Successful transmission by viruses in the face of vigorous innate and acquired host immunity requires the ability to evade, obstruct, or subvert critical elements that mediate host antiviral responses. To that end, viruses with larger genomes, such as poxviruses, encode multiple classes of immunomodulatory proteins that have evolved specifically to inhibit such diverse processes as apoptosis, the production of interferons, chemokines, and inflammatory cytokines, and the activity of cytotoxic T lymphocytes (CTLs), natural killer (NK) cells, complement, and antibodies. Often, the evolutionary origins of these virus-encoded immunomodulatory proteins are difficult to trace. The obvious sequence similarity between some immunomodulatory poxvirus genes and the cDNA versions of related cellular counterparts suggests that they were once captured by ancestral retrotranscription and/or recombination events and then reassorted into individual virus isolates during coevolution with vertebrate hosts. However, other poxviral immunomodulators have no known cellular counterpart or have putative functions that cannot be predicted based on similarity to known cellular proteins. The origins of these orphan regulators may be obscure, but their potential for immune subversion can be profound.
ADVANCES IN VIRAL GENOMICS: GENES APLENTY
In general, poxvirus genes that are centrally located in the genome tend to be relatively conserved within members of the Chordopoxvirinae family and encode proteins that perform common essential molecular functions such as replication and virion assembly. Terminally located genes tend to be more variable and express an array of proteins that mediate the biological specificity of infections through mechanisms such as host range restriction and modulation of host antiviral responses (63). To date, nearly two dozen poxvirus genomes have been fully sequenced or are in the process of being sequenced, and the number of protein families with potential immunomodulatory roles continues to grow rapidly. (More comprehensive information on these genes can be obtained online at www.poxvirus.org.)
So diverse are these genes that no single immunomodulatory ortholog that is common to every poxvirus has ever been identified, a property that points to marked differences in pathogenesis and host tropism among individual viruses. For example, orthopoxviruses such as Cowpox virus (CPV) and Vaccinia virus (VV) express multiple homologs of receptors for tumor necrosis factor (TNF) and interleukin-1β (IL-1β), whereas comparable orthologs are absent from members of the Capripoxvirus, Leporipoxvirus, Suipoxvirus, and Yatapoxvirus genera (9, 64, 82). Instead, these last families encode related proteins that are predicted to regulate pathways such as major histocompatibility complex (MHC)-restricted antigen presentation, a feature that is shared with many herpesviruses (115). Other poxviruses also possess apparently unique regulatory genes such as the homologs of Bcl-2, nerve growth factor beta, and tumor growth factor beta, found to date only in fowlpox virus (1), or the viral FLICE inhibitory proteins (vFLIPs) of molluscum contagiosum virus (MCV) (88). Despite this variability, all poxviruses target host immune pathways mediated by interferons (IFNs) and chemokines, although individual members intervene at mechanistically distinct steps in these pathways.
Poxvirus immunomodulatory proteins can be operationally divided by function into a trinity of distinct strategic classes, which we will refer to here as virostealth, virotransduction, and viromimicry (70) (Fig. 1). Virostealth is characterized by masking of the visible signals associated with virus infection, for example, by reducing the capacity of effector leukocytes to recognize and eliminate infected cells. Virotransducers are intracellular viral proteins that inhibit innate antiviral pathways, such as apoptosis, proinflammatory cascades, or the induction of the antiviral state. Virotransducers can also target host signal transduction pathways that influence host range. Viromimicry is exemplified by virokines and viroreceptors, which are virus-encoded proteins that mimic host cytokines or their receptors, respectively. These proteins block extracellular communication signals and promote a protected microenvironment for the virus within normally immuno-exposed tissues. This commentary focuses on a few select examples within each strategy and the reader is referred to other recent reviews for more comprehensive exegeses on this expanding subject (4, 59, 63, 64, 70, 82, 91, 93, 105, 116).
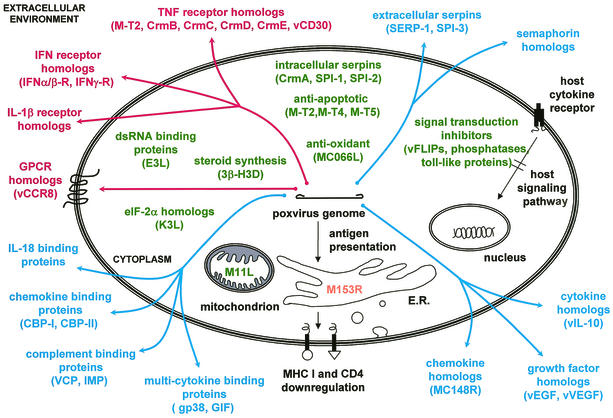
FIG. 1.
Diagrammatical representation of select poxvirus-encoded immunomodulatory proteins. Poxvirus proteins that participate in virostealth (orange), virotransduction (green), and viromimicry (viroreceptors [red] and virokines [blue]) are indicated. GPCR, G-protein-coupled receptor; GIF, granulocyte-macrophage colony-stimulating factor/IL-2 factor; IMP, inflammation modulatory protein; vEGF, viral epidermal growth factor; vVEGF, viral vascular endothelial growth factor.
VIROSTEALTH: SLEEPING WITH THE ENEMY
The participation of innate and educated cytolytic immune cells, especially NK cells and CTLs, is critical for the rapid identification and clearance of virus-infected cells (7). Thus, both small and large viruses attempt to subvert the non-self-discrimination pathway, most commonly by downregulating recognition receptors and/or blocking the presentation of viral antigens to immune cells (72, 115). The capacity to decrease expression of the class I MHC receptors that normally present endogenous viral antigens to circulating CD8+ CTLs correlates with the extent of systemic spread and replication within diverse tissues. For example, the rabbit-specific pathogen myxoma virus (MV) causes severe systemic infections and induces extensive MHC I depletion, whereas VV only moderately downregulates MHC I expression and, in fact, presents antigens efficiently as a vaccine vector (63, 70).
Although receptor downregulation can be a secondary effect of other immune evasion strategies such as extracellular blockade of proinflammatory cytokines, at least in the case of MV, loss of MHC I can be attributed to the product of the M153R gene (39). M153R is expressed as an early protein that contains a leukemia-associated protein (LAP) domain, hence its designation, MV-LAP (39). Viral proteins with LAP domains have also been identified in herpesviruses (human herpesvirus type 8 [HHV-8] and murine gammaherpesvirus 68) (22) and several other poxviruses such as Shope fibroma virus, swinepox virus (SPV), Yaba-like disease virus (YLDV), and lumpy skin disease virus (LSDV) (34). The MHC I depletion induced by MV infection has been shown to involve increased endosomal and/or lysosomal retention and degradation (116, 117), a finding that has led to the recent suggestion that M153R activity is linked to the MHC I trafficking pathway (39). In this model, which is similar to that proposed for the K3 and K5 LAP domain-containing proteins of HHV-8 (22, 54), M153R is postulated to localize to the endoplasmic reticulum (ER) and to target β2-microglobulin-associated MHC I molecules both at the cell surface and in the post-Golgi compartment for retention and degradation in lysosomes (39).
Rapid downregulation of cell surface CD4, a coreceptor in MHC II-restricted antigen-induced T-cell activation, has also been observed after MV infection of CD4+ T lymphocytes (8). This phenomenon was shown to be a function of an early viral protein and involved the active targeting of CD4 to lysosomes for degradation and the attendant uncoupling of CD4-mediated signaling events involving the associated kinase p56lck (8). Recent evidence has indicated that M153R/MV-LAP also mediates MV-induced CD4 downregulation, localizing to endosomes and the trans-Golgi network rather than to the ER and functioning as a membrane-bound ubiquitin ligase (58). As with HHV-8 K5, the LAP domain of M153R is thought to catalyze the ubiquitination of CD4 and to promote the lysosomal internalization and degradation of the receptor (58). M153R has also been implicated in the depletion of another cell surface receptor, CD95/Fas ligand, in MV-infected cells (39), suggesting that this protein may function in downregulating a broad spectrum of cell surface molecules relevant to controlling poxvirus infections.
Although downregulation of host receptors that participate in antigen recognition and presentation has the potential to protect infected cells from the killing activity of CTLs, it should be noted that this strategy also has problematic consequences. For example, loss of MHC I reduces the capacity for infected cells to generate the MHC I-dependent inhibitory signal that prevents killing by NK cells (72, 115). MCV has been shown to encode an MHC I homolog, MC080R, with the potential to act as a ligand for an inhibitory NK cell receptor (86). Although MC080R is retained in the ER and the Golgi apparatus and associates with β2-microglobulin, its effects on NK cell function remain unclear. The recently published genomes of SPV (2) and YLDV (51) have also revealed proteins with sequence similarity to MHC I domains, suggesting that this strategy may be a fairly common accessory virostealth mechanism.
VIROMIMICRY: VIROKINES AND VIRORECEPTORS
Poxviruses also encode mimics of diverse host immune molecules which are referred to as virokines and viroreceptors. These molecules target specific extracellular pathways by which the host coordinates and regulates early inflammatory responses, particularly at the level of complement, IFNs, proinflammatory cytokines, chemokines, and growth factors. Generally, viroreceptors, which can be either secreted or localized to the surfaces of infected cells, are related to cellular receptors and act by scavenging ligands that promote antiviral immune or inflammatory processes. In contrast, virokines are generally secreted viral proteins that mimic host regulatory molecules such as cytokines, complement regulators, or other sundry humoral inhibitors. The sheer volume of information that addresses these important immune evasion proteins renders a detailed analysis unfeasible within the scope of this review. Thus, we concentrate on only a few selected recent advances.
Viroreceptors.
All poxviruses are thought to employ extracellular and/or intracellular mechanisms to disrupt IFN activity, a fact that underscores the integral role of this cytokine family in host antiviral responses (85). Viral mimics of both IFN-α/β and IFN-γ receptors (IFN-Rs) represent the most common poxvirus extracellular strategies to evade the antiviral effects of these cytokines (reviewed in reference 92). Poxvirus IFN-Rs usually exhibit limited similarity to the extracellular domains of mammalian IFN-Rs and are thought to competitively prevent IFNs from binding to their native receptors. This sequestration function is likely facilitated by the oligomeric nature of these viral proteins. For example, the functional form of the MV IFN-γ-R is a stable trimer (49), while IFN-γ-Rs from the orthopoxviruses VV, camelpox virus, and CPV have recently been shown to form disulfide-linked homodimers (5).
IFN-γ is particularly critical for controlling poxvirus infection (85, 92), and a role for IFN-γ-R homologs in virus virulence is well-documented (70). However, the exact nature of this role is complicated by the fact that many poxviral IFN-γ-R homologs interact with multiple immune targets. For example, deletion of the VV IFN-γ-R homolog B8R, which does not bind or inhibit murine IFN-γ (65), has been reported to either enhance or not affect virulence in mice (99, 101, 108). In contrast, VV with a deletion of B8R is attenuated in other species such as rabbits (101), raising questions about whether the target host ligands and biological functions of B8R in infected hosts have been underestimated in the past. Ectromelia virus (EV), an orthopoxvirus that infects mice, has been shown to encode an IFN-γ-R homolog with the unique ability to inhibit murine IFN-γ (97). In addition, the IFN-α/β-R homolog of EV has a greater ability to inhibit human and murine IFN-α than does VV B8R (97). Targeted deletion analyses of these EV IFN viroreceptors may help resolve some of the issues regarding the host roles of these immunomodulators.
Tumor necrosis factor (TNF), a potent proinflammatory cytokine secreted by macrophages and activated T cells, is another critical extracellular immune regulator targeted by poxviruses. Like viral IFN-Rs, poxvirus-encoded TNF receptors (vTNFRs) resemble secreted versions of the extracellular domains of their counterpart cellular receptors and form functional oligomers that bind and sequester TNF (9, 23, 114). The poxviral TNFRs that have been best characterized to date are the T2-like TNFRs encoded by leporipoxviruses and the cytokine response modifier (Crm)-like TNFRs of orthopoxviruses. A family of Crm-like vTNFRs, including CrmB, CrmC, CrmD (23), the recently identified CrmE (80), and a putative new fifth member from CPV that closely resembles CD30 (73), have been identified in several orthopoxviruses. A related soluble CD30 homolog that blocks IFN-γ production and inhibits inflammatory responses mediated by Th1, but not Th2, cells has also been identified in EV (81), a virus that contains only a nonfunctional CrmE pseudogene (96).
Among orthopoxviruses, the distribution and activity of vTNFR-like proteins varies widely with the viral strain. CrmD is absent from most CPV strains and is usually found only in orthopoxviruses that lack CrmB and CrmC (3, 23). Functional versions of CrmE, which binds TNF from several species but only inhibits human TNF-mediated cytolysis, have been identified in CPV and VV (strain USSR) (77, 80). Interestingly, some VV strains carry discontinuous and nonfunctional TNFR homologs such as A53R (CrmC) and B28R (CrmB) (41). However, three VV strains (Lister, USSR, and Evans) exhibit both soluble and cell-associated TNF-binding activities that map to a functional A53R (CrmC) gene product in the Lister and USSR strains and to a dual-activity CrmE-like protein in the USSR strain (3, 77). The existence of so many viral TNF inhibitor genes, both functional and nonfunctional, suggests the occurrence of multiple discontinuous cycles of TNF-mediated selection events during the evolutionary history of most poxviruses.
Virokines.
In addition to exploiting IFN-R mimics, poxviruses also target the IFN pathway indirectly through proteins that scavenge IL-18, a potent inducer of IFN-γ that regulates cytokine synthesis, control of Th1 and Th2 responses, and activation of cytolytic effector cells (69). Mammalian IL-18 binding protein (IL-18BP) is a natural antagonist of IL-18 that forms inhibitory complexes which prevent IL-18 from interacting with its receptor (69). Many poxviruses (MCV, EV, VV, CPV) (17, 98, 113) have been shown to encode functional viral IL-18BPs, while several others (SPV, YLDV, monkeypox virus [MPV], LSDV) are now predicted to encode IL-18BP orthologs. For example, MCV expresses three putative IL-18BPs, designated MC51L, -53L, and -54L, but to date only MC54L has been shown to bind human and murine IL-18 (112, 113). This association with ligand is a high-affinity interaction involving conserved binding residues and kinetics comparable to those of the native IL-18BP of these species (112). Of note, neither MC51L nor MC53L possesses these conserved residues, nor does restoring them confer IL-18 binding function, suggesting that these proteins interact with other ligands (112). In vivo studies with mice infected with IL-18BP gene knockout constructs of EV (12) or VV (100) have shown decreased virulence and increased NK cell activity directed against infected cells.
Like IFN-γ, chemokines are also pivotal to limiting virus infections by virtue of their capacity to mobilize and activate diverse classes of leukocytes. All poxviruses employ strategies to modulate chemokine activity, including virus-encoded chemokine-binding proteins (CBP), receptor homologs, and ligand mimics (48, 66, 83). Poxvirus CBPs are classified as either type I (low affinity), of which the dual-function MV IFN-γ-R homolog (M-T7) is the sole member (49), or type II (high affinity), termed CBP-IIs or vCCIs (83). CBP-IIs specifically target CC chemokines and are considered orphan inhibitors because they lack sequence similarity to all known mammalian proteins (83). Resolution of the crystal structure of CPV CBP-II confirmed a unique structure featuring a β-sandwich topology that distantly resembled the collagen-binding domain of the Staphylococcus aureus adhesin molecule (18). More recent analyses of the VV 35-kDa CBP-II and the CC chemokine monocyte chemoattractant protein 1 indicate that CBP-IIs function by occluding the G-protein-coupled receptor binding site of the bound chemokine, thereby preventing interaction with the native receptor. The conserved nature of this binding site (10, 16, 84) likely accounts for the multiple-ligand targeting ability of CBP-IIs.
Chemokine ligand mimics have been identified for both MCV (15) and fowlpox virus (1). The MCV chemokine MC148R is a secreted viral version of the IL-11 receptor α-locus chemokine (43) that was initially reported to inhibit the activity of both CC and CXC chemokines (25). However, recent binding data suggest that MCV is a selective antagonist of human CCR8 (56). MC148R also exhibits the ability to inhibit allograft rejection in transgenic mice (28), a species in which it lacks any demonstrable chemokine-binding activity, suggesting that it has alternate targets or additional functions that remain to be uncovered.
Several viral mimics of IL-10, a multifunctional cytokine with both immunostimulatory and immunosuppressive effects (32), have also been identified among poxviruses, including Orf virus (ORFV), YLDV, and LSDV (33, 51, 104). ORFV IL-10, the only family member characterized to date, is an early protein with biological activity similar to that of ovine IL-10 (33). In vitro, ORFV IL-10 promotes thymocyte proliferation, costimulates mast cell growth, and suppresses macrophage activation (33, 42), suggesting a role in immune evasion that involves mimicking the suppressive effects of host IL-10 on Th1-mediated responses.
The complement system is an integrated network of cell-associated effector proteins and secreted regulatory proteins that participate in the identification and destruction of invading pathogens as well as the initiation and amplification of inflammatory responses (45). The prototypical poxviral complement regulatory protein is the VV complement control protein (VCP) (47). Additional VCP-like orthologs have been detected in other poxviruses such as MPV, variola virus (VarV), and CPV (61, 107). VCP is a highly stable monomeric secreted protein (94) that inhibits both the classical and alternative complement activation pathways by directly and indirectly promoting the decay of the C3 convertase (46, 79). VCP has also been shown to interact with cell surface glycosaminoglycans, inhibiting both chemokine-mediated leukocyte migration and antibody binding to MHC I (67, 95). The closely related smallpox inhibitor of complement enzymes (SPICE) is the most recently characterized VCP homolog (78). SPICE activity resembles that of VCP, but it is nearly 100-fold more potent at inhibiting human C3 activity (78), possibly contributing to the highly virulent nature of VarV in humans.
VIROTRANSDUCTION: THE SOUNDS OF SILENCE
The “holy grail” of virus infection is the generation of new progeny virions that can disseminate with minimal interference from the host's defenses. Consequently, poxviruses have developed multiple strategies designed to minimize the deleterious effects of proinflammatory signaling cascades and to create an environment for infected cells that is conducive to productive infection. A central paradigm in each of these strategies is the ability to micromanipulate elements critical to the infected host cell signal transduction machinery, particularly the pathways that activate one of the most ancestral antiviral responses, apoptosis (11). Thus, the interplay between the virus and cell signaling networks has important consequences for the pathogenic effects of viral infections.
After infection, poxviruses rapidly express proteins that target key regulatory points, such as the caspases, in the apoptotic cascade. These inhibitory viral proteins can be loosely divided by function into those that block caspase activation and those that serve as suicide caspase substrates. MCV encodes two related proteins, MC159L and MC160L, of which the former is thought to be the primary functional apoptosis inhibitor (88). Both MC159L and -160L are classified as vFLIPs and are related to cellular death effector domain (DED)-containing proteins that bind both caspase-8 and the Fas-associated death domain adaptor molecules (103). In this manner, they are postulated to prevent both the recruitment of pro-caspase-8 to death receptor complexes at the cell membrane and the initiation of the apoptotic cascade. Recent evidence has shown that the DEDs of MC159L cannot be functionally interchanged with those from other host proteins (35) and that mutations within regions of MC159L other than those within the DED motifs eliminate its antiapoptotic activity (36). This evidence suggests that MC159L may exert its inhibitory effects by interacting with cellular factors other than its predicted binding partners within the death receptor complex. For example, as has been observed for certain other poxvirus-infected cells (71), MCV MC159L prevents the activation of NF-κB (38), a critical intermediate in the signaling pathways of proinflammatory cytokines like TNF and IFN.
Poxviruses also encode intracellular serpins with antiapoptotic properties. The CPV serpin, CrmA, is a versatile apoptosis inhibitor that has the potential to target both intrinsic and extrinsic apoptotic pathways (76). By virtue of its ability to inhibit the serine protease granzyme B, CrmA protects cells from perforin-dependent apoptosis induced by CTLs and NK cells (74). In addition, CrmA is the prototypical poxvirus caspase suicide substrate, inhibiting the activity of caspase-8 and -10 and blocking apoptotic pathways that are initiated in response to such stimuli as serum and growth factor deprivation, hypoxia, detachment from the extracellular matrix, or TNF and Fas ligation (13, 40, 102). This unexpected cross-class caspase inhibition by CrmA is the product of its unique structure (89). The SPI-2 family of poxvirus serpins also has the potential to inhibit apoptosis, although some SPI-2 orthologs are apparently less effective than CrmA at inhibiting apoptosis. VV SPI-2 has been shown to protect cells from Fas- and TNF-mediated apoptosis (29), but disruption of this gene has little effect on virulence (44). In contrast, MV SERP-2 is an important virulence factor in myxomatosis that inhibits both caspase-1 and granzyme B to protect infected lymphoid cells from apoptosis (60), but it cannot functionally substitute for CrmA (106).
Mitochondria are also important checkpoints for the coordination of apoptotic signals initiated by cellular stress (20, 109). The MV M11L protein, an important virulence factor in lymphoid cells (57), is targeted to the mitochondria, where it inhibits pro-apoptotic changes in mitochondrial integrity such as the loss of inner mitochondrial membrane potential (30). Recently, M11L was shown to be closely associated with a component of the mitochondrial permeability transition pore, the peripheral benzodiazepine receptor (31). Related protection of mitochondrial membrane potential has also been observed after infection by VV (110). Since VV does not encode an obvious M11L ortholog, the identification of the relevant protein is awaited with interest.
The activities of poxvirus antiapoptotic proteins are closely intertwined with strategies that target intracellular elements in the IFN response pathway, including the IFN-inducible enzymes protein kinase R (PKR) and 2′,5′-oligoadenylate synthetase (OAS) (85). Both of these enzymes are activated by double-stranded RNA (dsRNA), which is copiously produced during poxviral transcription and known to initiate cascades that inhibit viral protein synthesis or induce apoptosis by activating caspase-8 (37). The VV E3L and K3L genes express the prototypical poxvirus inhibitors that target these enzymes, while several other poxviruses (MV, YLDV, VarV, Shope fibroma virus, EV, ORFV, SPV) express or are predicted to encode functional homologs of E3L and/or K3L. VV E3L is a dsRNA-binding protein that sequesters dsRNA and prevents activation of PKR and OAS (21, 27). E3L can also bind directly to PKR to inhibit its activity, thereby preventing the phosphorylation of eukaryotic initiation factor 2α (eIF-2α) and interferon response factors 3 and 7 (IRF-3 and -7) associated with cell cycle arrest (87, 90). Other activities ascribed to E3L include blocking the gene induction of IFN-α/β (111), reducing adenosine deaminase editing activity (53), and mediating virus host range (50). In comparison, the K3L gene product, a structural mimic of the eIF-2α subunit, functions as a suicide pseudosubstrate of PKR to competitively inhibit eIF-2α phosphorylation (19, 27). The crystal structures of both K3L (26) and the related M156R of MV (75) have recently been deduced and have revealed significant insights about the relevance of eIF-2α viral mimicry.
Elements within IFN pathways other than PKR and OAS, such as transcription factors that transduce the biological effect of IFN-inducible genes, are also subject to manipulation by poxviral proteins. For example, the VV H1L gene encodes a dual-specificity phosphatase that blocks IFN-induced activation of signal transducer and activator of transcription 1 (STAT-1) (68), while MCV, which lacks any obvious orthologs of E3L or K3L, may use MC159L to inhibit IFN-mediated, PKR-induced NF-κB activation (38). As was already shown for IFNs, some poxviruses also encode proteins that disrupt intracellular IL-1 receptor signaling. For example, the products of the VV A46R and A52R genes contain Toll-like/IL-1 receptor domains that enable these proteins to disrupt the IL-1 signaling pathway and inhibit IL-1-induced NF-κB activation (14).
FUTURE PERSPECTIVES
Advances in the field of viral immunomodulation continue to be made with increasing frequency, providing important clues about the selective pressures which drive the coevolution of virus and host. Moreover, our understanding of the mechanisms by which poxviruses modulate key components of the immune system has seen the advent of new avenues of research, most notably the exploitation of viral immunomodulatory proteins for therapeutic use. For example, several poxvirus proteins, including MV SERP-1 (62), M-T7 (52), MCV MC148R (28), and VCP (6), have been used in animal models to prevent allograft and xenograft transplant rejection. Additionally, SERP-1 and M-T7 have also been used to inhibit adverse inflammatory responses in models of arterial injury following balloon angioplasty (52, 55). Similarly, the anti-inflammatory properties of VV CBP-II have shown promise in inhibiting bronchospasm and cellular infiltration in models of asthma (24). It is likely that the list of poxviral immunomodulatory proteins with potential use as therapeutic agents will continue to grow as these proteins are better characterized in vivo and in vitro.
Acknowledgments
G.M. holds a Canada Research Chair in Molecular Virology. J.B.J. is a Robarts Research Fellow.
REFERENCES
- 1.Afonso, C. L., E. R. Tulman, Z. Lu, L. Zsak, G. F. Kutish, and D. L. Rock. 2000. The genome of fowlpox virus. J. Virol. 74:3815-3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Afonso, C. L., E. R. Tulman, Z. Lu, L. Zsak, F. A. Osorio, C. Balinsky, G. F. Kutish, and D. L. Rock. 2002. The genome of swinepox virus. J. Virol. 76:783-790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alcami, A., A. Khanna, N. L. Paul, and G. L. Smith. 1999. Vaccinia virus strains Lister, USSR and Evans express soluble and cell-surface tumor necrosis factor receptors. J. Gen. Virol. 80:949-959. [DOI] [PubMed] [Google Scholar]
- 4.Alcami, A., and U. H. Koszinowski. 2000. Viral mechanisms of immune evasion. Trends Microbiol. 8:410-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alcami, A., and G. L. Smith. 2002. The vaccinia virus soluble interferon-gamma receptor is a homodimer. J. Gen. Virol. 83:545-549. [DOI] [PubMed] [Google Scholar]
- 6.Anderson, J. B., S. A. Smith, and G. J. Kotwal. 2002. Vaccinia virus complement control protein inhibits hyperacute xenorejection. Transplant Proc. 34:1083-1085. [DOI] [PubMed] [Google Scholar]
- 7.Barry, M., and R. C. Bleackley. 2002. Cytotoxic T lymphocytes: all roads lead to death. Nat. Rev. Immunol. 2:401-409. [DOI] [PubMed] [Google Scholar]
- 8.Barry, M., S. F. Lee, L. Boshkov, and G. McFadden. 1995. Myxoma virus induces extensive CD4 downregulation and dissociation of p56(lck) in infected rabbit CD4+ T lymphocytes. J. Virol. 69:5243-5251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barry, M., and G. McFadden. 1997. Virokines and viroreceptors, p. 251-261. In D. J. Remick and J. S. Friedland (ed.), Cytokines in health and disease. Marcel Dekker Inc, New York, N.Y.
- 10.Beck, C. G., C. Studer, J. F. Zuber, B. J. Demange, U. Manning, and R. Urfer. 2001. The viral CC chemokine-binding protein vCCI inhibits monocyte chemoattractant protein-1 activity by masking its CCR2B-binding site. J. Biol. Chem. 276:43270-43276. [DOI] [PubMed] [Google Scholar]
- 11.Benedict, C. A., P. S. Norris, and C. F. Ware. 2002. To kill or be killed: viral evasion of apoptosis. Nat. Immunol. 3:1013-1018. [DOI] [PubMed] [Google Scholar]
- 12.Born, T. L., L. A. Morrison, D. J. Esteban, T. VandenBos, L. G. Thebeau, N. H. Chen, M. K. Spriggs, J. E. Sims, and R. M. L. Buller. 2000. A poxvirus protein that binds to and inactivates IL-18, and inhibits NK cell response. J. Immunol. 164:3246-3254. [DOI] [PubMed] [Google Scholar]
- 13.Boudreau, N., C. J. Sympson, Z. Werb, and M. J. Bissell. 1995. Suppression of ICE and apoptosis in mammary epithelial cells by extracellular matrix. Science 267:891-893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bowie, A., E. Kiss-Toth, J. A. Symons, G. L. Smith, S. K. Dower, and L. A. J. O'Neill. 2000. A46R and A52R from vaccinia virus are antagonists of host IL-1 and Toll-like receptor signaling. Proc. Natl. Acad. Sci. USA 97:10162-10167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bugert, J. J., C. Lohmuller, I. Damon, B. Moss, and G. Darai. 1998. Chemokine homolog of molluscum contagiosum virus—sequence conservation and expression. Virology 242:51-59. [DOI] [PubMed] [Google Scholar]
- 16.Burns, J. M., D. J. Dairaghi, M. Deitz, M. Tsang, and T. J. Schall. 2002. Comprehensive mapping of poxvirus vCCI chemokine-binding protein: expanded range of ligand interactions and unusual dissociation kinetics. J. Biol. Chem. 277:2785-2789. [DOI] [PubMed] [Google Scholar]
- 17.Calderara, S., Y. Xiang, and B. Moss. 2001. Orthopoxvirus IL-18 binding proteins: affinities and antagonist activities. Virology 279:22-26. [DOI] [PubMed] [Google Scholar]
- 18.Carfi, A., C. A. Smith, P. J. Smolak, J. McGrew, and D. C. Wiley. 1999. Structure of a soluble secreted chemokine inhibitor vCCI (p35) from cowpox virus. Proc. Natl. Acad. Sci. USA 96:12379-12383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carroll, K., O. Elroystein, B. Moss, and R. Jagus. 1993. Recombinant vaccinia virus K3L gene product prevents activation of double-stranded RNA-dependent, initiation factor-2-alpha-specific protein kinase. J. Biol. Chem. 268:12837-12842. [PubMed] [Google Scholar]
- 20.Castedo, M., J. L. Perfettini, and G. Kroemer. 2002. Mitochondrial apoptosis and the peripheral benzodiazepine receptor: a novel target for viral and pharmacological manipulation. J. Exp. Med. 196:1121-1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang, H. W., J. C. Watson, and B. L. Jacobs. 1992. The E3L gene of vaccinia virus encodes an inhibitor of the interferon-induced, double-stranded RNA-dependent protein kinase. Proc. Natl. Acad. Sci. USA 89:4825-4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coscoy, L., and D. Ganem. 2000. Kaposi's sarcoma-associated herpesvirus encodes two proteins that block cell surface display of MHC class I chains by enhancing their endocytosis. Proc. Natl. Acad. Sci. USA 97:8051-8056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cunnion, K. M. 1999. Tumor necrosis factor receptors encoded by poxviruses. Mol. Gen. Metab. 67:278-282. [DOI] [PubMed] [Google Scholar]
- 24.Dabbagh, K., Y. Xiao, C. Smith, P. Stepick-Biek, S. G. Kim, W. J. Lamm, D. H. Liggitt, and D. B. Lewis. 2000. Local blockade of allergic airway hyperreactivity and inflammation by the poxvirus-derived pan-CC-chemokine inhibitor vCCI. J. Immunol. 165:3418-3422. [DOI] [PubMed] [Google Scholar]
- 25.Damon, I., P. M. Murphy, and B. Moss. 1998. Broad spectrum chemokine antagonistic activity of a human poxvirus chemokine homolog. Proc. Natl. Acad. Sci. USA 95:6403-6407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dar, A. C., and F. Sicheri. 2002. X-ray crystal structure and functional analysis of vaccinia virus K3L reveals molecular determinants for PKR subversion and substrate recognition. Mol. Cell 10:295-305. [DOI] [PubMed] [Google Scholar]
- 27.Davies, M. V., H. W. Chang, B. L. Jacobs, and R. J. Kaufman. 1993. The E3L and K3L vaccinia virus gene products stimulate translation through inhibition of the double-stranded RNA-dependent protein kinase by different mechanisms. J. Virol. 67:1688-1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DeBruyne, L. A., K. Li, D. K. Bishop, and J. S. Bromberg. 2000. Gene transfer of virally encoded chemokine antagonists vMIP-II and MC148 prolongs cardiac allograft survival and inhibits donor-specific immunity. Gene Ther. 7:575-582. [DOI] [PubMed] [Google Scholar]
- 29.Dobbelstein, M., and T. Shenk. 1996. Protection against apoptosis by the vaccinia virus SPI-2 (B13R) gene product. J. Virol. 70:6479-6485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Everett, H., M. Barry, S. F. Lee, X. J. Sun, K. Graham, J. Stone, R. C. Bleackley, and G. McFadden. 2000. M11L: a novel mitochondria-localized protein of myxoma virus that blocks apoptosis of infected leukocytes. J. Exp. Med. 191:1487-1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Everett, H., M. Barry, X. Sun, S. F. Lee, C. Frantz, L. G. Berthiaume, G. McFadden, and R. C. Bleackley. 2002. The myxoma poxvirus protein, M11L, prevents apoptosis by direct interaction with the mitochondrial permeability transition pore. J. Exp. Med. 196:1127-1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fickenscher, H., S. Hor, H. Kupers, A. Knappe, S. Wittmann, and H. Sticht. 2002. The interleukin-10 family of cytokines. Trends Immunol. 23:89-96. [DOI] [PubMed] [Google Scholar]
- 33.Fleming, S. B., C. A. McCaughan, A. E. Andrews, A. D. Nash, and A. A. Mercer. 1997. A homolog of interleukin-10 is encoded by the poxvirus Orf virus. J. Virol. 71:4857-4861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fruh, K., E. Bartee, K. Gouveia, and M. Mansouri. 2002. Immune evasion by a novel family of viral PHD/LAP-finger proteins of gamma-2 herpesviruses and poxviruses. Virus Res. 88:55-69. [DOI] [PubMed] [Google Scholar]
- 35.Garvey, T., J. Bertin, R. Siegel, M. Lenardo, and J. Cohen. 2002. The death effector domains (DEDs) of the molluscum contagiosum virus MC159 v-FLIP protein are not functionally interchangeable with each other or with the DEDs of caspase-8. Virology 300:217-225. [DOI] [PubMed] [Google Scholar]
- 36.Garvey, T. L., J. Bertin, R. M. Siegel, G. H. Wang, M. J. Lenardo, and J. I. Cohen. 2002. Binding of FADD and caspase-8 to molluscum contagiosum virus MC159 v-FLIP is not sufficient for its antiapoptotic function. J. Virol. 76:697-706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gil, J., and M. Esteban. 2000. Induction of apoptosis by the dsRNA-dependent protein kinase (PKR): mechanism of action. Apoptosis 5:107-114. [DOI] [PubMed] [Google Scholar]
- 38.Gil, J., J. Rullas, J. Alcami, and M. Esteban. 2001. MC159L protein from the poxvirus molluscum contagiosum virus inhibits NF-kappa B activation and apoptosis induced by PKR. J. Gen. Virol. 82:3027-3034. [DOI] [PubMed] [Google Scholar]
- 39.Guerin, J. L., J. Gelfi, S. Boullier, M. Delverdier, F. A. Bellanger, S. Bertagnoli, I. Drexler, G. Sutter, and F. Messud-Petit. 2002. Myxoma virus leukemia-associated protein is responsible for major histocompatibility complex class I and Fas-CD95 down-regulation and defines scrapins, a new group of surface cellular receptor abductor proteins. J. Virol. 76:2912-2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gurevich, R. M., K. M. Regula, and L. A. Kirshenbaum. 2001. Serpin protein CrmA suppresses hypoxia-mediated apoptosis of ventricular myocytes. Circulation 103:1984-1991. [DOI] [PubMed] [Google Scholar]
- 41.Howard, S. T., Y. S. Chan, and G. L. Smith. 1991. Vaccinia virus homologues of the Shope fibroma virus inverted terminal repeat proteins and a discontinuous ORF related to the tumor necrosis factor receptor family. Virology 180:633-647. [DOI] [PubMed] [Google Scholar]
- 42.Imlach, W., C. A. McCaughan, A. A. Mercer, D. Haig, and S. B. Fleming. 2002. Orf virus-encoded interleukin-10 stimulates the proliferation of murine mast cells and inhibits cytokine synthesis in murine peritoneal macrophages. J. Gen. Virol. 83:1049-1058. [DOI] [PubMed] [Google Scholar]
- 43.Ishikawa-Mochizuki, I., M. Kitaura, M. Baba, T. Nakayama, D. Izawa, T. Imai, H. Yamada, K. Hieshima, R. Suzuki, H. Nomiyama, and O. Yoshie. 1999. Molecular cloning of a novel CC chemokine, interleukin-11 receptor alpha-locus chemokine (ILC), which is located on chromosome 9p13 and a potential homologue of a CC chemokine encoded by molluscum contagiosum virus. FEBS Lett. 460:544-548. [DOI] [PubMed] [Google Scholar]
- 44.Kettle, S., A. Alcami, A. Khanna, R. Ehret, C. Jassoy, and G. L. Smith. 1997. Vaccinia virus serpin B13r (SPI-2) inhibits interleukin-1-beta-converting enzyme and protects virus-infected cells from TNF- and FAS-mediated apoptosis, but does not prevent Il-1-beta-induced fever. J. Gen. Virol. 78:677-685. [DOI] [PubMed] [Google Scholar]
- 45.Kotwal, G. J. 2000. Poxviral mimicry of complement and chemokine system components: what's the end game? Immunol. Today 21:242-248. [DOI] [PubMed] [Google Scholar]
- 46.Kotwal, G. J., S. N. Isaacs, R. McKenzie, M. M. Frank, and B. Moss. 1990. Inhibition of the complement cascade by the major secretory protein of vaccinia virus. Science 250:827-830. [DOI] [PubMed] [Google Scholar]
- 47.Kotwal, G. J., and B. Moss. 1988. Vaccinia virus encodes a secretory polypeptide structurally related to complement control proteins. Nature 335:176-178. [DOI] [PubMed] [Google Scholar]
- 48.Lalani, A. S., J. W. Barrett, and G. McFadden. 2000. Modulating chemokines: more lessons from viruses. Immunol. Today 21:100-106. [DOI] [PubMed] [Google Scholar]
- 49.Lalani, A. S., K. Graham, K. Mossman, K. Rajarathnam, I. Clarklewis, D. Kelvin, and G. McFadden. 1997. The purified myxoma virus gamma interferon receptor homolog M-T7 interacts with the heparin-binding domains of chemokines. J. Virol. 71:4356-4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Langland, J., and B. Jacobs. 2002. The role of the PKR-inhibitory genes, E3L and K3L, in determining vaccinia virus host range. Virology 299:133-141. [DOI] [PubMed] [Google Scholar]
- 51.Lee, H. J., K. Essani, and G. L. Smith. 2001. The genome sequence of yaba-like disease virus, a yatapoxvirus. Virology 281:170-192. [DOI] [PubMed] [Google Scholar]
- 52.Liu, L. Y., A. Lalani, E. B. Dai, B. Seet, C. Macauley, R. Singh, L. Fan, G. McFadden, and A. Lucas. 2000. The viral anti-inflammatory chemokine-binding protein M-T7 reduces intimal hyperplasia after vascular injury. J. Clin. Investig. 105:1613-1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu, Y., K. C. Wolff, B. L. Jacobs, and C. E. Samuel. 2001. Vaccinia virus E3L interferon resistance protein inhibits the interferon-induced adenosine deaminase A-to-I editing activity. Virology 289:378-387. [DOI] [PubMed] [Google Scholar]
- 54.Lorenzo, M. E., J. U. Jung, and H. L. Ploegh. 2002. Kaposi's sarcoma-associated herpesvirus K3 utilizes the ubiquitin-proteasome system in routing class I major histocompatibility complexes to late endocytic compartments. J. Virol. 76:5522-5531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lucas, A., L. Liu, J. Macen, P. Nash, E. Dai, M. Stewart, K. Graham, W. Etches, L. Boshkov, P. N. Nation, D. Humen, M. L. Hobman, and G. McFadden. 1996. Virus-encoded serine proteinase inhibitor SERP-1 inhibits atherosclerotic plaque development after balloon angioplasty. Circulation 94:2890-2900. [DOI] [PubMed] [Google Scholar]
- 56.Luttichau, H. R., J. Gerstoft, and T. W. Schwartz. 2001. MC148 encoded by human molluscum contagiosum poxvirus is an antagonist for human but not murine CCR8. J. Leukoc. Biol. 70:277-282. [PubMed] [Google Scholar]
- 57.Macen, J. L., K. A. Graham, S. F. Lee, M. Schreiber, L. K. Boshkov, and G. McFadden. 1996. Expression of the myxoma virus tumor necrosis factor receptor homologue and M11L genes is required to prevent virus-induced apoptosis in infected rabbit T lymphocytes. Virology 218:232-237. [DOI] [PubMed] [Google Scholar]
- 58.Mansouri, M., E. Bartee, K. Gouveia, B. T. Hovey Nerenberg, J. Barrett, L. Thomas, G. Thomas, G. McFadden, and K. Fruh. 2003. The PHD/LAP-domain protein M153R of myxoma virus is a ubiquitin ligase that induces the rapid internalization and lysosomal destruction of CD4. J. Virol. 77:1427-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McFadden, G., and P. M. Murphy. 2000. Host-related immunomodulators encoded by poxviruses and herpesviruses. Curr. Opin. Microbiol. 3:371-378. [DOI] [PubMed] [Google Scholar]
- 60.Messud-Petit, F., J. Gelfi, M. Delverdier, M. F. Amardeilh, R. Py, G. Sutter, and S. Bertagnoli. 1998. Serp2, an inhibitor of the interleukin-1-beta-converting enzyme, is critical in the pathobiology of myxoma virus. J. Virol. 72:7830-7839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Miller, C. G., S. N. Shchelkunov, and G. J. Kotwal. 1997. The cowpox virus-encoded homolog of the vaccinia virus complement control protein is an inflammation modulatory protein. Virology 229:126-133. [DOI] [PubMed] [Google Scholar]
- 62.Miller, L. W., E. Dai, P. Nash, L. Liu, C. Icton, D. Klironomos, L. Fan, P. N. Nation, R. Zhong, G. McFadden, and A. Lucas. 2000. Inhibition of transplant vasculopathy in a rat aortic allograft model after infusion of anti-inflammatory viral serpin. Circulation 101:1598-1605. [DOI] [PubMed] [Google Scholar]
- 63.Moss, B. 2001. Poxviridae: the viruses and their replication, p. 2849-2883. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 4th ed. Lippincott Williams and Wilkins, Philadelphia, Pa.
- 64.Moss, B., and J. L. Shisler. 2001. Immunology 101 at poxvirus U: immune evasion genes. Semin. Immunol. 13:59-66. [DOI] [PubMed] [Google Scholar]
- 65.Mossman, K., C. Upton, R. M. L. Buller, and G. McFadden. 1995. Species specificity of ectromelia virus and vaccinia virus interferon-gamma binding proteins. Virology 208:762-769. [DOI] [PubMed] [Google Scholar]
- 66.Murphy, P. M. 2001. Viral exploitation and subversion of the immune system through chemokine mimicry. Nat. Immunol. 2:116-122. [DOI] [PubMed] [Google Scholar]
- 67.Murthy, K. H. M., S. A. Smith, V. K. Ganesh, K. W. Judge, N. Mullin, P. N. Barlow, C. M. Ogata, and G. J. Kotwal. 2001. Crystal structure of a complement control protein that regulates both pathways of complement activation and binds heparan sulfate proteoglycans. Cell 104:301-311. [DOI] [PubMed] [Google Scholar]
- 68.Najarro, P., P. Traktman, and J. A. Lewis. 2001. Vaccinia virus blocks gamma interferon signal transduction: viral VH1 phosphatase reverses Stat1 activation. J. Virol. 75:3185-3196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nakanishi, K., T. Yoshimoto, H. Tsutsui, and H. Okamura. 2001. Interleukin-18 regulates both Th1 and Th2 responses. Annu. Rev. Immunol. 19:423-474. [DOI] [PubMed] [Google Scholar]
- 70.Nash, P., J. Barrett, J. X. Cao, S. Hota-Mitchell, A. S. Lalani, H. Everett, X. M. Xu, J. Robichaud, S. Hnatiuk, C. Ainslie, B. T. Seet, and G. McFadden. 1999. Immunomodulation by viruses: the myxoma virus story. Immunol. Rev. 168:103-120. [DOI] [PubMed] [Google Scholar]
- 71.Oie, K. L., and D. J. Pickup. 2001. Cowpox virus and other members of the orthopoxvirus genus interfere with the regulation of NF-kappa B activation. Virology 288:175-187. [DOI] [PubMed] [Google Scholar]
- 72.Orange, J. S., M. S. Fassett, L. A. Koopman, J. E. Boyson, and J. L. Strominger. 2002. Viral evasion of natural killer cells. Nat. Immunol. 3:1006-1012. [DOI] [PubMed] [Google Scholar]
- 73.Panus, J. F., C. A. Smith, C. A. Ray, T. D. Smith, D. D. Patel, and D. J. Pickup. 2002. Cowpox virus encodes a fifth member of the tumor necrosis factor receptor family: a soluble, secreted CD30 homologue. Proc. Natl. Acad. Sci. USA 99:8348-8353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Quan, L. T., A. Caputo, R. C. Bleackley, D. J. Pickup, and G. S. Salvesen. 1995. Granzyme B is inhibited by the cowpox virus serpin cytokine response modifier A. J. Biol. Chem. 270:10377-10379. [DOI] [PubMed] [Google Scholar]
- 75.Ramelot, T., J. Cort, A. Yee, F. Liu, M. Goshe, A. Edwards, R. Smith, C. Arrowsmith, T. Dever, and M. Kennedy. 2002. Myxoma virus immunomodulatory protein M156R is a structural mimic of eukaryotic translation initiation factor eIF2alpha. J. Mol. Biol. 322:943-954. [DOI] [PubMed] [Google Scholar]
- 76.Ray, C. A., R. A. Black, S. R. Kronheim, T. A. Greenstreet, P. R. Sleath, G. S. Salvesen, and D. J. Pickup. 1992. Viral inhibition of inflammation: cowpox virus encodes an inhibitor of the interleukin-1 beta converting enzyme. Cell 69:597-604. [DOI] [PubMed] [Google Scholar]
- 77.Reading, P. C., A. Khanna, and G. L. Smith. 2002. Vaccinia virus CrmE encodes a soluble and cell surface tumor necrosis factor receptor that contributes to virus virulence. Virology 292:285-298. [DOI] [PubMed] [Google Scholar]
- 78.Rosengard, A. M., Y. Liu, Z. Nie, and R. Jimenez. 2002. Variola virus immune evasion design: expression of a highly efficient inhibitor of human complement. Proc. Natl. Acad. Sci. USA 99:8808-8813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sahu, A., S. N. Isaacs, A. M. Soulika, and J. D. Lambris. 1998. Interaction of vaccinia virus complement control protein with human complement proteins—factor I-mediated degradation of C3b to Ic3b(1) inactivates the alternative complement pathway. J. Immunol. 160:5596-5604. [PubMed] [Google Scholar]
- 80.Saraiva, M., and A. Alcami. 2001. CrmE, a novel soluble tumor necrosis factor receptor encoded by poxviruses. J. Virol. 75:226-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Saraiva, M., P. Smith, P. G. Fallon, and A. Alcami. 2002. Inhibition of type 1 cytokine-mediated inflammation by a soluble CD30 homologue encoded by ectromelia (mousepox) virus. J. Exp. Med. 196:829-839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Seet, B. T., J. B. Johnston, C. R. Brunetti, J. W. Barrett, H. Everett, C. Cameron, J. Sypula, S. H. Nazarian, A. Lucas, and G. McFadden. 2003. Poxviruses and immune evasion. Annu. Rev. Immunol. 21:377-423. [DOI] [PubMed] [Google Scholar]
- 83.Seet, B. T., and G. McFadden. 2002. Viral chemokine-binding proteins. J. Leukoc. Biol. 72:24-34. [PubMed] [Google Scholar]
- 84.Seet, B. T., R. Singh, C. Paavola, E. K. Lau, T. M. Handel, and G. McFadden. 2001. Molecular determinants for CC-chemokine recognition by a poxvirus CC-chemokine inhibitor. Proc. Natl. Acad. Sci. USA 98:9008-9013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sen, G. C. 2001. Viruses and interferons. Annu. Rev. Microbiol. 55:255-281. [DOI] [PubMed] [Google Scholar]
- 86.Senkevich, T. G., and B. Moss. 1998. Domain structure, intracellular trafficking, and beta2-microglobulin binding of a major histocompatibility complex class I homolog encoded by molluscum contagiosum virus. Virology 250:397-407. [DOI] [PubMed] [Google Scholar]
- 87.Sharp, T. V., F. Moonan, A. Romashko, B. Joshi, G. N. Barber, and R. Jagus. 1998. The vaccinia virus E3L gene product interacts with both the regulatory and the substrate binding regions of PKR—implications for PKR autoregulation. Virology 250:302-315. [DOI] [PubMed] [Google Scholar]
- 88.Shisler, J. L., and B. Moss. 2001. Molluscum contagiosum virus inhibitors of apoptosis: the MC159 v-FLIP protein blocks Fas-induced activation of procaspases and degradation of the related MC160 protein. Virology 282:14-25. [DOI] [PubMed] [Google Scholar]
- 89.Simonovic, M., P. G. W. Gettins, and K. Volz. 2000. Crystal structure of viral serpin crmA provides insights into its mechanism of cysteine proteinase inhibition. Prot. Sci. 9:1423-1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Smith, E. J., I. Marie, A. Prakash, A. Garcia-Sastre, and D. E. Levy. 2001. IRF3 and IRF7 phosphorylation in virus-infected cells does not require double-stranded RNA-dependent protein kinase R or I kappa B kinase but is blocked by vaccinia virus E3L protein. J. Biol. Chem. 276:8951-8957. [DOI] [PubMed] [Google Scholar]
- 91.Smith, G. L. 2000. Secreted poxvirus proteins that interact with the immune system, p. 491-507. In M. W. Cunningham and R. S. Fujinami (ed.), Effects of microbes on the immune system. Lippincott Williams and Wilkins, Philadelphia, Pa.
- 92.Smith, G. L., J. A. Symons, and A. Alcami. 1998. Poxviruses: interfering with interferon. Semin. Virol. 8:409-418. [Google Scholar]
- 93.Smith, S. A., and G. J. Kotwal. 2002. Immune response to poxvirus infections in various animals. Crit. Rev. Microbiol. 28:149-185. [DOI] [PubMed] [Google Scholar]
- 94.Smith, S. A., G. Krishnasamy, K. H. Murthy, A. Cooper, K. Bromek, P. N. Barlow, and G. J. Kotwal. 2002. Vaccinia virus complement control protein is monomeric, and retains structural and functional integrity after exposure to adverse conditions. Biochim. Biophys. Acta 1598:55-64. [DOI] [PubMed] [Google Scholar]
- 95.Smith, S. A., N. P. Mullin, J. Parkinson, S. N. Shchelkunov, A. V. Totmenin, V. N. Loparev, R. Srisatjaluk, D. N. Reynolds, K. L. Keeling, D. E. Justus, P. N. Barlow, and G. J. Kotwal. 2000. Conserved surface-exposed K/R-X-K/R motifs and net positive charge on poxvirus complement control proteins serve as putative heparin binding sites and contribute to inhibition of molecular interactions with human endothelial cells: a novel mechanism for evasion of host defense. J. Virol. 74:5659-5666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Smith, V. P., and A. Alcami. 2000. Expression of secreted cytokine and chemokine inhibitors by ectromelia virus. J. Virol. 74:8460-8471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Smith, V. P., and A. Alcami. 2002. Inhibition of interferons by ectromelia virus. J. Virol. 76:1124-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Smith, V. P., N. A. Bryant, and A. Alcami. 2000. Ectromelia, vaccinia and cowpox viruses encode secreted interleukin-18-binding proteins. J. Gen. Virol. 81:1223-1230. [DOI] [PubMed] [Google Scholar]
- 99.Sroller, V., V. Ludvikova, L. Maresova, P. Hainz, and S. Nemeckova. 2001. Effect of IFN-gamma receptor gene deletion on vaccinia virus virulence. Arch. Virol. 146:239-249. [DOI] [PubMed] [Google Scholar]
- 100.Symons, J. A., E. Adams, D. C. Tscharke, P. C. Reading, H. Waldmann, and G. L. Smith. 2002. The vaccinia virus C12L protein inhibits mouse IL-18 and promotes virus virulence in the murine intranasal model. J. Gen. Virol. 83:2833-2844. [DOI] [PubMed] [Google Scholar]
- 101.Symons, J. A., D. C. Tscharke, N. Price, and G. L. Smith. 2002. A study of the vaccinia virus interferon-gamma receptor and its contribution to virus virulence. J. Gen. Virol. 83:1953-1964. [DOI] [PubMed] [Google Scholar]
- 102.Tewari, M., and V. M. Dixit. 1995. Fas- and tumor necrosis factor-induced apoptosis is inhibited by the poxvirus crmA gene product. J. Biol. Chem. 270:3255-3260. [DOI] [PubMed] [Google Scholar]
- 103.Thome, M., P. Schneider, K. Hofmann, H. Fickenscher, E. Meinl, F. Neipel, C. Mattmann, K. Burns, J. L. Bodmer, M. Schroter, C. Scaffidi, P. H. Krammer, M. E. Peter, and J. Tschopp. 1997. Viral FLICE-inhibitory proteins (FLIPs) prevent apoptosis induced by death receptors. Nature 386:517-521. [DOI] [PubMed] [Google Scholar]
- 104.Tulman, E. R., C. L. Afonso, Z. Lu, L. Zsak, G. F. Kutish, and D. L. Rock. 2001. Genome of lumpy skin disease virus. J. Virol. 75:7122-7130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Turner, P., and R. Moyer. 2002. Poxvirus immune modulators: functional insights from animal models. Virus Res. 88:35-53. [DOI] [PubMed] [Google Scholar]
- 106.Turner, P. C., M. C. Sancho, S. R. Thoennes, A. Caputo, R. C. Bleackley, and R. W. Moyer. 1999. Myxoma virus Serp2 is a weak inhibitor of granzyme B and interleukin-1 beta-converting enzyme in vitro and unlike CrmA cannot block apoptosis in cowpox virus-infected cells. J. Virol. 73:6394-6404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Uvarova, E. A., and S. N. Shchelkunov. 2001. Species-specific differences in the structure of orthopoxvirus complement-binding protein. Virus Res. 81:39-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Verardi, P. H., L. A. Jones, F. H. Aziz, S. Ahmad, and T. D. Yilma. 2001. Vaccinia virus vectors with an inactivated gamma interferon receptor homolog gene (B8R) are attenuated in vivo without a concomitant reduction in immunogenicity. J. Virol. 75:11-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wang, X. 2001. The expanding role of mitochondria in apoptosis. Genes Dev. 15:2922-2933. [PubMed] [Google Scholar]
- 110.Wasilenko, S. T., A. F. A. Meyers, K. Vander Helm, and M. Barry. 2001. Vaccinia virus infection disarms the mitochondrion-mediated pathway of the apoptotic cascade by modulating the permeability transition pore. J. Virol. 75:11437-11448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Xiang, Y., R. C. Condit, S. Vijaysri, B. Jacobs, B. R. G. Williams, and R. H. Silverman. 2002. Blockade of interferon induction and action by the E3L double-stranded RNA binding proteins of vaccinia virus. J. Virol. 76:5251-5259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Xiang, Y., and B. Moss. 2001. Correspondence of the functional epitopes of poxvirus and human interleukin-18-binding proteins. J. Virol. 75:9947-9954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Xiang, Y., and B. Moss. 1999. IL-18 binding and inhibition of interferon gamma induction by human poxvirus-encoded proteins. Proc. Natl. Acad. Sci. USA 96:11537-11542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Xu, X. M., P. Nash, and G. McFadden. 2000. Myxoma virus expresses a TNF receptor homolog with two distinct functions. Virus Genes 21:97-109. [PubMed] [Google Scholar]
- 115.Yewdell, J. W., and A. B. Hill. 2002. Viral interference with antigen presentation. Nat. Immunol. 3:1019-1025. [DOI] [PubMed] [Google Scholar]
- 116.Zuniga, M. 2002. A pox on thee! Manipulation of the host immune system by myxoma virus and implications for viral-host co-adaptation. Virus Res. 88:17-33. [DOI] [PubMed] [Google Scholar]
- 117.Zuniga, M. C., H. Wang, M. Barry, and G. McFadden. 1999. Endosomal/lysosomal retention and degradation of major histocompatibility complex class I molecules is induced by myxoma virus. Virology 261:180-192. [DOI] [PubMed] [Google Scholar]