Abstract
Lentiviruses have long been considered host-specific pathogens, but several recent observations demonstrated their capacity to conquer new hosts from different species, genera, and families. From these cross-species infections emerged new animal and human infectious diseases. The successful colonization and adaptation of a lentivirus to a nonnatural host depends on unspecific and specific host barriers. Some of those barriers exert a relative control of viral replication (i.e., cytotoxic T-lymphocyte response, viral inhibitory factors), but none of them was found able to totally clear the infection once the retrovirus is fully adapted in its host. In this study we examined the evolution of the host-lentivirus interactions occurring in an experimental animal model of cross-species infection in order to analyze the efficiency of those barriers in preventing the establishment of a persistent infection. Five newborn calves were inoculated with caprine arthritis-encephalitis virus (CAEV), and the evolution of infection was studied for more than 12 months. All the animals seroconverted in the first 0.75 to 1 month following the inoculation and remained seropositive for the remaining time of the experiment. Viral infection was productive during 4 months with isolation of replication competent virus from the blood cells and organs of the early euthanized animals. After 4 months of infection, neither replication-competent virus nor virus genome could be detected in blood cells or in the classical target organs, even after an experimental immunosuppression. No evidence of in vitro restriction of CAEV replication was observed in cells from tissues explanted from organs of these calves. These data provide the demonstration of a natural clearance of lentivirus infection following experimental inoculation of a nonnatural host, enabling perspectives of development of new potential vaccine strategies to fight against lentivirus infections.
Lentiviruses comprise a genus of complex retroviruses that infect a limited number of mammalian hosts from several taxonomically distant orders (primates, carnivores, and ungulates). Typically, the virus exists in the infected host as a swarm of related but divergent genetic variants called a quasispecies (9), and degenerative inflammatory disease develops only after months or years, if at all. No endogenous lentiviruses have been described, so the recognizable similarity between the different lentiviruses, despite their high within-species variability, suggests a common ancestor much more recent than the divergence of their host species. The presence of many variants in the viral swarm might facilitate cross-species infections. Human immunodeficiency virus type 2 (HIV-2) is almost certainly a human-adapted variant of the simian immunodeficiency virus (SIV) from sooty mangabeys (16), and HIV-1 may similarly be derived from a chimpanzee SIV (12). Again, caprine arthritis-encephalitis virus (CAEV) probably emerged after cross-species transfer of a closely related sheep virus in recent times (5, 30, 39). For cross-species infection to occur, contact between an active source of infectious virus and a susceptible individual of the new host species must occur, leading to infection of the new host target cells. If this infection is successful, and the new host cannot efficiently eliminate the infection by innate or instructive immunity, a phase of adaptation of the virus in its new host may follow. An immediate necessity for the virus is the establishment of a route of infection of other individuals of the new host species, while the host may undergo selection for resistance, or at least reduction of pathology.
In general, SIV infections cause no observable disease in their natural hosts but may become severe pathogens in novel host species (20, 27, 31), and feline immunodeficiency virus, which causes disease in pet cats, shows little effect on the life span or reproductive potential of wild felines (3, 32, 35). Similarly, transfer of bovine immunodeficiency virus, which is innocuous in Bos taurus, to Bos javanicus resulted in a severe epidemic of lethal Jembrana disease (11, 36). Even in cases where lentiviruses cause disease, many infected individuals remain infected but symptom free over their natural lifetime, with a continual production of infectious virus despite a vigorous immune response. Horses infected with equine infectious anemia virus may recover to become asymptomatic carriers, and recurrence of disease in low-frequency areas suggests the presence of undetected infections (8). Maedi-visna virus (MVV) and CAEV can cause severe symptoms in infected sheep and goats, but most infected individuals develop no disease (17). This may reflect host selection for resistance to disease rather than to lentiviral infection.
Suitable in vitro cell cultures from many species, including cattle but not humans, support replication of CAEV (6, 15, 24, 25, and personal observations), and cattle may easily be exposed to infectious goats on mixed farms. Antibodies from calf sera can react with CAEV and MVV proteins, although it is not clear whether these are a result of infection, contamination, or cross-reactivity. In this study we have inoculated calves kept in secure housing with a pathogenic strain of CAEV and studied the dynamics of the viral infection and the host's immune response. Newborn calves become infected by CAEV and rapidly developed a persistent specific immune response. Virus can be recovered from blood cells and tissues for about 4 months, and then, surprisingly, it disappears. No trace of CAEV as infectious virus or integrated provirus could be detected in blood samples or tissues on autopsy at 7 months postinfection (p.i.), even after immunosuppression by corticosteroids, which efficiently induces viral expression in latently infected goats.
MATERIALS AND METHODS
Experimental CAEV infection of calves.
Male cross-bred Holstein calves were purchased at birth from local suppliers and were immediately transported, together with maternal colostrum, to secure safety level 2 experimental animal facilities at the Lyon Veterinary School. All experimental procedures were approved by the veterinary school ethical committee and were performed by personnel certified for animal experimentation. Heat-treated colostrum (56°C, 30 min) was administered around 6 h after birth, and the calves were mock infected or were infected with CAEV at 48 h of age. In contrast to mice that are not immune competent at birth, the immune system of the calf develops early in fetal life and both humoral and cellular responses against antigens can be observed more than 3 months before birth (38). Calves I-1 to I-5 received 5 × 106 50% tissue culture infectious doses (TCID50) of CAEV 3112, prepared as described below, in 25 ml of serum-free RPMI 1640 medium (Gibco BRL, Cergy Pontoise, France) by two routes. The major portion (15 ml) was given intratracheally to simulate a probable route of potential natural transfer, and the remaining 10 ml were given intravenously, a procedure known to be efficient for infecting goats with this virus (data not shown) and used here as a backup to ensure infection. Calf I-1 failed to give a cough reflex on instillation of the intratracheal dose, suggesting failure of the procedure, so it was given a total of 5 × 106 TCID50 of CAEV 3112 intravenously to compensate. Three calves, NI-1 to NI-3, were mock infected by using fresh serum-free RPMI 1640. Animals underwent regular clinical examination and blood sampling for virological and immunological analyses.
In an initial study involving calves I-1 to I-3 and controls NI-1 and NI-2, animals I-1 and NI-1 were sacrificed at 6 to 7 months p.i., and tissues from a range of organs (see below) were sampled for virological and histopathological investigations. Calf I-5, infected in a second confirmatory experiment 12 months later, was sacrificed at 2 months p.i. and was similarly examined. Calf I-2 from the first experiment was challenged at 16 months with a further 107 TCID50 of CAEV 3112 before sacrifice and autopsy at 24 months. Other animals underwent complementary investigations as described below before sacrifice and autopsy at around 27 months p.i.
Virus and indicator cells.
CAEV 3112 was originally obtained from a synovial membrane explant from a French goat with severe arthritis (2) and multiplied on goat synovial membrane (GSM) cells established from an uninfected animal (31) to a titer of 106 to 107 TCID50/ml. The same cells, grown in minimal essential medium (MEM; Gibco BRL) with 10% fetal bovine serum, served as sensitive indicator cells for the detection of CAEV on coculture. Thawed stock cells were multiplied at a 1:3 split and were used for up to 14 passages. The absence of parainfluenza virus 3 or bovine viral diarrhea virus was confirmed by immunofluorescent and PCR analyses at the Laboratoire des études sur les Petits Ruminants (AFSSA, Nice, France).
Antibodies to CAEV p25 Gag.
Serum samples from the dams of the experimental animals and those taken from the calves at various times during the experiment were evaluated by using a commercial enzyme-linked immunosorbent assay kit (Institut Pourquier, Montpellier, France) in 96-well plates to detect CAEV-specific antibodies to recombinant p25 Gag produced in eukaryotic cells. The supplied conjugated anti-goat immunoglobulin G cross-reacted efficiently with bovine immunoglobulin G (data not shown).
Peripheral blood monocyte cultures.
Buffy coats were recovered from 200-ml blood samples drawn onto 2 mM EDTA after centrifugation at 600 × g for 15 min at 15°C and then were diluted three times in Hank's balanced salt solution before centrifugation through a Ficoll gradient (Histopaque 1.077; Sigma, Lavepillere, France) at 600 × g for 10 min at 15°C to recover the monocytes. After two washes in Hank's solution with 2 mg of EDTA/ml (600 × g; 10 min; 15°C), cells were resuspended in macrophage differentiation medium (MDM) consisting of glutamine (2 mM), HEPES buffer (10 mM), 2-mercaptoethanol (0.05 mM), gentamicin (0.05 mg/ml), and heat-inactivated newborn calf serum (20%; Gibco BRL) in RPMI 1640. After culture for 7 to 10 days at 37°C in 5% CO2, 12 × 106 cultured peripheral blood mononuclear cells (PBMC) were transferred into each well of 6-well plates and the nonadherent cells were removed by washing with serum-free medium 4 to 24 h later. The resulting approximately 3 × 105 blood monocyte-derived macrophages (BMDM) were observed for at least 15 days for the appearance of giant multinucleated cells, the typical cytopathic effect (CPE) of CAEV infection. To enhance the effect, GSM indicator cells were added to certain cultures at 105 cells/well. Filtered supernatants (100 μl) were titrated on 5 × 104 GSM cells in 24-well plates.
PCR detection of CAEV provirus.
DNA templates from 3 × 105 lysed BMDM (see above) or 12 × 106 PBMC were submitted to 35 rounds of PCR amplification by using highly conserved primers corresponding to bases 393 to 416 and 1268 to 1291 in the leader-gag region of the CAEV genome. Initial denaturation consisted of 94°C for 5 min and then denaturation at 94°C for 1 min, annealing 46°C for 1.5 min, and extension 65°C for 2.5 min. After a final extension for 15 min at 65°C, 5 μl of the initial reaction mixture was used as template for a nested PCR by using primers corresponding to bases 524 to 546 and 1013 to 1036 under identical conditions. One series of amplifications used an annealing temperature of 56°C and an extension at 70°C with comparable results.
Primers encompassing a variable region of the env gene (bases 6458 to 6486 and 7711 to 7731) were used to amplify proviral DNA from samples of at least 105 GSM cells inoculated with culture supernatants from cultured calf macrophages by using the above standard amplification conditions.
Reaction products were visualized by ethidium bromide staining after electrophoresis through 1 or 1.5% agarose gels. PCR efficiency was evaluated by amplification from the fourth exon of β-actin, where the commercial human primers correspond to the bovine homologue.
Radioimmunoprecipitation of viral proteins.
Cultured BDMC (a minimum of 3 × 105 cells) either alone or in coculture with GSM indicator cells were rinsed twice with serum-free MEM and then were held for 2 h in methionine-cysteine-depleted MEM. Labeling of newly synthesized proteins was effected by incubation for 16 h with 100 μCi of [35S]methionine-cysteine (Promix; Amersham, Saclay, France) in 1 ml of the same medium. Viral particles in the clarified culture supernatants and cell monolayers were lysed with radioimmunoprecipitation assay (RIPA) buffer (Tris-HCl [pH 7.5], 50 mM NaCl, 0.5% deoxycholic acid, 0.2% sodium dodecyl sulfate, and 10 mM EDTA) and were incubated overnight at 4°C with 10 μl of our hyperimmune goat serum (G9615) and Sepharose-protein A (Sigma). The beads were repeatedly washed in RIPA buffer, and the specifically bound proteins were eluted from the beads and analyzed by polyacrylamide gel electrophoresis and autoradiography.
CAEV infection of organs at necropsy.
Tissue fragments from potential target organs were recovered at necropsy from infected and control calves (synovial membrane and fluid; lung; cranial, caudal, and median lobes; bone marrow; spleen; thymus; prescapular lymph node; choroid plexus; and dermis). Minced fragments of solid organs or samples of soft tissue were placed in 6-well plates in MDM supplemented with penicillin (100 IU/ml) and streptomycin (0.1 mg/ml) but containing only 10% newborn calf serum (R10), and they were cultured at 37°C in 5% CO2. Cultures were regularly examined for at least 15 days for CPE, either alone or in the presence of 105 GSM indicator cells/well. Culture supernatants were tested for infectivity on GSM cells, and virus titers were determined as previously described (33). Cultured cells were tested by radioimmunoprecipitation for the presence of viral proteins and by nested PCR for proviral genomic sequences (see above). Organ fragments were conserved in formalin for histopathological investigations.
CAEV infection of organ explants in vitro.
Explant cultures (5 × 104 cells) of spleen, lung, thymus, choroid plexus, bone marrow, and synovial membrane from calves I-1, I-2, and NI-1 were incubated with 5 × 104 TCID50 of CAEV 3112 and then were monitored as monocultures or cocultures with GSM cells for 15 days to detect CPE. Culture supernatants were assayed on GSM cells for the presence of infectious cytopathic virus, and virus titers were determined as previously described (33).
Experimental immunosuppression.
Calves I-1 and I-3 and control NI-2 were subjected to daily intramuscular injections of dexamethasone (Dexadreson; 0.04 mg/kg of body weight; Intervet SA, Angers, France) for 3 days at a period between 5 and 6 months p.i. The animals received a preventive antibiotic cover of amoxicillin (0.05 mg/kg of body weight; Pfizer, Paris, France). Animals I-2 and NI-2 served as sham treated controls. Immunosuppression was evaluated by flow cytometry on blood leukocyte samples taken at various times after the start of treatment. Antibodies to the bovine markers listed below were obtained from VMRD (Pullman, Wash.) and were used according to the supplier's recommendations. Cells were labeled with goat anti-mouse immunoglobulin fluorescein isothiocyanate conjugate (Sigma) at 1:50 and were fixed in 1% paraformaldehyde. Monoclonal antibodies to the following bovine antigens were used: B cell (BAQ155A), CD2 (MUCC2A), CD4 (CACT138A), CD8α (CACT80C), CD62-L selectin (DU1-29), major histocompatibility complex class II (H42A), CD14 (MM61A), TCR1 (GB21A), and WC1 (BAQ4A).
RESULTS
Productive infection of calves by CAEV 3112.
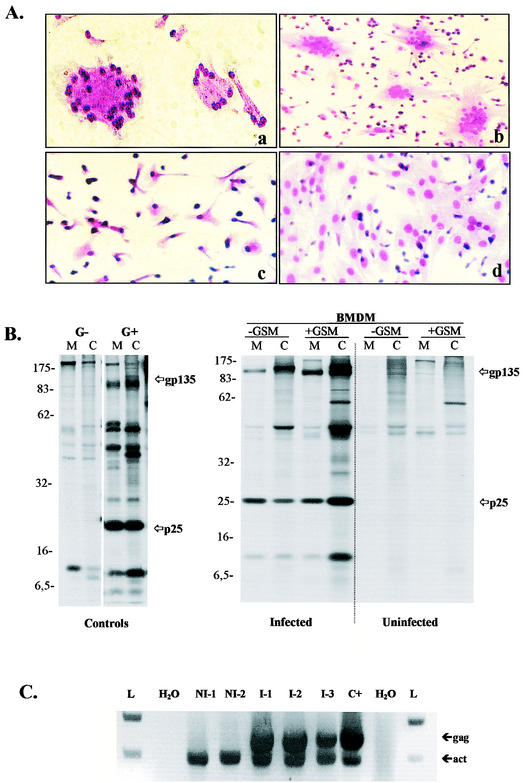
All five calves inoculated with CAEV 3112 as described in Materials and Methods but none of the three controls initially developed productive infection (Table 1). Blood macrophages isolated from the infected animals at 0.5 to 3 to 4 months p.i. regularly developed typical CPE when cocultured with GSM cells. BMDM from the uninfected control animals NI-1, NI-2, and NI-3 never showed any correlates of CAEV infection. CPE was occasionally observable on BMDM from inoculated calves alone (Fig. 1A, images a and c), but more often GSM indicator cells were necessary to amplify the phenomenon (Fig. 1A, images b and d). The quantities of free virus produced were often small even in the presence of clear CPE, but culture supernatants from calves I-1, I-2, and I-3 were able to transmit infection to inoculated fresh GSM cell cultures on occasion. Viral proteins could be detected in these supernatants by radioimmunoassay (Fig. 1B). Viral titers ranged from 102.75 TCID for I-3 at 2 months p.i. to 105.75 TCID for calf I-1 at 1 month p.i., a titer comparable to that of CAEV 3112 grown on GSM cells. Presence of the viral genome in the blood leukocytes was confirmed by PCR from gag region primers for all animals (Fig. 1C) at times ranging from 0.25 to 4 months p.i. (Table 1).
TABLE 1.
Indicators of CAEV infection in the blood of inoculated calves
| Indicator | Calfa | Presence of CAEV indicators at wk p.i.:
|
No. of samples negative for CAEV indicators/total no. of samples (17 to 54 wk p.i.) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | |||
| CPEb | I-1 | − | + | + | + | + | + | + | + | + | 3/3 (N) | |||||||
| I-2 | − | + | + | + | + | + | + | − | 5/5 | |||||||||
| I-3 | − | + | + | + | + | + | + | + | + | + | + | − | 6/6 | |||||
| I-4 | − | − | + | + | + | + | + | − | − | − | + | 1/1 | ||||||
| I-5 | − | − | + | + | + | + | − | + | Nh | |||||||||
| Infectivityc | I-1 | − | + | + | + | − | − | − | 3/3 (N) | |||||||||
| I-2 | − | − | + | − | − | − | 5/5 | |||||||||||
| I-3 | − | + | + | + | + | + | − | − | 6/6 | |||||||||
| I-4 | − | − | − | − | − | − | − | − | 1/1 | |||||||||
| I-5 | − | − | − | − | − | − | N | |||||||||||
| Viral proteinsd | I-1 | − | + | − | + | − | N | |||||||||||
| I-2 | − | − | ||||||||||||||||
| I-3 | − | − | + | + | + | + | 1/1 | |||||||||||
| I-4 | 1/1 | |||||||||||||||||
| I-5 | N | |||||||||||||||||
| Viral genome | I-1 | +f | +f,g | 3/3 (N) | ||||||||||||||
| I-2 | +f,g | 5/5 | ||||||||||||||||
| I-3 | +e,f | +f | +f,g | +e,g | 3/3 | |||||||||||||
| I-4 | −e | +e | +e | −e | +e | 1/1 | ||||||||||||
| I-5 | −e | +e | −e | N | ||||||||||||||
CAEV infection in calves was monitored for 2 (I-5), 4 (I-4), 7 (I-1), and 13 (I-2 and I-3) months p.i. with different methods. Calf I-1 was immunosuppressed at week 24; calf I-5 was immunosuppressed at week 27.
Presence of CAEV in coculture of BMDM with the indicator GSM cells was detected by observation of CPE development.
Culture supernatants of BMDM were tested on the indicator GSM cells.
Presence of viral proteins (gp135 and p25) was examined both in cell lysates and culture supernatants of BMDM cultures.
Presence of proviral genome was examined in PBMC lysates (13 × 106 cells).
Presence of proviral genome was examined in GSM cells inoculated with supernatants from cultured macrophage monolayers.
Presence of proviral genome was examined in BMDM (≥ 3 × 105 cells).
N, necropsy of steers I-5 and I-1.
FIG. 1.
Detection of CAEV in inoculated calves. (A) Observation of CPE. Blood samples were collected from infected and control animals at different times following the inoculation, and PBMC were isolated. BMDM were obtained after a step of in vitro differentiation (see Materials and Methods) and were cultivated with or without indicator GSM cells. Monocultures and cocultures were then examined for the development of giant multinucleated cell formations as CPE characteristic of CAEV infection. Monocultures (images a and c) and cocultures (b and d) of BMDM derived from the I-1 infected calf (a and b) and the NI-1 control calf (c and d) collected 0.25 month p.i. (B) Radioimmunoprecipitation of viral proteins. Cultures of BMDM collected 0.5 month p.i. were analyzed for expression of the major viral proteins gp135 Env and p25 Gag by using RIPA (see Materials and Methods). Autoradiographs present protein profiles obtained from cell lysates (C) and supernatants (M) of monocul-tures (−GSM) and cocultures (+GSM) of BMDM derived from the I-1 infected calf and the NI-1 control calf. Molecular sizes are expressed in kilodaltons. Uninfected GSM were used as negative controls (G−), and GSM inoculated in vitro with CAEV 3112 were used as positive controls (G+). (C) Detection of proviral genome. Amplification of CAEV proviral sequence was performed by using nested PCR (see Materials and Methods) from lysates of BMDM derived from infected (I-1, I-2, and I-3) and control (NI-1 and NI-2) calves. Primers used were specific for CAEV-gag (gag; 512-bp product) and for β-actin (act; 393-bp product). Samples lacking DNA provided a negative control (H2O), and a lysate of GSM infected with CAEV 3112 provided a positive control (C+). Molecular size ladder DNA (L) was run in both extremities of the gel.
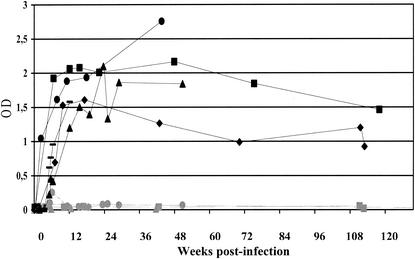
Active infection was also suggested by the rapid and sustained specific humoral antibody response to CAEV in the infected animals but not in the controls. Antibodies to p25 Gag developed at 0.75 to 1 month p.i., reached a peak at 4 months p.i., and then declined slowly thereafter (Fig. 2).
FIG. 2.
Detection of anti-p25 Gag antibodies in blood of CAEV-infected calves. The capacity of CAEV to infect newborn calves was determined by estimating the production of calf antibodies directed against the major viral protein p25 Gag. Sera were collected from infected (I-1 [black circles], I-2 [black diamonds], I-3 [black squares], I-4 [black triangles], and I-5 [black dash marks]) and control (NI-1 [gray circles], NI-2 [gray squares], and NI-3 [gray triangles]) animals at different times p.i. and were analyzed by using an enzyme-linked immunosorbent assay (see Materials and Methods). Results were expressed in optical densities (OD). The stippled line indicates the threshold derived from experimental measures that were considered positive.
Disappearance of CAEV infection around 4 months p.i.
Interestingly, after 4 months p.i., none of the samples examined showed any correlate of CAEV infection (Table 1). Calf I-5 was sacrificed at 2 months p.i., and 15 blood samples from the four surviving calves at dates ranging from 4 to 13 months p.i. showed no CPE or presence of viral protein in any condition (Table 1). Twelve of these samples were examined by PCR and showed no presence of CAEV provirus. Tissues from calf I-5, examined at sacrifice, showed active CAEV infection of the lungs, spleen, and prescapular lymph node, which all produced characteristic CPE on coculture with GSM cells (Table 2). Synovial liquid and bone marrow gave inconsistent results, but the synovial membrane and dermis never produced CPE. In contrast, tissues from calf I-1, which was sacrificed at 7 months p.i., were found to be negative for all correlates of CAEV infection, including PCR, although the animal had been subjected to experimental immunosuppression in an attempt to reactivate the virus (see below). A control animal (NI-1) was sacrificed at the same age as that of I-5, and as expected, none of the tissues sampled from a range of organs showed any sign of the presence of CAEV. The variable env region was chosen for amplification by PCR because of its divergence between CAEV-3112 and the molecularly cloned CAEV-CO. This minimizes the risk of contamination with plasmid DNA.
TABLE 2.
Dissemination of CAEV in calf organs
| Calf and tissue (passage no.)a | Detection of CAEV indicators
|
||||
|---|---|---|---|---|---|
| CPE in cultureb | CPE in coculturec | Viral proteins M and Cd | Pro- viruse | Infec- tivityf | |
| I-5 | |||||
| Synovial membrane (1) | − | − | − | − | − |
| Synovial liquid (1) | ++ | ++ | − | − | − |
| Cranial lobe (1) | +++ | +++ | + | + | +++ |
| Caudal lobe (1) | +++ | ++ | + | + | +++ |
| Median lobe (1) | +++ | +++ | + | + | +++ |
| Bone marrow (1) | + | − | − | − | − |
| Spleen (1) | +++ | +++ | + | + | +++ |
| Thymus (1) | − | − | − | − | − |
| Pre-scapular lymph node (1) | +++ | + | − | + | + |
| Derma (1) | − | − | − | − | − |
| I-1 | |||||
| Synovial membrane (3) | − | − | NDg | − | − |
| Median lobe (3) | − | − | − | − | − |
| Bone marrow (3) | − | − | −g | − | − |
| Spleen (3) | − | − | ND | − | − |
| Thymus (3) | − | − | ND | − | − |
| Choroïd plexus (3) | − | − | ND | − | − |
CAEV-infected calves I-5 and I-1 were autopsied at 2 and 7 months, respectively. Explant cultures were derived from various organs of infected calves and were examined after different passages.
CPE were examined in explant cultures (+, presence; −, absence).
CPE were examined in cocultures of explant cultures with GSM cells (+, presence; −, absence).
Viral proteins in cell lysates (C) and culture medium (M) were examined by RIPA.
Proviral genome was detected by nested PCR by using CAEV-specific primers.
Infectious cytopathic virus in the supernatants was detected following infection of the indicator GSM cells (ND, not determined).
Examined after passage 4.
Experimental immunosuppression does not reactivate the virus.
In an attempt to reactivate the viral infection, calves I-1, I-3, and NI-2 were immunosuppressed by using a protocol, shown to be effective in latently infected goats (4), at 6 to 7 months p.i. (about 3 months after the last positive viral sample for the infected animals). Blood monocytes rose under corticosteroid treatment from (0.45 ± 0.25) × 106/ml (range, 0.17 to 0.63) on day 0 to a level of (1.67 ± 0.43) × 106/ml (range, 1.22 to 2.07) on day 3. On day 8, 5 days after the last treatment, they still showed (1.30 ± 0.63) × 106 monocytes/ml (range, 0.76 to 2.0). Simultaneously, lymphocyte counts fell from (4.86 ± 0.19) × 106/ml to (2.06 ± 0.17) × 106/ml on day 3. Untreated animals I-2 and NI-1 had monocyte counts of (0.70 ± 0.11) × 106 monocytes/ml at day 0 and (0.32 ± 0.18) × 106/ml on day 3 and lymphocyte counts of (4.87 ± 0.29) × 106/ml at day 0 and (2.81 ± 0.15) × 106/ml on day 3. Blood samples taken at times during or after this immunosuppression remained negative for any correlates of CAEV infection in calves I-1 and I-3. The untreated animal and controls likewise remained CAEV negative (see Table 1).
Clinical and histopathological correlates of infection.
All calves, infected and controls, were regularly clinically examined during the 14-month experimental period, and they never showed any significant symptoms. Specifically, no arthritis, lung disease, or encephalitis was ever observed. Tissues from classical target organs for CAEV recovered from calves I-1 and I-5 at necropsy showed no signs of typical inflammatory lesions, such as monocyte or lymphocyte infiltration or lymphoid follicle formation.
Permissivity of cultures from infected animals.
In order to test whether the disappearance of virus from circulating blood at 4 months p.i. was due to an acquired refractory state of the target cells, we cultured explants of tissues from noninfected calf NI-1 and from infected calves I-1 and I-2. These infected calves had passed into the silent phase of infection at autopsy. Cells from the organs of all animals showed a similar sensitivity to CAEV infection in vitro as previously observed (Table 3).
TABLE 3.
Susceptibility of cells derived from calf tissues to CAEV
| Cell origin | Passage no. and CAEV detection results for calfa:
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NI-3
|
I-1
|
I-2
|
||||||||||
| Passage no. | CPE in culture | CPE in coculture | Infectivity | Passage no. | CPE in culture | CPE in coculture | Infectivity | Passage no. | CPE in culture | CPE in coculture | Infectivity | |
| Spleen | ND | ND | ND | ND | 6 | +++ | +++ | + | 2 | +++ | +++ | + |
| Median lobe | 4 | +++ | +++ | + | 5 | + | ++ | + | 3 | +++ | +++ | + |
| Thymus | 2 | +++ | +++ | + | ND | ND | ND | ND | ND | ND | ND | ND |
| Choroid plexus | 3 | + | + | + | 5 | + | + | + | 3 | + | + | + |
| Bone marrow | 4 | +++ | +++ | + | 5 | +++ | +++ | + | 2 | +++ | +++ | + |
| Synovial membrane | 3 | ++ | ++ | + | 5 | +++ | +++ | + | 2 | +++ | +++ | + |
Calves NI-3, I-1, and I-2 were autopsied at 6, 7, and 24 months of age, respectively. Explant cultures were derived from various tissues and were serially passaged before CAEV inoculation at the indicated passage numbers. Cytopathic effects in cultures, or cocultures with the indicators GSM cells and the presence of infectious cytopathic virus in the supernatants of infected explant cultures were examined. (+, presence of CPE or infectious virus; ND, not determined).
DISCUSSION
CAEV has been shown to possess a wide host tropism in vitro (6, 15, 24, 25), and bovine cells are readily infected (23). CAEV is present in many goat flocks worldwide (10, 13, 21, 37) and could spread to cattle in mixed farms; indeed, antibodies reacting with CAEV antigens have occasionally been found in bovine sera, although it is not clear if these represent inapparent infection, antigenic stimulation, or even cross-reactivity. Lentiviruses are known to cross species barriers, sometimes with dramatic consequences (5, 12, 16, 39), but because many natural lentiviral infections cause little overt disease, some transfers may go unobserved. Simple contact with a fresh potential host is not sufficient to ensure transfer, and the simian and feline lentiviruses show a patchy distribution among possible host species and populations.
After initial infection a lentivirus, like other retroviruses, is stably maintained as a DNA copy, a provirus in the chromosome of the host cell, and can be propagated by cellular division. Establishment in the germ line can lead to emergence of endogenous retrovirus sequences, but no endogenous lentivirus has yet been reported. Proviruses capable of reactivation in monocytes (28, 29), epithelial cells (26), lymphoid cells, and others are the main reservoirs maintaining a lentiviral infection in the face of a vigorous immune response by the host (34, 40). Even so, the immune response can often control or limit lentiviral infections; baboons infected with strain UC12 of HIV-2 can clear virus from their blood after an initial infection lasting about 6 months, although the lymphoid organs remain infected (23). Some humans apparently control HIV-1 infections for considerable periods without eliminating the virus (18, 19). A very rare instance of elimination of a lentiviral infection was reported for Rhesus macaques infected with SIVmacA11, where the virus disappeared completely after a short period of cell-associated viremia and presence of replication-competent virus in the lymphoid tissues (22).
All five calves inoculated with CAEV 3112 became infected, with replication-competent virus present in their blood leukocytes and tissues. They mounted a specific humoral response with kinetics very similar to those seen in infected goats (the natural host) (1) or mouflons (15). One animal, which received a supplementary intravenous dose of virus, showed viral replication in blood macrophages with similar titers to those seen for infected goats. Other animals, which received part of the infectious dose intratracheally, showed a lower level of active replication. For a period of 3 to 4 months the virus remained easily detectable in blood macrophage cultures and became undetectable thereafter. One animal sacrificed during the active phase of infection had virus in the lungs, spleen, and lymph nodes, suggesting virus diffusion to target organs. No virus could be recovered from tissues taken at autopsy from animals sacrificed after 4 months p.i., even after attempts to reactivate the virus by immunosuppression. Steroid treatment efficiently reactivates latent infections in goats (4, 14). Cultures established from organs of calves after 4 months p.i. were fully susceptible to CAEV infection in vitro.
It is unlikely that the virus was eliminated by the humoral response, because the calves' antibodies, like goat antibodies against CAEV, were not neutralizing in vitro (data not shown), but the reservoir of cells carrying a potentially active provirus may differ between the species. We were unable to demonstrate viral presence in the synovial membranes, choroid plexus, or bone marrow of the calf sacrificed during the active phase of infection, although the cells could be experimentally infected in vitro (Table 2). It is also possible that the virus is expressed to a lesser extent on maturation of monocytes to macrophages in calves than in goats. This has been described for a sheep infected with the closely related MVV (7) and may suggest additional factors involved in viral expression. The vigorous humoral response of our infected calves to different viral antigens nevertheless suggests a good production of viral proteins.
Further dissection of the immune response of calves infected with CAEV 3112 should provide insights into the protective mechanisms limiting cross-species spread of lentiviruses, and the system could provide a much-needed model for the study of the clearance of a lentiviral infection after initial successful establishment.
Acknowledgments
This work was supported by grants from INRA and DGER. Support of Thierry Morin is from the “Ministère de l'Education Nationale, de la Recherche et de la Technologie (MENRT).”
We sincerely thank Sylvain Bruyas and Barbara Gineys for technical assistance and animal care.
REFERENCES
- 1.Adams, D. S., T. B. Crawford, K. L. Banks, T. C. McGuire, and L. E. Perryman. 1980. Immune responses of goats persistently infected with caprine arthritis-encephalitis virus. Infect. Immun. 28:421-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blondin, I., C. Grillet, and Y. Thiogane. 1989. Syncytia formation in cultures and analysis of the protein composition of various strains of caprine arthritis encephalitis virus (CAEV). Ann. Rech. Vet. 20:153-158. [PubMed] [Google Scholar]
- 3.Brown, E. W., N. Yuhki, C. Packer, and S. J. O'Brien. 1994. A lion lentivirus related to feline immunodeficiency virus: epidemiologic and phylogenetic aspects. J. Virol. 68:5953-5968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buonavoglia, C., M. Tempesta, A. Cavalli, V. Voigt, D. Buonavoglia, A. Conserva, and M. Corrente. 1996. Reactivation of caprine herpesvirus 1 in latently infected goats. Comp. Immun. Microbiol. Infect. Dis. 19:275-281. [DOI] [PubMed] [Google Scholar]
- 5.Castro, R. S., T. Greenland, R. C. Leite, A. Gouveia, J. F. Mornex, and G. Cordier. 1999. Conserved sequence motifs involving the tat reading frame of Brazilian caprine lentiviruses indicate affiliations to both caprine arthritis-encephalitis virus and Visna-Maedi virus. J. Gen. Virol. 80:1583-1589. [DOI] [PubMed] [Google Scholar]
- 6.Chebloune, Y., D. Sheffer, B. M. Karr, E. Stephens, and O. Narayan. 1996. Restrictive type of replication of ovine/caprine lentiviruses in ovine fibroblast cell cultures. Virology 222:21-30. [DOI] [PubMed] [Google Scholar]
- 7.Chebloune, Y., B. Karr, D. Sheffer, K. Leung, and O. Narayan. 1996. Variations in lentiviral gene expression in monocyte-derived macrophages from naturally infected sheep. J. Gen. Virol. 77:2037-2051. [DOI] [PubMed] [Google Scholar]
- 8.Cheevers, W. P., and T. C. McGuire. 1985. Equine infectious anemia virus: immunopathogenesis and persistence. Rev. Infect. Dis. 7:83-88. [DOI] [PubMed] [Google Scholar]
- 9.Cichutek, K., H. Merget, S. Norlay, R. Linde, W. Kreuz, M. Gahr, and R. Kurth. 1992. Development of a quasispecies of human immunodeficiency virus type 1 in vivo. Proc. Natl. Acad. Sci. USA 89:7365-7369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cutlip, R. C., H. D. Lehmkuhl, J. M. Sacks, and A. L. Weaver. 1992. Prevalence of antibody to caprine arthritis-encephalitis virus in goats in the United States. J. Am. Vet. Med. Assoc. 200:802-805. [PubMed] [Google Scholar]
- 11.Flaming, K., M. van der Maaten, C. Whetstone, S. Carpenter, D. Frank, and J. Roth. 1993. Effect of bovine immunodeficiency-like virus infection on immune function in experimentally infected cattle. Vet. Immunol. Immunopathol. 36:91-105. [DOI] [PubMed] [Google Scholar]
- 12.Gao, F., E. Bailes, D. L. Robertson, Y. Chen, C. M. Rodenburg, S. F. Michael, L. B. Cummins, L. O. Arthur, M. Peeters, G. M. Shaw, P. M. Sharp, and B. H. Hahn. 1999. Origin of HIV-1 in the chimpanzee Pan troglodytes. Nature 397:436-444. [DOI] [PubMed] [Google Scholar]
- 13.Greenwood, P. L., R. N. North, and P. D. Kirkland. 1995. Prevalence, spread and control of caprine arthritis-encephalitis virus in dairy goat herds in New South Wales. Aust. Vet. J. 72:341-345. [DOI] [PubMed] [Google Scholar]
- 14.Guiguen, F., C. Lerondelle, C. Favier. 1990. Responses of kids to monocytes infected in vitro by the caprine arthritis-encephalitis virus. Ann. Rech. Vet. 21:179-185. [PubMed] [Google Scholar]
- 15.Guiguen, F., L. Mselli-Lakhal, J. Durand, J. Du, C. Favier, C. Fornazero, D. Grezel, S. Balleydier, E. Hausmann, and Y. Chebloune. 2000. Experimental infection of mouflon-domestic sheep hybrids with caprine arthritis-encephalitis virus. Am. J. Vet. Res. 61:456-461. [DOI] [PubMed] [Google Scholar]
- 16.Hirsch, V. M., R. A. Olmsted, M. Murphey-Corb, R. H. Purcell, and P. R. Jonhson. 1989. An African primate lentivirus (SIVsm) closely related to HIV-2. Nature 339:389-392. [DOI] [PubMed] [Google Scholar]
- 17.Jubb, K. F., P. C. Kennedy, and N. Palmer. 1991. Caprine arthritis encephalitis, p. 175-176. In K. V. Jubb and P. C. Kennedy (ed.), Pathology of domestic animals, vol 1. Academic Press Inc., San Diego, Calif.
- 18.Kaul, R., S. L. Rowland-Jones, J. Kimani, T. Dong, H. B. Yang, P. Kiama, T. Rostron, E. Njagi, J. J. Bwayo, K. S. MacDonald, A. J. McMichael, and F. A. Plummer. 2001. Late seroconversion in HIV-resistant Nairobi prostitutes despite pre-existing HIV-specific CD8+ responses. J. Clin. Investig. 107:341-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaul, R., F. A. Plummer, J. Kimani, T. Dong, P. Kiama, T. Rostron, E. Njagi, K. S. MacDonald, J. J. Bwayo, A. J. McMichael, and S. L. Rowland-Jones. 2000. HIV-1-specific mucosal CD8+ lymphocyte responses in the cervix of HIV-1-resistant prostitutes in Nairobi. J. Immunol. 164:1602-1611. [DOI] [PubMed] [Google Scholar]
- 20.Kaur, A., R. M. Grant, R. E. Means, H. McClure, M. Feinberg, and R. P. Johnson. 1998. Diverse host responses and outcomes following simian immunodeficiency virus sivMAC239 infection in sooty mangabeys and Rhesus macaques. J. Virol. 72:9597-9611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krieg, A., and E. Peterhans. 1990. Caprine arthritis-encephalitis in Switzerland: epidemiologic and clinical studies. Schweiz Arch. Tierheilkd 132:345-352. [PubMed] [Google Scholar]
- 22.Lackner, A. A., P. Vogel, R. A. Kluge, and M. Marthas. 1994. Early events in tissues during infection with pathogenic (SIVmac239) and nonpathogenic (SIVmac1A11) molecular clones of simian immunodeficiency virus. Am. J. Pathol. 145:428-439. [PMC free article] [PubMed] [Google Scholar]
- 23.Locher, C. P., D. J. Blackbourn, B. G. Herndier, G. Reyes-Teran, S. W. Barnett, K. K. Murthy, and J. A. Levy. 1998. Transient virus infection and pathogenesis of a new HIV type 2 isolate, UC12, in baboons. AIDS Res. Hum. Retrovir. 14:79-82. [DOI] [PubMed] [Google Scholar]
- 24.Morin, T., L. Mselli-Lakhal, B. Bouzar, S. Hoc, F. Guiguen, D. Grezel, T. Alogninouwa, T. Greenland, J. F. Mornex, and Y. Chebloune. 2002. Le virus de l'Arthrite et de l'Encéphalite Caprine (CAEV) et la barrière d'espèce. Virologie 6:279-291. [Google Scholar]
- 25.Mselli-Lakhal, L., C. Favier, K. Leung, F. Guiguen, D. Grezel, P. Miossec, J. F. Mornex, O. Narayan, G. Querat, and Y. Chebloune. 2000. Lack of functional receptors is the only barrier that prevents caprine arthritis-encephalitis virus from infecting human cells. J. Virol. 74:8343-8348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mselli-Lakhal, L., F. Guiguen, C. Fornazero, J. Du, C. Favier, J. Durand, D. Grezel, S. Balleydier, J. F. Mornex, and Y. Chebloune. 1999. Goat milk epithelial cells are highly permissive to CAEV infection in vitro. Virology 259:67-73. [DOI] [PubMed] [Google Scholar]
- 27.Murphey-Corb, M., L. N. Martin, S. R. Rangan, G. B. Baskin, B. J. Gormus, R. H. Wolf, W. A. Andes, M. West, and R. C. Montelaro. 1986. Isolation of an HTLV-III-related retrovirus from macaques with simian AIDS and its possible origin in asymptomatic mangabeys. Nature 321:435-437. [DOI] [PubMed] [Google Scholar]
- 28.Narayan, O., F. S. Kennedy Stoskop, D. Sheffer, D. Griffin, and J. E. Clements. 1983. Activation of caprine arthritis-encephalitis virus expression during maturation of monocytes to macrophages. Infect. Immun. 41:67-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Narayan, O., J. S. Wolinsky, J. E. Clements, J. D. Standberg, D. E. Griffin, and L. C. Cork. 1982. Slow virus replication. The role of macrophages in the persistence and expression of visna viruses of sheep and goats. J. Gen. Virol. 59:345-356. [DOI] [PubMed] [Google Scholar]
- 30.Narayan, O., J. E. Clements, J. D. Strandberg, L. C. Cork, and D. E. Griffin. 1980. Biological characterization of the virus causing leukoencephalitis and arthritis in goats. J. Gen. Virol. 50:69-79. [DOI] [PubMed] [Google Scholar]
- 31.Novembre, F. J., V. M. Hirsch, H. M. McClure, P. N. Fultz, and P. R. Johnson. 1992. SIV from stump-tailed macaques: molecular characterization of a highly transmissible primate lentivirus. Virology 186:783-787. [DOI] [PubMed] [Google Scholar]
- 32.Pedersen, N. C., E. W. Ho, M. L. Brown, and J. K. Yamamoto. 1987. Isolation of a T-lymphotropic virus from domestic cats with an immunodeficiency-like syndrome. Science 325:790-793. [DOI] [PubMed] [Google Scholar]
- 33.Reed, L., and H. Muench. 1938. A simple method for estimating fifty per cent points. Am. J. Hyg. 27:413-497. [Google Scholar]
- 34.Schmitz, J. E., M. J. Kuroda, S. Santra, V. G. Sasseville, M. A. Simon, M. A. Lifton, P. Racz, K. Tenner-Racz, M. Dalesandro, B. J. Scallon, J. Ghrayeb, M. A. Forman, D. C. Montefiori, E. P. Rieber, N. L. Letvin, and K. A. Reimann. 1999. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 283:857-860. [DOI] [PubMed] [Google Scholar]
- 35.Shelton, G. H., M. L. Linenberger, C. K. Grant, and J. L. Abkowitz. 1990. Hematologic manifestations of feline immunodeficiency virus infection. Blood 76:1104-1109. [PubMed] [Google Scholar]
- 36.Soeharsono, S., G. E. Wilcox, D. M. Dharma, N. Hartaningsih, G. Kertayadnya, and A. Budiantono. 1995. Species differences in the reaction of cattle to Jembrana disease virus infection. J. Comp. Pathol. 112:391-402. [DOI] [PubMed] [Google Scholar]
- 37.Surman, P. G., E. Daniels, and B. R. Dixon. 1987. Caprine arthritis-encephalitis virus infection of goats in South Australia. Aus. Vet. J. 64:266-271. [DOI] [PubMed] [Google Scholar]
- 38.Tizard, I. R. 2000. Veterinary immunology: an introduction, 6th ed. W. B. Saunders Company, Philadelphia, Pa.
- 39.Valas, S., C. Benoit, C. Guionaud, G. Perrin, and R. Z. Mamoun. 1997. North American and French caprine arthritis-encephalitis viruses emerge from ovine Maedi-Visna viruses. Virology 237:307-318. [DOI] [PubMed] [Google Scholar]
- 40.Walker, C. M., D. J. Moody, D. P. Stites, and J. A. Levy. 1986. CD8+ lymphocytes can control HIV infection in vitro by suppressive virus replication. Science 23:1563-1566. [DOI] [PubMed] [Google Scholar]