Abstract
Respiratory syncytial virus (RSV) infection in the neonate can alter respiratory rates, i.e., lead to episodes of apnea. We show that RSV G glycoprotein reduces respiratory rates associated with the induction of substance P (SP) and G glycoprotein-CX3CR1 interaction, an effect that is inhibited by treatment with anti-G glycoprotein, anti-SP, or anti-CX3CR1 monoclonal antibodies. These data suggest new approaches for treating some aspects of RSV disease.
Respiratory syncytial virus (RSV) is recognized as the most important cause of serious lower respiratory tract illness in infants and young children worldwide (17). Most children are infected with RSV by age two, and repeated infections can occur throughout life, with serious complications most often occurring in elderly patients and patients with compromised cardiac, immune, or pulmonary systems (7, 8, 10, 13, 38). RSV infection in infants and young children is often associated with bronchiolitis and an increase in respiratory rates; apnea can also occur (3). In one study of 274 infants under 6 months of age, 56 infants (20.4%) had apnea with RSV infection (3). The mechanisms by which RSV infection causes apnea are not understood. One potential mechanism for RSV-associated alteration in respiratory rates is induction of pulmonary substance P (SP). SP has been associated previously with excitatory effects related to respiratory rates (1, 2, 9, 12, 28), and pulmonary levels of SP are increased during RSV infection in the BALB/c mouse (46). In addition, there is some indication that SP has an important role in bronchial hypersensitivity reactions in children (25), and elevated levels of tachykinins have been recovered from the airways of patients with asthma and chronic obstructive pulmonary disease (18, 31, 42). RSV is the predominant cause of bronchiolitis (29), and bronchiolitis has clinical features of obstructed airway disease. SP is present in sensory, parasympathetic, and sympathetic neurons in airways and has been shown elsewhere to have proinflammatory effects on immune cells and to mediate mucus production, vascular leakage or edema, and smooth muscle contraction (5, 23, 30, 32, 33, 36). In the mouse model, induction of SP during RSV infection appears to be related to the presence of RSV G and/or SH glycoprotein (46). The RSV G glycoprotein has been shown elsewhere to be associated with a number of proinflammatory effects (43, 45, 46) and may contribute to the pathogenesis of RSV disease in several ways. Studies of mice have shown that RSV G glycoprotein modifies chemokine (43) and cytokine (45) expression, alters pulmonary leukocyte recruitment (45), and sensitizes for pulmonary eosinophilia (11, 40, 41). BALB/c mice immunized with vaccinia virus expressing G glycoprotein, purified G glycoprotein, or formalin-treated RSV produce an exaggerated CD4+ T-cell response with increased Th2-type cytokine expression and pulmonary eosinophilia when challenged with RSV (11, 27).
Structurally, the RSV G glycoprotein is of particular interest as it contains a CX3C motif at amino acid positions 182 to 186 in the central conserved region and has the ability to functionally mimic the CX3C chemokine fractalkine (FKN) (44). FKN has been shown previously to bind to neurons and microglia expressing its receptor, CX3CR1 (21, 24), and induces an excitatory effect that mediates the release of neuronal products such as SP. Accordingly, CX3C chemokine mimicry by the RSV G glycoprotein may alter signal transduction between the immune system and the nervous system, alter immune responses, and modulate disease pathogenesis associated with RSV infection. We hypothesized that RSV infection might alter respiratory rates through G glycoprotein CX3C interaction with CX3CR1 and subsequent induction of SP.
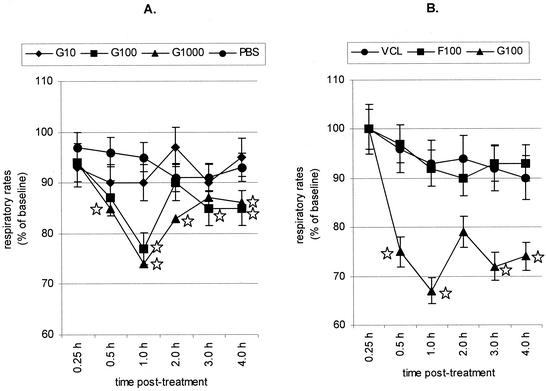
To address the possibility that RSV G glycoprotein might alter respiratory rates, we examined respiratory rates in unrestrained, spontaneously breathing 6- to 10-week-old female BALB/c mice, free of specific pathogens (Jackson Laboratories, Bar Harbor, Maine), maintained at the National Jewish Medical and Research Center under a protocol approved by the Institutional Animal Care and Use Committee. Respiratory rates were determined by barometric whole-body plethysmography with single-chamber whole-body plethysmographs (Buxco, Troy, N.Y.) (37) at intervals between 0.25 and 4 h posttreatment (Fig. 1A). Mice were intravenously (i.v.) injected with 100 μl of purified, endotoxin-free RSV G glycoprotein at concentrations of 10 nM (G10), 100 nM (G100), or 1,000 nM (G1000) via the tail vein and intermittently placed in the individual plethysmographs for subsequent monitoring of respiratory rates at the designated time points of the study. The changes in respiratory rates were expressed as percentages of baseline values for each animal. The obtained values were then averaged for each group, and the mean values were expressed for each treatment. Statistical significance with a P value of <0.05 was determined by analysis of variance with the Bonferroni correction for multiple comparisons of the means. Baseline respiratory rates (350 to 370 breaths/min) did not differ significantly among any of the groups examined. Purification of RSV G glycoproteins was carried out as previously described (44). Western blot analysis of the G glycoprotein preparations with anti-G glycoprotein monoclonal antibody (131-2G) yielded two distinct bands at approximately 90 and 45 kDa, and no detectable bands were revealed with anti-F glycoprotein monoclonal antibody (131-2A). Treatment with 10 nM G glycoprotein or phosphate-buffered saline (PBS) had no significant effect on respiratory rates; however, treatment with 100 or 1,000 nM G glycoprotein dramatically reduced respiratory rates beginning at 0.5 h postinjection, suggesting a dose-dependent effect on respiratory rates. The peak reduction in respiratory rate occurred at 1 h postinjection, but some reduction in respiratory rate was observed through the 4-h-postinjection period. The respiratory rates in mice treated with 100 nM G glycoprotein returned to baseline at 2 h posttreatment, i.e., respiratory rates of PBS-treated mice, but were reduced below the baseline rate at 3 and 4 h postinjection. Experiments examining the duration of this effect through 12 h postinjection showed that respiratory rates returned to baseline by 6 h postinjection. Mice treated i.v. with 100 nM purified, endotoxin-free F glycoprotein (F100), the other RSV major surface glycoprotein, or 1 μg of control, endotoxin-free uninfected Vero cell lysate (VCL) isolated in a similar manner as F and G glycoproteins showed no decrease in respiratory rate (Fig. 1B). Purification of RSV F glycoprotein was carried out as previously described (20). Western blot analysis of the purified F glycoprotein detected by anti-F glycoprotein monoclonal antibody (131-2A) yielded a distinct band at approximately 70 kDa and no distinct bands detected by anti-G glycoprotein monoclonal antibody (131-2G). These results indicate that RSV G glycoprotein rapidly (within 1 h) decreases respiratory rates in mice.
FIG. 1.
Respiratory rates associated with G and F glycoproteins. (A) G glycoprotein. BALB/c mice were i.v. treated with purified RSV G glycoprotein isolated from RSV-infected Vero cells (G10, G100, and G1000, respectively) or PBS. ⋆, significant differences (P < 0.05) compared with PBS-treated group. (B) Comparison of F and G glycoproteins. BALB/c mice were i.v. treated with purified RSV F (F100) or G (G100) glycoprotein or uninfected VCL control. ⋆, significant differences (P < 0.05) compared with VCL-treated group. The mean respiratory rates ± standard errors from eight individual BALB/c mice were determined at time points between 0.25 and 4 h posttreatment.
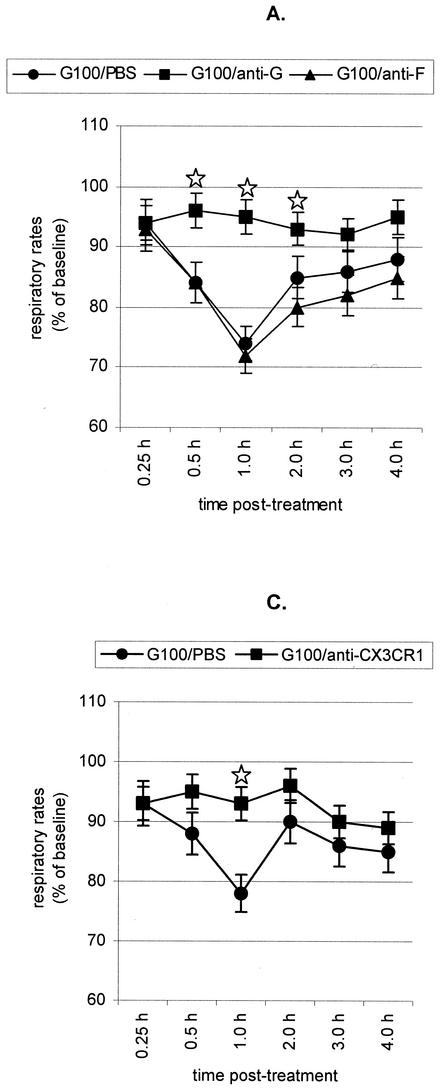
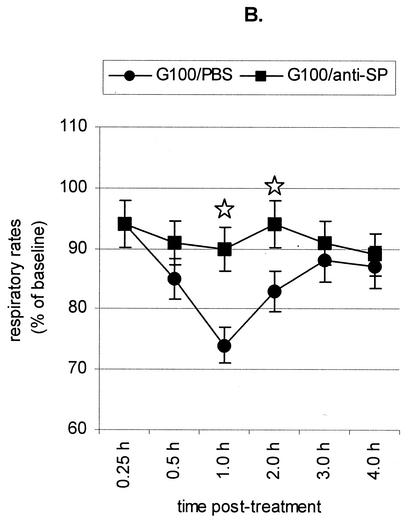
To better understand the mechanisms associated with G glycoprotein depression of respiratory rates, mice were intraperitoneally (i.p.) administered 10 μg of anti-SP (clone NC1/34; PharMingen, San Diego, Calif.) or anti-G glycoprotein (clone131-2G) or anti-CX3CR1 (clone 2A9-1; MBL, Nagoya, Japan) monoclonal antibodies diluted in PBS prior to treatment with 100 nM G glycoprotein (Fig. 2). Eight mice were examined for each treatment. Neither PBS nor 10 μg of anti-F glycoprotein monoclonal antibody altered the G glycoprotein-associated reduction in respiratory rates (Fig. 2A). In contrast, treatment with anti-G glycoprotein (Fig. 2A), anti-SP (Fig. 2B), or anti-CX3CR1 monoclonal antibodies (Fig. 2C) abolished G glycoprotein-associated reduction in respiratory rates. A study of SP levels in cell-free bronchoalveolar lavage (BAL) specimens from the monoclonal antibody-treated mice supports the role of SP in G glycoprotein-mediated reduction in respiratory rates. SP levels in cell-free BAL fluid were analyzed by a competitive enzyme-linked immunoassay kit per the manufacturer's instructions (Cayman Chemical, Ann Arbor, Mich.) as previously described (46). The assay is based on the competition between free SP and an SP tracer for a limited number of SP-specific binding sites. SP levels were examined prior to treatment and between 1 and 2 h posttreatment. The baseline level of SP in BAL specimens from naïve or PBS-treated naïve mice ranged from 200 to 350 pg/ml. SP levels posttreatment with 10 nM G glycoprotein increased slightly and ranged from 300 to 750 pg/ml; however, SP levels dramatically increased to 2,200 to 2,800 pg/ml in mice treated with 100 nM G glycoprotein. In contrast, SP levels were considerably reduced in 100 nM G glycoprotein-treated mice pretreated with anti-SP (250 to 500 pg/ml), anti-G glycoprotein (400 to 800 pg/ml), or anti-CX3CR1 (250 to 800 pg/ml). These results indicate that G glycoprotein treatment induces SP through CX3CR1 and that SP expression contributes to lower respiratory rates.
FIG. 2.
Antibody treatment and respiratory rates. (A) BALB/c mice were i.v. treated with purified RSV G glycoprotein (G100) following i.p. administration of either PBS (G100/PBS) or anti-G glycoprotein (G100/anti-G) or anti-F glycoprotein monoclonal antibody (G100/anti-F). ⋆, significant differences (P < 0.05) compared with G100/PBS-treated group. (B) BALB/c mice were i.v. treated with purified RSV G glycoprotein (G100) following i.p. administration of either PBS (G100/PBS) or anti-SP monoclonal antibody (G100/anti-SP). ⋆, significant differences (P < 0.05) compared with G100/PBS-treated group. (C) BALB/c mice were i.v. treated with purified RSV G glycoprotein (G100) following i.p. administration of either PBS (G100/PBS) or anti-CX3CR1 monoclonal antibody (G100/anti-CX3CR1). ⋆, significant differences (P < 0.05) compared with G100/PBS-treated group. The mean respiratory rates ± standard errors from eight individual BALB/c mice were determined at time points between 0.25 and 4 h posttreatment.
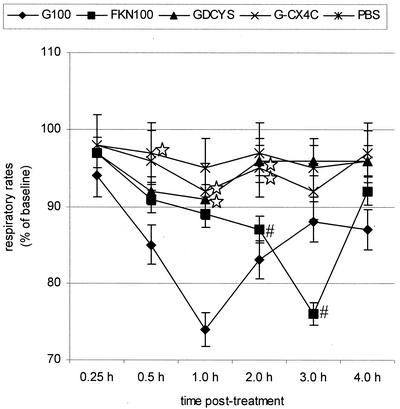
To determine if the G glycoprotein-associated reduction in respiratory rates was linked to the CX3C motif in G glycoprotein, we examined the respiratory rates in mice given (i.v.) 100 nM FKN (R&D Systems, Minneapolis, Minn.) or 100 nM RSV G glycoproteins with mutations at the CX3C motif, i.e., a deletion in the CX3C motif (GDCYS) or an Ala insertion in the CX3C motif (G-CX4C) (Fig. 3). Purified G glycoprotein mutants, i.e., GDCYS and G-CX4C, were prepared from Vero cells stably transfected with plasmid DNA encoding the G glycoprotein mutants under G418 selection as previously described (44). As hypothesized, FKN-treated mice had reduced respiratory rates beginning at 2.0 h posttreatment that lasted through 4 h posttreatment, while the mutated G glycoproteins (GDCYS and G-CX4C) showed no reduction in respiratory rates (Fig. 3). The slight decrease in respiratory rates that occurred with G-CX4C treatment suggests that addition of an Ala residue to the CX3C motif does not completely inhibit CX3CR1 interaction. This is similar to earlier results showing that G glycoprotein peptides lacking a CX3C motif did not block G glycoprotein or FKN binding to CX3CR1 but that a peptide with a CX4C motif partially blocked G glycoprotein or FKN binding to CX3CR1 (44). The SP levels in BAL specimens of mice treated with the G-CX4C protein were between 1,600 and 2,000 pg/ml, and those for mice treated with GDCYS were between 800 and 1,100 pg/ml, demonstrating that alteration of the CX3C motif in the G glycoprotein results in less SP production. As noted above, the baseline levels of SP are 200 to 350 pg/ml in naïve mice and 2,200 to 2,800 pg/ml in mice treated with intact G glycoprotein.
FIG. 3.
G glycoprotein with an altered CX3C site does not reduce respiratory rates. BALB/c mice were i.v. treated with FKN, G glycoprotein with a deletion in the CX3C motif (GDCYS), or G glycoprotein with an Ala insertion in the CX3C motif (G-CX4C). ⋆, significant differences (P < 0.05) compared with G100/PBS-treated group. #, significant differences (P < 0.05) compared with PBS-treated group. The mean respiratory rates ± standard errors from eight individual BALB/c mice were determined at time points between 0.25 and 4 h posttreatment.
The results from this study show that both FKN and RSV G glycoprotein can reduce respiratory rates in mice, although with different timing. Possible explanations for differences in G glycoprotein and FKN effects include conformational differences between the proteins; differences in CX3CR1 binding avidity; and induction of other cytokines, chemokines, or mediators that may affect respiratory rates. The results suggest a possible mechanism for RSV-induced apnea, i.e., reduction in respiratory rates associated with G glycoprotein binding to CX3CR1 and induction of SP. Neurons and microglia have been shown elsewhere to express CX3CR1 and SP receptors (14, 24, 26), and G glycoprotein has been shown previously to contain a CX3C motif and bind to CX3CR1 (44). Since FKN has been shown previously to bind CX3CR1 on neurons (14), this binding may mediate release of neuronal products such as SP, an effect that may also be mediated by G glycoprotein. The results from this study link the previous observations that the RSV G glycoprotein mimics CX3C chemokine actions through CX3CR1 interaction (44) and also induces SP (46), i.e., the G glycoprotein CX3C-CX3CR1 interaction induces SP. Furthermore, we have shown that anti-SP antibody treatment decreases pulmonary inflammation associated with primary RSV infection within 24 h of treatment and up to 6 days after RSV infection (15). Thus, RSV G glycoprotein CX3C-CX3CR1 interaction and associated induction of SP might contribute to several aspects of RSV disease.
Several viruses have now been shown to express determinants that mimic host proteins. Poxviruses offer an example of structural and functional virus mimicry of host immunoregulatory proteins, particularly cytokines, chemokines, and their receptors, a phenomenon that is likely related to viral evasion of host defenses (19, 22, 39). Viral proteins also mediate molecular mimicry of host proteins, particularly herpesvirus proteins. One example is molecular mimicry of host antigens by a determinant in the coat protein of herpes simplex virus type 1 that triggers self-reactive T-cell clones to destroy host tissue; however, mutant herpes simplex virus type 1 viruses with deletions in the amino acids coding for this determinant do not induce autoimmune disease (47). We similarly found that altering the CX3C site in the G glycoprotein reduced or eliminated G glycoprotein binding to CX3CR1 (44) and the associated reduction in respiratory rates.
The reduced respiratory rates associated with G glycoprotein or FKN CX3C interaction with CX3CR1 and SP expression identify a mechanism by which FKN expression may contribute to inflammatory diseases. FKN expression has been associated elsewhere with several inflammatory diseases including glomerulonephritis (4), cardiac allograft rejection (34), human renal inflammation (6, 16), rheumatoid arthritis and rat adjuvant-induced arthritis (35), and acute and chronic central nervous system inflammation in the rodent (35). It is possible that some of the proinflammatory effects associated with the expression of FKN may occur through induction of SP.
The link established by the data in this study among G glycoprotein binding to CX3CR1, induction of SP, and subsequent depressed respiratory rates and the previously established link between SP and increased pulmonary inflammation (46) support the concept that the G glycoprotein CX3C motif is likely important to some aspects of RSV disease and suggest new approaches for preventing and treating RSV disease. The apparent dose-dependent effect of G glycoprotein on respiratory rates suggests that attenuated RSV vaccine candidates would be less likely to alter respiratory rates through this mechanism. Structural modifications to the G glycoprotein CX3C motif to prevent binding to CX3CR1 may improve the safety of live and/or subunit RSV vaccines. In addition, administration of antibodies, drugs, or agents that inhibit the interaction between G glycoprotein and CX3CR1 or the actions of SP may be beneficial in treating some aspects of RSV disease.
Acknowledgments
R. A. Tripp and A. Dakhama contributed equally to the work from their respective laboratories.
This research was supported in part by grants from the National Institutes of Health (HL-60015 and HL-36577) and by Environmental Protection Agency grant R825702 to E.W.G.
We thank Annette Balhorn (National Jewish Medical and Research Center, Denver, Colo.) for her technical assistance.
REFERENCES
- 1.Balzamo, E., P. Joanny, J. G. Steinberg, C. Oliver, and Y. Jammes. 1996. Mechanical ventilation increases substance P concentration in the vagus, sympathetic, and phrenic nerves. Am. J. Respir. Crit. Care Med. 153:153-157. [DOI] [PubMed] [Google Scholar]
- 2.Bonham, A. C. 1995. Neurotransmitters in the CNS control of breathing. Respir. Physiol. 101:219-230. [DOI] [PubMed] [Google Scholar]
- 3.Bruhn, F. W., S. T. Mokrohisky, and K. McIntosh. 1977. Apnea associated with respiratory syncytial virus infection in young infants. J. Pediatr. 90:382-386. [DOI] [PubMed] [Google Scholar]
- 4.Chen, S., K. B. Bacon, L. Li, G. E. Garcia, Y. Xia, D. Lo, D. A. Thompson, M. A. Siani, T. Yamamoto, J. K. Harrison, and L. Feng. 1998. In vivo inhibition of CC and CX3C chemokine-induced leukocyte infiltration and attenuation of glomerulonephritis in Wistar-Kyoto (WKY) rats by vMIP-II. J. Exp. Med. 188:193-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi, D. C., and O. J. Kwon. 1998. Neuropeptides and asthma. Curr. Opin. Pulm. Med. 4:16-24. [DOI] [PubMed] [Google Scholar]
- 6.Cockwell, P., S. J. Chakravorty, J. Girdlestone, and C. O. Savage. 2002. Fractalkine expression in human renal inflammation. J. Pathol. 196:85-90. [DOI] [PubMed] [Google Scholar]
- 7.Falsey, A. R., J. J. Treanor, R. F. Betts, and E. E. Walsh. 1992. Viral respiratory infections in the institutionalized elderly: clinical and epidemiologic findings. J. Am. Geriatr. Soc. 40:115-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Falsey, A. R., and E. E. Walsh. 2000. Respiratory syncytial virus infection in adults. Clin. Microbiol. Rev. 13:371-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fuller, R. W., D. L. Maxwell, C. M. Dixon, G. P. McGregor, V. F. Barnes, S. R. Bloom, and P. J. Barnes. 1987. Effect of substance P on cardiovascular and respiratory function in subjects. J. Appl. Physiol. 62:1473-1479. [DOI] [PubMed] [Google Scholar]
- 10.Glezen, W. P., S. B. Greenberg, R. L. Atmar, P. A. Piedra, and R. B. Couch. 2000. Impact of respiratory virus infections on persons with chronic underlying conditions. JAMA 283:499-505. [DOI] [PubMed] [Google Scholar]
- 11.Graham, B. S., T. R. Johnson, and R. S. Peebles. 2000. Immune-mediated disease pathogenesis in respiratory syncytial virus infection. Immunopharmacology 48:237-247. [DOI] [PubMed] [Google Scholar]
- 12.Gray, P. A., J. C. Rekling, C. M. Bocchiaro, and J. L. Feldman. 1999. Modulation of respiratory frequency by peptidergic input to rhythmogenic neurons in the pre-Botzinger complex. Science 286:1566-1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hall, C. B. 2001. Respiratory syncytial virus and parainfluenza virus. N. Engl. J. Med. 344:1917-1928. [DOI] [PubMed] [Google Scholar]
- 14.Harrison, J. K., Y. Jiang, S. Chen, Y. Xia, D. Maciejewski, R. K. McNamara, W. J. Streit, M. N. Salafranca, S. Adhikari, D. A. Thompson, P. Botti, K. B. Bacon, and L. Feng. 1998. Role for neuronally derived fractalkine in mediating interactions between neurons and CX3CR1-expressing microglia. Proc. Natl. Acad. Sci. USA 95:10896-10901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haynes, L. M., J. Tonkin, L. J. Anderson, and R. A. Tripp. 2002. Neutralizing anti-F glycoprotein and anti-substance P antibody treatment effectively reduces infection and inflammation associated with respiratory syncytial virus infection. J. Virol. 76:6873-6881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holdsworth, S. R., A. R. Kitching, and P. G. Tipping. 2000. Chemokines as therapeutic targets in renal disease. Curr. Opin. Nephrol. Hypertens. 9:505-511. [DOI] [PubMed] [Google Scholar]
- 17.Institute of Medicine. 1986. New vaccine development: establishing priorities, diseases of importance in developing countries. National Academy Press, Washington, D.C. [PubMed]
- 18.Joos, G. F., K. O. De Swert, and R. A. Pauwels. 2001. Airway inflammation and tachykinins: prospects for the development of tachykinin receptor antagonists. Eur. J. Pharmacol. 429:239-250. [DOI] [PubMed] [Google Scholar]
- 19.Kotwal, G. J. 2000. Poxviral mimicry of complement and chemokine system components: what's the end game? Immunol. Today 21:242-248. [DOI] [PubMed] [Google Scholar]
- 20.Kurt-Jones, E. A., L. Popova, L. Kwinn, L. M. Haynes, L. P. Jones, R. A. Tripp, E. E. Walsh, M. W. Freeman, D. T. Golenbock, L. J. Anderson, and R. W. Finberg. 2000. Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nat. Immunol. 1:398-401. [DOI] [PubMed] [Google Scholar]
- 21.Maciejewski-Lenoir, D., S. Chen, L. Feng, R. Maki, and K. B. Bacon. 1999. Characterization of fractalkine in rat brain cells: migratory and activation signals for CX3CR-1-expressing microglia. J. Immunol. 163:1628-1635. [PubMed] [Google Scholar]
- 22.McFadden, G., A. Lalani, H. Everett, P. Nash, and X. Xu. 1998. Virus-encoded receptors for cytokines and chemokines. Semin. Cell Dev. Biol. 9:359-368. [DOI] [PubMed] [Google Scholar]
- 23.McGillis, J. P., M. L. Organist, and D. G. Payan. 1987. Substance P and immunoregulation. Fed. Proc. 46:196-199. [PubMed] [Google Scholar]
- 24.Meucci, O., A. Fatatis, A. A. Simen, and R. J. Miller. 2000. Expression of CX3CR1 chemokine receptors on neurons and their role in neuronal survival. Proc. Natl. Acad. Sci. USA 97:8075-8080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakai, S., Y. Iikura, K. Akimoto, and K. Shiraki. 1991. Substance P-induced cutaneous and bronchial reactions in children with bronchial asthma. Ann. Allergy 66:155-161. [PubMed] [Google Scholar]
- 26.Oh, S. B., P. B. Tran, S. E. Gillard, R. W. Hurley, D. L. Hammond, and R. J. Miller. 2001. Chemokines and glycoprotein 120 produce pain hypersensitivity by directly exciting primary nociceptive neurons. J. Neurosci. 21:5027-5035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Openshaw, P. J. 1995. Immunopathological mechanisms in respiratory syncytial virus disease. Semin. Immunopathol. 17:187-201. [DOI] [PubMed] [Google Scholar]
- 28.Palmer, J. B., and P. J. Barnes. 1987. Neuropeptides and airway smooth muscle function. Am. Rev. Respir. Dis. 136:S50-S54. [DOI] [PubMed] [Google Scholar]
- 29.Panitch, H. B. 2001. Bronchiolitis in infants. Curr. Opin. Pediatr. 13:256-260. [DOI] [PubMed] [Google Scholar]
- 30.Pernow, B. 1985. Role of tachykinins in neurogenic inflammation. J. Immunol. 135:812s-815s. [PubMed] [Google Scholar]
- 31.Piedimonte, G. 2001. Neural mechanisms of respiratory syncytial virus-induced inflammation and prevention of respiratory syncytial virus sequelae. Am. J. Respir. Crit. Care Med. 163:S18-S21. [DOI] [PubMed] [Google Scholar]
- 32.Rameshwar, P. 1997. Substance P: a regulatory neuropeptide for hematopoiesis and immune functions. Clin. Immunol. Immunopathol. 85:129-133. [DOI] [PubMed] [Google Scholar]
- 33.Reynolds, P. N., M. D. Holmes, and R. Scicchitano. 1997. Role of tachykinins in bronchial hyper-responsiveness. Clin. Exp. Pharmacol. Physiol. 24:273-280. [DOI] [PubMed] [Google Scholar]
- 34.Robinson, L. A., C. Nataraj, D. W. Thomas, D. N. Howell, R. Griffiths, V. Bautch, D. D. Patel, L. Feng, and T. M. Coffman. 2000. A role for fractalkine and its receptor (CX3CR1) in cardiac allograft rejection. J. Immunol. 165:6067-6072. [DOI] [PubMed] [Google Scholar]
- 35.Ruth, J. H., M. V. Volin, G. K. Haines III, D. C. Woodruff, K. J. Katschke, Jr., J. M. Woods, C. C. Park, J. C. Morel, and A. E. Koch. 2001. Fractalkine, a novel chemokine in rheumatoid arthritis and in rat adjuvant-induced arthritis. Arthritis Rheum. 44:1568-1581. [DOI] [PubMed] [Google Scholar]
- 36.Said, S. I. 1987. Influence of neuropeptides on airway smooth muscle. Am. Rev. Respir. Dis. 136:S52-S58. [DOI] [PubMed] [Google Scholar]
- 37.Schwarze, J., M. Makela, G. Cieslewicz, A. Dakhama, M. Lahn, T. Ikemura, A. Joetham, and E. W. Gelfand. 1999. Transfer of the enhancing effect of respiratory syncytial virus infection on subsequent allergic airway sensitization by T lymphocytes. J. Immunol. 163:5729-5734. [PubMed] [Google Scholar]
- 38.Shay, D. K., R. C. Holman, G. E. Roosevelt, M. J. Clarke, and L. J. Anderson. 2001. Bronchiolitis-associated mortality and estimates of respiratory syncytial virus-associated deaths among US children, 1979-1997. J. Infect. Dis. 183:16-22. [DOI] [PubMed] [Google Scholar]
- 39.Smith, G. L. 1999. Vaccinia virus immune evasion. Immunol. Lett. 65:55-62. [DOI] [PubMed] [Google Scholar]
- 40.Sparer, T. E., S. Matthews, T. Hussell, A. J. Rae, B. Garcia-Barreno, J. A. Melero, and P. J. Openshaw. 1998. Eliminating a region of respiratory syncytial virus attachment protein allows induction of protective immunity without vaccine-enhanced lung eosinophilia. J. Exp. Med. 187:1921-1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tebbey, P. W., M. Hagen, and G. E. Hancock. 1998. Atypical pulmonary eosinophilia is mediated by a specific amino acid sequence of the attachment (G) protein of respiratory syncytial virus. J. Exp. Med. 188:1967-1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tomaki, M., M. Ichinose, M. Miura, Y. Hirayama, H. Yamauchi, N. Nakajima, and K. Shirato. 1995. Elevated substance P content in induced sputum from patients with asthma and patients with chronic bronchitis. Am. J. Respir. Crit. Care Med. 151:613-617. [DOI] [PubMed] [Google Scholar]
- 43.Tripp, R. A., L. Jones, and L. J. Anderson. 2000. Respiratory syncytial virus G and/or SH glycoproteins modify CC and CXC chemokine mRNA expression in the BALB/c mouse. J. Virol. 74:6227-6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tripp, R. A., L. P. Jones, L. M. Haynes, H. Zheng, P. M. Murphy, and L. J. Anderson. 2001. CX3C chemokine mimicry by respiratory syncytial virus G glycoprotein. Nat. Immunol. 2:732-738. [DOI] [PubMed] [Google Scholar]
- 45.Tripp, R. A., D. Moore, L. Jones, W. Sullender, J. Winter, and L. J. Anderson. 1999. Respiratory syncytial virus G and/or SH protein alters Th1 cytokines, natural killer cells, and neutrophils responding to pulmonary infection in BALB/c mice. J. Virol. 73:7099-7107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tripp, R. A., D. Moore, J. Winter, and L. J. Anderson. 2000. Respiratory syncytial virus infection and G and/or SH protein expression contribute to substance P, which mediates inflammation and enhanced pulmonary disease in BALB/c mice. J. Virol. 74:1614-1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao, Z. S., F. Granucci, L. Yeh, P. A. Schaffer, and H. Cantor. 1998. Molecular mimicry by herpes simplex virus-type 1: autoimmune disease after viral infection. Science 279:1344-1347. [DOI] [PubMed] [Google Scholar]