Abstract
The chemokine receptors CCR5 and CXCR4 are the major coreceptors for human immunodeficiency virus (HIV) and simian immunodeficiency virus (SIV). At least 12 other chemokine receptors or close relatives support infection by particular HIV and SIV strains on CD4+ transformed indicator cell lines in vitro. However, the role of these alternative coreceptors in vivo is presently thought to be insignificant. Infection of cell lines expressing high levels of recombinant CD4 and coreceptors thus does not provide a true indication of coreceptor use in vivo. We therefore tested primary untransformed cell cultures that lack CCR5 and CXCR4, including astrocytes and brain microvascular endothelial cells (BMVECs), for naturally expressed alternative coreceptors functional for HIV and SIV infection. An adenovirus vector (Ad-CD4) was used to express CD4 in CD4− astrocytes and thus confer efficient infection if a functional coreceptor is present. Using a large panel of viruses with well-defined coreceptor usage, we identified a subset of HIV and SIV strains able to infect two astrocyte cultures derived from adult brain tissue. Astrocyte infection was partially inhibited by several chemokines, indicating a role for the chemokine receptor family in the observed infection. BMVECs were weakly positive for CD4 but negative for CCR5 and CXCR4 and were susceptible to infection by the same subset of isolates that infected astrocytes. BMVEC infection was efficiently inhibited by the chemokine vMIP-I, implicating one of its receptors as an alternative coreceptor for HIV and SIV infection. Furthermore, we tested whether the HIV type 1 and type 2 strains identified were able to infect peripheral blood mononuclear cells (PBMCs) via an alternative coreceptor. Several strains replicated in Δ32/Δ32 CCR5 PBMCs with CXCR4 blocked by AMD3100. This AMD3100-resistant replication was also sensitive to vMIP-I inhibition. The nature and potential role of this alternative coreceptor(s) in HIV infection in vivo is discussed.
Human immunodeficiency virus (HIV) and simian immunodeficiency virus (SIV) infect cells by inducing fusion of viral and cellular membranes and releasing the viral core into the cytoplasm. Fusion is initiated upon interaction of the trimeric viral envelope glycoprotein and the primary host cell receptor CD4 (21, 42). A conformational change in gp120 structure reveals a coreceptor-binding site, and the subsequent interaction with a coreceptor instigates further structural changes in gp41 and membrane fusion.
The seven-transmembrane G-protein-coupled chemokine receptors CCR5 and CXCR4 are the major coreceptors used for HIV and SIV infection in vivo (2, 24, 30). Cell tropism of HIV and SIV strains is largely determined by the expression of CD4 and these coreceptors. Cell tropism plays a role in both virus transmission and disease progression (39). The majority of transmitted viruses are R5- or M-tropic, highlighted by the substantial protection from infection observed in individuals homozygous for a 32-bp deletion in CCR5 (23, 47, 58). CXCR4-using variants emerge late in disease in up to 50% of AIDS patients (72). This change in coreceptor use correlates with disease progression in infected individuals (19, 60), although it is not a prerequisite, as not all infected individuals demonstrate a coreceptor switch (26). Although primary X4 strains can infect macrophages via CXCR4 (67, 75, 77), these variants primarily target new populations of T cells that express CXCR4 but not CCR5, e.g., naive T-cells (7, 52).
Although CCR5 and CXCR4 are the major coreceptors used in vivo, there are at least 12 other members of the chemokine receptor family, and related “orphan” receptors, that can support infection of indicator cell lines in vitro (5, 16, 27, 55). These include CCR3 (14, 28), CCR8 (55), GPR1 (34, 63), GPR15 (34), CXCR6 (3, 25), Apj (13, 31), and RDC1 (64). In general, HIV type 2 (HIV-2) and SIV strains use a wider range of these alternative coreceptors than HIV-1, frequently as efficiently as they use CCR5 and/or CXCR4 (16, 53). For HIV-1, there is little current evidence to indicate that alternative coreceptors (other than CCR5 and CXCR4) contribute to viral replication in vivo (79). The ability of HIV-1 strains to exploit alternative coreceptors on the surfaces of cell lines therefore does not provide a true indication of coreceptor usage properties in vivo. The capacity of naturally expressed coreceptors (other than CCR5 and CXCR4) to support HIV infection of primary cell cultures may provide a stronger indication for their use in vivo. Thus, it has been reported that a maternal isolate used CXCR6 in addition to CCR5 and CXCR4 on indicator cell lines and replicated in CCR5− peripheral blood mononuclear cells (PBMCs) in the presence of a CXCR4 inhibitor (61, 79). Similarly, Lee et al. reported that CCR8 supported infection of primary thymocytes by particular HIV-1 isolates (45).
In this study, we have investigated whether functional coreceptors can be detected on different primary or untransformed human cell cultures. We report the identification of a subset of HIV and SIV strains that are able to exploit an unknown coreceptor naturally expressed on brain microvascular endothelial cells (BMVECs) and astrocyte cultures. Moreover several of these HIV-1 and HIV-2 strains were able to replicate in CCR5− PBMCs with CXCR4 blocked by the bicyclam AMD3100. Replication of such strains was inhibited by the chemokine vMIP-I, implicating an unknown vMIP-I receptor as the coreceptor involved.
MATERIALS AND METHODS
Viruses.
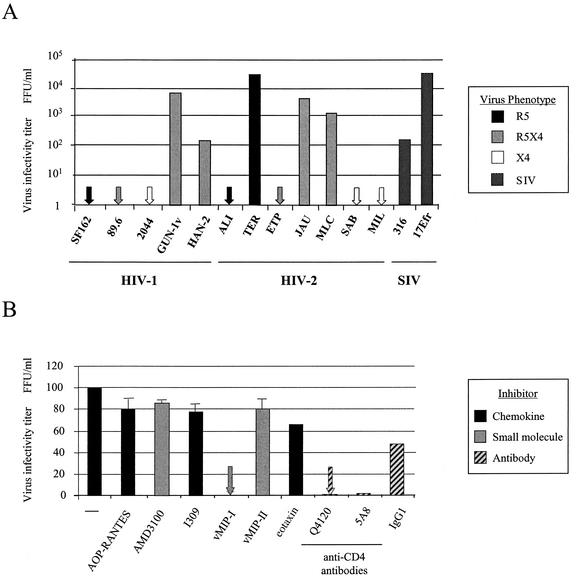
SF162 is a non-syncytium-inducing molecular clone of HIV-1 which uses primarily CCR5 as a coreceptor (12). The HIV-1 isolate 2044 utilizes CXCR4 as a coreceptor and has been previously described (68). GUN-1v is a variant of the T-cell-line-adapted HIV-1 isolate GUN-1WT, which was isolated by its ability to infect brain-derived BT cells derived from human meningioma tissue (71). The HIV-1 strain 89.6 is an R5X4 molecular clone of HIV-1 (18), as is the isolate HAN-2 (59). The R5X4 HIV-1 isolate P1019 is a pediatric strain isolated from a 3-year-old child (36). All isolates of HIV-1 used in this study are clade B. The HIV-2 isolates used here are all Portuguese primary isolates from individuals of West African descent (50, 54). ALI was derived from a patient with AIDS-related complex. TER, JAU, MIL, and SAB were isolated from AIDS patients, and the R5X4 isolates JAU, MLC, and ETP originated from symptomatic patients (54). The SIV strain SIVmac17Efr is a molecular clone of the isolate SIVmac239, containing the env and nef genes and the entire 3′ long terminal repeat from the neurovirulent SIVmac239 clone SIV/17E-Br (35). SIVman4 is a T-cell-line-adapted variant of SIVsmB670 (51). The coreceptor use of most of the virus isolates used in this study has been previously described (46, 54) and is summarized in Table 1. All virus stocks were propagated in PBMCs purified from whole blood by density gradient centrifugation and stimulated for 2 days in phytohemagglutinin (1 μg/ml) (Sigma) and for 2 days in human recombinant IL-2 (20 U/ml) (Roche, Inc.), with the exception of GUN-1v, which was passaged in the T-cell line MOLT4 clone 8. The replication-defective adenovirus vector carrying the human CD4 gene (Ad-CD4) (76) was propagated in the cell line 293. When cytopathicity was observed, cells were pelleted by centrifugation, the supernatant was discarded, and the cells were resuspended in fresh Dulbecco's modified Eagle's medium (DMEM) (Gibco Invitrogen Corporation) with 10% fetal bovine serum (FBS). Cells were lysed by freeze-thawing three times to release intracellular virus, and cell debris was removed by centrifugation. Ad-CD4 was titrated on NP2/CCR5 cells (see below) and subsequently challenged with serially diluted SF162. The Ad-CD4 dilution that conferred maximum SF162 infection was used in all further experiments.
TABLE 1.
Coreceptor use by HIV and SIV isolatesa
| VIRUS | Titer (FFU/ml) with coreceptor:
|
|||||
|---|---|---|---|---|---|---|
| CCR5 | CXCR4 | CCR3 | CCR8 | GPR1 | Apj | |
| HIV-1 | ||||||
| SF162 | 7.2 × 105 | 0 | 8.8 × 103 | 2.1 × 102 | 0 | 0 |
| 2044 | 0 | 1.0 × 105 | 0 | 1.0 × 102 | 0 | 0 |
| 89.6 | 3.0 × 103 | 5.0 × 103 | 8.0 × 03 | 2.6 × 105 | 0 | 1.3 × 104 |
| GUN-1v | 2.2 × 105 | 5.5 × 105 | 8.0 × 103 | 2.5 × 104 | 3.0 × 105 | 0 |
| HAN-2 | 1.0 × 104 | 2.4 × 104 | 1.5 × 103 | 3.6 × 104 | 2.0 × 102 | 0 |
| P1019 | 2.2 × 104 | 2.0 × 103 | 1.7 × 103 | 1.7 × 103 | 4.8 × 102 | 0 |
| HIV-2 | ||||||
| TER | 2.3 × 105 | 0 | 1.6 × 103 | 4.6 × 104 | 6.7 × 102 | 0 |
| ETP | 1.7 × 105 | 2.5 × 104 | 0 | 4.6 × 104 | 0 | 2.3 × 103 |
| JAU | 4.5 × 103 | 4.7 × 102 | 1.3 × 103 | 2.7 × 103 | 1.7 × 103 | 2.5 × 102 |
| ALI | 8.0 × 104 | 1.0 × 102 | 0 | 0 | 0 | NTb |
| SAB | 0 | 1.0 × 105 | 8.0 × 103 | 0 | 0 | NT |
| SIV (Man4) | 2.1 × 104 | 1.4 × 103 | 1.3 × 103 | 0 | 3.0 × 102 | 1.5 × 102 |
Coreceptor use by HIV and SIV isolates that use an alternative coreceptor on BMVEC and astrocyte cultures was determined by titration on the cell line NP2 (for all of the HIV-1 isolates, HIV-2 TER, ETP, and JAU, and SIVman4), U87 (for HIV-2 ALI and HIV-2 SAB with all coreceptors except CCR3), or GHOST (for HIV-2 SAB with CCR3), stably expressing CD4 and a range of coreceptors. Cells were incubated with 100 μl of 10-fold dilutions of virus stock for 3 h before being washed and incubated at 37°C for 72 h. Infected cells were detected by immunostaining for intracellular p24. The data as presented represent results compiled from several experiments.
NT, not tested.
Cell lines.
The human glioma-derived cell lines U87/CD4, stably expressing the chemokine receptor CCR2b (6, 25), and NP2/CD4, stably expressing CCR3, CCR5, CCR8, and GPR1 (69), were cultured in DMEM supplemented with 10% FBS, gentamicin (10 μg/ml) (Gibco Invitrogen Corporation), and puromycin (1 μg/ml). NP2/CD4/Apj cells were made by transfecting parental NP2/CD4 cells with a pBabe (puro)-Apj construct and selecting for stable transfectants in puromycin-containing selection medium. The T-cell line MOLT4 clone 8 was obtained from the National Institutes of Health (NIH) AIDS Research and Reference Reagent Program (22, 41) and were maintained in RPMI 1640 medium (Gibco Invitrogen Corporation) containing gentamicin (10 μg/ml) (Gibco Invitrogen Corporation) and 10% FBS.
Primary cells.
PBMCs from an individual homozygous for wild-type (wt) CCR5 or for the 32-bp deletion were prepared from whole blood, drawn under procedures approved by the University of Massachusetts Medical School Institutional Review Board, and stimulated with phytohemagglutinin and interleukin-2 as described above. Primary BMVECs (Clonetics Inc.) were maintained in endothelial cell basal medium 2 supplemented with EGM-2 additives (Clonetics Inc.). Fetal astrocytes were prepared from primary human fetal brain tissue, while human adult astrocytes were from temporal lobectomy samples. The use of fetal brain samples was approved by the University of Massachusetts Institutional Review Board (no. 10335). Following dissection and removal of blood vessels, tissue was resuspended in DMEM and passed through a cell strainer until all cell clumps were removed. The cell suspension was centrifuged for 5 min at 210 × g before the pellet was resuspended in DMEM-10% FBS and seeded into T25 tissue culture flasks. After cells had adhered, astrocytes were purified from mixed brain cultures by agitation on a plate shaker at 37°C for 1 to 2 h. Cells were washed in serum-free medium before incubation for 1 to 2 days in DMEM-10% FBS and reshaking as described above. Following a further 2-day incubation, the purity of astrocytes was determined by staining for the astrocytic marker glial fibrillary acidic protein as described below. Astrocyte cultures were maintained in DMEM with gentamicin and 10% FBS. Fetal astrocytes showed high plating efficiencies, whereas recovery from dispersed temporal lobectomy samples was markedly reduced.
Inhibitors and antibodies.
The chemokine RANTES was purchased from PeproTech Inc., Rocky Hill, N.J. The N-terminally modified RANTES analogue AOP-RANTES was provided by Amanda Proudfoot, Serono Inc, Geneva, Switzerland. The chemokines I309 and eotaxin and the human herpesvirus 8 (HHV8)-encoded chemokines vMIP-I and vMIP-II were purchased from R&D Systems, Inc. The CXCR4 antagonist AMD3100 was provided by AnorMED Inc., Langley, Canada. The small-molecule inhibitor TAK-779 was obtained through the AIDS Research and Reference Reagent Program, National Institute of Allergy and Infectious Diseases, NIH (4). The glial fibrillary acidic protein (GFAP) monoclonal antibody (MAb) used was a rabbit anti-cow GFAP from Dako. The CCR5 MAb 2D7 was from the NIH AIDS Research and Reference Reagent Program. The CXCR4 MAb 12G5 was provided through the NIH AIDS Research and Reference Reagent Program from James Hoxie (32). The CD4 MAb 5A8, directed to the D2 domain of CD4, has been described previously (9). Q4120 is directed to the N-terminal domain of CD4 and was provided through the Centralised Facility for AIDS Research from Quentin Sattentau (38).
Immunostaining for fluorescence microscopy.
Glass coverslips (13-mm diameter) were placed in the wells of a 24-well plate, sterilized by washing in 70% ethanol, and rinsed in sterile phosphate-buffered saline (PBS). Cells were plated at the appropriate density (primary astrocytes at 1 × 105/ml and cell lines at 4 × 104/ml) and left overnight at 37°C to adhere. Cells for GFAP staining were washed once in PBS before fixation in a cold (−40°C) 1:1 methanol-acetone mix for 5 to 10 min. Immunostaining for cell surface proteins was carried out at 4°C before fixation in a cold (−40°C) 1:1 methanol-acetone mix. Cells were gently rinsed in PBS before incubation with the appropriate antibody diluted to 5 μg/ml in PBS-1% FBS. Antigen was detected with a goat anti-mouse fluorescein isothiocyanate conjugate (Dako) diluted 1:40 or a swine F(ab′)2 anti-rabbit fluorescein isothiocyanate conjugate (Dako) diluted 1:15 in PBS-1% FBS. Images were captured on a confocal microscope and analyzed with the Confocal Assistant software.
Infectivity assays.
Cells were plated the day before use in a 48-well tray at 3 × 104 cells/ml in 500 μl of the appropriate medium. On the day of infection, medium was removed from the wells, and cells were infected with 100 μl of 10-fold serially diluted cell-free virus. After incubation for 3 h, cells were gently washed twice with growth medium and incubated for 72 h in 500 μl of fresh medium. For adenovirus expression of CD4, cells were plated at 3 × 104 cells/ml in 500 μl of the appropriate medium. The following day, medium was removed and cells were exposed to 100 μl of Ad-CD4 for 3 h. The amount of Ad-CD4 added corresponded to the dose of virus that conferred maximum SF162 infectivity to NP2/CCR5 cells. Cells were then rinsed and left overnight at 37°C. The next day, medium was removed and cells were exposed to HIV as described above. Infected cells were detected by immunostaining for intracellular p24 as described below.
Inhibition assays.
Adherent astrocytes and BMVECs were seeded the day before infection in 48-well plates at 3 × 104 cells/ml in 500 μl of the appropriate medium. On the day of infection, medium was removed and cells were incubated for 1 h with 75 μl of inhibitor (i.e., chemokines, AMD3100, or antibodies) at a 2× final concentration before being exposed to approximately 100 focus-forming units (FFU) of virus in 75 μl for 3 h. Cells were washed once in medium before the inhibitor was replaced at a 1× concentration (chemokines and small molecules at 500 nM and MAbs at 10 μg/ml) and cells were left at 37°C for 72 h. PBMCs were seeded in 100 μl in V-bottom 96-well plates at 106 cells/ml before centrifugation for 5 min at 210 × g and resuspension in 50 μl of medium alone or 2× inhibitor. Following incubation for 1 h at 37°C, 50 μl of virus at titers of 104 FFU/ml or higher was added and mixed, and cells were incubated at 37°C for 3 h. After incubation, cells were washed three times by centrifugation for 5 min at 210 × g in medium and resuspended in 150 μl of 1× inhibitor. Cells were left at 37°C, and cell-free supernatant was harvested on days 0, 3, 6, 9, 12, 15, and 18 of infection to monitor virus production. The inhibitor was replenished at each harvest. Inhibition assays that tested the effect of mouse MAbs, e.g., the anti-CCR5 2D7, and anti-CD4 Q4120, were directly compared with those for isotype antibody controls (immunoglobulin G1 [IgG1]). These antibodies were used at 5 μg/ml in these inhibition assays. This high concentration of isotype control nonspecifically reduced the infectivity of tested viruses by up to 50%.
Measurement of virus infectivity.
HIV infection of PBMCs was determined by measuring reverse transcriptase (RT) activity in cell supernatants by an RT enzyme-linked immunosorbent assay (CavidiTech, Uppsala, Sweden). Infected adherent cells were detected by immunostaining for intracellular p24 as previously described (17, 49). In brief, cells were rinsed in PBS before being fixed in a cold (−40°C) 1:1 methanol-acetone mix for 5 to 10 min and rinsed once in PBS and once in PBS-1% FBS. For HIV-1, cells were stained with a 1:1 mix of anti-HIV-1 Gag MAbs 38:96K and EF7 (Medical Research Council [MRC] AIDS Reagent Program, Potters Bar, England) diluted 1:40, while HIV-2-infected cells were stained with a mix of six HIV-2-positive serum samples (WHO panel C; MRC AIDS Reagent Program) diluted 1:4,000. Infected cells were detected with a 1:400 dilution of a goat anti-mouse or goat anti-human β-galactosidase conjugate for HIV-1 and HIV-2, respectively (Southern Biotechnology Associates, Inc.) and revealed with an X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) substrate (PBS with 3 mM potassium ferricyanide, 3 mM potassium ferrocyanide, 1 mM magnesium chloride, and 0.5 mg of X-Gal per ml).
RT-PCR.
Total cellular RNA was extracted from 1 × 106 to 5 × 106 cells by using the RNeasy kit from Qiagen, and mRNA was isolated by using the Oligotex kit from Qiagen. Specific mRNA sequences were amplified with the primers described in Table 2, using the Titanium one-step RT-PCR kit (Clontech). The housekeeping glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene was amplified from all cell samples as a positive control, and negative controls were primers for a gene known not be expressed in that cell type. All reactions were carried out in duplicate, with control reactions taking place on untranscribed mRNA to demonstrate the absence of contaminating genomic DNA. All reaction products were run on a 1.4% agarose gel with a 100-bp DNA ladder (New England Biolabs).
TABLE 2.
Oligonucleotide sequences used for the amplification of HIV and SIV receptors and coreceptors
| Primer | Sequence (5′→3′) | Product size (bp) |
|---|---|---|
| GAPDH | TGGATATTGCCATCAATGACC | 457 |
| GATGGCATGGACTGTGGTCATG | ||
| CD4 | TCAGGGAAAGAAAGTGGTGC | 170 |
| AAGAAGGAGCCCTGATTTCC | ||
| CCR5 | CTTCATTACACCTGCAGCTCT | 182 (wild type), 150 (Δ32) |
| CACAGCCCTGTGCCTCTTCTT | ||
| CXCR4 | TAGATATCTTACCATGGAGGGGATCAG | 1,044 |
| TAGCGGCGCTTAGCTGGAGTGAAAACTTG | ||
| CCR8 | GCAAGTTGCTCCTTGCTGTC | 710 |
| CATGGGTGGCATAAGTCAGC | ||
| CCR3 | GCTGATACCAGAGCACTGATG | 834 |
| CAACAAAGGCGTAGATCACCG | ||
| GPR1 | CCAGCTGGGAGTTGTTGTTCACT | 1,068 |
| GCTGTTTCCAGGAGACACAG | ||
| GPR15 | ATGGACCCAGAAGAAACTTC | 1,100 |
| TTAGAGTGACACAGACCTC | ||
| CXCR6 | CAGGCATCCATGAATGGGTGT | 766 |
| CAAGGCTATAACTGGAACATGCTG | ||
| RDC1 | AAGAAGATGGTACGCCGTGTCGTCTGCATCCTG | 280 |
| CTGCTGTGCTTCTCCTGGTCACTGGACGCCGAG |
RESULTS
Lack of CCR5 and CD4 on cultured astrocytes by immunostaining.
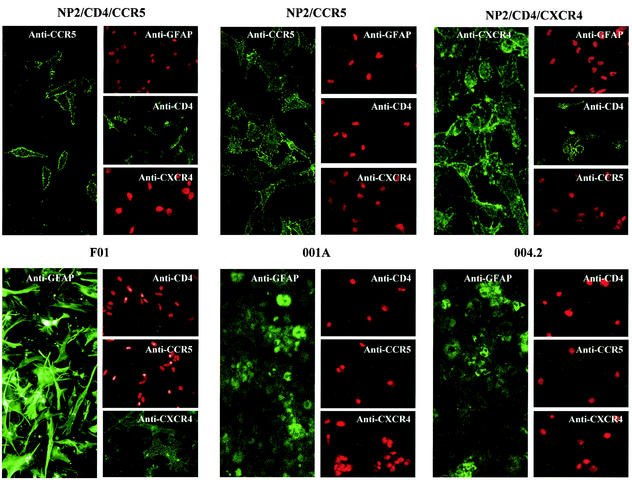
Untransformed cultured fetal astrocytes (F01) and cells derived from adult temporal lobectomies (samples 001A and 004.2) were tested for their expression of the astrocyte marker GFAP. Figure 1 shows that fetal astrocytes were strongly positive, while both adult cultures showed weaker and more diffuse staining, consistent with the observations of Marriott et al. (48). We also tested for CCR5, CXCR4, and CD4 expression on each of the untransformed astrocyte cultures and found that purified fetal astrocytes (F01) cultured for 2 weeks expressed neither CCR5 nor CD4 but were weakly positive for CXCR4, as shown previously (Fig. 1) (53). CD4, CCR5, and CXCR4 were not detected on either of the adult brain-derived cultures 001A and 004.2.
FIG. 1.
Immunostaining of astrocyte cultures. All astrocyte cultures and control cells were seeded onto 13-mm-diameter glass coverslips the day before being stained for CD4 (Q4120), CCR5 (2D7), CXCR4 (12G5), and GFAP, a marker for astrocytes. Nuclei of antigen-negative cells were stained with propidium iodide (red).
CD4 expression on cultured astrocytes confers sensitivity to a subset of HIV and SIV strains.
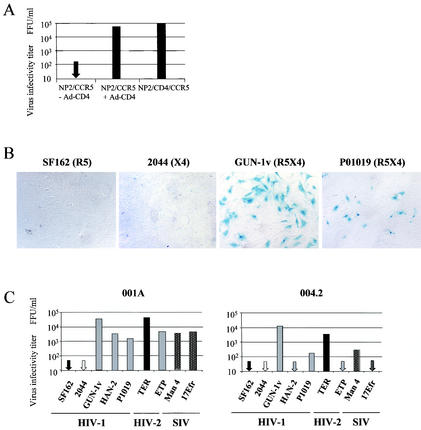
Astrocytes do not usually express CD4 and therefore support only an inefficient infection by particular HIV-1 strains (56, 74). The chemokine receptor expression profile of astrocytes is controversial, and the role of coreceptors in the observed low-level infection is unclear (8, 56). In order to determine if the cultured fetal and adult GFAP-positive cultures express functional coreceptors, we used an Ad-CD4 vector to express CD4 on these cells. As seen in Fig. 2A, infection of the cell line NP2/CCR5 with Ad-CD4 is sufficient to confer infection by R5 viruses to levels comparable to those seen with NP2 cells stably expressing both CD4 and CCR5. By the same principle, infection of astrocyte cultures with Ad-CD4 enables the screening of a range of HIV and SIV isolates for their capacity to use functional coreceptors expressed on astrocytes that lack CCR5 (fetal) or both CCR5 and CXCR4 (adult). Adult astrocyte cultures (001A and 004.2) preinfected with Ad-CD4 were resistant to HIV-1 strains that predominantly used CCR5 (SF162) or CXCR4 (2044). In contrast, three R5X4 HIV-1 strains (P1019, HAN-2, and GUN-1v) and several HIV-2 and SIV strains (TER, ETP, SIVman4, and SIVmac17Efr) were able to infect adult astrocytes 001A and, to various extents, 004.2 (Fig. 2B and C). The fetal astrocyte culture F01 weakly expressed CXCR4 (Fig. 1), and adenovirus-mediated expression of CD4 conferred sensitivity to all isolates that use CXCR4, including the HIV-1 X4 isolate 2044 (Fig. 2D). However, it is likely that the HIV-2 strain TER and SIVman4 exploit an alternative coreceptor for infection of fetal astrocytes, since TER does not use CXCR4 on standard CD4+ indicator cell lines (54), and CXCR4 use by SIVman4 is inefficient and at least 1,000 times less than CCR5 use (data not shown).
FIG. 2.
CD4 expression on astrocytes confers susceptibility to infection with HIV and SIV. (A) The activity of Ad-CD4 was tested by pretreating NP2/CCR5 cells with Ad-CD4 for 3 h, rinsing, and incubating overnight. Ad-CD4-pretreated and untreated NP2/CCR5 cells, as well as NP2 cells stably expressing CD4 and CCR5, were exposed to the R5-using HIV-1 strain SF162 for 3 h, and infected cells detected by p24 immunostaining were counted after 72 h. (B) Untransformed GFAP-positive adult astrocytes 001A and 004.2 were pretreated with Ad-CD4 before infection with the HIV-1, HIV-2, and SIV strains shown. After 72 h, cells were fixed and stained for intracellular viral antigens. (C and D) The titer of each HIV or SIV isolate was assessed on adult astrocytes 001A and 004.2 (C) and fetal astrocytes F01 (D). Cells were exposed to Ad-CD4 and infected as described above. All HIV and SIV isolates tested had titers of between 1 × 105 and 5 × 105 FFU/ml on NP2/CD4 coreceptor-expressing indicator cells (Table 1). Infected cells were detected by immunostaining for viral antigens and counted. All data are representative of those from at least three independent experiments.
Infection of fetal and adult astrocytes by HIV-1, HIV-2, and SIV strains in the absence of CD4 expression.
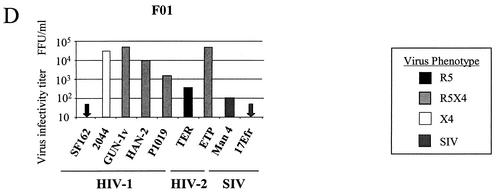
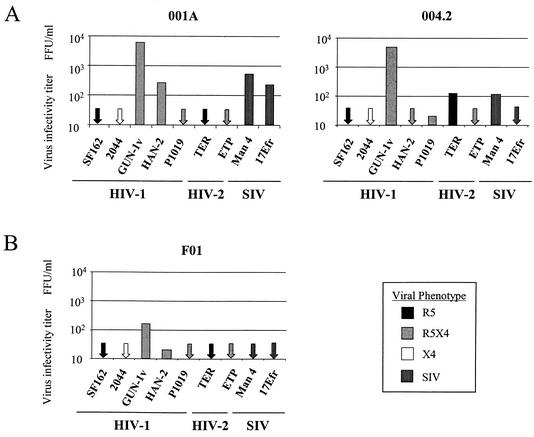
Low-level CD4-independent infection of brain cultures has been reported for various HIV and SIV strains (15, 56). Therefore, we also tested whether fetal and adult astrocytes supported infection by the same panel of HIV and SIV isolates in the absence of adenovirus-mediated CD4 expression. These cultures were negative for CD4 expression by immunohistochemical methods, and infection by most strains was weak, as expected (Fig. 3). The HIV-1 strains SF162 (R5) and 2044 (X4) did not infect either the adult or fetal astrocytes. The R5X4 isolates HAN-2 and P1019, as well as the HIV-2 strain TER and the SIV isolates Man4 and 17Efr, gave variable but low infectivity titers on all three astrocyte cultures. The HIV-1 isolate GUN-1v was able to infect both adult astrocyte cultures 004.2 and 001A, even though infection of coreceptor indicator cell lines in the absence of CD4 was negative (data not shown).
FIG. 3.
Infection of fetal and adult astrocytes by HIV-1, HIV-2, and SIV without Ad-CD4 pretreatment. Adult 004.2 and 001A astrocytes (A) and fetal F01 astrocytes (B) were seeded the day before infection with a panel of HIV and SIV isolates for 3 h. Infected cells were detected by immunostaining for intracellular viral p24 after 72 h. Titers are representative of those from three independent experiments. All viruses tested had infectivity titers of between 1 × 105 and 5 × 105 FFU/ml on NP2/CD4 coreceptor-expressing indicator cells.
Infection of fetal and adult astrocytes is reduced by chemokine receptor ligands.
In order to identify the possible coreceptor(s) being exploited for infection, we tested a range of chemokine receptor ligands for their capacity to block infection by HIV-1 GUN-1v. A common feature of the HIV and SIV strains that infected astrocytes was their capacity to exploit CCR3, CCR8, and GPR1 as coreceptors on indicator cell lines (in addition to CCR5 and sometimes CXCR4). We therefore included ligands that bind these chemokine receptors in the inhibition assays. The activities of all coreceptor-specific chemokines and antibodies were determined by carrying out control inhibitions on appropriate coreceptor-expressing cell lines. CCR3- and CCR8-mediated HIV infection was selectively inhibited by the ligands eotaxin and I309, respectively (data not shown). In comparison, vMIP-I (an HHV8-encoded chemokine) inhibited infection of control cell lines expressing CCR8, GPR1, and CXCR6, as previously reported (66), and weakly blocked CCR3-mediated infection (data not shown).
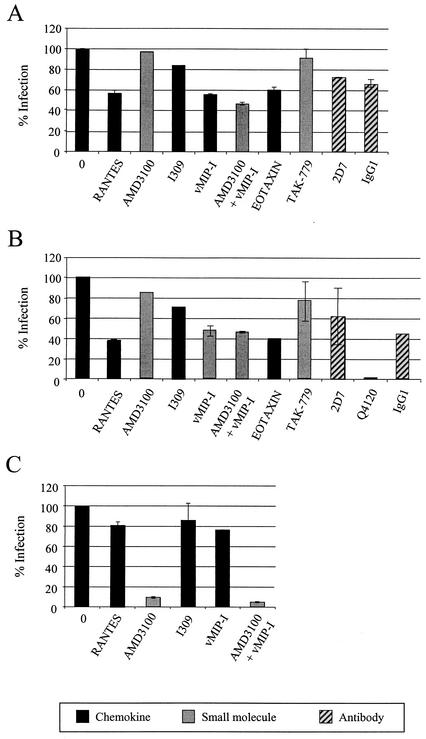
GUN-1v infection of adult astrocytes 001A pretreated with Ad-CD4 was reduced to approximately 50% of the levels seen in the absence of inhibitors by both vMIP-I (which binds CCR8, GPR1, and CXCR6) and RANTES (which binds CCR5, CCR1, CCR3, and CCR9). The ligand eotaxin (CCR3, D6) also reduced infectivity to approximately 60%. (Fig. 4A). TAK-779, the CCR5 small-molecule inhibitor, had no effect on 001A infection despite inhibiting GUN-1v infection of CCR5-expressing indicator cell lines by 100% (data not shown). In addition, the CCR5-specific MAb 2D7 failed to block astrocyte infection in comparison to an IgG1 isotype control. Taken together, these data confirm that the inhibition observed by RANTES is not due to the expression and use of CCR5 on these cells. Astrocyte infection was not affected by the CXCR4 antagonist AMD3100 or the CCR8-specific ligand I309. The same chemokines that weakly reduced infection of CD4+ adult astrocytes 001A (vMIP-I, eotaxin, and RANTES) also inhibited infection in the absence of CD4 by between 50 and 60% (Fig. 4B). Intriguingly, infection of CD4− 001A cells was completely blocked by the CD4 MAb Q4120, indicating the presence of low levels of CD4 undetectable by immunostaining (Fig. 1) but sufficient to explain the observed infection by the HIV-1 strains GUN-1v and HAN-2 in the absence of adenovirus-mediated CD4 expression. As seen in Fig. 4A, CD4− astrocyte infection was also unaffected by 2D7. Infection of the fetal astrocyte preparation F01 by GUN-1v was reduced to less than 10% of control levels by AMD3100 and was minimally affected by RANTES, I309, and vMIP-I, confirming that CXCR4 conferred the majority of infection in these cells (Fig. 4C). The observation that adult astrocyte infection can be inhibited by chemokines implies a role for a chemokine receptor(s) in virus entry into these cells. However, weak inhibition was observed with several chemokines, all of which interact with a range of chemokine receptors (vMIP-I, RANTES, and eotaxin), suggesting that the chemokine receptor exploited for infection of astrocytes is not a major target for these chemokines.
FIG. 4.
Infection of CD4+ astrocytes is partially inhibited by vMIP-I and RANTES. Adult-derived astrocytes 001A either pretreated with Ad-CD4 (A) or untreated (B) and fetal astrocytes F01 (C) were preincubated with a range of chemokines or small-molecule inhibitors at 500 nM or with antibodies at 5 μg/ml before being exposed to the HIV-1 isolate GUN-1v. Infected cells were detected by immunostaining for p24 at 72 h postinfection. One hundred percent infection represents the titer in the absence of inhibitor and was approximately 150 FFU. All values were calculated with respect to this control. All samples were done in duplicate, and the error bars represent the standard errors of the means.
BMVECs support infection by the same viruses shown to infect astrocytes via an unidentified coreceptor.
We next tested the ability of primary BMVECs to support infection by a range of HIV-1, HIV-2, and SIV isolates. No infection by HIV-1 and HIV-2 isolates that predominantly use CCR5 or CXCR4 was observed. However, the same subset of viruses that infected adult astrocytes also replicated in BMVECs. These included R5X4 HIV-1 strains (GUN-1v and HAN-2), HIV-2 strains (TER), and SIV strains (17Efr) (Fig. 5A). In addition, the R5X4 HIV-2 isolates JAU and MLC and the SIV variant SIVmac316 infected BMVECs. BMVEC infection by GUN-1v was completely inhibited by vMIP-I (Fig. 5B) but was unaffected by ligands for CCR5 (AOP-RANTES), CXCR4 (AMD3100), and CCR8 (I309), despite their ability to block infection of coreceptor-expressing cell lines (data not shown). The CCR3 ligand eotaxin reduced BMVEC infection by approximately 30%; however, the chemokine analogue vMIP-II, a more potent inhibitor of CCR3, had no effect on infection (43, 66). BMVEC infection was reduced to less than 5% by the CD4 MAbs Q4120 (D1) and 5A8 (D2/3), indicating that CD4 was present on the cell surface at levels high enough to support the efficient infection observed by the HIV and SIV strains shown in Fig. 5. The corresponding antibody isotype control, IgG1, had no effect on BMVEC infection. Infection of BMVECs therefore identifies the same subset of HIV-1, HIV-2, and SIV strains that infect GFAP+ astrocytes. Complete inhibition of BMVEC infection by vMIP-I, as well as the lack of inhibition by potent ligands for CCR5 and CXCR4, confirms that an alternative coreceptor is responsible for virus entry.
FIG. 5.
BMVECs support infection by the same subset of HIV and SIV isolates that infect astrocytes. (A) Untransformed BMVECs were exposed to HIV and SIV strains, and intracellular viral antigens were detected by immunostaining following a 72-h incubation. (B) The sensitivity of BMVEC infection to chemokine inhibition was determined by preincubating cells with chemokines or small molecule inhibitors at 200 nM or with CD4 MAbs at 5 μg/ml before incubation with the HIV-1 isolate GUN-1v. Infections were performed in duplicate, and error bars represent the standard errors of the means. Results are representative of those from at least two independent experiments.
A subset of HIV and SIV isolates can infect Δ32/Δ32 CCR5 PBMCs with CXCR4 blocked by AMD3100.
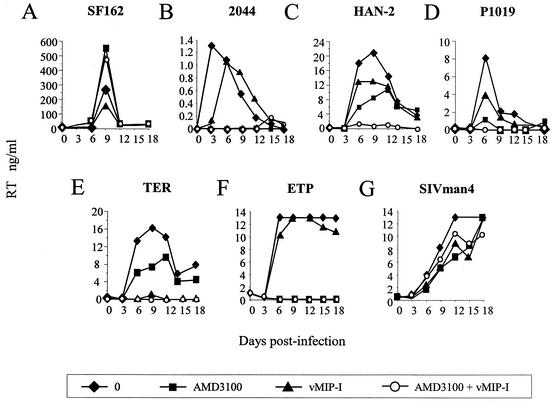
The experiments described above provide evidence that BMVECs and astrocytes naturally express alternative coreceptors that are functional for a subset of HIV-1 strains. Since these cells are not the major targets for HIV-1 in vivo, we tested whether the HIV and SIV isolates had the capacity to exploit an alternative coreceptor(s) on PBMCs. For this we used PBMCs prepared from an individual homozygous for the 32-bp deletion in CCR5 (Δ32/Δ32 CCR5) and blocked CXCR4 by treatment with AMD3100. The R5 HIV-1 isolate SF162 was unable to infect these cells, since they do not express CCR5 (data not shown). Infection of wt/wt CCR5 PBMCs by SF162 was not inhibited by the ligand AMD3100 (CXCR4) or vMIP-I (CCR8, GPR1, and CXCR6), either alone or in combination (Fig. 6A). Together these data demonstrate that SF162 infection of PBMCs is entirely CCR5 dependent. On Δ32/Δ32 CCR5 PBMCs, the X4 strain 2044 was efficiently inhibited by AMD3100 but not vMIP-I, although a slight delay in the rate of replication of this virus was noted (Fig. 6B). The HIV-1 isolate HAN-2 and HIV-2 TER replicated in the presence of AMD3100 (Fig. 6C and E). A second HIV-1 isolate, P1019, consistently replicated in the presence of AMD3100, albeit at low levels (Fig. 6D). Replication by each of these viruses was completely inhibited when cells were pretreated with vMIP-I in addition to AMD3100, yet pretreatment with I309 in addition to AMD3100 had no effect on any of the isolates tested (data not shown). We also tested GUN-1v; however, this T-cell-line-adapted virus replicated poorly in both wt/wt and Δ32/Δ32 CCR5 PBMCs. GUN-1v replication eventually ensued after a long lag phase; however, the impact of adaptive changes occurring during this lag time on GUN-1v coreceptor use is unclear. GUN-1v data were therefore omitted from this study. The results described above thus show that the primary HIV-1 and HIV-2 strains that infect astrocytes and BMVECs are also able to exploit an alternative coreceptor on PBMCs, a major target for HIV infection in vivo.
FIG. 6.
Productive infection of Δ32/Δ32 CCR5 PBMCs by HIV and SIV strains with CXCR4 blocked. Δ32/Δ32 CCR5 or wt/wt CCR5 PBMCs were preincubated for 1 h with medium alone or the inhibitor AMD3100, vMIP-I, or AMD3100 plus vMIP-I at 1,000 nM before addition of virus. (A) wt/wt CCR5 PBMCs were infected in triplicate with the R5 HIV-1 strain SF162. (B to G) Δ32/Δ32 CCR5 PBMCs were infected in triplicate with all other viruses (2044, HAN-2, P1019, TER, ETP, and SIVman4). All virus stocks had titers of between 104 and 105 FFU/ml on NP2/CD4/CCR5 or NP2/CD4/CXCR4 indicator cell lines. Supernatant was removed every 3 days up to day 18, and virus production was measured by RT enzyme-linked immunosorbent assay. All points represent the average for triplicate samples, and infections are representative of at least two independent infections.
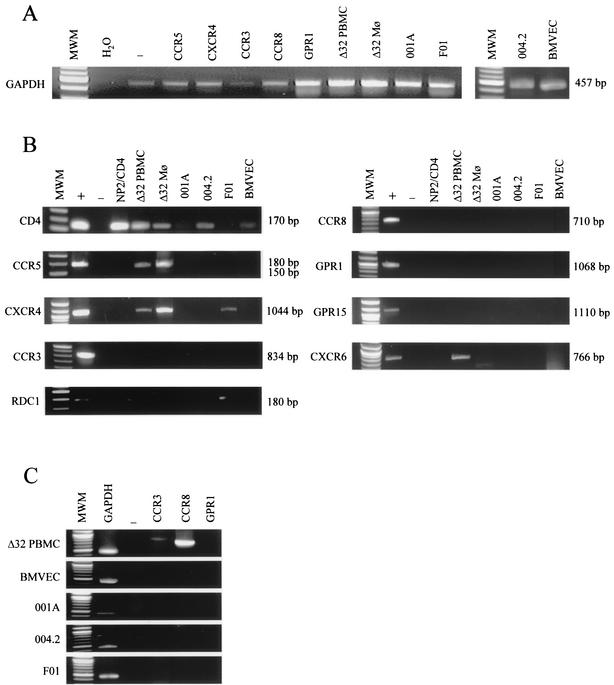
Analysis of HIV and SIV receptor expression on untransformed cell types.
RT-PCR was carried out on mRNA extracted from the three astrocyte cultures, BMVECs, Δ32/Δ32 PBMCs, and macrophages, to determine the pattern of HIV coreceptor expression. All mRNA preparations were positive for the housekeeping GAPDH gene (Fig. 7A). CD4 was found to be strongly expressed in Δ32/Δ32 CCR5 PBMCs and macrophages, as expected, but, surprisingly, also in the adult astrocytes 004.2 (Fig. 7B). BMVECs and the adult astrocyte culture 001A were very weakly positive for CD4, explaining the inhibition observed with anti-CD4 antibodies (Fig. 4B and 5B, respectively). A truncated CCR5 signal was detected for Δ32/Δ32 PBMCs and macrophages but not for any of the other cell cultures tested. CXCR6 expression was detected in PBMCs only. CXCR4 mRNA was expressed in Δ32/Δ32 PBMCs and macrophages, as well as in the fetal astrocyte culture F01. Although CCR3 and CCR8 were undetectable on Δ32/Δ32 PBMCs after 25 cycles, a signal was detected after amplification for 35 cycles (Fig. 7C). All other coreceptors screened for, including CCR3, CCR8, GPR1, and GPR15, were negative on all other primary cell cultures after 25 and 35 cycles (Fig. 7C). The orphan receptor RDC1 was detected in Δ32/Δ32 PBMCs, in the fetal F01 astrocytes, and weakly in the adult 004.2 astrocytes. In contrast to other studies (64), the parental NP2/CD4 cell line was also positive for this receptor. mRNA detection for each of the coreceptors tested here therefore did not correlate with HIV or SIV infection via the alternative coreceptor. We thus conclude that an as-yet-unidentified receptor for vMIP-I is partially (astrocytes) or wholly (BMVECs and PBMCs) responsible for infection of several untransformed cell cultures.
FIG. 7.
mRNA expression of HIV and SIV receptors and coreceptors in primary cell types. RT-PCR was used to test for chemokine receptor mRNA expression. (A) Amplification of GAPDH served as a control for intact mRNA. (B) The presence of chemokine receptor mRNA in Δ32/Δ32 CCR5 PBMCs and macrophages, astrocytes, and BMVECs was determined, with mRNA from coreceptor-expressing cell lines NP2/CD4 (CCR5, CXCR4, CCR3, CCR8, and GPR1) and GHOST (CXCR6 and GPR15) acting as positive controls. NP2/CD4 cells naturally expressed RDC1. The primers used are shown in Table 2. Lanes MWM, 100-bp molecular size DNA ladder. Each experiment was carried out with negative (water) (lanes −) and positive (mRNA from cells expressing the specific receptor) (lanes +) controls, as well as with the parental NP2/CD4 cells as a cellular negative control. The absence of contaminating genomic DNA was confirmed by carrying out all RT-PCRs on untranscribed mRNA (data not shown).
DISCUSSION
A wide range of different seven-transmembrane G-protein-coupled chemokine receptors function as coreceptors for HIV infection of indicator cell lines in vitro. Many HIV-2 and SIV strains are particularly promiscuous for different coreceptors in such assays; however, HIV-1 strains can also use a variety of alternative coreceptors (16, 53), e.g., CCR3, CCR8, and CXCR6. Although some of these coreceptors are expressed on CD4+ primary cells (1, 34, 61), there is little current evidence to suggest they are used by HIV-1 in vivo. Thus, the ability of particular HIV-1 strains to exploit alternative coreceptors for infection of cell lines in vitro does not provide a true indication of virus coreceptor use in vivo. We therefore analyzed several untransformed cell cultures for natural expression of functional alternative coreceptors that support HIV and SIV infection.
Astrocytes established from adult temporal lobectomy samples and untransformed BMVEC cultures do not express either CCR5 or CXCR4 and thus represent suitable target cells to test for the presence of alternative coreceptors. BMVECs and adult astrocytes expressing CD4 (via Ad-CD4) were resistant to infection by HIV and SIV strains that predominantly utilize CCR5 or CXCR4 alone. Both BMVECs and adult astrocytes were susceptible to infection by a subset of R5X4 HIV-1 strains, including two primary isolates (HAN-2 and P1019) as well as GUN-1v. GUN-1v is a variant virus isolated in vitro by its capacity to infect a wider range of host cells, including BT cells (human CD4+ meningioma-derived cells) (65, 71). GUN-1v infection of BMVECs was sensitive to inhibition by vMIP-I (an HHV8-encoded chemokine), suggesting that a receptor for this ligand is important for infection of these cells. In contrast, astrocyte infection by GUN-1v was reduced only approximately 50% by vMIP-I. This lack of complete inhibition by vMIP-I suggests either that the vMIP-I receptor (implicated for infection of BMVECs) is not solely responsible for astrocyte infection or that vMIP-I only weakly inhibits this receptor as expressed on astrocytes.
Infection of astrocytes was also reduced approximately 50% by the chemokines RANTES and eotaxin. These ligands each bind several chemokine receptors (CCR1, CCR3, CCR5, and CCR9 for RANTES and CCR3 and D6 for eotaxin), indicating a role for this subclass of receptor in the infection of astrocytes. Although CCR5 expression was not detected on these astrocytes, several groups have reported expression on astrocytes in situ in the brain, which is lost rapidly in culture (8, 29, 44). The lack of inhibition by the CCR5 small-molecule inhibitor TAK-779, as well as by the CCR5-specific MAb 2D7, confirms that the weak inhibition by RANTES was not due to the presence of low levels of CCR5.
There is evidence that astrocytes become persistently infected by HIV-1 in vivo, particularly in pediatric AIDS (57, 70, 74). Since R5 viruses are predominant in the brain (1, 37, 62), it is possible that these astrocytes are infected in vivo via CCR5. Nevertheless, the alternative coreceptor demonstrated on these CCR5− cultured astrocytes may potentially confer infection of astrocyte subsets critical for brain homeostasis and thus have an impact on neuropathology. In this study we used MAbs that detect p24 as a marker for HIV-1 infection. Since p24 is a late gene product, our results show that the astrocyte infection observed here in vitro is not restricted to the early phase of viral replication and expression of early gene products, thus confirming the observations of Canki et al. (10).
Since BMVECs and astrocytes are not major targets for HIV-1 in vivo, we sought evidence for use of the alternative coreceptor on primary PBMCs. Other studies have demonstrated the ability of some SIV strains to infect PBMCs lacking CCR5 via an alternative coreceptor, in a donor-dependent manner (11, 78). In some instances CXCR6 was implicated as the coreceptor involved (79, 80). In confirmation, several HIV-1 and HIV-2 isolates identified here that exploit an alternative coreceptor(s) for BMVEC and astrocyte infection were able to replicate in PBMCs that lack CCR5, with CXCR4 blocked by AMD3100. The X4 isolate 2044 was consistently sensitive to inhibition by AMD3100, although low-level residual replication was sometimes detected even in the presence of AMD3100. However, AMD3100-resistant replication in PBMCs by the isolates HAN-2, P1019, TER, and Man4 was not due to residual use of CXCR4, since such replication was inhibited by vMIP-I, a chemokine that does not interact with CXCR4 (20, 67). Our observations therefore provide evidence that an alternative coreceptor is active for infection of PBMCs by the HIV and SIV strains described.
The identity of the alternative coreceptor remains to be conclusively elucidated. A hallmark of the BMVEC and PBMC infection via an alternative coreceptor demonstrated here was the sensitivity to inhibition by vMIP-I. To date, vMIP-I has been reported to inhibit HIV infection mediated through CCR8 (20, 33) as well as GPR1 and CXCR6 (66). GUN-1v and the other HIV and SIV strains, identified here by their capacity to use alternative coreceptors, were able to use CCR8 and GPR1 (as well as other coreceptors) in addition to CCR5 and CXCR4 as coreceptors on cell lines. Furthermore, GUN-1v has previously been reported to use alternative coreceptors for infection of astroglial U87 cells, primary mesengial kidney cells, and BT cells. CCR8 was implicated as a coreceptor for BT infection (40) and GPR1 was implicated as a coreceptor for mesengial cell infection (73); however, inhibition by ligands to these coreceptors was not demonstrated. Our observations that the CCR8 ligand I309 had no effect on the replication of any HIV or SIV isolate in BMVECs or Δ32/Δ32 PBMCs, alone or in combination with AMD3100, despite being able to inhibit infection of a CCR8-expressing indicator cell line (data not shown) implies that vMIP-I is able to inhibit HIV infection of PBMCs via another chemokine receptor.
We also attempted to correlate coreceptor mRNA detection in PBMCs, BMVECs, and astrocytes with infection by this subset of HIV and SIV strains. None of the presently known vMIP-I receptors (CCR8, GPR1, and CXCR6) were detected by RT-PCR in BMVECs. mRNA for CCR8 was detected in the Δ32/Δ32 PBMCs; however, the CCR8-specific chemokine I309 had no effect on viral replication in either PBMCs or BMVECs. Δ32/Δ32 CCR5 PBMCs were also positive for expression of CXCR6, a coreceptor previously implicated as being responsible for replication of a maternal HIV-1 isolate (61, 79, 80). However, the CXCR6 ligand CXCL16 failed to block replication of vMIP-I-sensitive virus strains on Δ32/Δ32 CCR5 PBMCs even though infection of CXCR6-expressing cell lines was efficiently inhibited (data not shown). Finally, mRNA for GPR1 was not detected in either BMVECs or PBMCs. We therefore believe that an as-yet-unidentified vMIP-I receptor is responsible for the HIV and SIV infection of these cells. An alternative, albeit less likely, explanation is that vMIP-I induces down-modulation of a different receptor or induces intracellular signaling responses that negatively affect viral replication. However, previous studies have demonstrated that vMIP-I has no effect on chemokine-induced intracellular calcium signaling via the chemokine receptors CCR2, CCR4, CCR5, CCR7, CXCR2, CXCR3, and CXCR4 on PBMCs (20). Although vMIP-I did desensitize CCR8-mediated signaling, its ineffectiveness against such a large range of chemokine receptors suggests that receptor desensitization is not the mechanism of inhibition observed here.
All viruses that we found to use an alternative coreceptor(s) are R5X4, with the exception of HIV-2 TER, an isolate that readily acquires CXCR4 use in culture. We believe that an alternative coreceptor(s) may be exploited late in disease in addition to, or instead of, CXCR4 (like TER). The role of the expansion in coreceptor use may therefore contribute to the broadening of cell tropism late in disease, while a role in neuropathogenesis should also be considered. Moreover, the alternative coreceptor should be considered as a potential escape route from new therapeutic CCR5 and CXCR4 inhibitors currently undergoing clinical trials (34).
Acknowledgments
This work was supported by grants from amFAR (02802-30-RG) and the NIH (MH64408-01). S.J.W. is supported by Pfizer Global Research and Development, United Kingdom. A.M. is a recipient of a Wellcome Trust Fellowship, United Kingdom. P.R.C. is an Elizabeth Glaser Research Scientist.
We are grateful for the support, encouragement, and excellent discussion from Patrick Dorr and Manos Perros at Pfizer Global Research and Development, Ltd. Hiroo Hoshino very generously provided the HIV-1 strain GUN-1v and coreceptor-expressing NP2 cells. We thank Brad Poulos at the Albert Einstein Human Tissue Repository for fetal brain samples for astrocyte culture. We are grateful to the EU Programme EVA/MRC Centralised Facility for AIDS Reagents, NIBSC, United Kingdom (grant no. QLK2-CT-1999-00609 and GP828102), and the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH, for reagents used in this study.
REFERENCES
- 1.Albright, A. V., J. T. Shieh, T. Itoh, B. Lee, D. Pleasure, M. J. O'Connor, R. W. Doms, and F. Gonzalez-Scarano. 1999. Microglia express CCR5, CXCR4, and CCR3, but of these, CCR5, is the principal coreceptor for human immunodeficiency virus type 1 dementia isolates. J. Virol. 73:205-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alkhatib, G., E. A. Berger, P. M. Murphy, and J. E. Pease. 1997. Determinants of HIV-1 coreceptor function on CC chemokine receptor 3. Importance of both extracellular and transmembrane/cytoplasmic regions. J. Biol. Chem. 272:20420-20426. [DOI] [PubMed] [Google Scholar]
- 3.Alkhatib, G., F. Liao, E. A. Berger, J. M. Farber, and K. W. Peden. 1997. A new SIV co-receptor, STRL33. Nature 388:238.. [DOI] [PubMed] [Google Scholar]
- 4.Baba, M., O. Nishimura, N. Kanzaki, M. Okamoto, H. Sawada, Y. Iizawa, M. Shiraishi, Y. Aramaki, K. Okonogi, Y. Ogawa, K. Meguro, and M. Fujino. 1999. A small-molecule, nonpeptide CCR5 antagonist with highly potent and selective anti-HIV-1 activity. Proc. Natl. Acad. Sci. USA 96:5698-5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berger, E. A., P. M. Murphy, and J. M. Farber. 1999. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu. Rev. Immunol. 17:657-700. [DOI] [PubMed] [Google Scholar]
- 6.Bjorndal, A., H. Deng, M. Jansson, J. R. Fiore, C. Colognesi, A. Karlsson, J. Albert, G. Scarlatti, D. R. Littman, and E. M. Fenyo. 1997. Coreceptor usage of primary human immunodeficiency virus type 1 isolates varies according to biological phenotype. J. Virol. 71:7478-7487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blaak, H., A. B. van't Wout, M. Brouwer, B. Hooibrink, E. Hovenkamp, and H. Schuitemaker. 2000. In vivo HIV-1 infection of CD45RA(+)CD4(+) T cells is established primarily by syncytium-inducing variants and correlates with the rate of CD4(+) T cell decline. Proc. Natl. Acad. Sci. USA 97:1269-1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boutet, A., H. Salim, Y. Taoufik, P. M. Lledo, J. D. Vincent, J. F. Delfraissy, and M. Tardieu. 2001. Isolated human astrocytes are not susceptible to infection by M- and T-tropic HIV-1 strains despite functional expression of the chemokine receptors CCR5 and CXCR4. Glia 34:165-177. [PubMed] [Google Scholar]
- 9.Burkly, L. C., D. Olson, R. Shapiro, G. Winkler, J. J. Rosa, D. W. Thomas, C. Williams, and P. Chisholm. 1992. Inhibition of HIV infection by a novel CD4 domain 2-specific monoclonal antibody. Dissecting the basis for its inhibitory effect on HIV-induced cell fusion. J. Immunol. 149:1779-1787. [PubMed] [Google Scholar]
- 10.Canki, M., J. N. Thai, W. Chao, A. Ghorpade, M. J. Potash, and D. J. Volsky. 2001. Highly productive infection with pseudotyped human immunodeficiency virus type 1 (HIV-1) indicates no intracellular restrictions to HIV-1 replication in primary human astrocytes. J. Virol. 75:7925-7933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen, Z., P. Zhou, D. D. Ho, N. R. Landau, and P. A. Marx. 1997. Genetically divergent strains of simian immunodeficiency virus use CCR5 as a coreceptor for entry. J. Virol. 71:2705-2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng-Mayer, C., C. Weiss, D. Seto, and J. A. Levy. 1989. Isolates of human immunodeficiency virus type 1 from the brain may constitute a special group of the AIDS virus. Proc. Natl. Acad. Sci. USA 86:8575-8579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choe, H., M. Farzan, M. Konkel, K. Martin, Y. Sun, L. Marcon, M. Cayabyab, M. Berman, M. E. Dorf, N. Gerard, C. Gerard, and J. Sodroski. 1998. The orphan seven-transmembrane receptor apj supports the entry of primary T-cell-line-tropic and dualtropic human immunodeficiency virus type 1. J. Virol. 72:6113-6118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choe, H., M. Farzan, Y. Sun, N. Sullivan, B. Rollins, P. D. Ponath, L. Wu, C. R. Mackay, G. LaRosa, W. Newman, N. Gerard, C. Gerard, and J. Sodroski. 1996. The beta-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell 85:1135-1148. [DOI] [PubMed] [Google Scholar]
- 15.Clapham, P., A. McKnight, S. J. Talbot, and D. Wilkinson. 1996. HIV entry into cells by CD4-independent mechanisms, p. 83-95. In J.-M. Sabatier (ed.), Perspectives in drug discovery and design, vol. 5. ESCOM Science Publishers B.V., Leiden, The Netherlands.
- 16.Clapham, P. R., and A. McKnight. 2002. Cell surface receptors, virus entry and tropism of primate lentiviruses. J. Gen. Virol. 83:1809-1829. [DOI] [PubMed] [Google Scholar]
- 17.Clapham, P. R., A. McKnight, and R. A. Weiss. 1992. Human immunodeficiency virus type 2 infection and fusion of CD4-negative human cell lines: induction and enhancement by soluble CD4. J. Virol. 66:3531-3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Collman, R., J. W. Balliet, S. A. Gregory, H. Friedman, D. L. Kolson, N. Nathanson, and A. Srinivasan. 1992. An infectious molecular clone of an unusual macrophage-tropic and highly cytopathic strain of human immunodeficiency virus type 1. J. Virol. 66:7517-7521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Connor, R. I., K. E. Sheridan, D. Ceradini, S. Choe, and N. R. Landau. 1997. Change in coreceptor use correlates with disease progression in HIV-1-infected individuals. J. Exp. Med. 185:621-628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dairaghi, D. J., R. A. Fan, B. E. McMaster, M. R. Hanley, and T. J. Schall. 1999. HHV8-encoded vMIP-I selectively engages chemokine receptor CCR8. Agonist and antagonist profiles of viral chemokines. J. Biol. Chem. 274:21569-21574. [DOI] [PubMed] [Google Scholar]
- 21.Dalgleish, A. G., P. C. Beverley, P. R. Clapham, D. H. Crawford, M. F. Greaves, and R. A. Weiss. 1984. The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature 312:763-767. [DOI] [PubMed] [Google Scholar]
- 22.Daniel, M. D., Y. Li, Y. M. Naidu, P. J. Durda, D. K. Schmidt, C. D. Troup, D. P. Silva, J. J. MacKey, H. W. Kestler III, P. K. Sehgal, et al. 1988. Simian immunodeficiency virus from African green monkeys. J. Virol. 62:4123-4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dean, M., M. Carrington, C. Winkler, G. A. Huttley, M. W. Smith, R. Allikmets, J. J. Goedert, S. P. Buchbinder, E. Vittinghoff, E. Gomperts, S. Donfield, D. Vlahov, R. Kaslow, A. Saah, C. Rinaldo, R. Detels, S. J. O'Brien, et al. 1996. Genetic restriction of HIV-1 infection and progression to AIDS by a deletion allele of the CKR5 structural gene. Science 273:1856-1862. [DOI] [PubMed] [Google Scholar]
- 24.Deng, H., R. Liu, W. Ellmeier, S. Choe, D. Unutmaz, M. Burkhart, P. Di Marzio, S. Marmon, R. E. Sutton, C. M. Hill, C. B. Davis, S. C. Peiper, T. J. Schall, D. R. Littman, and N. R. Landau. 1996. Identification of a major co-receptor for primary isolates of HIV-1. Nature 381:661-666. [DOI] [PubMed] [Google Scholar]
- 25.Deng, H. K., D. Unutmaz, V. N. KewalRamani, and D. R. Littman. 1997. Expression cloning of new receptors used by simian and human immunodeficiency viruses. Nature 388:296-300. [DOI] [PubMed] [Google Scholar]
- 26.de Roda Husman, A. M., R. P. van Rij, H. Blaak, S. Broersen, and H. Schuitemaker. 1999. Adaptation to promiscuous usage of chemokine receptors is not a prerequisite for human immunodeficiency virus type 1 disease progression. J. Infect. Dis. 180:1106-1115. [DOI] [PubMed] [Google Scholar]
- 27.Dimitrov, D. S., X. Xiao, D. J. Chabot, and C. C. Broder. 1998. HIV coreceptors. J. Membr. Biol. 166:75-90. [DOI] [PubMed] [Google Scholar]
- 28.Doranz, B. J., J. Rucker, Y. Yi, R. J. Smyth, M. Samson, S. C. Peiper, M. Parmentier, R. G. Collman, and R. W. Doms. 1996. A dual-tropic primary HIV-1 isolate that uses fusin and the beta-chemokine receptors CKR-5, CKR-3, and CKR-2b as fusion cofactors. Cell 85:1149-1158. [DOI] [PubMed] [Google Scholar]
- 29.Dorf, M. E., M. A. Berman, S. Tanabe, M. Heesen, and Y. Luo. 2000. Astrocytes express functional chemokine receptors. J. Neuroimmunol. 111:109-121. [DOI] [PubMed] [Google Scholar]
- 30.Dragic, T., V. Litwin, G. P. Allaway, S. R. Martin, Y. Huang, K. A. Nagashima, C. Cayanan, P. J. Maddon, R. A. Koup, J. P. Moore, and W. A. Paxton. 1996. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature 381:667-673. [DOI] [PubMed] [Google Scholar]
- 31.Edinger, A. L., J. E. Clements, and R. W. Doms. 1999. Chemokine and orphan receptors in HIV-2 and SIV tropism and pathogenesis. Virology 260:211-221. [DOI] [PubMed] [Google Scholar]
- 32.Endres, M. J., P. R. Clapham, M. Marsh, M. Ahuja, J. D. Turner, A. McKnight, J. F. Thomas, B. Stoebenau-Haggarty, S. Choe, P. J. Vance, T. N. Wells, C. A. Power, S. S. Sutterwala, R. W. Doms, N. R. Landau, and J. A. Hoxie. 1996. CD4-independent infection by HIV-2 is mediated by fusin/CXCR4. Cell 87:745-756. [DOI] [PubMed] [Google Scholar]
- 33.Endres, M. J., C. G. Garlisi, H. Xiao, L. Shan, and J. A. Hedrick. 1999. The Kaposi's sarcoma-related herpesvirus (KSHV)-encoded chemokine vMIP-I is a specific agonist for the CC chemokine receptor (CCR)8. J. Exp. Med. 189:1993-1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Farzan, M., H. Choe, K. Martin, L. Marcon, W. Hofmann, G. Karlsson, Y. Sun, P. Barrett, N. Marchand, N. Sullivan, N. Gerard, C. Gerard, and J. Sodroski. 1997. Two orphan seven-transmembrane segment receptors which are expressed in CD4-positive cells support simian immunodeficiency virus infection. J. Exp. Med. 186:405-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Flaherty, M. T., D. A. Hauer, J. L. Mankowski, M. C. Zink, and J. E. Clements. 1997. Molecular and biological characterization of a neurovirulent molecular clone of simian immunodeficiency virus. J. Virol. 71:5790-5798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Forte, S. E., K. S. Byron, J. L. Sullivan, and M. Somasundaran. 1994. Non-syncytium-inducing HIV type 1 isolated from infected individuals replicates in MT-2 cells. AIDS Res. Hum. Retroviruses 10:1613-1618. [DOI] [PubMed] [Google Scholar]
- 37.He, J., Y. Chen, M. Farzan, H. Choe, A. Ohagen, S. Gartner, J. Busciglio, X. Yang, W. Hofmann, W. Newman, C. R. Mackay, J. Sodroski, and D. Gabuzda. 1997. CCR3 and CCR5 are co-receptors for HIV-1 infection of microglia. Nature 385:645-649. [DOI] [PubMed] [Google Scholar]
- 38.Healey, D., L. Dianda, J. P. Moore, J. S. McDougal, M. J. Moore, P. Estess, D. Buck, P. D. Kwong, P. C. Beverley, and Q. J. Sattentau. 1990. Novel anti-CD4 monoclonal antibodies separate human immunodeficiency virus infection and fusion of CD4+ cells from virus binding. J. Exp. Med. 172:1233-1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang, Y., W. A. Paxton, S. M. Wolinsky, A. U. Neumann, L. Zhang, T. He, S. Kang, D. Ceradini, Z. Jin, K. Yazdanbakhsh, K. Kunstman, D. Erickson, E. Dragon, N. R. Landau, J. Phair, D. D. Ho, and R. A. Koup. 1996. The role of a mutant CCR5 allele in HIV-1 transmission and disease progression. Nat. Med. 2:1240-1243. [DOI] [PubMed] [Google Scholar]
- 40.Jinno, A., N. Shimizu, Y. Soda, Y. Haraguchi, T. Kitamura, and H. Hoshino. 1998. Identification of the chemokine receptor TER1/CCR8 expressed in brain-derived cells and T cells as a new coreceptor for HIV-1 infection. Biochem. Biophys. Res. Commun. 243:497-502. [DOI] [PubMed] [Google Scholar]
- 41.Kikukawa, R., Y. Koyanagi, S. Harada, N. Kobayashi, M. Hatanaka, and N. Yamamoto. 1986. Differential susceptibility to the acquired immunodeficiency syndrome retrovirus in cloned cells of human leukemic T-cell line Molt-4. J. Virol. 57:1159-1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Klatzmann, D., E. Champagne, S. Chamaret, J. Gruest, D. Guetard, T. Hercend, J. C. Gluckman, and L. Montagnier. 1984. T-lymphocyte T4 molecule behaves as the receptor for human retrovirus LAV. Nature 312:767-768. [DOI] [PubMed] [Google Scholar]
- 43.Kledal, T. N., M. M. Rosenkilde, F. Coulin, G. Simmons, A. H. Johnsen, S. Alouani, C. A. Power, H. R. Luttichau, J. Gerstoft, P. R. Clapham, I. Clark-Lewis, T. N. C. Wells, and T. W. Schwartz. 1997. A broad-spectrum chemokine antagonist encoded by Kaposi's sarcoma-associated herpesvirus. Science 277:1656-1659. [DOI] [PubMed] [Google Scholar]
- 44.Klein, R. S., K. C. Williams, X. Alvarez-Hernandez, S. Westmoreland, T. Force, A. A. Lackner, and A. D. Luster. 1999. Chemokine receptor expression and signaling in macaque and human fetal neurons and astrocytes: implications for the neuropathogenesis of AIDS. J. Immunol. 163:1636-1646. [PubMed] [Google Scholar]
- 45.Lee, S., H. L. Tiffany, L. King, P. M. Murphy, H. Golding, and M. B. Zaitseva. 2000. CCR8 on human thymocytes functions as a human immunodeficiency virus type 1 coreceptor. J. Virol. 74:6946-6952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu, H. Y., Y. Soda, N. Shimizu, Y. Haraguchi, A. Jinno, Y. Takeuchi, and H. Hoshino. 2000. CD4-dependent and CD4-independent utilization of coreceptors by human immunodeficiency viruses type 2 and simian immunodeficiency viruses. Virology 278:276-288. [DOI] [PubMed] [Google Scholar]
- 47.Liu, R., W. A. Paxton, S. Choe, D. Ceradini, S. R. Martin, R. Horuk, M. E. MacDonald, H. Stuhlmann, R. A. Koup, and N. R. Landau. 1996. Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell 86:367-377. [DOI] [PubMed] [Google Scholar]
- 48.Marriott, D. R., W. D. Hirst, and M. C. Ljungberg. 2000. Astrocytes, p. p85-96. In J. Cohen (ed.), Neural cell culture. Oxford University Press, Oxford, United Kingdom.
- 49.McKnight, A., P. R. Clapham, and R. A. Weiss. 1994. HIV-2 and SIV infection of nonprimate cell lines expressing human CD4: restrictions to replication at distinct stages. Virology 201:8-18. [DOI] [PubMed] [Google Scholar]
- 50.McKnight, A., M. T. Dittmar, J. Moniz-Periera, K. Ariyoshi, J. D. Reeves, S. Hibbitts, D. Whitby, E. Aarons, A. E. Proudfoot, H. Whittle, and P. R. Clapham. 1998. A broad range of chemokine receptors are used by primary isolates of human immunodeficiency virus type 2 as coreceptors with CD4. J. Virol. 72:4065-4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Murphey-Corb, M., L. N. Martin, S. R. Rangan, G. B. Baskin, B. J. Gormus, R. H. Wolf, W. A. Andes, M. West, and R. C. Montelaro. 1986. Isolation of an HTLV-III-related retrovirus from macaques with simian AIDS and its possible origin in asymptomatic mangabeys. Nature 321:435-437. [DOI] [PubMed] [Google Scholar]
- 52.Ostrowski, M. A., T. W. Chun, S. J. Justement, I. Motola, M. A. Spinelli, J. Adelsberger, L. A. Ehler, S. B. Mizell, C. W. Hallahan, and A. S. Fauci. 1999. Both memory and CD45RA+/CD62L+ naive CD4+ T cells are infected in human immunodeficiency virus type 1-infected individuals. J. Virol. 73:6430-6435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reeves, J. D., and R. W. Doms. 2002. Human immunodeficiency virus type 2. J. Gen. Virol. 83:1253-1265. [DOI] [PubMed] [Google Scholar]
- 54.Reeves, J. D., S. Hibbitts, G. Simmons, A. McKnight, J. M. Azevedo-Pereira, J. Moniz-Pereira, and P. R. Clapham. 1999. Primary human immunodeficiency virus type 2 (HIV-2) isolates infect CD4-negative cells via CCR5 and CXCR4: comparison with HIV-1 and simian immunodeficiency virus and relevance to cell tropism in vivo. J. Virol. 73:7795-7804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rucker, J., A. L. Edinger, M. Sharron, M. Samson, B. Lee, J. F. Berson, Y. Yi, B. Margulies, R. G. Collman, B. J. Doranz, M. Parmentier, and R. W. Doms. 1997. Utilization of chemokine receptors, orphan receptors, and herpesvirus-encoded receptors by diverse human and simian immunodeficiency viruses. J. Virol. 71:8999-9007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sabri, F., E. Tresoldi, M. Di Stefano, S. Polo, M. C. Monaco, A. Verani, J. R. Fiore, P. Lusso, E. Major, F. Chiodi, and G. Scarlatti. 1999. Nonproductive human immunodeficiency virus type 1 infection of human fetal astrocytes: independence from CD4 and major chemokine receptors. Virology 264:370-384. [DOI] [PubMed] [Google Scholar]
- 57.Saito, Y., L. R. Sharer, L. G. Epstein, J. Michaels, M. Mintz, M. Louder, K. Golding, T. A. Cvetkovich, and B. M. Blumberg. 1994. Overexpression of nef as a marker for restricted HIV-1 infection of astrocytes in postmortem pediatric central nervous tissues. Neurology 44:474-481. [DOI] [PubMed] [Google Scholar]
- 58.Samson, M., F. Libert, B. J. Doranz, J. Rucker, C. Liesnard, C. M. Farber, S. Saragosti, C. Lapoumeroulie, J. Cognaux, C. Forceille, G. Muyldermans, C. Verhofstede, G. Burtonboy, M. Georges, T. Imai, S. Rana, Y. Yi, R. J. Smyth, R. G. Collman, R. W. Doms, G. Vassart, and M. Parmentier. 1996. Resistance to HIV-1 infection in Caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene. Nature 382:722-725. [DOI] [PubMed] [Google Scholar]
- 59.Sauermann, U., J. Schneider, J. Mous, U. Brunckhorst, I. Schedel, K. D. Jentsch, and G. Hunsmann. 1990. Molecular cloning and characterization of a German HIV-1 isolate. AIDS Res. Hum. Retroviruses 6:813-823. [DOI] [PubMed] [Google Scholar]
- 60.Scarlatti, G., E. Tresoldi, A. Bjorndal, R. Fredriksson, C. Colognesi, H. K. Deng, M. S. Malnati, A. Plebani, A. G. Siccardi, D. R. Littman, E. M. Fenyo, and P. Lusso. 1997. In vivo evolution of HIV-1 co-receptor usage and sensitivity to chemokine-mediated suppression. Nat. Med. 3:1259-1265. [DOI] [PubMed] [Google Scholar]
- 61.Sharron, M., S. Pohlmann, K. Price, E. Lolis, M. Tsang, F. Kirchhoff, R. W. Doms, and B. Lee. 2000. Expression and coreceptor activity of STRL33/Bonzo on primary peripheral blood lymphocytes. Blood 96:41-49. [PubMed] [Google Scholar]
- 62.Shieh, J. T., A. V. Albright, M. Sharron, S. Gartner, J. Strizki, R. W. Doms, and F. Gonzalez-Scarano. 1998. Chemokine receptor utilization by human immunodeficiency virus type 1 isolates that replicate in microglia. J. Virol. 72:4243-4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shimizu, N., Y. Soda, K. Kanbe, H. Y. Liu, A. Jinno, T. Kitamura, and H. Hoshino. 1999. An orphan G protein-coupled receptor, GPR1, acts as a coreceptor to allow replication of human immunodeficiency virus types 1 and 2 in brain-derived cells. J. Virol. 73:5231-5239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shimizu, N., Y. Soda, K. Kanbe, H. Y. Liu, R. Mukai, T. Kitamura, and H. Hoshino. 2000. A putative G protein-coupled receptor, RDC1, is a novel coreceptor for human and simian immunodeficiency viruses. J. Virol. 74:619-626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shimizu, N. S., N. G. Shimizu, Y. Takeuchi, and H. Hoshino. 1994. Isolation and characterization of human immunodeficiency virus type 1 variants infectious to brain-derived cells: detection of common point mutations in the V3 region of the env gene of the variants. J. Virol. 68:6130-6135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Simmons, G., J. D. Reeves, S. Hibbitts, J. T. Stine, P. W. Gray, A. E. Proudfoot, and P. R. Clapham. 2000. Co-receptor use by HIV and inhibition of HIV infection by chemokine receptor ligands. Immunol. Rev. 177:112-126. [DOI] [PubMed] [Google Scholar]
- 67.Simmons, G., J. D. Reeves, A. McKnight, N. Dejucq, S. Hibbitts, C. A. Power, E. Aarons, D. Schols, E. De Clercq, A. E. Proudfoot, and P. R. Clapham. 1998. CXCR4 as a functional coreceptor for human immunodeficiency virus type 1 infection of primary macrophages. J. Virol. 72:8453-8457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Simmons, G., D. Wilkinson, J. D. Reeves, M. T. Dittmar, S. Beddows, J. Weber, G. Carnegie, U. Desselberger, P. W. Gray, R. A. Weiss, and P. R. Clapham. 1996. Primary, syncytium-inducing human immunodeficiency virus type 1 isolates are dual-tropic, and most can use either Lestr or CCR5 as coreceptors for virus entry. J. Virol. 70:8355-8360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Soda, Y., N. Shimizu, A. Jinno, H. Y. Liu, K. Kanbe, T. Kitamura, and H. Hoshino. 1999. Establishment of a new system for determination of coreceptor usages of HIV based on the human glioma NP-2 cell line. Biochem. Biophys. Res. Commun. 258:313-321. [DOI] [PubMed] [Google Scholar]
- 70.Takahashi, K., S. L. Wesselingh, D. E. Griffin, J. C. McArthur, R. T. Johnson, and J. D. Glass. 1996. Localization of HIV-1 in human brain using polymerase chain reaction/in situ hybridization and immunocytochemistry. Ann. Neurol. 39:705-711. [DOI] [PubMed] [Google Scholar]
- 71.Takeuchi, Y., M. Akutsu, K. Murayama, N. Shimizu, and H. Hoshino. 1991. Host range mutant of human immunodeficiency virus type 1: modification of cell tropism by a single point mutation at the neutralization epitope in the env gene. J. Virol. 65:1710-1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tersmette, M., R. E. de Goede, B. J. Al, I. N. Winkel, R. A. Gruters, H. T. Cuypers, H. G. Huisman, and F. Miedema. 1988. Differential syncytium-inducing capacity of human immunodeficiency virus isolates: frequent detection of syncytium-inducing isolates in patients with acquired immunodeficiency syndrome (AIDS) and AIDS-related complex. J. Virol. 62:2026-2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tokizawa, S., N. Shimizu, L. Hui-Yu, F. Deyu, Y. Haraguchi, T. Oite, and H. Hoshino. 2000. Infection of mesangial cells with HIV and SIV: identification of GPR1 as a coreceptor. Kidney Int. 58:607-617. [DOI] [PubMed] [Google Scholar]
- 74.Tornatore, C., R. Chandra, J. R. Berger, and E. O. Major. 1994. HIV-1 infection of subcortical astrocytes in the pediatric central nervous system. Neurology 44:481-487. [DOI] [PubMed] [Google Scholar]
- 75.Valentin, A., H. Trivedi, W. Lu, L. G. Kostrikis, and G. N. Pavlakis. 2000. CXCR4 mediates entry and productive infection of syncytia-inducing (X4) HIV-1 strains in primary macrophages. Virology 269:294-304. [DOI] [PubMed] [Google Scholar]
- 76.Yasukawa, M., Y. Inoue, H. Ohminami, E. Sada, K. Miyake, T. Tohyama, T. Shimada, and S. Fujita. 1997. Human herpesvirus 7 infection of lymphoid and myeloid cell lines transduced with an adenovirus vector containing the CD4 gene. J. Virol. 71:1708-1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yi, Y., S. Rana, J. D. Turner, N. Gaddis, and R. G. Collman. 1998. CXCR-4 is expressed by primary macrophages and supports CCR5-independent infection by dual-tropic but not T-tropic isolates of human immunodeficiency virus type 1. J. Virol. 72:772-777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang, Y., B. Lou, R. B. Lal, A. Gettie, P. A. Marx, and J. P. Moore. 2000. Use of inhibitors to evaluate coreceptor usage by simian and simian/human immunodeficiency viruses and human immunodeficiency virus type 2 in primary cells. J. Virol. 74:6893-6910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang, Y. J., T. Dragic, Y. Cao, L. Kostrikis, D. S. Kwon, D. R. Littman, V. N. KewalRamani, and J. P. Moore. 1998. Use of coreceptors other than CCR5 by non-syncytium-inducing adult and pediatric isolates of human immunodeficiency virus type 1 is rare in vitro. J. Virol. 72:9337-9344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang, Y. J., and J. P. Moore. 1999. Will multiple coreceptors need to be targeted by inhibitors of human immunodeficiency virus type 1 entry? J. Virol. 73:3443-3448. [DOI] [PMC free article] [PubMed] [Google Scholar]