Abstract
Equine arteritis virus (EAV) is an enveloped, positive-stranded RNA virus belonging to the family Arteriviridae of the order Nidovirales. Six transmembrane proteins have been identified in EAV particles: the nonglycosylated membrane protein M and the glycoprotein GP5 (previously named GL), which occur as disulfide-bonded heterodimers and are the major viral envelope proteins; the unglycosylated small envelope protein E; and the minor glycoproteins GP2b (formerly designated GS), GP3, and GP4. Analysis of the appearance of the GP2b, GP3, and GP4 proteins in viral particles by gel electrophoresis under reducing and nonreducing conditions revealed the occurrence of two different covalently linked oligomeric complexes between these proteins, i.e., heterodimers of GP2b and GP4 and heterotrimers of GP2b, GP3, and GP4. Shortly after their release from infected cells, virions contained mainly cystine-linked GP2b/GP4 heterodimers, which were subsequently converted into disulfide-bonded GP2b/GP3/GP4 trimers through the covalent recruitment of GP3. This process occurred faster at a higher pH but was arrested at 4°C. Furthermore, the conversion was almost instantaneous in the presence of the thiol oxidant diamide. In contrast, the sulfhydryl-modifying agent N-ethylmaleimide inhibited the formation of disulfide-bonded GP2b/GP3/GP4 trimers. Using sucrose density gradients, we could not demonstrate a noncovalent association of GP3 with the cystine-linked GP2b/GP4 dimer in freshly released virions, nor did we observe higher-order structures of the GP2b/GP4 or GP2b/GP3/GP4 complexes. Nevertheless, the instantaneous diamide-induced formation of disulfide-bonded GP2b/GP3/GP4 heterotrimers at 4°C suggests that the three minor glycoproteins of EAV are assembled as trimeric complexes. The existence of a noncovalent interaction between the cystine-linked GP2b/GP4 dimer and GP3 was also inferred from coexpression experiments showing that the presence of GP3 increased the electrophoretic mobility of the disulfide-bonded GP2b/GP4 dimers. Our study reveals that the minor envelope proteins of arteriviruses enter into both covalent and noncovalent interactions, the function of which has yet to be established.
Equine arteritis virus (EAV) is a single-stranded RNA virus which belongs to the genus Arterivirus. Other members of this single genus in the family Arteriviridae are lactate dehydrogenase-elevating virus (LDV), porcine reproductive and respiratory syndrome virus (PRRSV), and simian hemorrhagic fever virus. Despite marked differences in their biophysical properties, genome sizes, and structural protein compositions, the Arteriviridae were joined together with the Coronaviridae and the recently established family Roniviridae in the order Nidovirales on the basis of similarities in their genomic organizations and replication strategies.
EAV virions have a diameter of 40 to 60 nm and consist of a putatively icosahedral core that is surrounded by a lipid-containing envelope with small surface protrusions (20, 24). The EAV genome is an unsegmented, positive-stranded RNA molecule of 12.7 kb, encapsidated by a 14-kDa phosphorylated nucleocapsid protein (N) (3, 21, 39). The genome contains nine open reading frames (ORFs). The first two, ORF1a and -1b, are located within the 5′ three-quarters of the genome and code for proteins involved in viral RNA replication and transcription (4). Downstream of ORF1a and -1b, seven small ORFs (ORF2a, -2b, and -3 through -7) have been identified, which are expressed from a 3′-coterminal nested set of six subgenomic mRNAs (5, 33) and encode viral structural proteins (6, 31, 38). The N protein is specified by ORF7; the other small ORFs code for the six viral transmembrane proteins. As discussed earlier (10), the nomenclature of the envelope glycoproteins of arteriviruses has not been standardized yet. For the sake of clarity, the EAV glycoproteins will hereafter be referred to as GPX, with x indicating the number of the ORF from which the protein is derived. The 16-kDa nonglycosylated membrane protein (M) encoded by ORF6 and the GP5 (previously GL) protein of 30 to 42 kDa (6) associate into disulfide-linked heterodimers, which are incorporated into virus particles in large amounts (6, 7). The 8-kDa unglycosylated envelope protein (E) specified by ORF2a is present in intermediate amounts (31), while the 25-kDa GP2b (previously GS) protein and the recently discovered membrane proteins GP3 and GP4, of 37 to 42 and 28 kDa, respectively, are minor virion components (8, 38).
Of the three minor envelope proteins, both GP2b and GP4 are type I membrane glycoproteins, containing one and three functional N-glycosylation sites, respectively. The GP3 protein is a heavily glycosylated integral membrane protein with an uncleaved amino-terminal signal sequence. The GP3 protein is anchored by either or both of its hydrophobic terminal domains and has no parts detectably exposed cytoplasmically (19, 38). Endoglycosidase digestions have shown that not all N-linked oligosaccharide side chains of the GP3 and GP4 glycoproteins in extracellular virions are biochemically mature, whereas the single N-linked glycan of GP2b becomes sialylated during transport of the virus particles through the secretory pathway (8, 38).
In this report, we describe the analysis by ultracentrifugation, immunoprecipitation, and gel electrophoresis of the interactions between the three minor envelope glycoproteins of EAV in extracellular virions and virus-infected cells. In addition, we studied complex formation between GP2b, GP3, and GP4 after the independent expression of the corresponding ORFs by using the vaccinia virus-T7 RNA polymerase transfection system. Our data indicate that these proteins occur in two different oligomeric structures: (i) disulfide-bonded GP2b/GP4 heterodimers that are loosely associated with GP3 or (ii) covalently linked heterotrimers of GP2b, GP3, and GP4. Shortly after their release from infected cells, EAV particles contain mainly disulfide-bonded GP2b/GP4 heterodimers, which are subsequently converted into cystine-linked GP2b/GP3/GP4 trimers through the covalent recruitment of GP3. The influence of temperature, pH, and oxidizing and alkylating agents on this process was examined. Our results highlight the unique architecture of arterivirus particles, which differs from that of other RNA viruses not only by the large number of different envelope proteins but also by the complex interactions in which these protein molecules are engaged.
MATERIALS AND METHODS
Cells, viruses, and antisera.
Two baby hamster kidney cell lines were used, BHK-21 C13 (BHK-21; American Type Culture Collection) and BSR T7/5 (2). The latter cells constitutively express the gene encoding the bacteriophage T7 RNA polymerase. Both of these cell types were cultured in Glasgow minimal essential medium (GMEM) (Invitrogen-Life Technologies) containing 10% heat-inactivated fetal bovine serum (FBS), 100 IU of penicillin per ml, and 100 μg of streptomycin per ml (GMEM-10% FBS) and supplemented in the case of BSR T7/5 cells with 1 mg of G-418 (Geneticin; Invitrogen-Life Technologies) per ml. The Utrecht variant of the Bucyrus strain of EAV (EAV Utr) was propagated in BHK-21 cells. For the production of stocks of the recombinant vaccinia virus vTF7.3 expressing the bacteriophage T7 RNA polymerase gene, rabbit kidney (RK-13) cells were used (14). The production and characterization of rabbit antisera specific for the extreme carboxy terminus of the GP2b and the ectodomain of the GP3 protein (raised against a synthetic peptide corresponding to residues 211 to 227 and residues 129 to 145, respectively) and for the ectodomain of the GP4 protein, designated αGP2b, αGP3, and αGP4, respectively, have been reported earlier (6, 38).
Plasmids.
The construction of the plasmids pAVI02, pAVI13, and pMRI14, expressing EAV ORF2b, -3, and -4, respectively, has been described elsewhere (6, 37, 38).
EAV infections.
Subconfluent monolayers of BHK-21 cells were infected with EAV at a multiplicity of infection (MOI) of ≥10 PFU as described earlier (38).
Independent expression studies using the vaccinia virus-T7 RNA polymerase transfection system.
Subconfluent monolayers of BSR T7/5 cells were washed with GMEM and infected with vTF7.3 in GMEM for 50 min at 37°C at an MOI of ≥10 PFU per cell. The cells were then transfected with plasmid DNA by using Lipofectin (Life Technologies), as described previously (38), and further incubated at 37°C. At 3 h postinfection (p.i.), 2 ml of prewarmed GMEM-10% FBS was added to the cells.
Metabolic labeling of intracellular proteins.
At the indicated time points, the culture fluid was removed and the cells were washed with prewarmed starvation medium (Dulbecco's modified Eagle's medium without l-cysteine and l-methionine [Invitrogen-Life Technologies] and supplemented with 5% dialyzed fetal calf serum, 10 mM HEPES [pH 7.4], and 0.2 mM l-methionine) and subsequently incubated in 800 μl of fresh starvation medium. Following an incubation period of 30 min, 80 μCi of [35S]cysteine (ICN) was added, and the cells were labeled for the indicated lengths of time at 39°C (EAV infections) or 37°C (independent expression). After the labeling, the cells were placed on ice and washed with ice-cold phosphate-buffered saline containing 50 mM CaCl2, 50 mM MgCl2, and 20 mM of the sulfhydryl-modifying agent N-ethylmaleimide (NEM) (Sigma-Aldrich). Next, the cells were lysed in ice-cold lysis buffer (20 mM Tris-HCl [pH 7.6], 150 mM NaCl, 1% Nonidet P-40 [NP-40], 0.5% sodium deoxycholate [DOC], and 0.1% sodium dodecyl sulfate [SDS] containing 1 μg each of aprotinin, leupeptin, and pepstatin A per ml) with 20 mM NEM. The lysate was cleared by centrifugation in a microcentrifuge for 15 min at 4°C and 20,000 × g. The pellet was discarded, and the supernatant was supplemented with EDTA to a final concentration of 5 mM. Alternatively, the labeling was followed by a rapid wash with prewarmed chase medium (GMEM-10% FCS containing 1 mM l-methionine, 2 mM l-cysteine hydrochloride monohydrate, and 10 mM HEPES [pH 7.4]). Subsequently, the cells were incubated in chase medium for different time periods, and the samples were further processed as described above.
Preparation of radiolabeled EAV particles.
Subconfluent monolayers of BHK-21 cells were infected with EAV at a high MOI, and labeled with [35S]cysteine as described previously (38). At 11 h p.i., the medium was harvested and cell debris was removed by low-speed centrifugation (10 min at 4,000 rpm and room temperature [RT] in a microcentrifuge). The supernatant was then mixed with 1/4 volume of 5× lysis buffer (100 mM Tris-HCl [pH 7.6], 150 mM NaCl, 5% NP-40, 2.5% DOC, 0.5% SDS, and 100 mM NEM containing 5 μg each of aprotinin, leupeptin, and pepstatin A per ml). The solution was cleared by centrifugation for 15 min at 20,000 × g in a microcentrifuge. The pellet was discarded, and the supernatant was supplemented with EDTA to a final concentration of 5 mM. Alternatively, the labeled virus was pelleted through a cushion of 20% (wt/wt) sucrose as described previously (38). The pellet was then dissolved in 1 ml of ice-cold 1× lysis buffer containing the aforementioned protease inhibitors and further processed as described above.
Virus incubation experiments.
[35S]cysteine-labeled EAV particles were prepared as described above. At 11 h p.i., the medium was harvested and cleared by centrifugation for 10 min at 1,700 × g and RT in a microcentrifuge. Equal aliquots of the supernatant were incubated in 1.5-ml microvials at 39, 4, or 39°C in the presence of 20 mM NEM or at 39°C in the presence of 100 mM diamide (Sigma-Aldrich). To further investigate the effect of diamide, equal portions of the supernatant were incubated in the presence of 0, 1, 3, 9, 27, and 81 mM diamide for 1 min at 4°C or at RT. Immediately after each treatment, the samples were mixed with 1/4 volume of 5× lysis buffer with protease inhibitors. To study the influence of pH, equal-size samples of the supernatants were mixed either with 9 volumes of 120 mM Tris-100 mM NaCl-1 mM EDTA adjusted with HCl to a pH of 6.3, 7.0, 7.8, or 8.6 or with 9 volumes of 60 mM acetic acid-100 mM NaCl-1 mM EDTA, pH 5.1. After a 30-min incubation at 39°C, the supernatants were loaded on a 2-ml cushion of ice-cold 20% (wt/wt) sucrose in 20 mM Tris-HCl (pH 7.6)-20 mM MgCl2 and centrifuged for 2 h in an SW50.1 rotor at 28,000 rpm and 4°C. The pellets were then lysed in 300 μl of ice-cold 1× lysis buffer and further processed as described above.
Sucrose density gradients.
For sucrose density gradient analysis, labeled virus was mixed with an equal volume of ice-cold 16% polyethylene glycol 6000 (Serva) in phosphate-buffered saline and stirred slowly overnight at 4°C. The pellet obtained after centrifugation for 30 min at 20,000 × g in a microcentrifuge at 4°C was gently resuspended in 20 mM 2-(N-morpholino)ethanesulfonic acid-30 mM Tris-100 mM NaCl-2.5 mM EDTA-2 mM EGTA (MNT) (pH 5.8) containing 0.4% Triton X-100 (TX-100) or in 20 mM Tris-100 mM NaCl-2.5 mM EDTA-2 mM EGTA (NT) (pH 7.6) containing 0.4% TX-100. When indicated, these solutions were supplemented with 5 mM dithiothreitol (DTT) to disrupt disulfide bonds. Aliquots of 200 μl of the samples were loaded onto 5 to 20% (wt/wt) sucrose gradients made up in MNT (pH 5.8)-0.1% TX-100 or in NT (pH 7.4)-0.1% TX-100 and were immediately subjected to centrifugation at 38,000 rpm for 52 h at 4°C with an SW41 rotor (Beckman). The gradients were collected in 15 serial fractions of 750 μl from the bottoms of the centrifugation tubes. Next, 1/4 volume of 5× lysis buffer with protease inhibitors was added to each fraction, and the samples were cleared by centrifugation for 15 min at 14,000 rpm and 4°C in a microcentrifuge. The pellets were discarded, and the supernatants were subjected to immunoprecipitations after the addition of EDTA to a final concentration of 5 mM. The pellets present at the bottom of the SW41 centrifugation tubes were dissolved in 1× lysis buffer and further processed as described above.
For sucrose gradient analysis of marker proteins, 25 μg of ovalbumin (Sigma) (45 kDa) and 25 μg of bovine albumin (Sigma) (66 kDa) were dissolved in 200 μl of MNT (pH 5.8)-0.4% TX-100, loaded onto a 5 to 20% sucrose gradient, and subjected to centrifugation under the conditions described above. After gradient fractionation, the proteins in each sample were precipitated by addition of 15 μl of 50% myoglobin (Calbiochem) and 235 μl of trichloroacetic acid (Merck) and incubation for 1 h on ice. The pellets obtained after centrifugation for 30 min at 14,000 rpm in a microcentrifuge at 4°C were resuspended in Laemmli sample buffer (LSB) (23) containing 50 mM DTT and analyzed by SDS-polyacrylamide (PAA) gel electrophoresis (SDS-PAGE). The gel was subsequently stained with GelCode blue stain reagent (Pierce) according to the instructions of the manufacturer.
Immunoprecipitation and gel electrophoresis.
Proteins were immunoprecipitated from cell lysates, solubilized virions, and sucrose gradient fractions as described previously (38). The washed immune complexes were resuspended in 20 μl of LSB without DTT for analysis of the proteins under nonreducing conditions or with 50 mM DTT for analysis in a reducing environment and were incubated for 5 min at 96°C. After centrifugation for 15 min at 14,000 rpm and 4°C in a microcentrifuge, the supernatants were analyzed in SDS-15% PAA gels. Following electrophoresis, the gels were fixed in 10% acetic acid-50% methanol-0.005% Coomassie brilliant blue R250 for 30 min at RT and incubated for another 30 min in 1 M sodium salicylate. Finally, the gels were dried on Whatman 3MM paper and exposed at −80°C to Kodak X-ray films or, for quantitative analyses, to storage phosphorimaging plates (Molecular Dynamics).
Extraction of radiolabeled proteins from PAA gels.
Autoradiographs were used to determine the positions in the dried SDS-PAA gels of the protein complexes that were to be extracted from the gel matrix. Next, the parts of the gels containing these protein complexes were excised and shred into pieces, which were subsequently taken up in LSB without DTT, heated for 5 min at 96°C, and incubated overnight at RT. The next day, the samples were centrifuged for 5 min at 14,000 rpm and RT in a microcentrifuge, and the supernatants were pipetted off and split into two equal portions. To one sample of each pair, DTT was added to a final concentration of 50 mM. The samples were again heated for 5 min at 96°C and analyzed again by SDS-PAGE as described above.
Endoglycosidase treatment.
Washed immunoprecipitates were resuspended in 0.5% SDS and heated for 5 min at 96°C. For endoglycosidase H (endo H) treatment, each sample was subsequently mixed with 1/9 volume of 10× G5 buffer (New England Biolabs) containing 10 μg each of aprotinin, leupeptin, and pepstatin A per ml. Next, 10 U of endo H (New England Biolabs), diluted in 1× G5 buffer, was added, and the mixtures were incubated for ≥16 h at 37°C. Finally, 1/2 volume of 3× LSB with or without 50 mM DTT was added to the samples, which were further processed as described above. N-Glycosidase F (PNGase F) digestions were carried out under the same conditions after addition of 1/9 volume of 10× G7 buffer (New England Biolabs) containing 10% NP-40 and 10 μg each of aprotinin, leupeptin, and pepstatin A per ml to the denatured protein samples. Ten units of PNGase F (New England Biolabs) per digestion mixture was used. Negative controls consisted of identical immunoprecipitates incubated in parallel under the same conditions but without enzyme.
RESULTS
Appearance of the GP2b, GP3, and GP4 proteins in EAV virions.
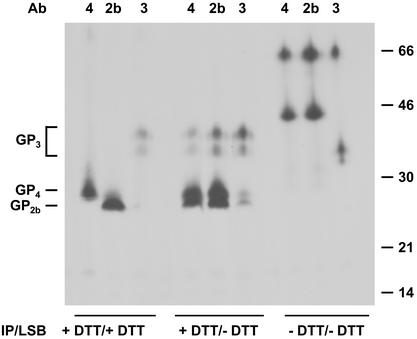
It was previously established that the GP2b, GP3, and GP4 proteins are minor structural components of EAV (6, 38). To study the possible interactions between these proteins and their oligomeric structure in virions, EAV-infected BHK-21 cells were labeled with [35S]cysteine, and the virions released into the culture medium were concentrated by sedimentation through a 20% (wt/wt) sucrose cushion. The GP2b, GP3, and GP4 proteins were immunoprecipitated from solubilized virus particles with specific antisera in the absence or presence of a 5 mM concentration of the reducing agent DTT and analyzed by SDS-PAGE under reducing and nonreducing conditions. As shown in Fig. 1, the three proteins were each specifically precipitated by the appropriate antiserum when the entire procedure was carried out under reducing conditions (lanes +DTT/+DTT). Thus, protein molecules of about 28 and 25 kDa were observed for GP4 and GP2b, respectively, while the two typical glycoprotein species of about 38 and 42 kDa were seen for GP3. After immunoprecipitation and analysis under nonreducing conditions (lanes −DTT/−DTT), however, the patterns obtained with the three different antisera were remarkably similar. In all cases, a complex with an apparent molecular mass of 66 kDa was observed. With the GP2b- and GP4-specific antisera an additional complex of 45 kDa was detected, and with αGP3 two extra protein species of around 40 kDa appeared. Based on their appearance and electrophoretic mobility, the latter material most probably represents monomeric forms of the GP3 protein. As indicated by the analysis of the immunocomplexes under reducing conditions (lanes −DTT/+DTT), the 66-kDa protein complexes are most likely composed of GP2b, GP3, and GP4, whereas the 45-kDa protein complexes probably consist of GP2b and GP4. These observations indicate that the GP2b, GP3, and GP4 proteins interact with each other.
FIG. 1.
Appearance of the GP2b, GP3, and GP4 proteins in EAV particles. EAV-infected BHK-21 cells were labeled with l-[35S]cysteine from 6.5 to 11 h p.i. After removal of cell debris from the cell culture medium by low-speed centrifugation, the extracellular virions were pelleted through a cushion of 20% (wt/wt) sucrose. The pellet was dissolved in lysis buffer, and immunoprecipitations (IP) with αGP2b (2b), αGP3 (3), or αGP4 (4) in the presence or absence of 5 mM DTT were carried out. The samples were finally dissolved in LSB with or without 50 mM DTT and analyzed in SDS-15% PAA gels. The numbers on the right are the molecular masses, in kilodaltons, of marker proteins analyzed in the same gel. Ab, antibody.
Composition of the 66- and 45-kDa complexes.
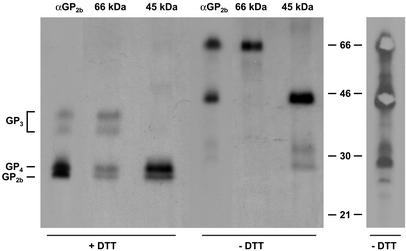
To study the composition of the 66- and 45-kDa complexes in more detail, concentrated [35S]cysteine-labeled EAV particles were dissolved in lysis buffer and incubated with αGP2b in the absence of a reducing agent. The immunoprecipitated material was separated by SDS-PAGE under nonreducing conditions, and the parts of the gel containing the 66- and 45-kDa protein complexes were excised (Fig. 2, right panel). The protein complexes were subsequently eluted from the gel slices and analyzed by SDS-PAGE under reducing (lanes +DTT) as well as nonreducing (lanes −DTT) conditions (Fig. 2, left panel). For comparison, the protein species precipitated by αGP2b under nonreducing conditions were also directly applied to the gel. Analysis by SDS-PAGE under nonreducing conditions (lanes −DTT) demonstrated that the gel extraction procedure had not affected the mobility of the 66- and 45-kDa protein complexes. After reduction, the 45-kDa complex resolved into two different products comigrating with the GP2b and GP4 proteins, while the 66-kDa complex disassembled into four species which comigrated with the GP2b, GP3, and GP4 proteins. Quantitative analyses revealed that the 45-kDa complex contains equimolar amounts of GP2b and GP4 and that the ratio between GP2b, GP3, and GP4 in the 66-kDa complex is 1:1:1 (data not shown). Collectively, these results demonstrate that the 66-kDa complex is a disulfide-bonded heterotrimer of GP2b, GP3, and GP4 and redefine the 45-kDa complex, previously identified as a homodimer of the GP2b protein (8), as a covalently linked heterodimer of GP2b and GP4.
FIG. 2.
Composition of the 66- and 45-kDa complexes. EAV-infected BHK-21 cells were labeled with l-[35S]cysteine from 6.5 to 11 h p.i. After removal of cell debris from the culture medium by low-speed centrifugation, the extracellular virions were pelleted through a cushion of 20% (wt/wt) sucrose. The pellet was dissolved in lysis buffer, and the 66- and 45-kDa complexes were immunoprecipitated with αGP2b and separated in an SDS-15% PAA gel under nonreducing conditions. Subsequently, the 66- and 45-kDa complexes were purified from the dried gel (right panel) and analyzed by SDS-PAGE under reducing (+DTT) and nonreducing (−DTT) conditions (left panel, lanes 66 kDa and 45 kDa). For comparison, the immune complexes obtained after incubation of aliquots of the solubilized virus pellet with αGP2b were run in parallel (left panel, lanes αGP2b). The numbers between the panels are the molecular masses, in kilodaltons, of marker proteins analyzed in the same gel.
Covalently linked GP2b/GP3/GP4 trimers in EAV-infected cells.
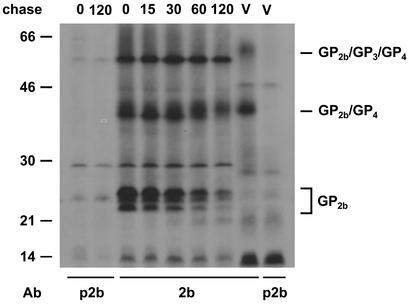
To investigate whether covalently linked GP2b/GP4 dimers and GP2b/GP3/GP4 trimers are formed intracellularly, EAV-infected BHK-21 cells were pulse-labeled for 15 min with l-[35S]cysteine starting at 8.25 h p.i. and then chased for different time periods. Next, the cells were lysed, and proteins were immunoprecipitated with αGP2b and analyzed by SDS-PAGE under nonreducing conditions. For comparison, immunoprecipitates prepared with the αGP2b serum from solubilized virions were run in parallel (Fig. 3). Consistent with a previous study (8), after the pulse-labeling the different monomeric conformations of GP2b as well as the 45-kDa GP2b-containing complexes were detected. During the chase the amount of monomeric GP2b protein declined, while the 45-kDa complex was gradually converted into a species with a slightly higher apparent molecular mass. Because this complex comigrates with the GP2b/GP4 dimer occurring in virus particles, it is likely to also represent such heterodimers. Further support for this interpretation comes from observations (described below) showing the occurrence of a similar complex in EAV-infected cells that can be precipitated both with anti-GP4 and anti-GP2b sera (see Fig. 9A). Moreover, the complexes detected after coexpression of the two proteins and in EAV-infected cells still comigrate in gels after their oligosaccharides have been removed by endo H digestion (see Fig. 9A). However, no 66-kDa GP2b/GP3/GP4 heterotrimers could be distinguished in the infected cells. The product of approximately 60 kDa presumably represents a host cell protein, since it was resistant to endo H (see Fig. 9) and PNGase F digestion and because a product with a similar electrophoretic mobility was observed after gel electrophoresis under reducing conditions.
FIG. 3.
Presence of covalently linked GP2b/GP3/GP4 trimers in EAV-infected cells. EAV-infected BHK-21 cells were pulse-labeled for 15 min with l-[35S]cysteine starting at 8.25 h p.i. and chased for the indicated time periods (in minutes). Next, cell lysates were prepared and immunoprecipitations were performed with αGP2b (2b) or with the corresponding preimmune serum (p2b). The immunoprecipitates were analyzed under nonreducing conditions in SDS-15% PAA gels. As a reference, the immune complexes obtained after incubation of an aliquot of solubilized EAV particles with αGP2b or the corresponding preimmune serum were run in parallel (lanes V). The numbers on the left are the molecular masses, in kilodaltons, of marker proteins analyzed in the same gel. Ab, antibody.
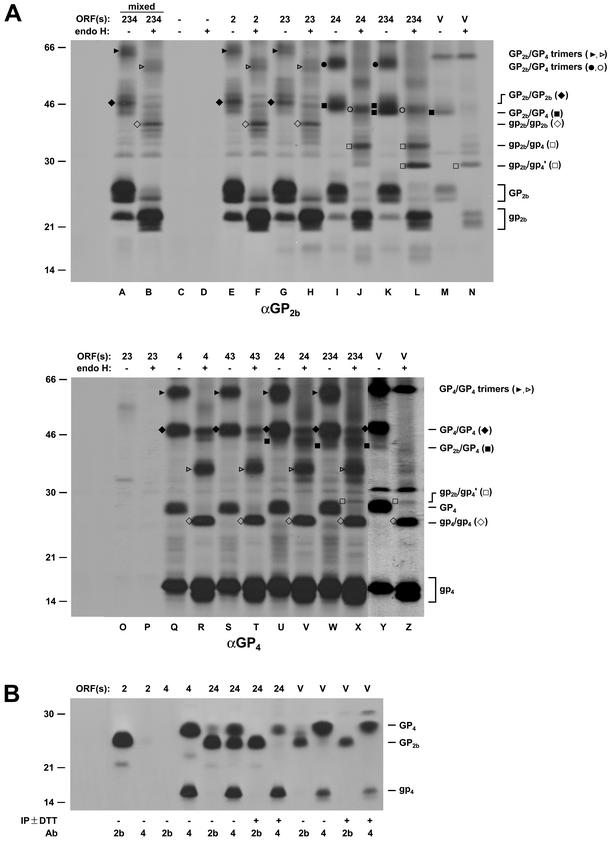
FIG. 9.
Coexpression of the ORFs encoding the GP2b, GP3, and GP4 proteins. EAV ORF2b, -3, and -4 were expressed in BSR T7/5 cells alone or together, as indicated at the top of the autoradiographs, by using the vaccinia virus-T7 RNA polymerase transfection system. The cells were pulse-labeled for 30 min with l-[35S]cysteine starting at 4.5 h p.i. (A) Cell lysates were subjected to immunoprecipitations with the αGP2b serum (upper panel) or the αGP4 serum (lower panel). The immunoprecipitates were treated (lanes +) or mock treated (lanes −) with endo H. As a control, lysates of cells that expressed only ORF2b, ORF3, or ORF4 were mixed in a 1:1:1 ratio and incubated with the αGP2b serum (lanes 234 mixed). The samples were analyzed in SDS-15% PAA gels under nonreducing conditions. For comparison, EAV-infected BHK-21 cells (lanes V) were pulse-labeled for 30 min with l-[35S]cysteine starting at 8.5 h after infection and further processed as described above. (B) Alternatively, immunoprecipitates (IP) prepared with the αGP2b (lanes 2) or αGP4 (lanes 4) serum in the presence or absence of 5 mM DTT were analyzed by SDS-PAGE under reducing conditions. Glycosylated protein species are indicated in uppercase letters and/or closed with symbols. Deglycosylated protein species are indicated in lowercase letters and/or with open symbols. The numbers on the left are the molecular masses, in kilodaltons, of marker proteins analyzed in the same gel. Ab, antibody.
Formation of the covalently linked GP2b/GP3/GP4 trimers in virions.
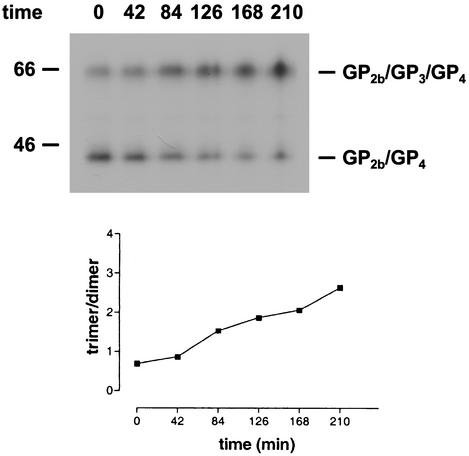
Our inability to detect disulfide-linked GP2b/GP3/GP4 trimers in EAV-infected cells suggested that these complexes are formed after virus release. To investigate this process, the culture supernatant of [35S]cysteine-labeled EAV-infected cells was split in equal portions, which were subsequently incubated at 39°C for different time periods. Next, the virions were solubilized and immunoprecipitations with αGP2b were carried out. The immunoprecipitates were analyzed by SDS-PAGE under nonreducing conditions (Fig. 4, upper panel). During the incubation at 39°C the amount of the disulfide-bonded GP2b/GP4 complex gradually decreased, with a concomitant increase of the amount of covalently linked GP2b/GP3/GP4 trimers. A linear correlation was observed when the ratio between the radiolabeling intensities of the GP2b/GP3/GP4 and the GP2b/GP4 complexes was plotted against time, indicating a constant rate of conversion from dimer to trimer during the period examined (Fig. 4, lower panel). Thus, shortly after their release from infected cells, EAV particles contain mainly disulfide-bonded GP2b/GP4 heterodimers, which are subsequently converted into cystine-linked GP2b/GP3/GP4 trimers through the covalent recruitment of GP3.
FIG. 4.
Formation of covalently linked GP2b/GP3/GP4 trimers. (Upper panel) The culture supernatant of [35S]cysteine-labeled EAV-infected cells was split into equal fractions and incubated at 39°C for the indicated time periods (in minutes). Next, the virions were dissolved by the addition of concentrated lysis buffer, and immunoprecipitations were performed with the serum directed against the GP2b protein. The numbers on the left are the molecular masses, in kilodaltons, of marker proteins analyzed in the same gel. (Lower panel) The ratio between the amounts of radiolabel incorporated into the GP2b/GP3/GP4 trimers and into the GP2b/GP4 dimers was determined by phosphorimager analysis and plotted against time.
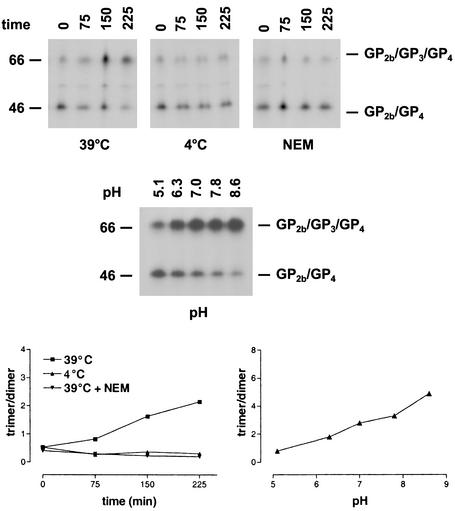
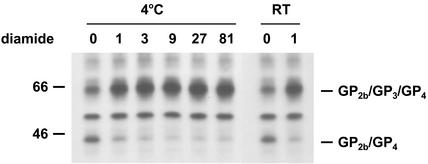
We further investigated this unusual postassembly process by testing the effect of different conditions on the covalent association of GP3 with the disulfide-bonded GP2b/GP4 heterodimers (Fig. 5). To study the effect of temperature, equal volumes of the cell culture supernatant from [35S]cysteine-labeled EAV-infected cells were incubated at the standard temperature of 39°C or at 4°C for different time periods. It turned out that no covalently linked GP2b/GP3/GP4 trimers were formed at 4°C. In another experiment, [35S]cysteine-labeled EAV particles were incubated for 30 min at different pH values. It appeared that within the pH range investigated, the conversion of the disulfide-bonded GP2b/GP4 heterodimers into cystine-linked GP2b/GP3/GP4 trimers occurred more efficiently under alkaline conditions. The effect of some compounds reactive to sulfhydryl groups was also investigated. Incubation of [35S]cysteine-labeled EAV particles with the thiol-blocking agent NEM for different lengths of time completely blocked the formation of the covalently linked GP2b/GP3/GP4 trimers. Likewise, equal-size samples of the cell culture supernatant from [35S]cysteine-labeled EAV-infected cells were incubated for 1 min at RT in the presence of a 1 mM concentration of the oxidizing agent diamide (22). Under these conditions, the disulfide-bonded GP2b/GP4 dimers were rapidly and almost completely converted to cystine-linked GP2b/GP3/GP4 trimers (Fig. 6). However, a small fraction of the GP2b/GP4 complexes remained as disulfide-bonded dimers even when higher concentrations of diamide or longer incubation times were used (data not shown). Very similar results were obtained when the experiments were repeated at 4°C (Fig. 6). Finally, we studied the stability of the heteromeric complexes by heating the immunoprecipitates in the presence of 2, 5, and 8% SDS. It appeared that the covalently linked GP2b/GP4 and GP2b/GP3/GP4 complexes stayed intact under each of these conditions (data not shown).
FIG. 5.
Effect of different conditions on the conversion of disulfide-bonded GP2b/GP4 dimers into covalently linked GP2b/GP3/GP4 trimers. (Upper panel) The culture supernatant of [35S]cysteine-labeled EAV-infected cells was split into equal fractions and incubated at 4, 39, or 39°C in the presence of 20 mM NEM for the indicated times. Alternatively, equal-size aliquots of the same culture were adjusted to different pHs and incubated for 30 min at 39°C. The samples were subsequently processed as described in the legend to Fig. 4. The numbers on the left are the molecular masses, in kilodaltons, of marker proteins analyzed in the same gel. (Lower panel) The ratio between the amounts of radiolabel incorporated into the GP2b/GP3/GP4 trimers and into the GP2b/GP4 dimers was determined by phosphorimager analysis and plotted against time.
FIG. 6.
Effect of the oxidizing agent diamide on the conversion of disulfide-bonded GP2b/GP4 dimers into covalently linked GP2b/GP3/GP4 trimers. The culture supernatant of [35S]cysteine-labeled EAV-infected cells was split into equal fractions and incubated in the presence of different concentrations of diamide for 1 min at 4°C or RT. The samples were subsequently processed as described in the legend to Fig. 4. The numbers on the left are molecular masses in kilodaltons.
Higher-order structure of the covalently linked GP2b/GP4 and GP2b/GP3/GP4 complexes.
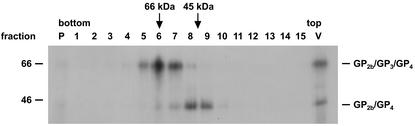
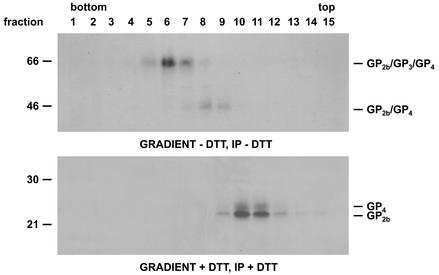
The very rapid conversion of the disulfide-bonded GP2b/GP4 dimers into cystine-linked GP2b/GP3/GP4 trimers in the presence of diamide suggested that the GP3 protein is already noncovalently associated with the former complex prior to the formation of the latter. To address this issue and to investigate whether the disulfide-linked GP2b/GP4 and GP2b/GP3/GP4 complexes occur as higher-order structures, concentrated [35S]cysteine-labeled EAV particles were dissolved in 0.4% TX-100 at pH 5.8 and loaded onto a 5 to 20% (wt/wt) sucrose density gradient of the same pH and containing 0.1% TX-100. After centrifugation, the gradients were fractionated, immunoprecipitations were performed with the αGP2b serum, and the samples were analyzed by SDS-PAGE under nonreducing conditions (Fig. 7). The results showed that the disulfide-bonded GP2b/GP4 and GP2b/GP3/GP4 complexes occupied different positions in the gradient, peaking in fractions 8/9 and 6/7, respectively. These observations imply that, under the analytic conditions used, GP3 molecules are not associated with the covalently linked GP2b/GP4 heterodimers.
FIG. 7.
Higher-order structure of the covalently linked GP2b/GP4 and GP2b/GP3/GP4 complexes. Concentrated [35S]cysteine-labeled EAV particles were dissolved in MNT-0.4% Triton (pH 5.8) and loaded onto a 5 to 20% (wt/wt) sucrose density gradient. After centrifugation for 52 h at 38,000 rpm and 4°C in an SW41 rotor, fractions were collected and subjected to immunoprecipitations with αGP2b. The immunoprecipitates were analyzed in SDS-15% PAA gels under nonreducing conditions. Fraction numbers are indicated above the autoradiograph. In lane P, the material from the bottom of the gradient was analyzed. As a reference, the immune complexes obtained after incubation of an aliquot of solubilized EAV particles with αGP2b was run in parallel (lane V). Arrows indicate the positions of the marker proteins ovalbumin (45 kDa) and bovine albumin (66 kDa) in another 5 to 20% (wt/wt) sucrose density gradient of the same spin. The numbers on the left are the molecular masses, in kilodaltons, of marker proteins analyzed in the same gel.
In order to determine the oligomeric state of the covalently linked GP2b/GP4 and GP2b/GP3/GP4 complexes, their sedimentation rates were compared with those of two marker proteins. The GP2b/GP4 complex appeared to peak in the same fractions as the 45-kDa ovalbumin marker, while the GP2b/GP3/GP4 complex cosedimented with the bovine albumin marker of 66 kDa. Accordingly, under the analytic conditions tested, neither of the two complexes occurs as multimers or is noncovalently associated with GP3. Equivalent results were obtained when the complexes had been dissolved in TX-100 at pH 7.4 (Fig. 8, upper panel).
FIG. 8.
Stability of the GP2b/GP4 and GP2b/GP3/GP4 complexes after disulfide bond disruption. Concentrated [35S]cysteine-labeled EAV particles were dissolved in NT-0.4% Triton (pH 7.4) in the presence or absence of 5 mM DTT and loaded onto a 5 to 20% (wt/wt) sucrose density gradient. After centrifugation for 52 h at 38,000 rpm and 4°C in an SW41 rotor, fractions were collected and subjected to immunoprecipitations (IP) with a mixture of αGP2b and αGP4 in the presence (lower panel) or absence (upper panel) of 5 mM DTT. The immunoprecipitates were analyzed by SDS-PAGE under reducing (lower panel) or nonreducing (upper panel) conditions. Fraction numbers are indicated above the autoradiographs. The numbers on the left are the molecular sizes, in kilodaltons, of marker proteins analyzed in the same gel.
To investigate the consequences of disulfide bond disruption for the stability of complexes between GP2b, GP3 and GP4, concentrated [35S]cysteine-labeled EAV particles were dissolved in 0.4% TX-100 at pH 7.4 in the presence or absence of DTT, and loaded onto a 5 to 20% (wt/wt) sucrose density gradient. After centrifugation and collection of gradient fractions, immunoprecipitations with αGP2b and αGP4 were performed, and the samples were analyzed by SDS-PAGE (Fig. 8). The covalently linked GP2b/GP3/GP4 and GP2b/GP4 complexes peaked again in fractions 6 and 7 and fractions 8 and 9, respectively (Fig. 8, upper panel). In contrast, when the covalent interactions had been disrupted with DTT before centrifugation, the GP2b and GP4 proteins sedimented less deeply into the gradient, accumulating in fractions 11 and 12 (Fig. 8, lower panel). These are sedimentation rates to be expected for their monomeric forms, which indicates that after disruption of the disulfide bridges, the GP2b/GP3/GP4 trimers as well as the GP2b/GP4 dimers fall apart.
Coexpression of the ORFs encoding the GP2b, GP3, and GP4 proteins.
To study the interactions between the minor envelope glycoproteins of EAV after independent expression of the corresponding ORFs, BSR T7/5 cells were infected with vTF7.3 and transfected with various combinations of plasmids carrying the GP2b-, GP3-, or GP3-coding sequences behind a bacteriophage T7 RNA polymerase promoter. At 4.5 h p.i., the proteins were metabolically labeled during a 30-min pulse. Next, the proteins were dissolved in lysis buffer, immunoprecipitated with αGP2b or αGP4, and analyzed by SDS-PAGE under reducing and nonreducing conditions. When indicated, the glycosylation status of the immunoprecipitated proteins was monitored biochemically by assaying the susceptibility of their N-linked oligosaccharide chains to endo H cleavage. Immunoprecipitates obtained after incubation of lysates from pulse-labeled EAV-infected BHK-21 cells with αGP2b or αGP4 were analyzed in parallel (Fig. 9).
When produced in the absence of any other EAV protein and analyzed by SDS-PAGE under nonreducing conditions, the GP2b protein appeared mainly in its characteristic monomeric conformational forms. In addition, a small fraction of the protein was found as homomultimers (i.e., homodimers and homotrimers, etc.) with various conformations (Fig. 9A, lane E). Endo H digestion reduced the apparent molecular masses of the different GP2b species proportionately to their number of oligosaccharide side chains (Fig. 9A, lane F). After analysis under reducing conditions, the GP2b protein migrated as a single product of 25 kDa (Fig. 9B).
After independent expression of EAV ORF4, the unprocessed 15-kDa GP4 protein, the N-glycosylated 28-kDa GP4 monomer without signal sequence, and a collection of N-glycosylated GP4-containing multimers were observed in an SDS-PAA gel run under nonreducing conditions (compare lanes Q and R of Fig. 9A). Gel electrophoresis under reducing conditions yielded two protein species of 15 and 28 kDa, respectively, which indicates that the GP4 multimers represent homomeric disulfide-bonded complexes of the GP4 protein (Fig. 9B).
Upon coexpression of the ORFs coding for the GP2b and GP4 proteins, a considerable portion of GP2b remained monomeric (Fig. 9A, lanes I and J). In addition, multiple protein complexes were immunoprecipitated, which migrated slightly faster than the cystine-linked GP2b homomultimers. Several observations indicate that these complexes, which had apparent molecular masses of approximately 44 and 57 kDa, respectively, represent disulfide-bonded GP2b/GP4 assemblies. Endo H treatment reduced the size of the 44- and 57-kDa complexes to approximately 35 and 41 kDa, respectively, which again migrated somewhat faster than the deglycosylated GP2b multimers. The reduction of the size of the 44-kDa complex after endo H digestion to 35 kDa is consistent with the removal of four N-linked glycans, matching a GP2b/GP4 heterodimer. Moreover, gel electrophoresis under reducing conditions showed that the αGP2b serum pulled down a protein comigrating with GP4, while a product with the same electrophoretic mobility as GP2b was coprecipitated by αGP4. These coprecipitations were not observed when the incubations with the antisera were carried out in the presence of 5 mM DTT (Fig. 9B). The 44- and 35-kDa complexes precipitated with αGP2b could not be identified by using the αGP4 serum (Fig. 9A, lanes U and V), since their presence was obscured by the disulfide-bonded GP4 homodimers and by a background band, respectively.
No covalently linked heterotrimeric complexes of GP2b, GP3, and GP4 were detected after coexpression of EAV ORF2b, -3, and -4 (Fig. 9A, lanes K, L, W, and X). Interestingly, however, after endo H digestion, the disulfide-bonded GP2b/GP4 dimers migrated as two species. One of them had the same electrophoretic mobility as the GP2b/GP4 complex formed after coexpression of ORF2b and ORF4 (GP2b/GP4), whereas the other migrated considerably faster (GP2b/GP4′ in Fig. 9A, lanes L and X).
The electrophoretic mobilities of the protein species immunoprecipitated by αGP2b from lysates of cells expressing ORF2b alone or together with ORF3 were identical (Fig. 9A, lanes G and H). Likewise, no differences were observed between the apparent molecular masses of the proteins pulled down with αGP4 from lysates of cells expressing ORF4 alone or in combination with ORF3 (Fig. 9A, lanes S and T). As a control, a mixture of lysates from cells expressing either ORF2b, ORF3, or ORF4 was prepared and incubated with the αGP2b serum. Under these conditions, no complexes between GP2b and GP4 were immunoprecipitated (Fig. 9A, lanes A and B), indicating that the disulfide-bonded GP2b/GP4 dimers observed following the coexpression of ORF2b, -3, and 4 were not formed during or after cell lysis.
For comparison, immunoprecipitates from lysates of EAV-infected cells that had been pulse-labeled for 30 min with l-[35S]cysteine were analyzed in parallel. SDS-PAGE under nonreducing conditions revealed that the covalently linked GP2b/GP4 dimers were immunoprecipitated with the αGP2b as well as the αGP4 serum (Fig. 9A, lanes M and Y). Strikingly, after endo H treatment the disulfide-bonded GP2b/GP4 complex comigrated with GP2b/GP4′ (Fig. 9A, lanes N and Z). With the αGP4 serum, the unprocessed GP4 protein and the N-glycosylated GP4 monomers and homomultimers were immunoprecipitated as well, while the αGP2b serum also pulled down the different monomeric conformations of GP2b. Gel electrophoresis under reducing conditions confirmed the precipitation of part of the GP4 molecules by αGP2b and of a fraction of the GP2b protein by αGP4 (Fig. 9B). The coprecipitation of GP2b and GP4 was prevented when the immunoprecipitations were performed in the presence of 5 mM DTT. Finally, the GP2b and GP4 proteins produced by cells transfected with ORF2b and/or ORF4 expression plasmids showed an electrophoretic mobility under reducing conditions that was similar to the mobility of those proteins in EAV virions (data not shown). From these data, we conclude that the 45-kDa complexes observed in EAV-infected cells represents GP2b/GP4 heterodimers.
To investigate whether the increase of the electrophoretic mobility of the cystine-linked GP2b/GP4 dimers in the presence of the GP3 protein is caused by a different extent of N glycosylation of these complexes, PNGase F digestions were carried out. In contrast to endo H, PNGase F cleaves off N-linked oligosaccharide side chains from proteins irrespective of their maturation state. Furthermore, while PNGase F removes the entire N-linked glycans, endo H leaves the first monosaccharide, i.e., the N-acetylglucosamine that tethers each oligosaccharide to the Asn residue, attached to the polypeptide chain. In comparison to endo H digestion, PNGase F treatment caused a slight increase in the mobility of the disulfide-bonded GP2b/GP4 dimers as well as of the cystine-linked GP2b/GP4′ complexes, consistent with the different specificities of both enzymes (data not shown). Thus, because the difference in their mobility remained the same, the two different-sized covalently linked GP2b/GP4 complexes apparently do not differ in their N-glycosylation patterns.
Based on these data, we conclude that when the GP2b and GP4 proteins are produced together, they can form disulfide-bonded heterodimers but that no covalently linked GP2b/GP3/GP4 trimers are formed when GP3 is also cosynthesized. The presence of GP3, however, apparently affects the conformation of the cystine-linked GP2b/GP4 dimers, resulting in GP2b/GP4 molecules with an electrophoretic mobility indistinguishable from that of the disulfide-bonded GP2b/GP4 dimers assembled in EAV-infected cells. The endoglycosidase digestions showed that the compaction of the GP2b/GP4 complexes under the influence of GP3 takes place in a pre-Golgi compartment.
DISCUSSION
The minor structural glycoproteins GP2b, GP3, and GP4 occur in mature EAV particles as covalently linked heterotrimeric complexes. Surprisingly, the GP3 protein seems not to be covalently associated with the disulfide-linked GP2b/GP4 heterodimers when the virions leave the cells. The protein appears, however, to noncovalently interact with these heterodimers already within the infected cell, but disulfide linkage takes place only after virus particles have been released. These observations reveal an unusual type of postassembly biochemical virus modification.
The covalent recruitment of GP3 into the cystine-linked GP2b/GP4 complex is a relatively slow process. The half-life of the disulfide-bonded GP2b/GP4 dimer in virions at 39°C was approximately 2.5 h under the experimental conditions used. Furthermore, the formation of the covalently linked heterotrimeric complexes appeared to be strongly temperature dependent, i.e., the conversion of the GP2b/GP4 complex into disulfide-bonded GP2b/GP3/GP4 trimers was not detectable at 4°C. The trimerization process was also sensitive to pH, with the rate of conversion being reduced at lower pH. The formation in EAV-infected cells of covalently linked GP2b/GP3/GP4 trimers may thus be suppressed by the acidic conditions in the late compartments of the exocytic pathway. The alkylating agent NEM, which preferentially reacts with free thiol groups, also inhibited the generation of disulfide-bonded GP2b/GP3/GP4 trimers. In contrast, the oxidizing reagent diamide strongly promoted the trimerization process. At concentrations of ≥1 mM the GP3 protein apparently becomes covalently bound to the cystine-linked GP2b/GP4 dimer almost instantaneously. The presence in the virus preparation of damaged EAV particles may explain the failure to convert all disulfide-bonded GP2b/GP4 dimers into covalently linked GP2b/GP3/GP4 trimers after treatment of virions with diamide.
Much effort was spent to establish whether the GP3 protein is already associated with the GP2b/GP4 complex in a noncovalent manner before being covalently recruited into the trimeric complex. The instantaneous formation of disulfide-bonded GP2b/GP3/GP4 trimers in the presence of diamide at 4°C is a strong indication that such an interaction does exist, given the restricted lateral diffusion of membrane proteins at that temperature. However, the existence of noncovalently linked complexes between the disulfide-bonded GP2b/GP4 dimers and GP3 could not be confirmed by sedimentation analysis of detergent-solubilized virions in sucrose gradients, most likely because these complexes were disrupted during solubilization of the EAV particles. Independent support for a noncovalent interaction of GP3 with the cystine-linked GP2b/GP4 dimers came from coexpression studies. Although the combined expression in cells of the ORFs encoding the three minor EAV envelope glycoproteins did not result in any detectable formation of covalently linked heterotrimeric structures, the presence of GP3 led to an increase of the electrophoretic mobility of the disulfide-bonded GP2b/GP4 dimers under nonreducing but not under reducing conditions. The fast-migrating GP2b/GP4 complexes formed in the presence of GP3 comigrated with the disulfide-bonded GP2b/GP4 dimers of virions. These results suggest that the association, transiently or permanently, of the GP3 protein with the initially formed GP2b/GP4 complexes mediates a conformational maturation of these dimeric structures involving the formation of additional or alternative disulfide linkages. Together with the results of the diamide experiments, these observations imply that the GP3 protein does interact with the disulfide-bonded GP2b/GP4 dimers intracellularly and that they are thus collectively incorporated into virus particles. Further support for this conclusion came from the recent finding that the incorporation of the GP2b and GP4 proteins in EAV particles is fully dependent on the presence of the GP3 protein (R. Wieringa et al., unpublished data).
Until now, no heteromeric complexes of the GP2b, GP4, and GP3 proteins have been found in virions of the other three arteriviruses. However, the GP4 protein of the IAF-Klop strain of PRRSV could be coprecipitated with the GP3 protein from lysates of virus-infected cells (25). Interestingly, while Western blot analyses have suggested that the GP3 protein of the Lelystad strain of PRRSV is incorporated into or associated with the viral envelope (34), studies on the ORF3 product of the IAF-Klop strain of PRRSV indicate that it is rather a soluble or weakly membrane-associated protein (18, 25), as is the case for the GP3 protein of LDV (12).
Nothing is known yet about the functions of the minor glycoproteins in the arteriviral life cycle, except that all three are essential. (27, 31). Since it has been established here that these proteins occur in virions as a complex, it is conceivable that they function collectively. A role in viral targeting seems most plausible now that this role can no longer be attributed to the GP5/M complex. Exchange of the ectodomain of the EAV GP5 protein with that of LDV or PRRSV in the context of a full-length EAV cDNA construct was found not to alter viral tropism (10). Similarly, recombinant PRRSV particles in which the ectodomain of the M protein had been replaced by that of EAV or LDV still displayed the host cell range of PRRSV (35) An involvement of the heterotrimeric complex in the actual cell entry process of EAV is also feasible. For several viruses, it has been proposed that free thiol groups or specific disulfide bonds are involved in thiol-disulfide interchanges triggering membrane fusion (1, 13, 15, 26, 29, 32). Accordingly, the infectivity of a number of viruses (1, 13, 15, 17, 26, 29, 32), including arteriviruses (data not shown) (11), can be inhibited by thiol-blocking or reducing agents, although such observations have to be considered with caution due to possible side effects of these agents. The sensitivity of LDV to DTT, for example, was interpreted to imply a critical function for the M/VP-3P disulfide linkage in virus infectivity, but the present results open the alternative explanation that the disruption of cystine bridges within or between the minor envelope glycoproteins of LDV may have led to the inactivation of the virus. We were unable to establish whether the covalent attachment of GP3 to the disulfide-bonded GP2b/GP4 complex is essential for EAV infectivity. Treatment of freshly released virions with diamide, which catalyzes the instantaneous covalent linkage of GP3 to the disulfide-bonded GP2b/GP4 dimer, did not measurably enhance viral infectivity (data not shown). In fact, we cannot exclude the possibility that the formation of the cystine-linked GP2b/GP3/GP4 trimers following the release of EAV particles from BHK-21 cells is an artifact of the in vitro culture system. This does not rule out the possibility that under normal circumstances, the formation of disulfide-bonded GP2b/GP3/GP4 trimers takes place at a later stage of the infectious cycle, e.g., during virus attachment or entry.
The presence in EAV particles of heterotrimers of the minor envelope glycoproteins prompts us to reconsider the arterivirus structure. EAV particles presumably contain an isometric nucleocapsid (20, 24). In the surrounding membrane the proteins GP5 and M are predominant and occur in equimolar amounts. As was suggested earlier (7), dimers of these proteins constitute the basic matrix of the viral envelope. This study indicates that virions also contain equal numbers of the three minor envelope glycoproteins. While the molar ratios between GP3 or GP4 and either of the two major envelope proteins in virions have not been determined, earlier calculations revealed that the molar ratio of GP2b compared to GP5 and M is approximately 1:25 (6). Assuming that the GP2b/GP4/GP3 trimers adopt regular positions in the viral envelope, these estimations lead us to propose that these trimeric complexes, possible assembled as higher-order structures, are positioned at the vertices of the isometric nucleocapsid.
The maturation of virions after their assembly is not uncommon among viruses. This process often involves proteolytic cleavages catalyzed by viral or host cell proteases. Well-studied examples are the retroviruses, which are released from the cells as immature particles (28). Shortly after budding from the plasma membrane, the particles undergo a proteolytic maturation step that is essential for viral infectivity and results in the condensation of the inner virion structure and capsid shell (36) Other examples are found among nonenveloped viruses. Nodaviruses, for instance, are initially assembled as immature virions, which are converted to infectious virus particles by postassembly cleavage of the structural precursor protein α. This maturation results in increased particle stability and is essential for virus infectivity (16, 30). Likewise, adenovirus infectivity is critically dependent on the cleavage of multiple virion proteins by the 23-kDa viral protease (9). However, to our knowledge, the extracellular formation of a new disulfide bond(s) in viral membrane proteins has not been described earlier. It represents yet another interesting feature of the arteriviruses.
Acknowledgments
We are grateful to Bernard Moss for providing the recombinant vaccinia virus vTF7.3 and to Karl-Klaus Conzelmann for making the BSR T7/5 cell line available. We thank Linda Cox for critical reading of the manuscript.
REFERENCES
- 1.Abell, B. A., and D. T. Brown. 1993. Sindbis virus membrane fusion is mediated by reduction of glycoprotein disulfide bridges at the cell surface. J. Virol. 67:5496-5501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buchholz, U. J., S. Finke, and K. K. Conzelmann. 1999. Generation of bovine respiratory syncytial virus (BRSV) from cDNA: BRSV NS2 is not essential for virus replication in tissue culture, and the human RSV leader region acts as a functional BRSV genome promoter. J. Virol. 73:251-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.den Boon, J. A., M. F. Kleijnen, W. J. M. Spaan, and E. J. Snijder. 1996. Equine arteritis virus subgenomic mRNA synthesis: analysis of leader-body junctions and replicative-form RNAs. J. Virol. 70:4291-4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.den Boon, J. A., E. J. Snijder, E. D. Chirnside, A. A. F. de Vries, M. C. Horzinek, and W. J. M. Spaan. 1991. Equine arteritis virus is not a togavirus but belongs to the coronaviruslike superfamily. J. Virol. 65:2910-2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Vries, A. A. F., E. D. Chirnside, P. J. Bredenbeek, L. A. Gravestein, M. C. Horzinek, and W. J. M. Spaan. 1990. All subgenomic mRNAs of equine arteritis virus contain a common leader sequence. Nucleic Acids Res. 18:3241-3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Vries, A. A. F., E. D. Chirnside, M. C. Horzinek, and P. J. M. Rottier. 1992. Structural proteins of equine arteritis virus. J. Virol. 66:6294-6303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Vries, A. A. F., S. M. Post, M. J. B. Raamsman, M. C. Horzinek, and P. J. M. Rottier. 1995. The two major envelope proteins of equine arteritis virus associate into disulfide-linked heterodimers. J. Virol. 69:4668-4674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Vries, A. A. F., M. J. B. Raamsman, H. A. van Dijk, M. C. Horzinek, and P. J. M. Rottier. 1995. The small envelope glycoprotein (GS) of equine arteritis virus folds into three distinct monomers and a disulfide-linked dimer. J. Virol. 69:3441-3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D'Halluin, J. C. 1995. Virus assembly. Curr. Top. Microbiol. Immunol. 199:47-66. [PubMed] [Google Scholar]
- 10.Dobbe, J. C., Y. van der Meer, W. J. M. Spaan, and E. J. Snijder. 2001. Construction of chimeric arteriviruses reveals that the ectodomain of the major glycoprotein is not the main determinant of equine arteritis virus tropism in cell culture. Virology 288:283-294. [DOI] [PubMed] [Google Scholar]
- 11.Faaberg, K. S., C. Even, G. A. Palmer, and P. G. Plagemann. 1995. Disulfide bonds between two envelope proteins of lactate dehydrogenase-elevating virus are essential for viral infectivity. J. Virol. 69:613-617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Faaberg, K. S., and P. G. Plagemann. 1997. ORF 3 of lactate dehydrogenase-elevating virus encodes a soluble, nonstructural, highly glycosylated, and antigenic protein. Virology 227:245-251. [DOI] [PubMed] [Google Scholar]
- 13.Fenouillet, E., R. Barbouche, J. Courageot, and R. Miquelis. 2001. The catalytic activity of protein disulfide isomerase is involved in human immunodeficiency virus envelope-mediated membrane fusion after CD4 cell binding. J. Infect. Dis. 183:744-752. [DOI] [PubMed] [Google Scholar]
- 14.Fuerst, T. R., E. G. Niles, F. W. Studier, and B. Moss. 1986. Eukaryotic transient-expression system based on recombinant vaccinia virus that synthesizes bacteriophage T7 RNA polymerase. Proc. Natl. Acad. Sci. USA 83:8122-8126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gallagher, T. M. 1996. Murine coronavirus membrane fusion is blocked by modification of thiols buried within the spike protein. J. Virol. 70:4683-4690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gallagher, T. M., and R. R. Rueckert. 1988. Assembly-dependent maturation cleavage in provirions of a small icosahedral insect ribovirus. J. Virol. 62:3399-3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glomb-Reinmund, S., and M. Kielian. 1998. The role of low pH and disulfide shuffling in the entry and fusion of Semliki Forest virus and Sindbis virus. Virology 248:372-381. [DOI] [PubMed] [Google Scholar]
- 18.Gonin, P., H. Mardassi, C. A. Gagnon, B. Massie, and S. Dea. 1998. A nonstructural and antigenic glycoprotein is encoded by ORF3 of the IAF-Klop strain of porcine reproductive and respiratory syndrome virus. Arch. Virol. 143:1927-1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hedges, J. F., U. B. R. Balasuriya, and N. J. MacLachlan. 1999. The open reading frame 3 of equine arteritis virus encodes an immunogenic glycosylated, integral membrane protein. Virology 264:92-98. [DOI] [PubMed] [Google Scholar]
- 20.Horzinek, M. C., J. Maess, and R. Laufs. 1971. Studies on the substructure of togaviruses. II. Analysis of equine arteritis, rubella, bovine viral diarrhea, and hog cholera viruses. Arch. Gesamte Virusforsch. 33:306-318. [PubMed] [Google Scholar]
- 21.Hyllseth, B. 1973. Structural proteins of equine arteritis virus. Arch. Gesamte Virusforsch. 40:177-188. [DOI] [PubMed] [Google Scholar]
- 22.Kosower, N. S., and E. M. Kosower. 1987. Formation of disulfides with diamide. Methods Enzymol. 143:264-270. [DOI] [PubMed] [Google Scholar]
- 23.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 24.Magnusson, P., B. Hyllseth, and H. Marusyk. 1970. Morphological studies on equine arteritis virus. Arch. Gesamte Virusforsch. 30:105-112. [DOI] [PubMed] [Google Scholar]
- 25.Mardassi, H., P. Gonin, C. A. Gagnon, B. Massie, and S. Dea. 1998. A subset of porcine reproductive and respiratory syndrome virus GP3 glycoprotein is released into the culture medium of cells as a non-virion-associated and membrane-free (soluble) form. J. Virol. 72:6298-6306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mirazimi, A., M. Mousavi-Jazi, V. A. Sundqvist, and L. Svensson. 1999. Free thiol groups are essential for infectivity of human cytomegalovirus. J. Gen. Virol. 80:2861-2865. [DOI] [PubMed] [Google Scholar]
- 27.Molenkamp, R., H. van Tol, B. C. D. Rozier, Y. van der Meer, W. J. M. Spaan, and E. J. Snijder. 2000. The arterivirus replicase is the only viral protein required for genome replication and subgenomic mRNA transcription. J. Gen. Virol. 81:2491-2496. [DOI] [PubMed] [Google Scholar]
- 28.Nermut, M. V., and D. J. Hockley. 1996. Comparative morphology and structural classification of retroviruses. Curr. Top. Microbiol. Immunol. 214:1-24. [DOI] [PubMed] [Google Scholar]
- 29.Ryser, H. J., E. M. Levy, R. Mandel, and G. J. DiSciullo. 1994. Inhibition of human immunodeficiency virus infection by agents that interfere with thiol-disulfide interchange upon virus-receptor interaction. Proc. Natl. Acad. Sci. USA 91:4559-4563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schneemann, A., W. Zhong, T. M. Gallagher, and R. R. Rueckert. 1992. Maturation cleavage required for infectivity of a nodavirus. J. Virol. 66:6728-6734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Snijder, E. J., H. van Tol, K. W. Pedersen, M. J. B. Raamsman, and A. A. F. de Vries. 1999. Identification of a novel structural protein of arteriviruses. J. Virol. 73:6335-6345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sturman, L. S., C. S. Ricard, and K. V. Holmes. 1990. Conformational change of the coronavirus peplomer glycoprotein at pH 8.0 and 37°C correlates with virus aggregation and virus-induced cell fusion. J. Virol. 64:3042-3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Berlo, M. F., P. J. M. Rottier, W. J. M. Spaan, and M. C. Horzinek. 1986. Equine arteritis virus-induced polypeptide synthesis. J. Gen. Virol. 67:1543-1549. [DOI] [PubMed] [Google Scholar]
- 34.van Nieuwstadt, A. P., J. J. M. Meulenberg, A. van Essen-Zanbergen, A. Petersen-den Besten, R. J. Bende, R. J. M. Moormann, and G. Wensvoort. 1996. Proteins encoded by open reading frames 3 and 4 of the genome of Lelystad virus (Arteriviridae) are structural proteins of the virion. J. Virol. 70:4767-4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Verheije, M. H., T. J. M. Welting, H. T. Jansen, P. J. M. Rottier, and J. J. M. Meulenberg. Chimeric arteriviruses generated by swapping of the M protein ectodomain rule out a role of this domain in viral targeting. Virology 302:364-373. [DOI] [PubMed]
- 36.Vogt, V. M. 1996. Proteolytic processing and particle maturation. Curr. Top. Microbiol. Immunol. 214:95-131. [DOI] [PubMed] [Google Scholar]
- 37.Weiland, E., S. Bolz, F. Weiland, W. Herbst, M. J. B. Raamsman, P. J. M. Rottier, and A. A. F. de Vries. 2000. Monoclonal antibodies directed against conserved epitopes on the nucleocapsid protein and the major envelope glycoprotein of equine arteritis virus. J. Clin. Microbiol. 38:2065-2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wieringa, R., A. A. F. de Vries, M. J. B. Raamsman, and P. J. M. Rottier. 2002. Characterization of two new structural glycoproteins, GP3 and GP4, of equine arteritis virus. J. Virol. 76:10829-10840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zeegers, J. J. W., B. A. M. van der Zeijst, and M. C. Horzinek. 1976. The structural proteins of equine arteritis virus. Virology 73:200-205. [DOI] [PubMed] [Google Scholar]