Abstract
We employed a newly developed genotyping technique with direct representational detection of LMP-1 gene sequences to study the molecular epidemiology of Epstein-Barr virus (EBV) infection in healthy individuals. Infections with up to five different EBV genotypes were found in two of nine individuals studied. These results support the hypothesis that multiple EBV infections of healthy individuals are common. The implications for the development of an EBV vaccine are discussed.
Multiple Epstein-Barr virus (EBV) infections are common among immunocompromised individuals (21, 29, 31, 39, 41, 43, 44, 46), but the origin of the multiple EBV strains remains a mystery. Multiple EBV strains could accumulate as superinfections in individuals who have lost previous protective immunity to EBV. Alternatively, they could represent the reactivation of latent EBV strains that were acquired prior to the onset of immunodeficiency. The reported prevalence of multiple EBV infections in healthy individuals ranges broadly between 0 and 100% (Table 1) (4, 7, 13, 16, 19, 20, 23, 30, 32, 35, 38, 45; M. L. Lung and R. S. Chang, Letter, J. Infect. Dis. 162:994-995, 1990), but differences among these studies in the molecular detection and definition of an EBV strain confound the interpretation of their results.
TABLE 1.
Literature review of multiple EBV infections in healthy individuals
| Author; yr (reference) | Prevalence (%) of multiple infectiona | Molecular epidemiology method |
|---|---|---|
| Lung et al.; 1988 (23) | 2 (1 of 46)* | Culture and RFLPd |
| Katz et al.; 1988 (19) | 29 (2 of 7)* | Culture and RFLP |
| Sixbey et al.; 1989 (32) | 9 (3 of 34)* | PCR: type 1 and type 2 |
| Lung et al.; 1990 (letter) | 0 (0 of 6)* | Culture and RFLP |
| Yao et al.; 1991 (45) | 0 (0 of 24)* | Culture and EBNAprint |
| Yao et al.; 1991 (45) | 0 (0 of 22) | PCR: type 1 and type 2 |
| Kunimoto et al.; 1992 (20) | 0 (0 of 21)* | PCR: type 1 and type 2 |
| Gratama et al.; 1994 (16) | 0 (0 of 39)* | Culture and EBNotype |
| Apolloni et al.; 1994 (4) | 14 (3 of 21)*b | PCR: type 1 and type 2 |
| Apolloni et al.; 1994 (4) | 27 (21 of 78)*c | PCR: type 1 and type 2 |
| Walling et al.; 1995 (38) | 67 (2 of 3)* | PCR-clone-sequence: LMP-1 |
| Falk et al.; 1997 (13) | 33 (1 of 3)*b | PCR: type 1 and type 2 |
| Falk et al.; 1997 (13) | 0 (0 of 15)*c | PCR: type 1 and type 2 |
| Srivastava et al.; 2000 (35) | 53 (8 of 15) | PCR: type 1 and type 2 |
| Srivastava et al.; 2000 (35) | 53 (8 of 15) | PCR and RFLP |
| Srivastava et al.; 2000 (35) | 93 (14 of 15)* | PCR: LMP-1 30-bp deletion |
| Srivastava et al.; 2000 (35) | 80 (12 of 15) | PCR: LMP-1 repeat region |
| Srivastava et al.; 2000 (35) | 71 (5 of 7) | PCR: BZLF1 repeat region |
| Brooks et al.; 2000 (7) | 20 (3 of 15)* | PCR-clone-sequence: EBNA-3C, EBNA-1 |
| Sitki-Green et al.; 2003 (30) | 100 (20 of 20)* | PCR-heteroduplex tracking assay: LMP-1 |
| Sitki-Green et al.; 2003 (30) | 87 (13 of 15)* | PCR-heteroduplex tracking assay: LMP-1 |
*, data were pooled and yielded a mean rate of multiple EBV infection of 23% (83 of 362).
Blood samples.
Saliva samples.
RFLP, restriction fragment length polymorphism.
Molecular epidemiologic studies requiring EBV isolation by B-lymphocyte transformation (16, 19, 23, 45; Lung and Chang, letter) suffer from selection bias toward transformation-competent EBV isolates (10, 27, 33). PCR amplification directly detects the EBV genome and avoids transformation selection bias, but the genetic definition of an EBV strain has been inconsistent across studies. Restriction fragment length polymorphisms detect either point mutations within restriction enzyme cleavage sites or variations of large repetitive regions within genome fragments (19, 23, 35; Lung and Chang, letter). Similarly, size variation in EBNA proteins (“EBNotype” or “EBNAprint”) (16, 45) and size variation in specific gene PCR products (LMP-1, BZLF1, EBNA-6) (13, 35) reflect variations in repetitive and other genome sequences. However, many EBV genome sequences are susceptible to intrastrain homologous and nonhomologous recombination during productive replication and the number of repeat units present may vary in different isolates of the same EBV strain (12, 38, 41-43). Studies examining the major sequence divergence between EBV types 1 and 2 have reported EBV coinfection rates ranging from 0 to 53% (4, 13, 20, 34, 35, 45). However, EBV types 1 and 2 can both be further subdivided into different strains (1, 24, 41) and only three studies to date have utilized EBV gene nucleotide sequence variation to define EBV strains in healthy individuals (7, 30, 38).
EBV genotyping assay.
A consistent approach is needed for the definition and nomenclature of EBV genomes. It is impractical or even impossible to physically isolate (culture) and fully characterize the EBV genome(s) in clinical infections. A reasonable goal would be to identify an EBV genetic marker that represents the broadest range of natural genetic heterogeneity while still distinguishing between evolutionarily stable genetic entities. In this context, the word “genotype” may be preferable to either “type” or “strain” to refer to a specific EBV genome that is capable of independently infecting a human host. Derivative entities arising from the originally infecting EBV genome through intrahost evolutionary genetic changes could be termed “substrains” or “variants.”
We have developed a highly sensitive and specific EBV genotyping technique based upon patterns of sequence variation in the EBV LMP-1 gene (43) that offers distinct advantages over previous molecular epidemiologic approaches. First, multiple EBV sequences are identified in a single tissue specimen by direct representational detection, thereby avoiding culture selection bias. Second, EBV genotypes are precisely defined at a single polymorphic genetic locus, based upon evolutionarily stable gene sequence patterns (11, 24, 43). Finally, this technique distinguishes between independent EBV infection events and intrahost EBV evolution, including point mutation and homologous and nonhomologous recombination events that may occur in the context of an LMP-1 sequence pattern (43).
(This work was presented in part at the Tenth Biennial Meeting of the International Association for Research on Epstein-Barr Virus and Associated Diseases, Cairns, Australia, July 2002.)
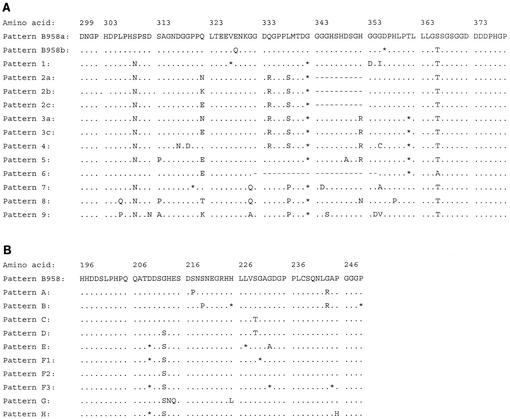
Nested PCR amplification of EBV LMP-1 gene sequences was achieved with primer set 1, 5′-AGTCATAGTAGCTTAGCTGAA-3′ (EBV coordinates 168160 to 168182) and 5′-CCATGGACAACGACACAGT-3′ (EBV coordinates 168763 to 168745), followed by primer set 2, 5′-AGTCATAGTAGCTTAGCTGAA-3′ (EBV coordinates 168160 to 168182) and 5′-CAGTGATGAACACCACCACG-3′ (EBV coordinates 168748 to 168729). The limit of detection of this LMP-1 gene nested PCR amplification was approximately 10 EBV genome copies per reaction, as determined by quantitative competitive PCR testing (unpublished data). PCR products visible on an ethidium bromide-stained agarose gel were cloned, and the LMP-1 sequence was determined for at least 8 to 10 clones from each specimen as previously described (43). The LMP-1 sequence patterns used to identify EBV genotypes are summarized in Fig. 1.
FIG. 1.
Defined sequence patterns in the carboxy terminus of the EBV LMP-1 gene are used to identify EBV genotypes. Each EBV genotype present represents an independent EBV infection of the host and has been validated for the multiple EBV infections and intrahost EBV evolutionary events that characterize individuals with human immunodeficiency virus and AIDS (43). Intrahost evolution of a previously infecting EBV genotype is recognizable as such and does not result in a change of genotype identity. (A) Fourteen numbered sequence patterns represent the region downstream of the 11-amino-acid repeat region of LMP-1. (B) Eleven-lettered sequence patterns represent the region upstream of the 11-amino-acid repeat region of LMP-1. All 25 sequence patterns represent EBV LMP-1 sequences identified in at least three different individuals and from at least two different geographic locations. EBV genotypes are named based upon the combination of the downstream and upstream patterns present conjointly in the LMP-1 gene (for example, 2a-F1). Symbols: dot, amino acid identity with pattern B958(a); *, synonymous nucleotide substitution; −, amino acid codon deletion. Amino acids are numbered on the basis of the LMP-1 gene of the lymphoblastoid cell line isolate B958.
This genotyping assay was tested to determine its ability to detect relative quantities of different coinfecting EBV genotypes. Two different clones of known different LMP-1 sequences (clones A and B) were mixed in vitro in various ratios ranging from 1:1 to 1:300. For each ratio combination, nested PCR amplification was performed by using 105 molecules of target LMP-1 sequence diluted into 1.0 μg of human genomic DNA. All PCR products were cloned. For each original ratio combination, the identity of multiple resultant clones was determined as matching the original clone A or clone B sequences. The ratios of the resultant clone sequences closely resembled the original sequence ratios before PCR (Table 2), suggesting that sequencing 8 to 10 clones per specimen is sufficient to detect all coinfecting EBV genotypes present in vivo at relative ratios of 1:10 or less.
TABLE 2.
LMP-1 genotype sequence ratios before and after PCR and cloning
| A:B ratio before PCR | No. of resultant clones A | No. of resultant clones B | A:B ratio after PCR |
|---|---|---|---|
| 1:1 | 15 | 26 | 1:1.7 |
| 2:1 | 44 | 13 | 3.4:1 |
| 4:1 | 45 | 6 | 7.5:1 |
| 10:1 | 18 | 2 | 9:1 |
| 30:1 | 28 | 2 | 14:1 |
| 100:1 | 14 | 0 | Not applicable |
| 300:1 | 40 | 0 | Not applicable |
EBV quantitation in healthy individuals.
Saliva and peripheral blood mononuclear cells from nine individuals were nonrandomly selected (based on detection of EBV in saliva for eight of the nine subjects and on the absence of EBV in the saliva for the remaining subject) from a cohort of 30 healthy human research subject volunteers enrolled in a long-term, prospective study of virus reactivation and shedding (22). Quantitative measurement of the EBV in each of 28 pairs of saliva and blood specimens was accomplished by real-time quantitative PCR of the EBER gene (Table 3) as previously described (28). EBV was detected in 25 of 28 saliva specimens by EBER PCR, and detectable quantities ranged from 6 to 2,220,000 EBV genome copies per 0.5 μg of DNA (Table 3). For subjects 2 and 4, the quantity of EBV detected in the saliva was remarkably high, approaching 30 EBV genomes per cell equivalent, a range similar to levels of productive EBV replication in oral hairy leukoplakia (15, 40).
TABLE 3.
Molecular epidemiology of EBV infection in healthy individuals
| Subject and specimen | Time point (mo) | No. of EBV genome copiesa | EBV LMP-1 genotype(s)b | No. of clones |
|---|---|---|---|---|
| 1 | ||||
| Saliva | 0 | ND | - | 0 |
| Blood | 0 | ND | - | 0 |
| Saliva | 2 | ND | 3c-C | 10 |
| Blood | 2 | ND | - | 0 |
| 2 | ||||
| Saliva | 0 | 10,400 | 3c-D | 10 |
| Blood | 0 | ND | - | 0 |
| Saliva | 2 | 2,220,000 | 3c-D | 10 |
| Blood | 2 | ND | - | 0 |
| Saliva | 4 | 328,000 | 3c-D | 9 |
| Blood | 4 | ND | - | 0 |
| 3 | ||||
| Saliva | 0 | 6 | 2a-G | 8 |
| Blood | 0 | ND | B958a-B958 | 10 |
| Saliva | 2 | ND | 3c-D | 10 |
| Blood | 2 | ND | 1-G | 10 |
| Saliva | 6 | 8 | - | 0 |
| Blood | 6 | 39 | - | 0 |
| Saliva | 8 | 14 | 3c-D | 8 |
| Blood | 8 | ND | - | 0 |
| Saliva | 10 | 51 | - | 0 |
| Blood | 10 | ND | - | 0 |
| 4 | ||||
| Saliva | 0 | 81,500 | 2a-G | 10 |
| Blood | 0 | ND | - | 0 |
| Saliva | 2 | 66,700 | 2a-G | 10 |
| Blood | 2 | ND | - | 0 |
| Saliva | 4 | 158,000 | 2a-G | 22 |
| Blood | 4 | ND | - | 0 |
| 5 | ||||
| Saliva | 0 | 2,920 | B958b-F2 | 9 |
| Blood | 0 | ND | - | 0 |
| Saliva | 2 | 365 | B958b-F2 | 10 |
| Blood | 2 | ND | - | 0 |
| Saliva | 4 | 4,240 | B958b-F2 | 10 |
| Blood | 4 | ND | - | 0 |
| 6 | ||||
| Saliva | 0 | 42 | - | 0 |
| Blood | 0 | 13 | - | 0 |
| Saliva | 2 | 17 | 2a-G | 10 |
| Blood | 2 | ND | - | 0 |
| Saliva | 4 | 161 | - | 0 |
| Blood | 4 | ND | - | 0 |
| 7 | ||||
| Saliva | 0 | 14,100 | B958b-F2 | 10 |
| Blood | 0 | ND | - | 0 |
| Saliva | 2 | 15 | - | 0 |
| Blood | 2 | ND | - | 0 |
| Saliva | 4 | 566 | B958b-F2 | 10 |
| Blood | 4 | ND | - | 0 |
| 8 | ||||
| Saliva | 0 | 428 | - | 0 |
| Blood | 0 | ND | - | 0 |
| Saliva | 2 | 690 | 3c-D | 8 |
| Blood | 2 | ND | - | 0 |
| Saliva | 4 | 4,210 | 3c-D | 10 |
| Blood | 4 | ND | - | 0 |
| 9 | ||||
| Saliva | 0 | 350 | 2a-F2 + 3c-D | 8 + 2 |
| Blood | 0 | 80 | B958a-B958 + B958b-F2 | 6 + 4 |
| Saliva | 2 | 276 | 2a-F2 + 3c-D | 3 + 7 |
| Blood | 2 | ND | - | 0 |
| Saliva | 8 | 320 | 1-G + 2a-F2 + 3c-D | 1 + 2 + 6 |
| Blood | 8 | ND | 2a-F2 | 10 |
In 0.5 μg of DNA extracted from salivary cells or peripheral blood mononuclear cells. ND, not detectable.
-, PCR and cloning unsuccessful.
EBV was detected in 3 of 28 blood specimens by EBER PCR, and detectable quantities ranged from 13 to 80 EBV genome copies per 0.5 μg of DNA (Table 3). Previous studies have indicated that healthy individuals carry EBV in the peripheral blood at 1 to 63 EBV genome copies per 106 B lymphocytes (25, 37). This quantity is at or below the limit of detection for this assay using up to 0.75 × 105 blood mononuclear cell genome equivalents of DNA per reaction.
Multiple EBV infections in healthy individuals.
In this pilot study, we tested the hypothesis that healthy individuals harbor infections with multiple LMP-1-defined EBV genotypes, representative of multiple independent EBV infections. We determined that an individual study subject harbored multiple EBV infections when one or more of the following three criteria were met: (i) two or more EBV genotypes are present in a single saliva or blood specimen; (ii) different EBV genotypes are present among simultaneously collected saliva and blood specimens from the same individual; (iii) temporal changes in the EBV genotype are present in sequentially collected saliva or blood specimens from the same individual.
Single-genotype EBV infection was identified in seven of the nine subjects, including five subjects that had a single EBV genotype repeatedly detected in saliva at multiple time points over periods of up to 4 months (Table 3). Two subjects were found to harbor multiple EBV infections (Table 3). Subject 3 harbored different EBV genotypes among simultaneously collected saliva and blood specimens at two different time points. Additionally, subject 3 exhibited temporal changes in the EBV genotypes present in sequentially collected saliva and blood specimens. In total, four different EBV genotypes were detected for subject 3 over a period of 2 months, with at least two detectable genotypes simultaneously infecting this subject at two separate time points. Subject 9 harbored two or more EBV genotypes present in four different saliva and blood specimens. Additionally, subject 9 harbored different EBV genotypes between simultaneously collected saliva and blood specimens at two different time points. Finally, subject 9 also exhibited temporal changes in the EBV genotypes present in sequentially collected saliva and blood specimens. In total, five different EBV genotypes were identified for subject 9 over a period of 8 months, with up to four detectable genotypes simultaneously infecting this subject at any single point in time.
Our data demonstrated multiple EBV infections in two of nine subjects. This prevalence rate of 22% is very close to the mean prevalence rate of 23% calculated from the pooled data (Table 1). However, the limitations of this study (small sample size, nonrandom selection, short duration, and low success rate for blood specimens) could tend to either underestimate or overestimate the true prevalence of multiple EBV infections. A large, well-designed, EBV LMP-1 genotyping study is warranted in order to accurately determine the prevalence of multiple EBV infection in healthy individuals.
Immunocompromised individuals routinely exhibit high-level oral EBV shedding (2, 14), coinfection with multiple EBV genotypes (39, 41, 43), and temporal changes in EBV populations (26, 31). Yet, even among this small sample of nine healthy individuals, we found examples of high salivary levels of EBV, infection with up to five different EBV genotypes, and temporal changes in the EBV populations in saliva and blood. The different magnitude, yet similar nature, of the EBV behavior in immunocompromised and in healthy individuals suggests that acquired immunodeficiency simply unmasks or exaggerates intrinsic aspects of the normal EBV-host relationship. More-frequent reactivations and higher levels of replication in immunocompromised individuals allow preexisting multiple EBV infections to be detected more easily.
If multiple EBV infections are common in healthy individuals, then the temporal nature of acquisition of these multiple infections remains to be determined. It is possible that multiple EBV genotypes are simultaneously acquired during primary EBV infection, through exposure to an individual (such as subject 9) who is orally shedding multiple EBV genotypes. In this single-event hypothesis, all of the coinfecting genotypes would simultaneously establish persistent latent infection prior to the development of EBV-specific immunity in the host. Once developed, host immunity may prevent further EBV superinfection.
Alternatively, it is possible that multiple EBV genotypes are sequentially acquired as successive superinfections from multiple exposures over the lifetime of the host. In this accumulation hypothesis, immunity to EBV that developed after primary infection may not protect the host against exogenous EBV superinfection. Each successively encountered genotype may establish persistent latent infection despite preexisting host immunity to EBV. This hypothesis is supported by molecular epidemiologic data from another human herpesvirus. Healthy individuals previously infected with cytomegalovirus are susceptible to superinfection with additional, genetically different cytomegalovirus strains (5, 6, 8).
The relationship between EBV infection and host immune response must be understood before designing an EBV vaccine. If natural EBV infection does not protect against subsequent EBV superinfection with a different genotype, then the goal of an EBV vaccine to prevent wild-type EBV infection may not be achievable. However, natural infection with EBV early in life appears to protect against later developing the infectious mononucleosis syndrome (17, 18, 36). A vaccination that induces an immunity similar to that obtained by wild-type infection may protect against developing the infectious mononucleosis syndrome if wild-type EBV infection does subsequently occur. The precedent for this concept has been established with another human herpesvirus. Vaccination with live, attenuated varicella-zoster virus does not prevent wild-type varicella-zoster virus superinfection but does prevent or greatly attenuate the clinical syndrome of chickenpox (3, 9).
Acknowledgments
This work was supported by a Public Health Service grant to Dennis M. Walling (NIH R01-DE12323) and a cooperative agreement NCC 9-58 with the National Space Biomedical Research Institute.
REFERENCES
- 1.Aitken, C., S. K. Sengupta, C. Aedes, D. J. Moss, and T. B. Sculley. 1994. Heterogeneity within the Epstein-Barr virus nuclear antigen 2 gene in different strains of Epstein-Barr virus. J. Gen. Virol. 75:95-100. [DOI] [PubMed] [Google Scholar]
- 2.Alsip, G. R., Y. Ench, C. V. Sumaya, and R. N. Boswell. 1988. Increased Epstein-Barr virus DNA in oropharyngeal secretions from patients with AIDS, AIDS-related complex, or asymptomatic human immunodeficiency virus infections. J. Infect. Dis. 157:1072-1076. [DOI] [PubMed] [Google Scholar]
- 3.Ampofo, K., L. Saiman, P. LaRussa, S. Steinberg, P. Annunziato, and A. Gershon. 2002. Persistence of immunity to live attenuated varicella vaccine in healthy adults. Clin. Infect. Dis. 34:774-779. [DOI] [PubMed] [Google Scholar]
- 4.Apolloni, A., and T. B. Sculley. 1994. Detection of A-type and B-type Epstein-Barr virus in throat washings and lymphocytes. Virology 202:978-981. [DOI] [PubMed] [Google Scholar]
- 5.Bale, J. F., Jr., S. J. Petheram, I. E. Souza, and J. R. Murph. 1996. Cytomegalovirus reinfection in young children. J. Pediatr. 128:347-352. [DOI] [PubMed] [Google Scholar]
- 6.Boppana, S. B., L. B. Rivera, K. B. Fowler, M. Mach, and W. J. Britt. 2001. Intrauterine transmission of cytomegalovirus to infants of women with preconceptional immunity. N. Engl. J. Med. 344:1366-1371. [DOI] [PubMed] [Google Scholar]
- 7.Brooks, J. M., D. S. Croom-Carter, A. M. Leese, R. J. Tierney, G. Habeshaw, and A. B. Rickinson. 2000. Cytotoxic T-lymphocyte responses to a polymorphic Epstein-Barr virus epitope identify healthy carriers with coresident viral strains. J. Virol. 74:1801-1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chandler, S. H., H. H. Handsfield, and J. K. McDougall. 1987. Isolation of multiple strains of cytomegalovirus from women attending a clinic for sexually transmitted disease. J. Infect. Dis. 155:655-660. [DOI] [PubMed] [Google Scholar]
- 9.Clements, D. A., C. B. Armstrong, A. M. Ursano, M. M. Moggio, E. B. Walter, and C. M. Wilfert. 1995. Over five year follow-up of Oka/Merck varicella vaccine recipients in 465 infants and adolescents. Pediatr. Infect. Dis. J. 14:874-879. [DOI] [PubMed] [Google Scholar]
- 10.Cohen, J. I., F. Wang, J. Mannick, and E. Kieff. 1989. Epstein-Barr virus nuclear protein 2 is a key determinant of lymphocyte transformation. Proc. Natl. Acad. Sci. USA 86:9558-9562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edwards, R. H., F. Seillier-Moiseiwitsch, and N. Raab-Traub. 1999. Signature amino acid changes in latent membrane protein 1 distinguish Epstein-Barr virus strains. Virology 261:79-95. [DOI] [PubMed] [Google Scholar]
- 12.Falk, K., J. W. Gratama, M. Rowe, J. Z. Zou, F. Khanim, L. S. Young, M. A. Oosterveer, and I. Ernberg. 1995. The role of repetitive DNA sequences in the size variation of Epstein-Barr virus (EBV) nuclear antigens, and the identification of different EBV isolates using RFLP and PCR analysis. J. Gen. Virol. 76:779-790. [DOI] [PubMed] [Google Scholar]
- 13.Falk, K. I., J. Z. Zou, E. Lucht, A. Linde, and I. Ernberg. 1997. Direct identification by PCR of EBV types and variants in clinical samples. J. Med. Virol. 51:355-363. [DOI] [PubMed] [Google Scholar]
- 14.Ferbas, J., M. A. Rahman, L. A. Kingsley, J. A. Armstrong, M. Ho, S. Y. Zhou, and C. R. Rinaldo, Jr. 1992. Frequent oropharyngeal shedding of Epstein-Barr virus in homosexual men during early HIV infection. AIDS 6:1273-1278. [DOI] [PubMed] [Google Scholar]
- 15.Gilligan, K., P. Rajadurai, L. Resnick, and N. Raab-Traub. 1990. Epstein-Barr virus small nuclear RNAs are not expressed in permissively infected cells in AIDS-associated leukoplakia. Proc. Natl. Acad. Sci. USA 87:8790-8794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gratama, J. W., M. A. Oosterveer, W. Weimar, K. Sintnicolaas, W. Sizoo, R. L. Bolhuis, and I. Ernberg. 1994. Detection of multiple ‘Ebnotypes’ in individual Epstein-Barr virus carriers following lymphocyte transformation by virus derived from peripheral blood and oropharynx. J. Gen. Virol. 75:85-94. [DOI] [PubMed] [Google Scholar]
- 17.Heath, C. W., A. L. Brodsky, and A. I. Potolsky. 1972. Infectious mononucleosis in a general population. Am. J. Epidemiol. 95:46-52. [DOI] [PubMed] [Google Scholar]
- 18.Henle, G., and W. Henle. 1970. Observations on childhood infections with the Epstein-Barr virus. J. Infect. Dis. 121:303-310. [DOI] [PubMed] [Google Scholar]
- 19.Katz, B. Z., J. C. Niederman, B. A. Olson, and G. Miller. 1988. Fragment length polymorphisms among independent isolates of Epstein-Barr virus from immunocompromised and normal hosts. J. Infect. Dis. 157:299-308. [DOI] [PubMed] [Google Scholar]
- 20.Kunimoto, M., S. Tamura, T. Tabata, and O. Yoshie. 1992. One-step typing of Epstein-Barr virus by polymerase chain reaction: predominance of type 1 virus in Japan. J. Gen. Virol. 73:455-461. [DOI] [PubMed] [Google Scholar]
- 21.Kyaw, M. T., L. Hurren, L. Evans, D. J. Moss, D. A. Cooper, E. Benson, D. Esmore, and T. B. Sculley. 1992. Expression of B-type Epstein-Barr virus in HIV-infected patients and cardiac transplant recipients. AIDS Res. Hum. Retrovir. 8:1869-1874. [DOI] [PubMed] [Google Scholar]
- 22.Ling, P. D., J. A. Lednicky, W. A. Keitel, D. G. Poston, Z. S. White, R. S. Peng, Z. Liu, S. K. Mehta, D. L. Pierson, C. M. Rooney, R. A. Vilchez, E. O. Smith, and J. S. Butel. The dynamics of herpesvirus and polyomavirus reactivation and shedding in healthy adults: a 14 month longitudinal study. J. Infect. Dis., in press. [DOI] [PubMed]
- 23.Lung, M. L., R. S. Chang, and J. H. Jones. 1988. Genetic polymorphism of natural Epstein-Barr virus isolates from infectious mononucleosis patients and healthy carriers. J. Virol. 62:3862-3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller, W. E., R. H. Edwards, D. M. Walling, and N. Raab-Traub. 1994. Sequence variation in the Epstein-Barr virus latent membrane protein 1. J. Gen. Virol. 75:2729-2740. (Erratum, 76:1305, 1995.) [DOI] [PubMed] [Google Scholar]
- 25.Miyashita, E. M., B. Yang, K. M. Lam, D. H. Crawford, and D. A. Thorley-Lawson. 1995. A novel form of Epstein-Barr virus latency in normal B cells in vivo. Cell 80:593-601. [DOI] [PubMed] [Google Scholar]
- 26.Palefsky, J. M., J. Berline, D. Greenspan, and J. Greenspan. 2002. Evidence for trafficking of Epstein-Barr virus strains between hairy leukoplakia and peripheral blood lymphocytes. J. Gen. Virol. 83:317-321. [DOI] [PubMed] [Google Scholar]
- 27.Rickinson, A. B., L. S. Young, and M. Rowe. 1987. Influence of the Epstein-Barr virus nuclear antigen EBNA 2 on the growth phenotype of virus-transformed B cells. J. Virol. 61:1310-1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Savoldo, B., M. H. Huls, Z. Liu, T. Okamura, H. D. Volk, P. Reinke, R. Sabat, N. Babel, J. F. Jones, J. Webster-Cyriaque, A. P. Gee, M. K. Brenner, H. E. Heslop, and C. M. Rooney. 2002. Autologous Epstein-Barr virus (EBV)-specific cytotoxic T-cells for the treatment of persistent active EBV infection. Blood 100:4059-4066. [DOI] [PubMed]
- 29.Sculley, T. B., A. Apolloni, L. Hurren, D. J. Moss, and D. A. Cooper. 1990. Coinfection with A- and B-type Epstein-Barr virus in human immunodeficiency virus-positive subjects. J. Infect. Dis. 162:643-648. [DOI] [PubMed] [Google Scholar]
- 30.Sitki-Green, D., M. Covington, and N. Raab-Traub. 2003. Compartmentalization and transmission of multiple Epstein-Barr virus strains in asymptomatic carriers. J. Virol. 77:1840-1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sitki-Green, D., R. H. Edwards, J. Webster-Cyriaque, and N. Raab-Traub. 2002. Identification of Epstein-Barr virus strain variants in hairy leukoplakia and peripheral blood by use of a heteroduplex tracking assay. J. Virol. 76:9645-9656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sixbey, J. W., P. Shirley, P. J. Chesney, D. M. Buntin, and L. Resnick. 1989. Detection of a second widespread strain of Epstein-Barr virus. Lancet ii:761-765. [DOI] [PubMed]
- 33.Sixbey, J. W., P. Shirley, M. Sloas, N. Raab-Traub, and V. Israele. 1991. A transformation-incompetent, nuclear antigen 2-deleted Epstein-Barr virus associated with replicative infection. J. Infect. Dis. 163:1008-1015. [DOI] [PubMed] [Google Scholar]
- 34.Sixbey, J. W., E. H. Vesterinen, J. G. Nedrud, N. Raab-Traub, L. A. Walton, and J. S. Pagano. 1983. Replication of Epstein-Barr virus in human epithelial cells infected in vitro. Nature 306:480-483. [DOI] [PubMed] [Google Scholar]
- 35.Srivastava, G., K. Y. Wong, A. K. Chiang, K. Y. Lam, and Q. Tao. 2000. Coinfection of multiple strains of Epstein-Barr virus in immunocompetent normal individuals: reassessment of the viral carrier state. Blood 95:2443-2445. [PubMed] [Google Scholar]
- 36.Tamir, D., A. Benderly, J. Levy, E. Ben-Porath, and A. Vonsover. 1974. Infectious mononucleosis and Epstein-Barr virus in childhood. Pediatrics 53:330-335. [PubMed] [Google Scholar]
- 37.Wagner, H. J., G. Bein, A. Bitsch, and H. Kirchner. 1992. Detection and quantification of latently infected B lymphocytes in Epstein-Barr virus-seropositive, healthy individuals by polymerase chain reaction. J. Clin. Microbiol. 30:2826-2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walling, D. M., N. M. Clark, D. M. Markovitz, T. S. Frank, D. K. Braun, E. Eisenberg, D. J. Krutchkoff, D. H. Felix, and N. Raab-Traub. 1995. Epstein-Barr virus coinfection and recombination in non-human immunodeficiency virus-associated oral hairy leukoplakia. J. Infect. Dis. 171:1122-1130. [DOI] [PubMed] [Google Scholar]
- 39.Walling, D. M., S. N. Edmiston, J. W. Sixbey, M. Abdel-Hamid, L. Resnick, and N. Raab-Traub. 1992. Coinfection with multiple strains of the Epstein-Barr virus in human immunodeficiency virus-associated hairy leukoplakia. Proc. Natl. Acad. Sci. USA 89:6560-6564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Walling, D. M., C. M. Flaitz, C. M. Nichols, S. D. Hudnall, and K. A. Storthz. 2001. Persistent productive Epstein-Barr virus replication in normal tongue epithelial cells in vivo. J. Infect. Dis. 184:1499-1507. [DOI] [PubMed] [Google Scholar]
- 41.Walling, D. M., A. G. Perkins, J. Webster-Cyriaque, L. Resnick, and N. Raab-Traub. 1994. The Epstein-Barr virus EBNA-2 gene in oral hairy leukoplakia: strain variation, genetic recombination, and transcriptional expression. J. Virol. 68:7918-7926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Walling, D. M., and N. Raab-Traub. 1994. Epstein-Barr virus intrastrain recombination in oral hairy leukoplakia. J. Virol. 68:7909-7917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Walling, D. M., N. Shebib, S. C. Weaver, C. M. Nichols, C. M. Flaitz, and J. Webster-Cyriaque. 1999. The molecular epidemiology and evolution of Epstein-Barr virus: sequence variation and genetic recombination in the latent membrane protein-1 gene. J. Infect. Dis. 179:763-774. [DOI] [PubMed] [Google Scholar]
- 44.Yao, Q. Y., D. S. Croom-Carter, R. J. Tierney, G. Habeshaw, J. T. Wilde, F. G. Hill, C. Conlon, and A. B. Rickinson. 1998. Epidemiology of infection with Epstein-Barr virus types 1 and 2: lessons from the study of a T-cell-immunocompromised hemophilic cohort. J. Virol. 72:4352-4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yao, Q. Y., M. Rowe, B. Martin, L. S. Young, and A. B. Rickinson. 1991. The Epstein-Barr virus carrier state: dominance of a single growth-transforming isolate in the blood and in the oropharynx of healthy virus carriers. J. Gen. Virol. 72:1579-1590. [DOI] [PubMed] [Google Scholar]
- 46.Yao, Q. Y., R. J. Tierney, D. Croom-Carter, D. Dukers, G. M. Cooper, C. J. Ellis, M. Rowe, and A. B. Rickinson. 1996. Frequency of multiple Epstein-Barr virus infections in T-cell-immunocompromised individuals. J. Virol. 70:4884-4894. [DOI] [PMC free article] [PubMed] [Google Scholar]