2 p24Gag in mice 2 weeks after two immunizations (weeks 0 and 4) with DNA (A) or 5 days postchallenge with rVVgagpol after a single DNA immunization at week 0 (B). Collected serum samples were analyzed by p24Gag ELISA as described in Materials and Methods. (A) The plasmid expressing only p55Gag (gag) was compared to GP1 and GP2. (B) Expression cassettes gag and gagFSpol were compared to nonframeshifted versions of gagpol. The values shown are the geometric mean antibody titers and the standard deviations of the midpoint antibody titers for each group.
Cellular immune responses to Pol.
For detection of cellular immune responses to Pol, studies were done with C3H/HeN mice. Spleen cells were stimulated with the H-2Kk-restricted nonamer TEMEKGEKI (35) and analyzed by flow cytometry for IFN-γ synthesis. Figure 7A and C compare the RT and protInaRT DNA vaccines with those encoding gag plus pol sequences. In general, the magnitude of the Pol responses was lower than that of the Gag responses. No significant differences were observed between the different antigens, with the exception of gagFSpol, which was not as potent as expected as a result of the low-level expression of the encoded Pol products. Figure 7B and D show that the p2pol cassette, in which the p2p7gag and p1p6pol sequences precede protInaRT, induced Pol-specific CD8+ T cells, even at low doses. Thus, in-frame insertions of p2p7p1p6 and protease upstream of RT did not seem to reduce RT-specific immunogenicity. To study this further, the complete gag coding region was inserted upstream of pol. As shown in Fig. 7, in-frame insertion of gag did not suppress the induction of RT-specific CD8+ T cells; however, if the wild-type frameshift was present (gagFSpol), the vaccine was less potent at inducing this Pol-specific response after a vaccinia virus boost for all doses (Fig. 7B) and no response was detectable after two DNA immunizations, even at the highest dose (Fig. 7D). As for immune responses to Gag, the differences in Pol expression in gagCpol constructs with functional and nonfunctional protease, as seen in vitro, did not result in differences in the observed immune potencies of these constructs. The cellular immune responses to Pol, as measured here, were not affected either by the activity of protease or by the addition of gag sequences upstream of pol.
DISCUSSION
For the design and development of an effective HIV-1 vaccine, the induction of T-cell responses with a large repertoire of specificities is essential. Inclusion of HIV-1 Pol in a vaccine would be expected to increase this repertoire significantly (54). Pol is well-conserved, broad CTL responses are found in the majority of infected patients, and these responses have been shown to be inversely correlated to the viral load (7, 21). Since the virus-encoded pol gene is expressed at very low levels compared to gag as a result of the translational frameshifting mechanism by which it is expressed, increasing pol expression by removal of the natural frameshift and removal of inhibitory sequences could result in the induction of a higher frequency of Pol-specific effector and memory CTL by pol-based DNA vaccines. In addition, because an effective HIV-1 vaccine would very likely be composed of at least gag and pol plus env, cost and practicability should also be considered. A multigenic DNA vaccine containing gag and pol on one plasmid would therefore be an advantage. Gene cassettes encoding gagpol have been used previously in vaccines with modest immunological outcomes with respect to the induction of Pol-specific T-cell responses in human and nonhuman primate studies (9, 15, 16). This could be explained by the use of the gagpol gene with an intact frameshift and/or native codon usage, which would be expected to provide lower levels of pol expression. Casimiro et al. reported recently for the first time strong Pol-specific cellular immune responses in nonhuman primates after immunization with synthetic pol DNA vaccines (10).
In this work, we analyzed immune responses to HIV-1 gag and a variety of pol sequences in separate and combined expression cassettes. Particular attention was given to the possible negative effect of pol on gag expression and immunogenicity. Immune responses to the well-characterized plasmid pCMVKm2.GagMod.SF2 (gag) (56) served as a benchmark for these studies. Results obtained with the sequence-modified pol gene indicated that the expression and immunogenicity of Gag using gagFSpol with an intact frameshift was not affected by the pol sequence (Fig. 5 and 6B). Also, after removal of the frameshift region from the gagpol cassettes, Pol expression was improved dramatically. While Pol expression could not be detected in Western blots of lysates and culture supernatants from cells transfected with gagFSpol (data not shown), plasmids encoding an in-frame gagpol cassette with nonfunctional protease showed high-level expression (Fig. 3C). This was also confirmed in mice immunized with gagFSpol versus gagCpol in-frame cassettes. Pol-specific CD8+ T-cell responses could only be detected in gagFSpol-immunized animals after an rVVpol boost, whereas gagCpol induced strong responses after two DNA doses (Fig. 7B and D). Interestingly, previously described cytotoxic effects of HIV-1 protease that were shown to affect the expression of additional genes in vivo (51) did not diminish CD8+ T-cell responses. The gagCpol (functional protease) and gagCpolIna (nonfunctional protease) DNA vaccines were indistinguishable in their abilities to induce cellular immune responses to Gag or Pol (Fig. 5 and 7B and D). However, reduced expression of the Gag and Pol proteins was observed in Western blots of transfected cells when the protease was functional (Fig. 3). Whether this effect was directly related to negative effects of protease or altered expression kinetics remains to be determined.
HIV-1 Gag is a major target with respect to the induction of CTL responses in HIV-1-infected patients, and p24Gag and p17Gag appear to have the highest epitope density, besides Nef, of all HIV-1 antigens (55). Recently, an important contribution of p15Gag to the overall CTL response in HIV-1-infected subjects also was reported (55). This result should be considered in a Gag-based vaccine design. Thus, to retain important epitopes for Gag, the gagCpol cassette, containing the complete gag coding sequences in addition to pol in frame, was designed. After removal of the frameshift by a single-base insertion, p1p6Gag protein expression was lost, resulting in a truncated Gag protein that was shortened by p1p6Gag at the frameshift site. The extension of gagpol to include p2p7p1p6Gag in the gagCpol construct had no negative influence on expression (Fig. 2C), and this cassette design was therefore selected for use in immunogenicity studies instead of the original gagpol construct.
Immune responses generated against Gag or Pol by using various Gag- and Pol-expressing DNA vaccines were evaluated by repeated experiments with either two DNA immunizations or one immunization followed by an rVV boost. Responses were scored by flow cytometric measurements of antigen-specific IFN-γ-secreting CD8+ cells with an ICS assay. Responses to Gag were detectable after two immunizations with amounts of DNA as small as 2 ng. No significant differences in Gag-specific CD8+ T-cell responses were found for any of the sequence-modified expression cassettes tested here. Cellular immune responses to Pol were analyzed by using C3H mice (H-2k), and spleen cells were stimulated by using the 9-mer CTL peptide described by Hosmalin et al. (25). Positive responses could be detected in the 20- to 200-ng DNA dose range, compared to 2 ng for Gag. This could be explained by the reduced recognition and assay sensitivity of this peptide as recently described (10). However, solid stimulation was demonstrated with this peptide epitope; up to 32% of RT-specific CD8+ cells responded after one 20-μg DNA prime and an rVV boost (Fig. 7A and B). As expected from the expression results, the gagFSpol DNA vaccine (i.e., Pol expressed with a frameshift) induced significantly lower levels of Pol-specific immune responses if DNA-primed mice were boosted with rVV expressing Pol (Fig. 7B) and no detectable Pol-specific responses after two DNA immunizations (Fig. 7D). As for Gag responses, no significant differences were found among the in-frame sequence-modified constructs with regard to the induction of Pol-specific CD8+ T-cell responses. Thus, it appears that efficient secretion of Gag antigens as VLP secretion, which is impaired in gagpol fusion constructs (28, 40), was not essential for the induction of potent Gag-specific CD8+ responses. Previous results obtained by another group using synthetic pol and gagpol genes also demonstrated improved expression of Pol when it was fused in frame with Gag (26). However, cellular immune responses to Gag and Pol were demonstrated for single and fusion gene cassettes when mice were immunized four times with 100 μg of DNA. In our experiments, we titrated the DNA doses down to 2 ng for Gag responses and 20 ng for Pol responses, which allowed us to more fully evaluate the relative potency of each construct. Moreover, in the present study, several additional versions of pol and gagpol, including those with an attenuated, functional and nonfunctional protease gene, were analyzed.
Altogether, the data presented in this study suggest that the highly efficient expression and immunogenicity of Gag are not impaired by Pol, and vice versa, if Gag and Pol are expressed as a multigenic fusion protein (gagCpol) in a DNA vaccine. Moreover, the expression and immunogenicity of the Pol antigen can be enhanced through removal of the frameshift and sequence modifications to remove inhibitory sequences and optimize codon usage. The improved gag-plus-pol DNA vaccine described here, when administered by using recently described enhanced DNA vaccine delivery technologies (38, 52), should prove to be a potent vaccine for the induction of T-cell immune responses. Furthermore, vaccine approaches that combine the gagCpol DNA vaccine for the induction of cellular immune responses with improved Env antigens for the induction of neutralizing antibodies (3, 13, 50) hold great promise for the next generation of HIV vaccines.
Acknowledgments
We thank Diana Atchley, Jacqueline Wilson, Pedro Benitez, Debbie Swinarski, and Charles Vitt for excellent help with the mouse immunization studies. We also thank Brian Munneke and Lynn Eudey for exquisite help with the statistical analyses and Kent Thudium for help with rVV constructs.
This work was supported by National Institute of Allergy and Infectious Diseases (National Institutes of Health) HIV Vaccine Design and Development Team contract NO1-AI-05396.
REFERENCES
- 1.Accola, M. A., B. Strack, and H. G. Gottlinger. 2000. Efficient particle production by minimal Gag constructs which retain the carboxy-terminal domain of human immunodeficiency virus type 1 capsid-p2 and a late assembly domain. J. Virol. 74:5395-5402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amara, R. R., F. Villinger, J. D. Altman, S. L. Lydy, S. P. O'Neil, S. I. Staprans, D. C. Montefiori, Y. Xu, J. G. Herndon, L. S. Wyatt, M. A. Candido, N. L. Kozyr, P. L. Earl, J. M. Smith, H. L. Ma, B. D. Grimm, M. L. Hulsey, J. Miller, H. M. McClure, J. M. McNicholl, B. Moss, and H. L. Robinson. 2001. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science 292:69-74. [DOI] [PubMed] [Google Scholar]
- 3.Barnett, S. W., S. Lu, I. Srivastava, S. Cherpelis, A. Gettie, J. Blanchard, S. Wang, I. Mboudjeka, L. Leung, Y. Lian, A. Fong, C. Buckner, A. Ly, S. Hilt, J. Ulmer, C. T. Wild, J. R. Mascola, and L. Stamatatos. 2001. The ability of an oligomeric human immunodeficiency virus type 1 (HIV-1) envelope antigen to elicit neutralizing antibodies against primary HIV-1 isolates is improved following partial deletion of the second hypervariable region. J. Virol. 75:5526-5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barouch, D. H., J. Kunstman, M. J. Kuroda, J. E. Schmitz, S. Santra, F. W. Peyerl, G. R. Krivulka, K. Beaudry, M. A. Lifton, D. A. Gorgone, D. C. Montefiori, M. G. Lewis, S. M. Wolinsky, and N. L. Letvin. 2002. Eventual AIDS vaccine failure in a rhesus monkey by viral escape from cytotoxic T lymphocytes. Nature 415:335-339. [DOI] [PubMed] [Google Scholar]
- 5.Barouch, D. H., S. Santra, J. E. Schmitz, M. J. Kuroda, T. M. Fu, W. Wagner, M. Bilska, A. Craiu, X. X. Zheng, G. R. Krivulka, K. Beaudry, M. A. Lifton, C. E. Nickerson, W. L. Trigona, K. Punt, D. C. Freed, L. Guan, S. Dubey, D. Casimiro, A. Simon, M. E. Davies, M. Chastain, T. B. Strom, R. S. Gelman, D. C. Montefiori, M. G. Lewis, E. A. Emini, J. W. Shiver, and N. L. Letvin. 2000. Control of viremia and prevention of clinical AIDS in rhesus monkeys by cytokine-augmented DNA vaccination. Science 290:486-492. [DOI] [PubMed] [Google Scholar]
- 6.Betts, M. R., J. Krowka, C. Santamaria, K. Balsamo, F. Gao, G. Mulundu, C. W. Luo, N. NGandu, H. Sheppard, B. H. Hahn, S. Allen, and J. A. Frelinger. 1997. Cross-clade human immunodeficiency virus (HIV)-specific cytotoxic T-lymphocyte responses in HIV-infected Zambians. J. Virol. 71:8908-8911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Betts, M. R., J. F. Krowka, T. B. Kepler, M. Davidian, C. Christopherson, S. Kwok, L. Louie, J. Eron, H. Sheppard, and J. A. Frelinger. 1999. Human immunodeficiency virus type 1-specific cytotoxic T lymphocyte activity is inversely correlated with HIV type 1 viral load in HIV type 1-infected long-term survivors. AIDS Res. Hum. Retrovir. 15:1219-1228. [DOI] [PubMed] [Google Scholar]
- 8.Borrow, P., H. Lewicki, B. H. Hahn, G. M. Shaw, and M. B. A. Oldstone. 1994. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J. Virol. 68:6103-6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boyer, J. D., K. E. Ugen, B. Wang, M. Agadjanyan, L. Gilbert, M. L. Bagarazzi, M. Chattergoon, P. Frost, A. Javadian, W. V. Williams, Y. Refaeli, R. B. Ciccarelli, D. McCallus, L. Coney, and D. B. Weiner. 1997. Protection of chimpanzees from high-dose heterologous HIV-1 challenge by DNA vaccination. Nat. Med. 3:526-532. [DOI] [PubMed] [Google Scholar]
- 10.Casimiro, D. R., A. Tang, H. C. Perry, R. S. Long, M. Chen, G. J. Heidecker, M. E. Davies, D. C. Freed, N. V. Persaud, S. Dubey, J. G. Smith, D. Havlir, D. Richman, M. A. Chastain, A. J. Simon, T. M. Fu, E. A. Emini, and J. W. Shiver. 2002. Vaccine-induced immune responses in rodents and nonhuman primates by use of a humanized human immunodeficiency virus type 1 pol gene. J. Virol. 76:185-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chakrabarti, S., K. Brechling, and B. Moss. 1985. Vaccinia virus expression vector: coexpression of β-galactosidase provides visual screening of recombinant virus plaques. Mol. Cell. Biol. 5:3403-3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chapman, B. S., R. M. Thayer, K. A. Vincent, and N. L. Haigwood. 1991. Effect of intron A from human cytomegalovirus (Towne) immediate-early gene on heterologous expression in mammalian cells. Nucleic Acids Res. 19:3979-3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cherpelis, S., X. Jin, A. Gettie, D. D. Ho, S. W. Barnett, I. Shrivastava, and L. Stamatatos. 2001. DNA-immunization with a V2 deleted HIV-1 envelope elicits protective antibodies in macaques. Immunol. Lett. 79:47-55. [DOI] [PubMed] [Google Scholar]
- 14.Clerici, M., and G. Shearer. 1996. Correlates of protection in HIV infection and the progression of HIV infection to AIDS. Immunol. Lett. 51:69-73. [DOI] [PubMed] [Google Scholar]
- 15.Evans, T. G., M. C. Keefer, K. J. Weinhold, M. Wolff, D. Montefiori, G. J. Gorse, B. S. Graham, M. J. McElrath, M. L. Clements-Mann, M. J. Mulligan, P. Fast, M. C. Walker, J. L. Excler, A. M. Duliege, and J. Tartaglia. 1999. A canarypox vaccine expressing multiple human immunodeficiency virus type 1 genes given alone or with rgp120 elicits broad and durable CD8+ cytotoxic T lymphocyte responses in seronegative volunteers. J. Infect. Dis. 180:290-298. [DOI] [PubMed] [Google Scholar]
- 16.Ferrari, G., C. Berend, J. Ottinger, R. Dodge, J. Bartlett, J. Toso, D. Moody, J. Tartaglia, W. Cox, E. Paoletti, and K. Weinhold. 1997. Replication-defective canarypox (ALVAC) vectors effectively activate anti-human immunodeficiency virus-1 cytotoxic T lymphocytes present in infected patients: implications for antigen-specific immunotherapy. Blood 90:2406-2416. [PubMed] [Google Scholar]
- 17.Girard, M., A. Habel, and C. Chanel. 1999. New prospects for the development of a vaccine against human immunodeficiency virus type 1: an overview. C. R. Acad. Sci. III 322:959-966. [DOI] [PubMed] [Google Scholar]
- 18.Gotch, F. M., N. Imami, and G. Hardy. 2001. Candidate vaccines for immunotherapy in HIV. HIV Med. 2:260-265. [DOI] [PubMed] [Google Scholar]
- 19.Gottlinger, H. G., T. Dorfman, J. G. Sodroski, and W. A. Haseltine. 1991. Effect of mutations affecting the p6 gag protein on human immunodeficiency virus particle release. Proc. Natl. Acad. Sci. USA 88:3195-3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Graham, B. S. 2002. Clinical trials of HIV vaccines. Annu. Rev. Med. 53:207-221. [DOI] [PubMed] [Google Scholar]
- 21.Haas, G., A. Samri, E. Gomard, A. Hosmalin, J. Duntze, J. M. Bouley, H. G. Ihlenfeldt, C. Katlama, and B. Autran. 1998. Cytotoxic T-cell responses to HIV-1 reverse transcriptase, integrase and protease. AIDS 12:1427-1436. [DOI] [PubMed] [Google Scholar]
- 22.Haas, J., E. Park, and B. Seed. 1996. Codon usage limitation in the expression of HIV-1 envelope glycoprotein. Curr. Biol. 6:315-324. [DOI] [PubMed] [Google Scholar]
- 23.Habel, A., C. Chanel, R. Le Grand, F. Martinon, L. Couillin, C. Moog, R. Doms, M. C. Gauduin, B. Hurtrel, J. G. Guillet, A. M. Aubertin, and M. Girard. 2000. DNA vaccine protection against challenge with simian/human immunodeficiency virus 89.6 in rhesus macaques. Dev. Biol. 104:101-105. [PubMed] [Google Scholar]
- 24.Horton, H., T. U. Vogel, D. K. Carter, K. Vielhuber, D. H. Fuller, T. Shipley, J. T. Fuller, K. J. Kunstman, G. Sutter, D. C. Montefiori, V. Erfle, R. C. Desrosiers, N. Wilson, L. J. Picker, S. M. Wolinsky, C. Wang, D. B. Allison, and D. I. Watkins. 2002. Immunization of rhesus macaques with a DNA prime/modified vaccinia virus Ankara boost regimen induces broad simian immunodeficiency virus (SIV)-specific T-cell responses and reduces initial viral replication but does not prevent disease progression following challenge with pathogenic SIVmac239. J. Virol. 76:7187-7202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hosmalin, A., M. Clerici, R. Houghten, C. D. Pendleton, C. Flexner, D. R. Lucey, B. Moss, R. N. Germain, G. M. Shearer, and J. A. Berzofsky. 1990. An epitope in human immunodeficiency virus 1 reverse transcriptase recognized by both mouse and human cytotoxic T lymphocytes. Proc. Natl. Acad. Sci. USA 87:2344-2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang, Y., W. P. Kong, and G. J. Nabel. 2001. Human immunodeficiency virus type 1-specific immunity after genetic immunization is enhanced by modification of Gag and Pol expression. J. Virol. 75:4947-4951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jacks, T., M. D. Power, F. R. Masiarz, P. A. Luciw, P. J. Barr, and H. E. Varmus. 1988. Characterization of ribosomal frameshifting in HIV-1 gag-pol expression. Nature 331:280-283. [DOI] [PubMed] [Google Scholar]
- 28.Karacostas, V., E. Wolffe, K. Nagashima, M. Gonda, and B. Moss. 1993. Overexpression of the HIV-1 gag-pol polyprotein results in intracellular activation of HIV-1 protease and inhibition of assembly and budding of virus-like particles. Virology 193:661-671. [DOI] [PubMed] [Google Scholar]
- 29.Kaul, R., S. L. Rowland-Jones, J. Kimani, K. Fowke, T. Dong, P. Kiama, J. Rutherford, E. Njagi, F. Mwangi, T. Rostron, J. Onyango, J. Oyugi, K. S. MacDonald, J. J. Bwayo, and F. A. Plummer. 2001. New insights into HIV-1 specific cytotoxic T-lymphocyte responses in exposed, persistently seronegative Kenyan sex workers. Immunol. Lett. 79:3-13. [DOI] [PubMed] [Google Scholar]
- 30.Kim, J. J., J. S. Yang, L. K. Nottingham, D. J. Lee, M. Lee, K. H. Manson, M. S. Wyand, J. D. Boyer, K. E. Ugen, and D. B. Weiner. 2001. Protection from immunodeficiency virus challenges in rhesus macaques by multicomponent DNA immunization. Virology 285:204-217. [DOI] [PubMed] [Google Scholar]
- 31.Klein, M. R., C. A. van Baalen, A. M. Holwerda, S. R. Kerkhof Garde, R. J. Bende, I. P. Keet, J. K. Eeftinck-Schattenkerk, A. D. Osterhaus, H. Schuitemaker, and F. Miedema. 1995. Kinetics of Gag-specific cytotoxic T lymphocyte responses during the clinical course of HIV-1 infection: a longitudinal analysis of rapid progressors and long-term asymptomatics. J. Exp. Med. 181:1365-1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Konvalinka, J., M. A. Litterst, R. Welker, H. Kottler, F. Rippmann, A. M. Heuser, and H. G. Kräusslich. 1995. An active-site mutation in the human immunodeficiency virus type 1 proteinase (PR) causes reduced PR activity and loss of PR-mediated cytotoxicity without apparent effect on virus maturation and infectivity. J. Virol. 69:7180-7186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koup, R. A., J. T. Safrit, Y. Cao, C. A. Andrews, G. McLeod, W. Borkowsky, C. Farthing, and D. D. Ho. 1994. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J. Virol. 68:4650-4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kozak, M. 1991. An analysis of vertebrate mRNA sequences: intimations of translational control. J. Cell Biol. 115:887-903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leggatt, G. R., A. Hosmalin, C. D. Pendleton, A. Kumar, S. Hoffman, and J. A. Berzofsky. 1998. The importance of pairwise interactions between peptide residues in the delineation of TCR specificity. J. Immunol. 161:4728-4735. [PubMed] [Google Scholar]
- 36.Letvin, N., D. Montefiori, Y. Yasutomi, H. Perry, M. Davies, C. Lekutis, M. Alroy, D. Freed, C. Lord, L. Handt, M. Liu, and J. Shiver. 1997. Potent, protective anti-HIV immune responses generated by bimodal HIV envelope DNA plus protein vaccination. Proc. Natl. Acad. Sci. USA 94:9378-9383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mascola, J. R., M. G. Lewis, G. Stiegler, D. Harris, T. C. VanCott, D. Hayes, M. K. Louder, C. R. Brown, C. V. Sapan, S. S. Frankel, Y. Lu, M. L. Robb, H. Katinger, and D. L. Birx. 1999. Protection of Macaques against pathogenic simian/human immunodeficiency virus 89.6PD by passive transfer of neutralizing antibodies. J. Virol. 73:4009-4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O'Hagan, D., M. Singh, M. Ugozzoli, C. Wild, S. Barnett, M. Chen, M. Schaefer, B. Doe, G. R. Otten, and J. B. Ulmer. 2001. Induction of potent immune responses by cationic microparticles with adsorbed human immunodeficiency virus DNA vaccines. J. Virol. 75:9037-9043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Palaniappan, C., M. Wisniewski, P. S. Jacques, S. F. Le Grice, P. J. Fay, and R. A. Bambara. 1997. Mutations within the primer grip region of HIV-1 reverse transcriptase result in loss of RNase H function. J. Biol. Chem. 272:11157-11164. [DOI] [PubMed] [Google Scholar]
- 40.Park, J., and C. D. Morrow. 1991. Overexpression of the gag-pol precursor from human immunodeficiency virus type 1 proviral genomes results in efficient proteolytic processing in the absence of virion production. J. Virol. 65:5111-5117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Patel, P. H., A. Jacobomolina, J. P. Ding, C. Tantillo, A. D. Clark, R. Raag, R. G. Nanni, S. H. Hughes, and E. Arnold. 1995. Insights into DNA polymerization mechanisms from structure and function analysis of HIV-1 reverse transcriptase. Biochemistry 34:5351-5363. [DOI] [PubMed] [Google Scholar]
- 42.Polacino, P., V. Stallard, D. C. Montefiori, C. R. Brown, B. A. Richardson, W. R. Morton, R. E. Benveniste, and S.-L. Hu. 1999. Protection of macaques against intrarectal infection by a combination immunization regimen with recombinant simian immunodeficiency virus SIVmne gp160 vaccines. J. Virol. 73:3134-3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Richmond, J. F., S. Lu, J. C. Santoro, J. Weng, S. L. Hu, D. C. Montefiori, and H. L. Robinson. 1998. Studies of the neutralizing activity and avidity of anti-human immunodeficiency virus type 1 Env antibody elicited by DNA priming and protein boosting. J. Virol. 72:9092-9100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rosenberg, E. S., J. M. Billingsley, A. M. Caliendo, S. L. Boswell, P. E. Sax, S. A. Kalams, and B. D. Walker. 1997. Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science 278:1447-1450. [DOI] [PubMed] [Google Scholar]
- 45.Ross, R. W., M. E. Wright, and J. A. Tavel. 2001. Ongoing trials of immune-based therapies for HIV infection in adults. Exp. Opin Biol. Ther. 1:413-424. [DOI] [PubMed] [Google Scholar]
- 46.Rowland-Jones, S., J. Sutton, K. Ariyoshi, T. Dong, F. Gotch, S. McAdam, D. Whitby, S. Sabally, A. Gallimore, T. Corrah, et al. 1995. HIV-specific cytotoxic T-cells in HIV-exposed but uninfected Gambian women. Nat. Med. 1:59-64. [DOI] [PubMed] [Google Scholar]
- 47.Rowland-Jones, S. L., T. Dong, K. R. Fowke, J. Kimani, P. Krausa, H. Newell, T. Blanchard, K. Ariyoshi, J. Oyugi, E. Ngugi, J. Bwayo, K. S. MacDonald, A. J. McMichael, and F. A. Plummer. 1998. Cytotoxic T cell responses to multiple conserved HIV epitopes in HIV-resistant prostitutes in Nairobi. J. Clin. Investig. 102:1758-1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schneider, R., M. Campbell, G. Nasioulas, B. K. Felber, and G. N. Pavlakis. 1997. Inactivation of the human immunodeficiency virus type 1 inhibitory elements allows Rev-independent expression of Gag and Gag/protease and particle formation. J. Virol. 71:4892-4903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shiver, J. W., T. M. Fu, L. Chen, D. R. Casimiro, M. E. Davies, R. K. Evans, Z. Q. Zhang, A. J. Simon, W. L. Trigona, S. A. Dubey, L. Huang, V. A. Harris, R. S. Long, X. Liang, L. Handt, W. A. Schleif, L. Zhu, D. C. Freed, N. V. Persaud, L. Guan, K. S. Punt, A. Tang, M. Chen, K. A. Wilson, K. B. Collins, G. J. Heidecker, V. R. Fernandez, H. C. Perry, J. G. Joyce, K. M. Grimm, J. C. Cook, P. M. Keller, D. S. Kresock, H. Mach, R. D. Troutman, L. A. Isopi, D. M. Williams, Z. Xu, K. E. Bohannon, D. B. Volkin, D. C. Montefiori, A. Miura, G. R. Krivulka, M. A. Lifton, M. J. Kuroda, J. E. Schmitz, N. L. Letvin, M. J. Caulfield, A. J. Bett, R. Youil, D. C. Kaslow, and E. A. Emini. 2002. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature 415:331-335. [DOI] [PubMed] [Google Scholar]
- 50.Srivastava, I. K., L. Stamatatos, H. Legg, E. Kan, A. Fong, S. R. Coates, L. Leung, M. Wininger, J. J. Donnelly, J. B. Ulmer, and S. W. Barnett. 2002. Purification and characterization of oligomeric envelope glycoprotein from a primary R5 subtype B human immunodeficiency virus. J. Virol. 76:2835-2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Strack, P. R., M. W. Frey, C. J. Rizzo, B. Cordova, H. J. George, R. Meade, S. P. Ho, J. Corman, R. Tritch, and B. D. Korant. 1996. Apoptosis mediated by HIV protease is preceded by cleavage of Bcl-2. Proc. Natl. Acad. Sci. USA 93:9571-9576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Widera, G., M. Austin, D. Rabussay, C. Goldbeck, S. W. Barnett, M. Chen, L. Leung, G. R. Otten, K. Thudium, M. J. Selby, and J. B. Ulmer. 2000. Increased DNA vaccine delivery and immunogenicity by electroporation in vivo. J. Immunol. 164:4635-4640. [DOI] [PubMed] [Google Scholar]
- 53.Wilson, W., M. Braddock, S. E. Adams, P. D. Rathjen, S. M. Kingsman, and A. J. Kingsman. 1988. HIV expression strategies: ribosomal frameshifting is directed by a short sequence in both mammalian and yeast systems. Cell 55:1159-1169. [DOI] [PubMed] [Google Scholar]
- 54.Yu, X. G., M. M. Addo, E. S. Rosenberg, W. R. Rodriguez, P. K. Lee, C. A. Fitzpatrick, M. N. Johnston, D. Strick, P. J. Goulder, B. D. Walker, and M. Altfeld. 2002. Consistent patterns in the development and immunodominance of human immunodeficiency virus type 1 (HIV-1)-specific CD8+ T-cell responses following acute HIV-1 infection. J. Virol. 76:8690-8701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yu, X. G., H. Shang, M. M. Addo, R. L. Eldridge, M. N. Phillips, M. E. Feeney, D. Strick, C. Brander, P. J. Goulder, E. S. Rosenberg, B. D. Walker, and M. Altfeld. 2002. Important contribution of p15 Gag-specific responses to the total Gag-specific CTL responses. AIDS 16:321-328. [DOI] [PubMed] [Google Scholar]
- 56.zur Megede, J., M. C. Chen, B. Doe, M. Schaefer, C. E. Greer, M. Selby, G. R. Otten, and S. W. Barnett. 2000. Increased expression and immunogenicity of sequence-modified human immunodeficiency virus type 1 gag gene. J. Virol. 74:2628-2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zybarth, G., and C. Carter. 1995. Domains upstream of the protease (PR) in human immunodeficiency virus type 1 Gag-Pol influence PR autoprocessing. J. Virol. 69:3878-3884. [DOI] [PMC free article] [PubMed] [Google Scholar]