Abstract
Cellular immune responses, particularly those associated with CD3+ CD8+ cytotoxic T lymphocytes (CTL), play a primary role in controlling viral infection, including persistent infection with human immunodeficiency virus type 1 (HIV-1). Accordingly, recent HIV-1 vaccine research efforts have focused on establishing the optimal means of eliciting such antiviral CTL immune responses. We evaluated several DNA vaccine formulations, a modified vaccinia virus Ankara vector, and a replication-defective adenovirus serotype 5 (Ad5) vector, each expressing the same codon-optimized HIV-1 gag gene for immunogenicity in rhesus monkeys. The DNA vaccines were formulated with and without one of two chemical adjuvants (aluminum phosphate and CRL1005). The Ad5-gag vector was the most effective in eliciting anti-Gag CTL. The vaccine produced both CD4+ and CD8+ T-cell responses, with the latter consistently being the dominant component. To determine the effect of existing antiadenovirus immunity on Ad5-gag-induced immune responses, monkeys were exposed to adenovirus subtype 5 that did not encode antigen prior to immunization with Ad5-gag. The resulting anti-Gag T-cell responses were attenuated but not abolished. Regimens that involved priming with different DNA vaccine formulations followed by boosting with the adenovirus vector were also compared. Of the formulations tested, the DNA-CRL1005 vaccine primed T-cell responses most effectively and provided the best overall immune responses after boosting with Ad5-gag. These results are suggestive of an immunization strategy for humans that are centered on use of the adenovirus vector and in which existing adenovirus immunity may be overcome by combined immunization with adjuvanted DNA and adenovirus vector boosting.
AIDS, caused by human immunodeficiency virus type 1 (HIV-1) infection, has become one of the largest infectious disease killers in the world, with over 24 million deaths to date and over 40 million people infected worldwide (http://www.unaids.org/barcelona/presskit/barcelona%20report/Global_estimate.pdf). The development of an effective HIV-1 vaccine has become increasingly important as the only means to arrest this growing epidemic. Recent studies in both humans and nonhuman primates suggest that cytotoxic T lymphocytes (CTL) control virus replication and delay disease progression (1, 3, 5, 15, 22, 23, 25). Given the relative conservation of the viral internal structural proteins, such as Gag, these antigens potentially represent a set of broadly recognized targets for a vaccine-elicited cellular immune response (4, 12, 17).
Host T-cell immunity against HIV-1 can, in principle, be induced by immunizing with a vector that can mediate the expression of selected HIV-1 genes in the appropriate antigen-presenting cells. The immunogenic potential in laboratory animals of several DNA and recombinant viral vectors and combinations thereof has been described in numerous reports (2, 3, 6, 8, 10, 13, 20, 21). Recently, we compared the efficacy of a DNA vector and two viral vectors, modified vaccinia virus Ankara (MVA) and replication-defective adenovirus serotype 5 that expressed a simian immunodeficiency virus (SIV) Gag protein to protect monkeys against simian-human immunodeficiency virus (SHIV) challenge (25). The adenovirus type 5 vector proved to be the most immunogenic for elicitation of a cellular immune response that effectively mitigated the pathogenic immunodeficiency virus challenge.
In the current report, we expand on this work by examining the immunogenicity in nonhuman primates of a clinically relevant HIV-1 gag gene delivered with a DNA plasmid vector and a recombinant MVA vector as well as an adenovirus type 5 vector. The data from these studies served as a basis for choosing vectors and immunization strategies for human clinical trials.
MATERIALS AND METHODS
Vaccine vectors.
A synthetic gene for Gag from HIV-1 CAM-1 was previously constructed with codons frequently used in humans (14, 16). The gene was inserted into the V1Jns plasmid (V1Jns-gag) under the control of the human cytomegalovirus-human intron A promoter and bovine growth hormone terminator (8, 26). V1Jns-gag was formulated at 5 mg/ml in normal phosphate-buffered saline or with selected adjuvants: (i) 7.5 to 45 mg of a nonionic block copolymer (CRL1005; CytRx Corp., Atlanta, Ga.) per ml or (ii) 700 μg of aluminum phosphate (alum from HCI Biosector) per ml.
The same synthetic gag gene was used for construction of the adenovirus type 5 vector. The adenovirus type 5 construct is a prototypic first-generation vector containing an E1 deletion replaced with the gag expression cassette. Since the E1 deletion renders it replication defective, the vector was propagated in 293 or PER.C6 (Crucell) cells, which provided E1 in trans. Recombinant virus was generated in the following manner (25): (i) cloning the expression cassette consisting of the human cytomegalovirus immediate-early promoter region, gag, and bovine growth hormone polyadenylation sequence into an E1 shuttle vector; (ii) homologous bacterial recombination to form the preadenoviral plasmid; and (iii) viral rescue in either 293 or PER.C6 cells. The virus yield was quantified by the Maizel spectroscopic method (18). Typical Ad5-gag preparations have 1 infectious particle for every 50 to 100 viral particles.
Vectors for generating recombinant MVA viruses (9) were generously provided by B Moss and V. M. Hirsch (National Institutes of Health); the method for generating the viruses has been described elsewhere (27). Briefly, gag was cloned into the pSC59 shuttle vector, which was designed to insert the transgene fragment into the viral thymidine kinase region and to drive transgene expression with a highly effective synthetic early-late vaccinia virus promoter (9). The virus was propagated in primary specific-pathogen-free chicken embryo fibroblasts; virus titers were measured by plaque assay on the same cell line.
Immunization.
Rhesus macaques used in all studies were between 3 and 10 kg in weight. It should be noted that in any given study, the monkeys were evenly distributed between cohorts on the basis of their body weights. In all cases, the total vaccine dose was suspended in 1 ml of phosphate-buffered saline. The macaques were anesthetized (ketamine-xylazine), and the vaccines were delivered intramuscularly in 0.5-ml aliquots into both deltoid muscles with tuberculin syringes (Becton-Dickinson, Franklin Lakes, N.J.). Sera and peripheral blood mononuclear cells (PBMCs) were prepared from blood samples collected at several time points during the immunization regimen. All animal care and treatment were in accordance with standards approved by the Institutional Animal Care and Use Committee according to the principles set forth in the Guide for Care and Use of Laboratory Animals, Institute of Laboratory Animal Resources, National Research Council.
Elispot and CTL assays.
The gamma interferon (IFN-γ) Elispot assays for rhesus macaques were conducted following a previously described protocol (7). For antigen-specific stimulation, a peptide pool was prepared from 20-amino-acid peptides that encompassed the entire HIV-1 Gag sequence with 10-amino-acid overlaps (Synpep Corp., Dublin, Calif.). To each well, 50 μl containing 2 × 105 to 4 × 105 PBMCs was added; the cells were counted with a Beckman Coulter Z2 particle analyzer with a lower size cutoff set at 80 fl. Either 50 μl of medium or the Gag peptide pool at 8 μg/ml concentration per peptide was added to the PBMCs. Mock wells were laid by adding the same final concentration of peptide solvent (1% dimethyl sulfoxide) to the cells. All samples were incubated at 37°C in 5% CO2 for 20 to 24 h. Spots were developed accordingly and counted under a dissecting microscope; the counts were normalized to 106 cell input.
The 51C -release CTL assay for rhesus macaques was conducted following a protocol described earlier with some modifications (11). Specifically, effector cells were prepared from a 2-week-long incubation with vaccinee PBMCs infected previously with recombinant vaccinia virus expressing the same synthetic gag gene (vaccinia virus-gag) or in some cases, with Ad5-gag. The harvested effector cells were tested against autologous B lymphoid cell lines (BLCL) sensitized overnight with the Gag peptide pool (at 4 μg/ml concentration per peptide).
Anti-p24 ELISA.
A modified competitive anti-p24 assay was developed with reagents from the Coulter p24 antigen assay kit (Beckman Coulter, Fullerton, Calif.). Briefly, to 250 μl of serum, 20 μl of lyse buffer and 15 μl of p24 antigen (9.375 pg) from the Coulter kit were added. After mixing, 200 μl of each sample was added to wells coated with a mouse anti-p24 monoclonal antibody from the Coulter kit and incubated for 1.5 h at 37°C. The wells were then washed, and 200 μl per well of biotin reagent (polyclonal anti-p24-biotin) from the Coulter kit was added. After a 1-h, 37°C incubation, detection was achieved with streptavidin-conjugated horseradish peroxidase and tetramethylbenzidine substrate as described in the Coulter kit. The optical density at 450 nm was recorded. A seven-point standard curve was generated with a serial twofold dilution of serum from an HIV-seropositive individual. The lower cutoff for the assay was arbitrarily set at 10 milli-Merck units (mMU)/ml, defined by a dilution of the seropositive human serum. This cutoff falls at approximately 65% of the maximum bound control defined by an HIV-seronegative human serum pool.
Intracellular cytokine staining.
Anti-human CD28 (clone L293; Becton-Dickinson) and anti-human CD49d (clone L25; Becton-Dickinson) monoclonal antibodies were added to a final concentration of 1 μg/ml to a tube containing 2 × 106 PBMCs in 1 ml of complete RPMI medium. Then 10 μl of the Gag 20-mer peptide pool (at 0.4 mg/ml per peptide) or dimethyl sulfoxide was added for antigen-specific or mock stimulation, respectively. The suspensions were incubated at 37°C for 1 h, after which brefeldin A (Sigma) was added to a final concentration of 100 μg/ml. The cells were incubated for 16 h at 37°C in 5% CO2 at 90% humidity. The cells were washed, resuspended in phosphate-buffered saline-2% fetal bovine serum, and stained (30 min, 4°C) for surface markers with several fluorescently tagged monoclonal antibodies: 20 μl per tube of anti-human CD3-allophycocyanin, clone FN-18 (Biosource); 20 μl of anti-human CD8-peridinin chlorophyll protein, clone SK1 (Becton Dickinson); and 20 μl of anti-human CD4-phycoerythrin, clone SK3 (Becton Dickinson). The cells were permeabilized in 750 μl of 1× FACS Perm buffer (Becton Dickinson) for 10 min at room temperature, resuspended in phosphate-buffered saline-2% fetal bovine serum, and stained for 30 min with 0.1 μg of fluorescein isothiocyanate-conjugated anti-human IFN-γ, clone MD-1 (Biosource). Samples were analyzed with all four color channels of the Becton Dickinson FACSCalibur instrument. To analyze the data, the low side and forward scatter CD3+ lymphocyte population was initially gated; a common fluorescence cutoff for cytokine-positive events was used for both the CD4+ and CD8+ populations and for both the mock-stimulated and Gag peptide-stimulated reaction tubes of a sample.
Antiadenovirus neutralization.
Monkey serum was initially diluted 1:5 and then serially diluted 1:3 in serum-free Dulbecco's modified Eagle's medium (Gibco, San Diego, Calif.); equal volumes of the diluted stocks and a 1-PFU/μl stock of the wild-type adenovirus type 5 virus were mixed for 1 h at 37°C. Wells were seeded with HEK-293 cells at 3 × 104 cells/100 μl a day prior and decanted. Then 200 μl of the virus-serum mixes was added to duplicate wells. Following 4 days of incubation at 37°C, 40 μl of MTS solution (Promega, Madison, Wis.) was added to the wells, and the optical density at 490 nm was measured. Endpoint titers corresponded to the last dilution well with purple color.
RESULTS
Immune responses to Ad5-gag vaccine.
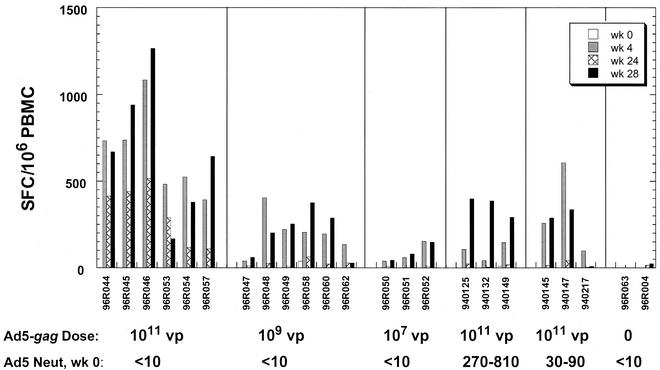
Cohorts of three to six rhesus monkeys were immunized intramuscularly with escalating doses (107, 109, and 1011 viral particles) of Ad5-gag at weeks 0 and 24. The frequencies of HIV-1 Gag-specific T cells were determined by Elispot assay with a peptide pool that encompassed the entire Gag sequence as described in Materials and Methods (Fig. 1). The magnitude of vaccine-induced T-cell responses was dependent on the dose of Ad5-gag, with the best responses observed at 1011 viral particles. These responses peaked at 4 weeks following the first immunization and declined gradually until the second immunization was given at week 24. Anamnestic responses were observed following the boost, with peak levels that were comparable to or exceeded those observed at week 4 (Fig. 1 and 3A).
FIG. 1.
Frequencies of Gag-specific IFN-γ-secreting cells from monkeys immunized with Ad5-gag. These values are expressed as the number of SFC per 106 PBMCs. Naïve monkeys (adenovirus type 5 neutralization (neut) titers < 10) were immunized with various doses of Ad5-gag at weeks 0 and 24. Monkeys that were pretreated with one or three 1010 viral particle (vp) doses of non-Gag-encoding adenovirus type 5 virus (producing neutralization titers of ≈30 to 90 and ≈270 to 810, respectively, at the time of the first Ad5-gag injection) were immunized with 1011 viral particles of Ad5-gag at weeks 0 and 24. PBMCs collected at weeks 0, 4, 24, and 28 were incubated in the absence (mock) or presence of the HIV-1 Gag peptide pool, and the mock-corrected response levels are shown here for each monkey. Mock-treated background responses averaged 3 SFC per 106 PBMCs (standard deviation, ≈5 SFC per 106 PBMCs).
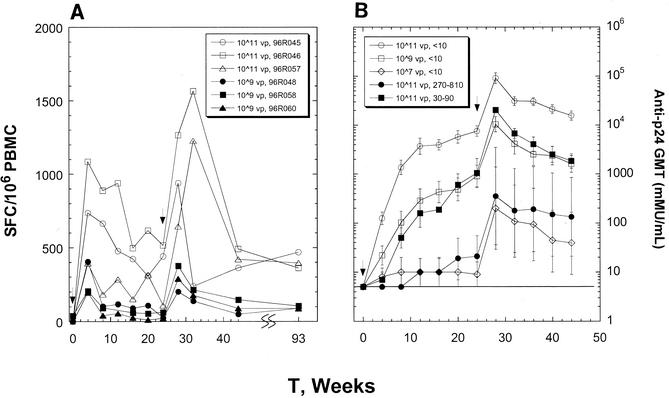
FIG. 3.
Time course of induction of Gag-specific T-cell responses from PBMCs of representative monkeys that received 1011 viral particles and 109 viral particles of Ad5-gag at week 0 and week 24 (A). Mock responses were subtracted to give the reported levels shown here. Kinetics of induction of p24-specific antibodies as a function of the Ad5-gag dose and adenovirus type 5 preexposure (B). The geometric mean titers (GMT) are shown with standard errors for each cohort of either three or six monkeys. Each curve is defined by the Ad5-gag dose (viral particles, vp) and adenovirus type 5 neutralization titers measured at the start of the immunization. Titers below the dection limit of 10 mMU/ml are scored at 5 mMU/ml (line).
To address the effects of preimmunity to adenovirus on immunization with the Ad5-gag vector, two additional groups of monkeys were given one or three injections of 1010 viral particles of an adenovirus type 5 virus that did not encode the vaccine antigen prior to administration of 1011 viral particles of Ad5-gag. Serum anti-adenovirus type 5 neutralization titers resulting from the preexposure ranged from 30 to 90 and from 270 to 810 for the single and multiple exposures of the irrelevant adenovirus type 5 vector, respectively. These levels appear typical of the range of antiadenovirus neutralizing antibody titers observed in adult, North American humans (our unpublished results). The presence of existing vector-specific immunity in the animals resulted in a partial reduction but not total ablation of the Gag-specific cellular immune response following immunization with two doses of Ad5-gag. These responses were comparable to those observed with the 109 virus particle dose of Ad5-gag in animals with no existing immunity to the vector (Fig. 1).
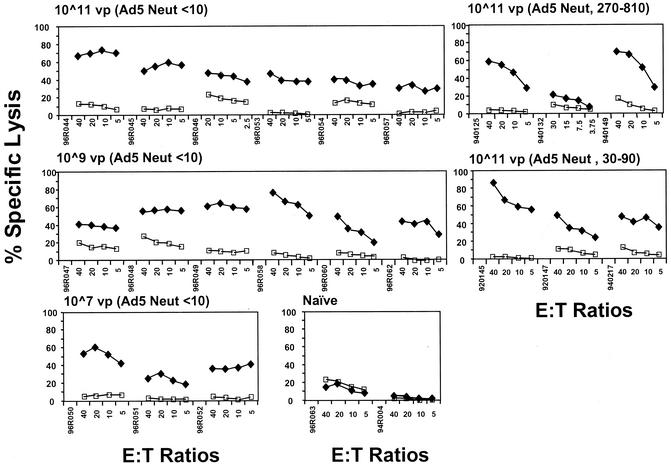
The T-cell responses observed against the Gag antigen in the experimental animals exhibited functional, cytotoxic activities in CTL assays. All vaccinees, including those with established adenovirus type 5-specific immunity prior to Ad5-gag immunization, exhibited moderate to strong cytotoxicity against autologous Gag peptide-pulsed BLCL after a single immunization (Fig. 2). Each animal was tested for CTL responses on a monthly basis for approximately a year following the first immunization. Cytotoxic responses were notably maintained in each animal throughout this period (data not shown).
FIG. 2.
CTL responses in monkeys immunized with Ad5-gag. Peptide-pulsed autologous BLCLs were used as target cells at the indicated effector-to-target cell (E:T) ratios, treated with PBMCs (collected at week 8) from Ad5-gag vaccinees restimulated for 2 weeks with gag-expressing vaccinia virus. The percentages of lysed target cells are shown for unpulsed cells (open squares) and cells pulsed with the HIV-1 Gag peptide pool (solid diamonds).
The stability of both T-cell and B-cell immune responses was observed throughout an extended period of time. Figure 3 shows longitudinal profiles of anti-Gag Elispot and anti-p24 Gag antibody responses. The levels of antigen-specific T cells remained significant for as long as a year following the booster. Ad5-gag also elicited substantial levels of circulating p24-specific antibodies (Fig. 3B). Based on the elicited humoral immunity against Gag, the animals that had preestablished adenovirus type 5 neutralization titers of 30 to 90 appeared to respond to the 1011 viral particle dose, similar to adenovirus-naïve monkeys that received 109 viral particles of Ad5-gag. Anti-Gag antibody titers induced by 1011 viral particles of Ad5-gag in animals with preimmunization neutralization titers of 270 to 810 were comparable to those induced by a 107 viral particle dose in naïve animals.
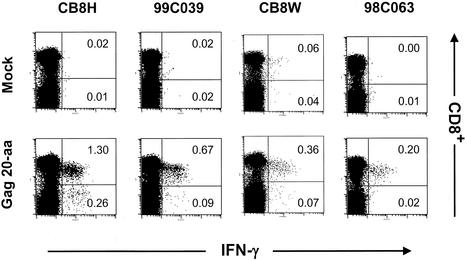
The levels of Gag-specific CD4+ and CD8+ T cells can be determined by intracellular cytokine staining (ICS). Figure 4 shows the staining of PBMCs from a different yet representative set of rhesus macaques immunized with 1011 viral particle doses of Ad5-gag at weeks 0, 4, and 24. In the absence of Gag antigen stimulation, very little IFN-γ production was observed (<0.03% of CD3+ T cells). Upon overnight stimulation with the Gag peptide pool, cytokine production was detected from both CD8+ and CD4+ T cells. Substantially more Gag-specific CD8+ T cells than CD4+ T cells were observed in each animal. These data also support the observation of pronounced cytotoxic activities of T cells derived from Ad5-gag vaccinees.
FIG. 4.
Intracellular IFN-γ staining of PBMCs from Ad5-gag vaccinees following overnight incubation in medium alone (mock) or in medium plus the Gag peptide pool (Gag 20-aa). CD3+ lymphocytes are shown and were characterized for CD8+ staining and IFN-γ production. Numbers reflect the percentage of CD3+ lymphocytes that were CD8+ IFN-γ+ (upper right quadrant) or CD4+ IFN-γ+ (lower right quadrant).
Comparison of Ad5-gag and MVA-gag.
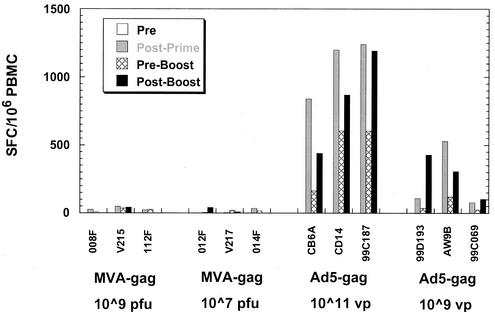
We compared the immunogenicity of the adenovirus type 5 vector to that of a recombinant MVA expressing the identical HIV-1 gag gene. MVA is considered a standard for the elicitation of cellular immune responses (2, 24, 25). Cohorts of three naïve monkeys were immunized with three doses of recombinant MVA expressing gag (at either 107 or 109 PFU) or Ad5-gag (at 1011 or 109 viral particles). The MVA vector had previously been confirmed for in vitro Gag expression in chicken embryo fibroblast cells and for Gag-specific immunogenicity in BALB/c mice (data not shown). Immunization of macaques with MVA-gag resulted in relatively weak antigen-specific T-cell responses; the levels did not exceed 150 spot-forming cells (SFC)/106 PBMCs after three doses (Fig. 5) and were significantly less than those observed in the Ad5-gag vaccinees.
FIG. 5.
Frequencies of Gag-specific IFN-γ-secreting cells from monkeys immunized with Ad5-gag and MVA-gag. Priming immunizations were administered at weeks 0 and 4, followed by a booster shot at week 24 with the same virus. Shown are the mock-corrected levels against the Gag peptide pool prior to the first dose (pre), 4 weeks after the second dose (post prime), time of the boost (preboost), and 4 weeks after the viral boost (postboost) for each animal.
It should be noted that two of three macaques that received MVA-gag at the higher dose (109 PFU) showed increased spot counts in their mock reaction mixes (109 to 238 SFC/106 PBMCs) as early as 4 weeks after the first dose (data not shown). In contrast to adenovirus type 5-treated monkeys, effector cells from the MVA-gag vaccinees generated by PBMCs restimulation with either vaccinia virus-gag or Ad5-gag did not exhibit any detectable bulk killing of Gag peptide pool-pulsed autologous BLCL at any time point (data not shown). Only one of six MVA-gag vaccinees elicited any detectable Gag-specific antibody response (140 mMU/ml at 4 weeks post-dose 3 for monkey V215).
Immune responses following DNA-adjuvant priming and viral boosting.
A potential limitation in the use of common adenovirus serotype vectors in human subjects may be the negative effect of existing neutralizing immunity against the viral vehicle, as observed in the preexposed monkeys (Fig. 1). Hence, we explored the possibility of priming the immune responses with HIV-1 gag delivered by an immunologically inert vehicle such as DNA. The primates were then given a booster immunization with a low dose of Ad5-gag (107 viral particles) selected to mimic the effect of existing adenovirus type 5-neutralizing immunity on a 1011 viral particle dose of Ad5-gag (Fig. 1 and 3B).
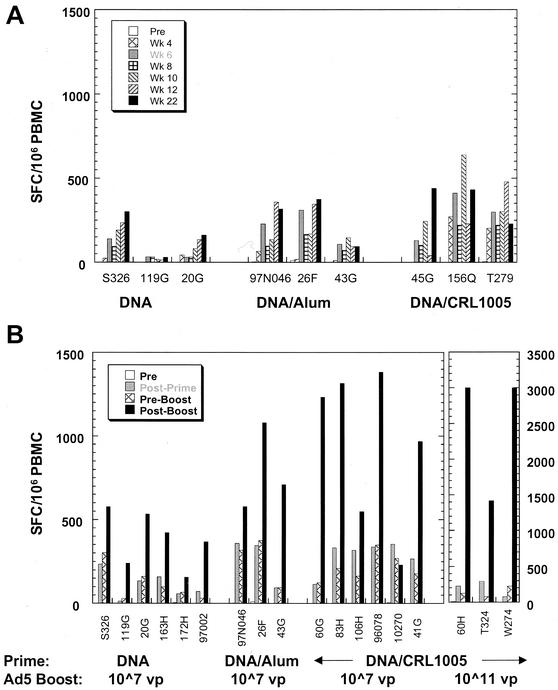
Cohorts of three to six macaques were given priming immunizations of 5.0 mg of gag-expressing DNA plasmid either in saline or formulated with adjuvants at weeks 0, 4, and 8. The DNA vector in saline was found to be immunogenic in macaques, with maximal postpriming responses averaging 111 ± 32 SFC/106 PBMCs (Fig. 6). Formulation of DNA with the CRL1005-based adjuvant resulted in much improved T-cell responses to HIV-1 Gag (322 ± 46 SFC/106 PBMCs), while the DNA-alum formulation elicited intermediate responses. The subsequent Ad5-gag booster immunization resulted in consistent increases in T-cell frequencies to levels higher than the postpriming peak values. The best responses following the boost were observed in the animals that were primed with the DNA-CRL1005 vaccine. The peak responses in these animals reached levels of between 1,000 and 1,500 SFC/106 PBMCs. These response levels were comparable to those observed with multiple high doses (1011 viral particles) of Ad5-gag in previously unexposed animals (Fig. 1).
FIG. 6.
Frequencies of Gag-specific IFN-γ-secreting cells from PBMCs of macaques immunized following various DNA prime-adenovirus type 5 boost regimens. (A) Macaques were given multiple doses of 5 mg of V1Jns-gag DNA intramuscularly in phosphate-buffered saline, with aluminum phosphate (alum) or with CRL1005 (7.5 mg) at weeks 0, 4, and 8. Shown are levels of antigen-specific T cells (mock corrected) at different time points during and after the priming immunizations. (B) Macaques were treated intramuscularly with three doses (weeks 0, 4, and 8) of 5 mg of V1Jns-gag with and without adjuvants (CRL1005 at 7.5 or 22.5 mg) and boosted with an intramuscular dose of Ad5-gag (107 viral particles or 1011 viral particles) at weeks 24 to 26. Shown are the mock-corrected levels prior to the first dose (pre), 4 weeks after the third dose (post prime), time of the boost (preboost), and 4 weeks after the viral boost (postboost) for each animal.
All six animals given nonadjuvanted DNA had maximum responses of ≈600 SFC/106 PBMCs following the booster immunization. Gag-specific antibody levels in all animals increased by 100-fold following boosting with Ad5-gag; the postboost levels in DNA-CRL1005- and DNA-alum-primed animals were also five- to eightfold elevated compared to those of DNA-primed animals (data not shown). The noted increases in immune responses with the DNA-adenovirus type 5 combination vaccine strongly suggest synergy between DNA and adenovirus type 5 modalities, given that this low adenovirus type 5 dose is capable of eliciting only weak responses when used alone in naïve monkeys (Fig. 1 and 3B). To determine the maximum potency of the DNA prime-adenovirus type 5 boost regimen, animals primed with the DNA-CRL1005-based vaccine were boosted with 1011 viral particles of Ad5-gag. The responses exceeded 2,500 SFC/106 PBMCs in two of three animals.
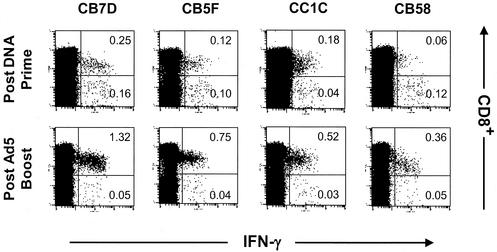
The phenotypes of the vaccine-induced anti-Gag T lymphocytes after the priming or booster immunization can be monitored by ICS methods. Figure 7 shows the results of such analyses for a representative cohort of macaques immunized with DNA-CRL1005 followed by the low-dose Ad5-gag booster. Analyses of PBMCs collected before the booster dose revealed that the ratios of antigen-specific CD4+/CD8+ T cells can vary, ranging from CD4+- to CD8+-biased population. However, after boosting with Ad5-gag, a pronounced CD8+-biased response was observed in all animals. Hence, DNA-CRL1005-adenovirus type 5 prime-boost immunization appeared to result in cellular immune responses that were comparable to those induced by multiple high doses of Ad5-gag alone on the basis of levels and CD4+/CD8+ distribution of antigen-specific T cells.
FIG. 7.
Intracellular IFN-γ staining of PBMCs from a representative cohort of macaques immunized with three doses of V1jns-gag formulated with CRL1005-based adjuvant (weeks 0, 4, and 8) followed by 107 viral particles of Ad5-gag boost (week 24). Samples were collected at week 10 (postprime) and week 28 (postboost). Shown are CD3+ lymphocytes following incubation with the HIV-1 Gag peptide pool that were characterized for CD8+ staining and IFN-γ production. For all samples, intracellular IFN-γ production in the absence of the peptide pool was minimal (data not shown). Numbers reflect the percentage of CD3+ lymphocytes that were CD8+ IFN-γ+ (upper right quadrant) or CD4+ IFN-γ+ (lower right quadrant) after subtraction of the mock levels.
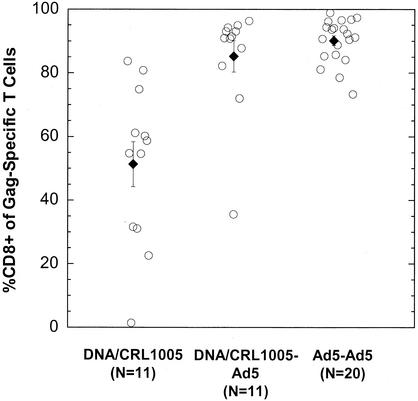
In order to corroborate the trends in the relative CD4+/CD8+ contributions to the T-cell responses observed from the limited cohort sizes described above, we collected data from several other immunization studies and summarize these results in Fig. 8 along with those described above. Homologous immunization with Ad5-gag at 109 and 1011 viral particle doses (administered at weeks 0, 4, and 24) elicited antigen-specific T-lymphocyte populations with CD8+ T-cell compositions ranging from 73% to 99% (mean ± standard error, 90% ± 2%); the CD8+ composition was not influenced by the dose used. In contrast, priming immunizations with DNA-CRL1005 produced a highly variable (2% to 84%) but generally balanced CD8+/CD4+ distribution (mean ± standard error, 51% ± 7.25%). The administration of an Ad5-gag boost to the same animals resulted in Gag-specific T-cell populations that were 35% to 97% CD8+ (mean ± standard error, 85% ± 4%).
FIG. 8.
Percentages of Gag-specific T cells that are CD3+ CD8+ in rhesus macaques immunized with various regimens. The adenovirus type 5 prime-adenovirus type 5 boost (Ad5-Ad5) cohort consisted of animals given two priming doses of the vector (109 or 1011 viral particles) followed by a booster shot of the same dose. The DNA-CRL1005 prime-adenovirus type 5 boost cohort comprised animals given three priming immunizations with 5 mg of V1Jns-gag formulated with CRL1005-based adjuvant, followed by 107 viral particles of Ad5-gag. The percentage values are reported for this cohort of 11 animals before (DNA-CRL1005) and after the boost (DNA-CRL1005-Ad5). The dark diamonds and bars are the cohort arithmetic means and standard errors of the mean, respectively. These data points include those for the animals in Fig. 4 and 7.
DISCUSSION
There is increasing interest in the use of viral and nonviral systems as vectors to elicit anti-HIV-1 immune responses. The human clinical testing of these vectors can be guided by results of comparative studies in appropriate nonhuman primate immunogenicity and challenge model systems. Recently, we reported on the ability of SIV Gag delivered by DNA, MVA, or adenovirus type 5 vectors to inhibit viral replication and disease progression in rhesus macaques following challenge with SHIV89.6P (25). The best degree of viremia control and CD4 cell count preservation was observed in animals that had been immunized with the adenovirus type 5 vector either by itself or in a prime-boost combination with DNA. In contrast, only 50% of the animals that received MVA alone or in combination with DNA were able to effectively control viremia.
In this report, we evaluated the cellular immune responses to HIV-1 Gag induced by similar DNA, MVA, and adenovirus vectors. The studies were conducted with macaques that were unselected on the basis of HLA haplotype. Our results showed that adenovirus type 5-mediated gene transfer provided an extremely potent means of inducing anti-HIV-1 T-cell responses, specifically CTL. Both antigen-specific CD4+ and CD8+ T cells were observed in the adenovirus type 5 vaccinees; the majority of responding cells were of the CD8+ phenotype, consistent with strong CTL activities. The induced CTL responses were long-lived in macaques, suggesting that substantial quantities of antigen-specific CD4+ T cells may not be necessary to generate stable responses (19, 29). The DNA vectors were notably less immunogenic, although immunogenicity was enhanced by formulation with adjuvants.
We found that immunization with the MVA vector as a single modality elicited minimal cellular immune responses. These results may appear unexpected given that MVA expressing SIV Gag has been reported to generate pronounced levels of CD8+ T cells directed against the immunodominant CM9 Gag epitope in monkeys expressing the MamuA*01+ HLA haplotype (2, 24, 25). Consistent with our observations, Franchini and coworkers observed Gag-specific CTL killing in only 1 of 18 (6%) mamuA*01− macaques immunized with multiple doses of another poxvirus (ALVAC) expressing SIV gag-pol-env (20). Our results likely reflect the outbred nature of the animals used in our study in which the potency of the immune response is not enhanced by being focused on a single immunogenic determinant.
The immunogenicity of a vaccine based on a common adenovirus serotype can be negatively influenced by preexisting immunity directed at the viral vector itself. In order to quantitatively address the effect of adenovirus type 5-specific immunity on HIV-1 Gag immunogenicity, anti-adenovirus type 5 serum antibody titers representative of the levels observed in humans were established in monkeys prior to immunization with the adenovirus type 5 vector. The presence of such high neutralizing activities only reduced, but did not abolish anti-Gag T-cell responses. Moreover, priming the immune system for anti-Gag responses with multiple doses of the DNA-CRL1005-based vaccine followed by low-dose adenovirus type 5 boosting resulted in levels of circulating Gag-specific T cells and CD4+/CD8+ distribution profiles that closely resembled those obtained when with high doses of the adenovirus type 5 vaccine in adenovirus type 5-seronegative animals. Similar effects have been very recently reported with vectors expressing the Ebola virus glycoprotein antigen (28). These data strongly suggest that the synergy observed between these two vaccines presents a viable strategy for circumventing the negative effects of high adenovirus type 5 immunity in humans.
In summary, these studies suggest the potential utility of adenovirus-vectored vaccines for producing cellular immunity in humans. Clinical trials have been designed based on the observations reported here. These trials will assess the tolerability and immunogenic potential in humans of adenovirus type 5 vectors expressing the HIV-1 Gag protein, either alone or in prime-boost regimens with adjuvanted DNA vectors.
Acknowledgments
We gratefully acknowledge Robert Druilhet and Rachel Colligan of the New Iberia Research Center for contributions to this research and Bernard Moss and Vanessa Hirsch of the NIH for providing the MVA vectors.
REFERENCES
- 1.Amara, R. R., F. Villinger, J. D. Altman, S. L. Lydy, S. P. O'Neil, S. I. Staprans, D. C. Montefiori, Y. Xu, J. G. Herndon, L. S. Wyatt, M. A. Candido, N. L. Kozyr, P. L. Earl, J. M. Smith, H. L. Ma, B. D. Grimm, M. L. Hulsey, J. Miller, H. M. McClure, J. M. McNicholl, B. Moss, and H. L. Robinson. 2001. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science 292:69-74. [DOI] [PubMed] [Google Scholar]
- 2.Barouch, D. H., S. Santra, M. J. Kuroda, J. E. Schmitz, R. Plishka, A. Buckler White, A. E. Gaitan, R. Zin, J. H. Nam, L. S. Wyatt, M. A. Lifton, C. E. Nickerson, B. Moss, D. C. Montefiori, V. M. Hirsch, and N. L. Letvin. 2001. Reduction of simian-human immunodeficiency virus 89.6P viremia in rhesus monkeys by recombinant modified vaccinia virus Ankara vaccination. J. Virol. 75:5151-5158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barouch, D. H., S. Santra, J. E. Schmitz, M. J. Kuroda, T. M. Fu, W. Wagner, M. Bilska, A. Craiu, X. X. Zheng, G. R. Krivulka, K. Beaudry, M. A. Lifton, C. E. Nickerson, W. L. Trigona, K. Punt, D. C. Freed, L. Guan, S. Dubey, D. Casimiro, A. Simon, M. E. Davies, M. Chastain, T. B. Strom, R. S. Gelman, D. C. Montefiori, and M. G. Lewis. 2000. Control of viremia and prevention of clinical AIDS in rhesus monkeys by cytokine-augmented DNA vaccination. Science 290:486-492. [DOI] [PubMed] [Google Scholar]
- 4.Betts, M. R., D. R. Ambrozak, D. C. Douek, S. Bonhoeffer, J. M. Brenchley, J. P. Casazza, R. A. Koup, and L. J. Picker. 2001. Analysis of total human immunodeficiency virus (HIV)-specific CD4+ and CD8+ T-cell responses: relationship to viral load in untreated HIV infection. J. Virol. 75:11983-11991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borrow, P., H. Lewicki, B. H. Hahn, G. M. Shaw, and M. B. Oldstone. 1994. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J. Virol. 68:6103-6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caley, I. J., M. R. Betts, D. M. Irlbeck, N. L. Davis, R. Swanstrom, J. A. Frelinger, and R. E. Johnston. 1997. Humoral, mucosal, and cellular immunity in response to a human immunodeficiency virus type 1 immunogen expressed by a Venezuelan equine encephalitis virus vaccine vector. J. Virol. 71:3031-3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Casimiro, D. R., A. Tang, H. C. Perry, R. S. Long, M. Chen, G. J. Heidecker, M. E. Davies, D. C. Freed, N. V. Persaud, S. Dubey, J. G. Smith, D. Havlir, D. Richman, M. A. Chastain, A. J. Simon, T. M. Fu, E. A. Emini, and J. W. Shiver. 2002. Vaccine-induced immune responses in rodents and nonhuman primates by use of a humanized human immunodeficiency virus type 1 pol gene. J. Virol. 76:185-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caulfield, M. J., S. Wang, J. G. Smith, T. W. Tobery, X. Liu, M.-E. Davies, D. R. Casimiro, T.-M. Fu, A. Simon, R. K. Evans, E. A. Emini, and J. Shiver. 2002. Sustained peptide-specific gamma interferon T-cell response in rhesus macaques immunized with human immunodeficiency virus gag DNA vaccines. J. Virol. 76:10038-10043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chakrabarti, S., J. R. Sisler, and B. Moss. 1997. Compact, synthetic, vaccinia virus early/late promoter for protein expression. BioTechniques 23:1094-1097. [DOI] [PubMed] [Google Scholar]
- 10.Chen, J. D., Q. Yang, A. G. Yang, W. A. Marasco, and S. Y. Chen. 1996. Intra- and extracellular immunization against HIV-1 infection with lymphocytes transduced with an AAV vector expressing a human anti-gp120 antibody. Hum. Gene Ther. 7:1515-1525. [DOI] [PubMed] [Google Scholar]
- 11.Fu, T. M., D. C. Freed, W. L. Trigona, L. Guan, L. Zhu, R. Long, N. V. Persaud, K. Manson, S. Dubey, and J. W. Shiver. 2001. Evaluation of cytotoxic T-lymphocyte responses in human and nonhuman primate subjects infected with human immunodeficiency virus type 1 or simian/human immunodeficiency virus. J. Virol. 75:73-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gea Banacloche, J. C., S. A. Migueles, L. Martino, W. L. Shupert, A. C. McNeil, M. S. Sabbaghian, L. Ehler, C. Prussin, R. Stevens, L. Lambert, J. Altman, C. W. Hallahan, J. C. de Quiros, and M. Connors. 2000. Maintenance of large numbers of virus-specific CD8+ T cells in HIV-infected progressors and long-term nonprogressors. J. Immunol. 165:1082-1092. [DOI] [PubMed] [Google Scholar]
- 13.Hanke, T., R. V. Samuel, T. J. Blanchard, V. C. Neumann, T. M. Allen, J. E. Boyson, S. A. Sharpe, N. Cook, G. L. Smith, D. I. Watkins, M. P. Cranage, and A. J. McMichael. 1999. Effective induction of simian immunodeficiency virus-specific cytotoxic T lymphocytes in macaques by with a multiepitope gene and DNA prime-modified vaccinia virus Ankara boost vaccination regimen. J. Virol. 73:7524-7532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Korber, B., C. Kuiken, B. Foley, B. Hahn, F. McCutchan, J. Mellors, and J. Sodroski. 1998. Human retroviruses and AIDS. Los Alamos National Laboratory, Los Alamos, N.Mex.
- 15.Koup, R. A., J. T. Safrit, Y. Cao, C. A. Andrews, G. McLeod, W. Borkowsky, C. Farthing, and D. D. Ho. 1994. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J. Virol. 68:4650-4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lathe, R. 1985. Synthetic oligonucleotide probes deduced from amino acid sequence data. Theoretical and practical considerations. J. Mol. Biol. 183:1-12. [DOI] [PubMed] [Google Scholar]
- 17.Lubaki, N. M., M. E. Shepherd, R. S. Brookmeyer, H. Hon, T. C. Quinn, M. Kashamuka, M. Johnson, R. Gottle, J. Devers, H. M. Lederman, and R. C. Bollinger. 1999. HIV-1-specific cytolytic T-lymphocyte activity correlates with lower viral load, higher CD4 count, and CD8+CD38−DR− phenotype: comparison of statistical methods for measurement. J. Acquired Immune Defic. Syndr. 22:19-30. [DOI] [PubMed] [Google Scholar]
- 18.Maizel, J. V., D. O. White, and M. D. Scharff. 1968. The polypeptides of adenovirus. I. Evidence for multiple protein components in the virion and a comparison of types 2, 7A, and 12. Virology 36:115-125. [DOI] [PubMed] [Google Scholar]
- 19.Matloubian, M., R. J. Concepcion, and R. Ahmed. 1994. CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection. J. Virol. 68:8056-8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pal, R., D. Venzon, N. L. Letvin, S. Santra, D. C. Montefiori, N. R. Miller, E. Tryniszewska, M. G. Lewis, T. C. VanCott, V. Hirsch, R. Woodward, A. Gibson, M. Grace, E. Dobratz, P. D. Markham, Z. Hel, J. Nacsa, M. Klein, J. Tartaglia, and G. Franchini. 2002. ALVAC-SIV gag-pol-env-based vaccination and macaque major histocompatibility complex class I (A*01) delay simian immunodeficiency virus SIVmac-induced immunodeficiency. J. Virol. 76:292-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pialoux, G., J. L. Excler, Y. Riviere, G. Gonzalez Canali, V. Feuillie, P. Coulaud, J. C. Gluckman, T. J. Matthews, B. Meignier, M. P. Kieny, et al. 1995. A prime-boost approach to HIV preventive vaccine with a recombinant canarypox virus expressing glycoprotein 160 (MN) followed by a recombinant glycoprotein 160 (MN/LAI). The AGIS Group and l'Agence Nationale de Recherche sur le SIDA. AIDS Res. Hum. Retroviruses 11:373-381. [DOI] [PubMed] [Google Scholar]
- 22.Rowland Jones, S. L., T. Dong, L. Dorrell, G. Ogg, P. Hansasuta, P. Krausa, J. Kimani, S. Sabally, K. Ariyoshi, J. Oyugi, K. S. MacDonald, J. Bwayo, H. Whittle, F. A. Plummer, and A. J. McMichael. 1999. Broadly cross-reactive HIV-specific cytotoxic T-lymphocytes in highly exposed persistently seronegative donors. Immunol. Lett. 66:9-14. [DOI] [PubMed] [Google Scholar]
- 23.Schmitz, J. E., M. J. Kuroda, S. Santra, V. G. Sasseville, M. A. Simon, M. A. Lifton, P. Racz, K. Tenner Racz, M. Dalesandro, B. J. Scallon, J. Ghrayeb, M. A. Forman, D. C. Montefiori, E. P. Rieber, N. L. Letvin, and K. A. Reimann. 1999. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 283:857-860. [DOI] [PubMed] [Google Scholar]
- 24.Seth, A., I. Ourmanov, J. E. Schmitz, M. J. Kuroda, M. A. Lifton, C. E. Nickerson, L. Wyatt, M. Carroll, B. Moss, D. Venzon, N. L. Letvin, and V. M. Hirsch. 2000. Immunization with a modified vaccinia virus expressing simian immunodeficiency virus (SIV) Gag-Pol primes for an anamnestic Gag-specific cytotoxic T-lymphocyte response and is associated with reduction of viremia after SIV challenge. J. Virol. 74:2502-2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shiver, J. W., T.-M. Fu, L. Chen, D. R. Casimiro, M. Davies, R. K. Evans, Z.-Q. Zhang, A. J. Simon, W. L. Trigona, S. A. Dubey, L. Huang, V. A. Harris, R. S. Long, X. Liang, L. Handt, W. A. Schleif, L. Zhu, D. C. Freed, N. V. Persaud, L. Guan, K. S. Punt, A. Tang, M. Chen, K. A. Wilson, K. B. Collins, G. J. Heidecker, V. R. Fernandez, H. C. Perry, J. G. Joyce, K. M. Grimm, J. C. Cook, P. M. Keller, D. S. Kresock, H. Mach, R. D. Troutman, L. A. Isopi, D. M. Williams, Z. Xu, K. E. Bohannon, D. B. Volkin, D. C. Montefiori, A. Miura, G. R. Krivulka, M. A. Lifton, M. J. Kuroda, J. E. Schmitz, N. L. Letvin, M. J. Caulfield, A. J. Bett, R. Youil, D. C. Kaslow, and E. A. Emini. 2002. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature 415:331-335. [DOI] [PubMed] [Google Scholar]
- 26.Shiver, J. W., H. C. Perry, M. E. Davies, and M. A. Liu. 1995. Immune responses to HIV gp120 elicited by DNA vaccination, p. 95. In R. M. Chanock, F. Brown, H. S. Ginsberg, and E. Norrby (ed.), Vaccines 95. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 27.Wyatt, L. S., S. T. Shors, B. R. Murphy, and B. Moss. 1996. Development of a replication-deficient recombinant vaccinia virus vaccine effective against parainfluenza virus 3 infection in an animal model. Vaccine 14:1451-1458. [DOI] [PubMed] [Google Scholar]
- 28.Yang, Z., L. S. Wyatt, W. Kong, Z. Moodie, B. Moss, and G. J. Nabel. 2003. Overcoming immunity to a viral vaccine by DNA priming before vector boosting. J. Virol. 77:799-803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zajac, A. J., J. N. Blattman, K. Murali-Krishna, D. J. D. Sourdive, M. Suresh, J. Altman, and R. Ahmed. 1998. Viral immune evasion due to persistence of activated T cells without effector function. J. Exp. Med. 188:2205-2213. [DOI] [PMC free article] [PubMed] [Google Scholar]