Abstract
The time course for delivery and transport of two major proteins of herpes simplex virus (HSV) has been determined for mature mouse retinal ganglion cell axons in vivo. Twenty-four hours after intravitreal injection of HSV, valacyclovir was introduced into the drinking water of the mice to inhibit subsequent viral replication. Without treatment, viral spread and replication in periaxonal glial cells confound study of axonal transport. At 2 to 5 days after infection, the animals were sacrificed and contiguous segments of the optic pathway were removed. Immunofluorescence microscopy indicated that the number of infected astrocytes was reduced in the proximal optic nerve and eliminated in the optic tract. Western blots of the retina with antibodies for envelope and capsid components, glycoprotein D (gD) and VP5, respectively, revealed that both components were expressed in retinal homogenates by 2 days. Results of reverse transcription-PCR indicated that there was no gD mRNA present in the treated optic tract 5 days after infection. Therefore, we conclude that gD is transcribed from viral mRNA in the retinal ganglion cell bodies. The gD accumulated in the proximal ganglion cell axon by 2 days and reached the most distal segment after 3 days. The VP5 first appeared in the proximal axons at 4 days, about 48 h after the appearance of gD. Thus, gD entered the axon earlier and independent of VP5. These finding confirm the subassembly model of viral transport in neurons and suggest that there is a 4- to 5-day window for initiation of effective antiviral treatment with valacyclovir.
Herpes simplex virus (HSV) type 1 has particular affinity for mucous membranes, such as the corneal epithelium of the eye. The virus gains access to the cornea through breaks in the outer layer of the epithelium, and from there it spreads to the underlying free nerve endings of sensory trigeminal ganglion cells. Initially, it attaches and fuses with the nerve terminal membrane and injects the nucleocapsid and tegument into the cytoplasm. The composite of capsid and a subset of tegument proteins is transported in a retrograde manner from the neuron periphery to the neuron nucleus (for a review, see reference 7). Ultimately, viral DNA replicates and may either kill the host cell in a lytic infection or abort replication and remain in a latent form as a relatively inactive episome (26). After some appropriate stimulus, such as stress to the neuron or organism, the latent virus begins to replicate. In the second, anterograde transport phase, the viral progeny are transported out of the neuron cell body in the same peripheral branches of the sensory axon. Thus, the intra-axonal transport of HSV may be bidirectional in the same peripheral axon. It is the regulation of the anterograde transport phase of viral movement that is the focus of this paper.
Despite an impressive amount of cellular and molecular information about HSV maturation in nonpolarized cells in vivo and in vitro, our information about the regulation of virion assembly, transport, and delivery to the surface of polarized cells is incomplete. A mixture of nucleocapsids and fully assembled virions accumulates in infected mucosal epithelial cells in vivo and is distributed throughout their cytoplasm (24). Similarly, a mixture of viral particles accumulates in the cell bodies of infected immature sensory neurons in vitro (23). In contrast, only incomplete virion components are found in the primitive axons (or neurites) of these cells, and no information is yet available about the composition of HSV in the immature dendritic processes. The axons of in vitro and immature neurons are generally about 200 nm in diameter (2, 25). Given the small caliber of these fibers, it is not surprising that enveloped virions (about 200 nm in diameter) are restricted to the cell bodies. The transport of mature virions would appear to be sterically impeded in the small neurites. Although adult optic axons vary in diameter, most are larger than 200 nm, and steric considerations should not play a role in excluding mature virus from the mature axon (27).
Moreover, embryonic in vitro neurites contain the cell machinery necessary for synthesis of new proteins. Thus, viral protein synthesis theoretically might also occur in situ within the neurite (1, 6). In contrast, mature uninjured vertebrate axons in vivo do not have ribosomes, mRNA, or several of the other factors necessary to produce proteins locally (10). Therefore, any viral protein that is to be delivered to the axon terminal is assumed to be synthesized in the neuron cell body.
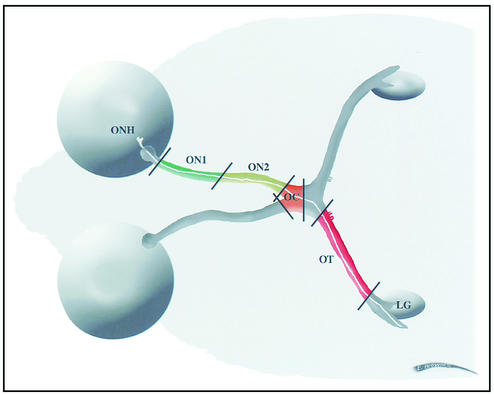
We have examined the time course of delivery of a subset of the HSV viral envelope and nucleocapsid proteins in retinal ganglion cell axons in adult mice. This animal model has several advantages for study of axonal transport. There are approximately 63,400 retinal ganglion cells in the BALB/c mouse retina (29). Each neuron contributes an axon to the optic nerve and extends a distance of about 15 mm to the lateral geniculate nucleus, one of its termination sites in the brain (Fig. 1). The collection of all axons is named sequentially the optic nerve, optic chiasm, and optic tract. These are portions of a relatively homogeneous population of axons and their surrounding glial cells. Thus, the direction of axonal transport of endogenous proteins (as well as viral proteins) is uniformly organized from retinal ganglion cell body to axon endings. The length of the axon pathway allows one to isolate individual portions which can be immunohistochemically and biochemically assayed.
FIG. 1.
Diagram of the path of retinal ganglion cell axons. A single retinal ganglion cell has an axon (indicated in white) about 15 mm long extending from the neuron cell body in the retina through the optic nerve head (ONH), optic nerve (ON1 [green] and ON2 [yellow]), optic chiasm (OC [orange]), and optic tract (OT [red]) toward the thalamus, where it synapses with neurons in the lateral geniculate nucleus (LG).
However, there are several disadvantages. One problem with examination of viral kinetics and axonal transport in vivo is that viral protein synthesis is not accomplished as a pulse but rather continues over the period of the experiment. In addition, glial cells, including the astrocytes, which surround retinal ganglion cell axons within the optic nerve, are not physically separable from the axons. Infectious virions spread from the axons to these surrounding glial cells (10, 19, 31). This spread from axon to glial cell and subsequent viral replication in glial cells confounds any attempt to study purely axonal transport.
To simplify the analysis, we used the oral prodrug of acyclovir, valacyclovir, to inhibit viral replication in glial cells in the optic nerve (21). Valacyclovir is a guanine analogue that acts within a few hours by interfering with viral DNA synthesis and thereby blocking viral replication and protein expression (19). The purpose of this study was twofold. First, we used valacyclovir treatment to study the anterograde transport of virus that replicated during the first 24 h of infection. Second, we used Western blots and reverse transcription-PCR (RT-PCR) to study biochemically the segregation of viral mRNA and capsid and envelope proteins in transit in a large population of homogeneous axons of various diameter within a mature nerve.
MATERIALS AND METHODS
Propagation of viral stocks.
African green monkey kidney (Vero) cells were grown in high-glucose Dulbecco's modified Eagle's medium (DME) supplemented with 10% fetal bovine serum, nonessential amino acids, and penicillin-streptomycin at 37°C. Cells grown to 80% confluency were infected with 0.001 PFU of F strain HSV (YBH-Mp3 substrain) per cell in DME H21 containing 1% fetal bovine serum, nonessential amino acids, and penicillin-streptomycin. After 48 h, the medium and extracellular virus were collected. All of the following steps were performed at 4°C. Cellular debris was removed by centrifugation at 3,000 rpm for 10 min with a Sorvall GSA rotor, and virus was pelleted by centrifugation of the supernatant with an SW28 rotor (Beckman Instruments, Inc., Palo Alto, Calif.) at 12,000 rpm for 90 min. For viral stocks, the resulting pellets were resuspended in minimal essential medium containing 5% (wt/vol) bovine serum albumin and stored at −80°C.
Antibodies and immunoreagents.
Two polyclonal rabbit antisera that were specific for HSV were obtained from Accurate Chemical & Scientific Corp. (Westbury, N.Y.). One of these was conjugated to horseradish peroxidase, and one was conjugated to fluorescein isothiocyanate. G. H. Cohen and R. J. Eisenberg (University of Pennsylvania) kindly provided a monoclonal antibody, MCA-gD 1D3, for gD. A monoclonal antibody specific for the 155-kDa viral capsid was obtained from Biodesign International (Saco, Maine). Sheep anti-mouse immunoglobulin G (IgG) conjugated to horseradish peroxidase (NA 931) was from Amersham Pharmacia Biotech Inc. (Piscataway, N.J.). Anti-glial fibrillary acidic protein (anti-GFAP) (clone G-A-5)-CY3 conjugate was from Sigma Aldrich (St. Louis, Mo.). For protein assays, we used a bicinchoninic acid (BCA) kit from Pierce (Rockford, Ill.). For immunoprecipitation assays, we used the monoclonal antibody to VP5 (HSV-ICP5) (Virusys Corp., North Berwick, Maine) with protein G PLUS-agarose beads (Santa Cruz Biotechnology, Santa Cruz, Calif.) for extraction.
Intraocular injection.
All procedures involving animals adhered to the Society for Neuroscience Guidelines for the Use of Animals in Research and the guidelines of the University of California San Francisco (UCSF) Committee on Animal Research. Male BALB/c mice (5 to 6 weeks of age) were anesthetized by intraperitoneal injection of Avertin (22), and the cornea was treated with a drop of 1% atropine-0.05% proparacaine (1:1) to prevent any discomfort. Solutions containing equivalent titers of plaque-purified virus in sterile phosphate-buffered saline (PBS) at concentrations of approximately 9 × 104 PFU/μl were drawn up into tubing connected to a 25-μl Hamilton syringe attached to a Hamilton repeating dispenser. After the tubing was connected to a 27-gauge needle, 2 μl of virus was injected into the vitreal chamber of each eye under a dissecting microscope. Approximately 30 s later, the needle was removed. There is some inherent variability in the amount of virus injected in different experiments. The number of ganglion cells that are infected from an intravitreal injection may vary between animals because of slight differences in the placement of the needle in the vitreous chamber.
Oral drug treatment.
We introduced valacyclovir hydrochloride (Valtrex; Glaxo Wellcome, Inc, Greenville, N.C.) in the drinking water 24 h after ocular infection. The drug was supplied ad libitum until the animals were sacrificed. This drug is normally prescribed for human patients and is known from previous studies to have no harmful effect in mice that are treated with it (8, 21). Our experimental goal was to “pulse infect” with HSV by allowing the virus to replicate for the first 24 h of the experiment and then to block further viral replication in the animal. We confirmed previous observations that the daily water consumption of each mouse was about 1.8 ml of water. The drug was supplied as a 1-mg/ml solution, corresponding to an average daily intake of 100 mg/kg (9). The drug ameliorated negative effects of the viral infection on the general health of the mice as measured by weight loss (Table 1). Drug treatment reversed the loss of weight over the 5-day experiment. In one control experiment, we began the oral drug treatment 24 h before the virus was inoculated into the vitreal chamber. No viral infection of the eye or optic pathway was observed with immunofluorescence microscopy (data not shown). Some variability was nevertheless found in the amount of viral protein expressed at different times after intraocular infection. One possible cause might be the time of day when the mice were infected. Mice are nocturnal and drink more during the night than during the day. Injections earlier in the day correlate with more time for viral replication before drinking the antiviral drug.
TABLE 1.
Effect of valacyclovir effect on body weight
| Treatment (no. of animals) | Days postinfection | Mean change in body wt (g) |
|---|---|---|
| Control (2) | 5 | +2.06 ± 0.08 |
| HSV alone (5) | 5 | −3.28 ± 0.96 |
| HSV + valacyclovir (5) | 3 | −0.65 ± 0.84 |
| 5 | +0.56 ± 0.47 |
Tissue isolation.
After 2 to 5 days, the animals were anesthetized and each mouse was intracardially perfused with 20 ml of saline. The optic nerves, extending from the orbit to the optic chiasm, were removed and cut into proximal and distal halves (each ∼3 mm in length), which were designated ON1 and ON2, respectively (Fig. 1). The optic chiasm was dissected from the hypothalamus (see below) (∼2 mm in length), and the optic tracts were removed to the point where they enter the dorsal thalamus (∼5 mm in length, pooled) (Fig. 1). As a negative control, uninfected mice were similarly prepared, and the optic pathway was collected for immunocytochemical and biochemical experiments. The same experiment was repeated on separate days three to four times for each time point. Each experiment represented results from pooled samples from four to six mice.
One possible source of viral proteins in the optic pathway was virally infected circulating lymphocytes. To test this hypothesis, we took samples of blood, liver, and hypothalamus near the optic chiasm from infected mice. Western blots of aliquots of the tissues were treated with the polyclonal antiserum to whole HSV. We found no evidence of viral proteins in the blood or liver samples. However, we did find HSV antigen on Western blots in the samples of the hypothalamus at 2 to 5 days after infection (data not shown). Some of the retina ganglion cells send an axon collateral branch to innervate neurons located in the suprachiasmatic nucleus of the hypothalamus (14, 18). We limited the dissection to the portion of the optic pathway which was not in direct contact with these hypothalamic nuclei (see Fig. 1).
Polyacrylamide gel electrophoresis and Western blotting.
Segments of the optic pathways from valacyclovir-treated mice were pooled in 500 μl of cold radioimmunoprecipitation assay buffer composed of 0.2 M Tris-Cl (pH 7.2), 10 mM EDTA, 0.3 M NaCl, 0.1% sodium dodecyl sulfate, and 0.05% Tween 20 with freshly added protease inhibitors (complete protease inhibitor cocktail; Roche Diagnostics GmbH, Mannheim, Germany). The samples were collected in homogenizers over ice and dispersed by manual homogenization. After 2 h, the samples were sonicated three times for 5 s each over ice and centrifuged at 9,500 × g for 10 min at 4°C. The supernatant was collected. Aliquots were removed to determine the protein concentration of each sample with the BCA assay (Pierce). From a sample of six experiments, we found that on average each ON1, ON2, optic chiasm, and optic tract segment contained about 69, 80, 47, and 121 μg of protein, respectively. The samples were snap frozen in liquid nitrogen and then stored at −80°C. The samples were thawed, and equal protein concentrations were loaded into the lanes of 4 to 20% gradient polyacrylamide gels under reducing conditions (10). The proteins were transferred to Immobilon P (Millipore Corp., Bedford, Mass.) by standard methods. After incubation with various antibodies, the viral antigens were visualized by using ECL (Amersham Corp.). A standard curve of viral protein was created by assaying dilutions of known viral concentrations on Western blots probed with gD antibody. The limit of detection was between 0.8 and 8 μg of protein/lane.
When we omitted the primary monoclonal antibody in Western blots and incubated with rabbit or goat anti-mouse antisera, we consistently found a band at ∼55 kDa in all lanes. This represents the nonspecific binding of the second antibody to the heavy chain of the IgG raised against virus in the mice. If we omitted the second antibody, we saw no bands on the gels. The rabbit polyclonal antiserum to HSV also precipitated bands at the molecular masses corresponding to gI and gE, because these polypeptides form a complex that has IgG Fc receptor activity. This binds rabbit IgG well but binds mouse IgG poorly (16).
Immunoprecipitation of viral antigen.
The optic pathways from five animals that were infected with HSV but not treated with an antiviral and from five animals that were infected with HSV and treated with valacyclovir 24 h later were pooled for each experiment. Samples were centrifuged at 9,500 × g for 10 min at 4°C, and the supernatant was collected in Eppendorf tubes. To 500 μl of whole nerve section lysate we added 40 μl of protein G-agarose beads (Zymed, South San Francisco, Calif.) and incubated the mixture for 10 min at 4°C with end-over-end rotation. Each sample was subsequently centrifuged to remove the beads. Small aliquots were taken for BCA protein analysis. Based on these results, equal amounts of protein were incubated with 2 to 3 μl of monoclonal antibody specific for VP5 overnight at 4°C. We added 20 to 30 μl of protein G-agarose beads to each tube and mixed the contents for 3 h at 4°C. The samples were centrifuged at 380 × g for 30 s at 4°C. After the supernatant was aspirated, the beads were resuspended in 800 μl of cold PBS. This wash was repeated three times. After the final centrifugation, the supernatant was discarded and the remaining pellet was suspended in a 40-μl aliquot of 2× sample buffer with β-mercaptoethanol. Each aliquot was boiled for 5 min and stored at 4°C until used for polyacrylamide gel electrophoresis and Western blotting. Relative estimates of the amounts of viral antigens in the lanes were obtained by using NIH Image to quantify the density of bands in the films. The experiment was repeated twice.
Immunocytochemistry.
Mice were injected intraocularly with HSV as described above and allowed to survive for 1, 2, 3, 4, or 5 days. The animals were anesthetized with Avertin (22) and killed by intracardiac perfusion with normal saline followed by a fixative containing 4% paraformaldehyde in 0.1 M sodium cacodylate buffer (pH 7.2). The tissues were immersed in fixative overnight. The following day the optic pathways were dissected and rinsed in 30% sucrose in 0.1 M sodium phosphate buffer (pH 7.2). Sections of the optic nerves, optic chiasms, and optic tracts were frozen in OCT compound (Tissue Tek) and cut at 6-μm thickness on a cryostat. Tissue sections were treated with blocking buffer (3% normal goat serum and 0.3% Triton X-100 in PBS, pH 7.2) for 90 min, followed by a 2-h incubation with the polyclonal rabbit anti-HSV conjugated to fluorescein isothiocyanate. In some cases, the sections were incubated in a second antibody to GFAP. The sections were then rinsed in PBS, and coverslips were applied. As a control for endogenous immunoreactivity, some sections were treated with antibody vehicle alone. Immunostaining was absent in these sections, as well as in sections taken from uninfected mice. The number of infected cells as a function of distance from the optic nerve head was counted in immunostained sections from animals treated with valacyclovir or untreated. NIH Image software was used to measure the areas of optic pathway segments. Based on the areas, we estimated the density of HSV-infected astrocytes in the segments. A one-way analysis of variance between treatment groups was used for statistical analysis.
EM immunocytochemistry.
The mice were anesthetized and perfused with a fixative composed of 4% paraformaldehyde and 1% glutaraldehyde in cacodylate buffer (pH 7.2). After 24 h at 4°C, the retina and optic pathway of each animal was dissected in one piece, and the ON1, ON2, optic chiasm, and optic tract segments were isolated with surgical thread ties to allow us to distinguish proximal and distal poles of each segment. The segments were then treated for electron microscopy (EM) immunocytochemistry according to standard procedures (24). The thin section had not been stained with heavy metals. This omission and the use of a relatively mild fixation are necessary to preserve and enhance the antibody signal. However, some features of ultrastructure are compromised.
Assay for gD mRNA.
A total of 15 mice were infected with HSV. At 24 h postinfection, 10 mice were treated with valacyclovir. The remaining five mice received no drug. At 5 days after infection, the retinas and optic tracts were isolated and pooled. The retinas and optic tracts from the animals that were infected with HSV but never treated with valacyclovir were used as a positive control; retinas and optic tracts from uninfected mice were assayed as negative controls. Tissues were snap frozen in TRIzol reagent (Invitrogen Corporation, Carlsbad, Calif.) and stored at −80°C. They were homogenized, and the RNA was extracted, DNase treated, and purified by using an RNeasy minikit (Qiagen, Valencia, Calif.). The RNA was quantified by using an Agilent 2100 Bioanalyzer, and equal amounts of RNA were used for cDNA synthesis and amplification by reverse transcription-PCR (RT-PCR). The RT-PCR was performed with viral gD primers ATGGGAGGCAACTGTGCTAT and CTCGGTGCTCCAGGATAAAC (Biomolecular Resource Center, UCSF) and with 15S ribosomal control primers TTCCGCAAGTTCACCTACC and CGGGCCGGCCATGCTTTACG (Ambion, Austin, Tex.). These steps were performed according to the Superscript One-Step RT-PCR with Platinum Taq kit and protocol (Invitrogen). The reverse transcription for cDNA synthesis was performed as one cycle of 50°C for 30 min followed by 94°C for 2 min. The PCR amplification step was performed for 30 cycles as follows: 94°C for 30 s, 60°C for 30 s, and 72°C for 60 s. A final extension was performed as one cycle at 72°C for 8 min. The PCR products were electrophoresed in a 2% agarose gel and visualized with a UV transilluminator. The experiment was repeated three times.
RESULTS
Effect of valacyclovir on infected retina and optic nerve.
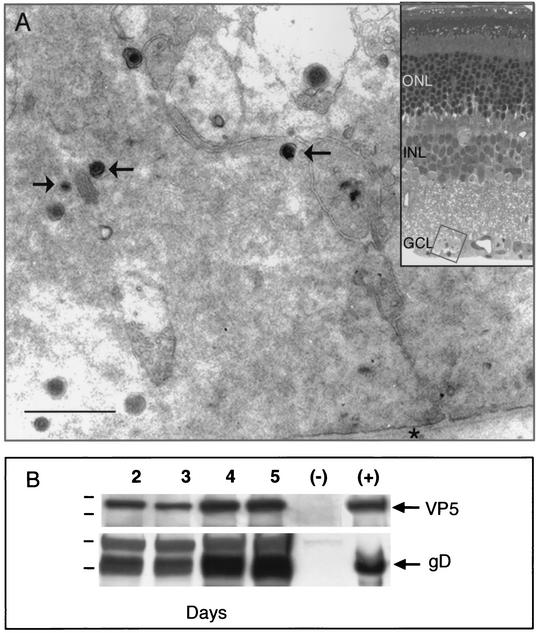
Following infection of retinas with HSV, we examined the tissue for signs of pathology to define the spread of HSV during the first 24 h after injection, i.e., at a time before the introduction of valacyclovir to the mice. Immunostaining was concentrated to a segment of about 400 μm of the retinal circumference. Within this segment, immunocytopathic changes in retinal ganglion cell bodies and Müller cell processes included the dispersion of the Nissl substance and proliferation of immunopositive lysosomal dense bodies (Fig. 2A). Lymphocytes had already invaded the retinal tissue (data not shown). In addition to retinal ganglion cells bodies, the axon profiles in the optic nerve layer also contained lysosomal dense bodies as well as capsid and small membrane organelles. Thus, there was clear evidence of retinal ganglion cell infection.
FIG. 2.
(A) EM immunostained section of retinal ganglion cell in the ganglion cell layer (GCL) 24 h after infection. The cell contains many immunoreactive viral capsids and virions (arrows). The inner limiting membrane (asterisk) underlies the ganglion cell bodies. Bar, 0.45 μm. Inset, light micrograph of the region of the GCL that was sampled. The thin section has not been stained with heavy metals. INL, inner nuclear layer; ONL, outer nuclear layer. (B) Western blots of retinas at 2, 3, 4, and 5 days after infection with equivalent titers of the F strain of HSV. The F strain expresses both gD and VP5 at each time point. Lane (−), retinal homogenates from an uninfected animal; lane (+), viral stock aliquots. Molecular markers for the VP5 gels are 176 and 113 kDa, and those for the gD gels are 64 and 49 kDa.
To ensure that any reduction in the accumulation of VP5 or gD in the optic nerve was not due to a reduction in the expression of these viral proteins in the retinas, we prepared Western blots of retinal homogenates from mice sacrificed at 2, 3, 4, or 5 days after infection (Fig. 2B). The animals were not treated with valacyclovir. Both the capsid protein, VP5, and the envelope component, gD, were expressed at each day. We also found that retinas from animals that were never exposed to virus expressed neither of the proteins.
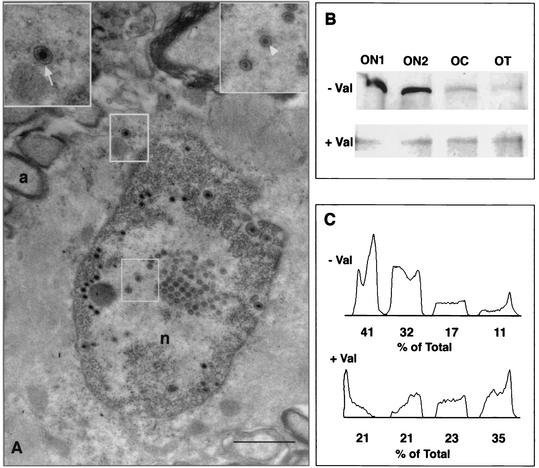
In the optic nerve head, there was a decreasing gradient from proximal to distal in the number of infected astrocytes and oligodendrocytes, with the highest frequency of infected glial cells in the region extending from the optic nerve layer in the orbit to about 3 mm into the optic nerve (Fig. 3A to C). There was no statistically significant difference in the number of infected cells between the segments of the retinal ganglion cell axons in animals that were not treated with valacyclovir. However, when we compared the number of infected astrocytes as a function of area of the optic nerve sections, we found that the density of infected cells decreased with distance from the nerve cell body (Table 2). There were about half as many infected glial cells in the ON2 segment as we found in the ON1 segment, i.e., about 3 mm farther from the optic nerve head, and about one-quarter the number of infected cells in the optic tract segment as in the ON1 segment, i.e., about 8 to 13 mm farther from the optic nerve head. Immunostained sections of the ON1 segment were also examined at the EM level. Infected astrocytes contained nuclei with many nucleocapsids and empty capsids (Fig. 4A). The same cells contained enveloped virions in the cytoplasm (Fig. 4A, inset). These results confirm those from previous experiments (20, 31).
FIG. 3.
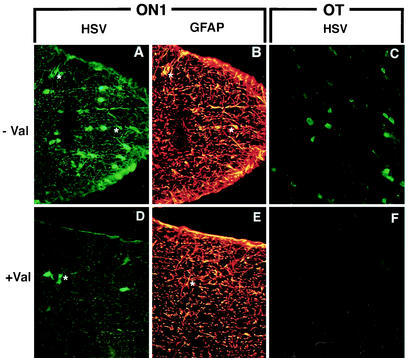
Five days after intraocular infection, we found HSV immunostained astroglial cells (asterisks) in the optic nerve in the ON1 segment of the animal that was not treated with valacyclovir (− Val) after infection (A and B). In contrast, there were relatively few HSV-positive glial cells (asterisk) in sections from the animal treated with the antiviral drug (+ Val) (D and E). Rare HSV-positive astroglial cells were identified in sections of the optic tracts from the animals that received no valacyclovir (C). In contrast, no HSV-positive glial cells were found in the optic tracts of animals that received valacyclovir 24 h after infection (F). For panels A, C, D, and F, sections were immunostained with a polyclonal antiserum to HSV. For panels B and E, sections were immunostained with a marker for astrocytes, GFAP. Magnification, ×400.
TABLE 2.
Density of infected astrocytes as a function of distance from the optic nerve head 3 days after infection
| Treatmenta | Segment (no. of sections) | Infected astrocytesb | Area (μm2)c | Densityd |
|---|---|---|---|---|
| Untreated | ON1 (4) | 49.5 ± 19.5 | 36,657 ± 12,128 | 1.35 |
| ON2 (3) | 41.0 ± 24.2 | 52,000 ± 12,243 | 0.78 | |
| Optic tract (3) | 24.7 ± 7.1 | 61,394 ± 732 | 0.40 | |
| Treated | ON1 (3) | 2.7 ± 3.01 | 61,576 ± 416 | 0.04 |
| ON2 (3) | 1.0 ± 1.7 | 61,817 ± 0 | 0.02 | |
| Optic tract (3) | 0.34 ± 0.6 | 61,596 ± 0 | 0.01 |
Valacyclovir was supplied in the drinking water 24 h after infection.
Mean (and standard deviation) number of infected astrocytes in each section.
Mean (and standard deviation) area of sections.
Number of infected astrocytes per 1,000 μm2.
FIG. 4.
(A) EM of infected astrocytes in ON1 3 days after intraocular infection. Without valacyclovir treatment, there is significant secondary infection of glial cells. Unenveloped capsids are found in the nucleus (lower inset), and enveloped virions (upper inset) are found in the cytoplasm of astrocytes in the ON1 segment. a, axon; n, nucleus. Bar, 2 μm. (B) Western blots of immunoprecipitated VP5 in the segments of the optic pathways of untreated (− Val) and valacyclovir-treated (+ Val) animals infected intraocularly with HSV 5 days before sacrifice. OC, optic chiasm; OT, optic tract. (C) Densitometric scans of the gels in panel B. The percentage of the total density is expressed per lane. There is significantly more capsid protein in the ON1 and ON2 segments of the pathway in the untreated animals. By contrast, there are more similar amounts of VP5 in all segments of the treated animals.
We next compared these results with the extracellular spread of HSV in the optic pathway in animals that were treated with valacyclovir 24 h after retinal infection. By 3 days there were relatively few HSV-positive glial cells in ON1 segments and essentially none in more distal segments (Table 2) (Fig. 3D to F). There were statistically significant differences between the numbers of infected astrocytes in ON1, ON2, and optic tract in treated and untreated animals (n = 7 and P = 0.01, n = 6 and P = 0.05, and n = 6 and P = 0.001, respectively). As a further test of the effectiveness of valacyclovir treatment, mice were injected intraocularly with HSV, and 5 days later the optic segments were prepared for immunoprecipitation assays by probing the lysate with an antibody to VP5 (Fig. 4B). There was significantly more capsid protein in the ON1 and ON2 segments of the pathway in the untreated animals. We scanned the immunoblot from one of the experiments with NIH Image to estimate the relative amounts of VP5 in these segments of the pathways from untreated and valacyclovir-treated animals (41 and 32% of the total density, respectively) (Fig. 4C). In contrast, there was less protein in the ON1 and ON2 segments of the antiviral-treated animals (21 and 21%). Thus, the antiviral drug treatment significantly reduced the viral replication and expression in secondarily infected astrocytes in the optic pathway.
Axonal transport of viral proteins.
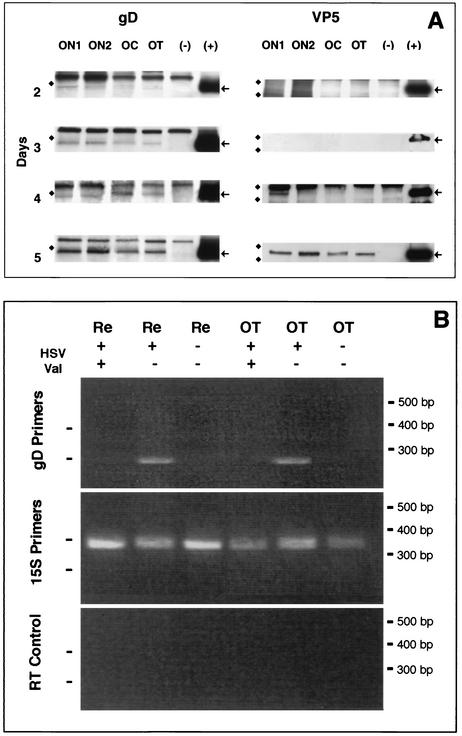
We examined the accumulation of VP5 and gD in the optic pathway after intraocular infection by using Western blots (Fig. 5A). By 2 days after infection (1 day after valacyclovir was introduced into the drinking water), we found gD in all four segments of the optic pathway, although the most immunoreactivity was found in the ON1 and ON2 segments. This difference was found despite the loading of equivalent amounts of protein in each of the lanes. By days 4 and 5, the four segments of the optic pathway contained greater amounts of gD than seen in gels loaded with the homogenates from shorter survival times.
FIG. 5.
(A) The viral envelope glycoprotein is delivered to the axon 2 days before the capsid protein is recognized in the axon. Western blots of optic pathways of animals at 2, 3, 4, and 5 days after intravitreal infection with HSV followed 24 h later with valacyclovir treatment are shown. The gD (arrows) reached the ON1 and ON2 segments in the first 48 h after infection. By 24 h later, it was recognizable in all four segments. The VP5 (arrows) was first identified at 4 days after infection and had reached the optic tract (OT) by 5 days. Lanes (−), optic pathway homogenates from an uninfected animal; lanes (+), viral stock aliquots. The markers are 50 kDa for gD gels and 182 and 114 kDa for VP5 gels. OC, optic chiasm. (B) At 5 days after intraocular injection of HSV, the mRNA for gD is present in infected retinas and optic tracts of animals not treated with valacyclovir (HSV+, Val−). No PCR products for gD were seen in the tissues taken from infected animals that received valacyclovir (HSV+, Val+). Retinas and optic tracts of animals with no infection were also negative (HSV−, Val−). The internal control, 15S RNA, was detected in all samples (15S primers). No bands were seen in control RT-PCRs for 15S PCR product when reverse transcriptase was omitted (RT control). HSV, infected animals; Val, animals treated with valacyclovir. Re, retina; OT, optic tract. On the left are markers for 250 bp (gD) and 360 bp (15S).
We found a different pattern when we examined the accumulation of VP5 along the optic pathway in the same optic nerves (Fig. 5A). By 2 days after infection, there was no evidence of VP5 in any of the segments of the retinal ganglion cell axons, despite the fact that the same axons had accumulated gD in all four segments and equivalent amounts of protein had been loaded into each lane. The results suggest that gD, the viral membrane protein, is loaded earlier and perhaps is transported at a higher rate than VP5, the capsid protein. In all experiments but one, we found no evidence of VP5 in the optic pathway at day 3; in one experiment, we found very small amounts of VP5 in ON1 and ON2 but not in the optic chiasm or optic tract. By day 4, we found VP5 in ON1 and ON2 in the remaining animals, and by day 5, the VP5 was found in all four segments of the pathway. Our failure to find VP5 in the proximal portions of the ganglion cell axons may be due to a limit to the sensitivity of the antibody. At the least, the results indicate that there is significantly more VP5 in the retinal portion of the ganglion cells (see above) than in the axonal portions at 2 to 3 days after infection.
By 5 days we found gD in all segments of the optic pathway. Therefore, it is possible that gD initially is transported separately from the capsid component (during the first 3 to 4 days) and then later is transported in vesicles containing nucleocapsids. However, if that is the case, it is a rare occurrence, based on our EM immunocytochemical examination of the optic pathways at 5 days (A. N. Tauscher and J. H. LaVail, unpublished data).
It is important to note that equal amounts of protein were loaded into each lane. Therefore, any differences that we saw with antibodies to VP5 and gD were not due to differences in amounts of total optic nerve protein. Furthermore, because multiple gels were obtained from the same experiment, the viral proteins recognized with different antibodies were eluted from the same pool of infected ganglion cells for each time point.
gD mRNA in infected retinal ganglion cell axons.
To test whether gD that was expressed in the axons might have been produced locally by mRNA within the axons, we assayed for mRNA by using RT-PCR (Fig. 5B). At 5 days after infection, gD mRNA was detected in infected retinas and optic tracts of animals that were not treated with valacyclovir. The mRNA could have be localized in glial cells or axons. However, no gD mRNA was detected in retinas and optic tracts from infected animals that were treated with valacyclovir; neither glial cells nor axons had mRNA. Tissues of animals with no infection and no valacyclovir treatment were also negative. Thus, we infer that viral RNA in the retina expresses the gD that is found along the optic pathway. Since we have analyzed the optic tracts only at 5 days after infection, it is also possible that gD mRNA was present earlier in the axon but was degraded before 5 days. We assume that the gD mRNA that is found in the optic tracts of infected animals that received no valacyclovir is a result of secondary viral infection of glial cells in the nerve. Future experiments will address the question of the possible localization of gD mRNA by in situ hybridization.
DISCUSSION
Although the specific details are poorly understood, it is clear that HSV can move from an infected epithelial cell, such as the corneal epithelial cell, to a neighboring cell rapidly and efficiently (5, 32). In the initially infected cells, a variety of viral particles are produced, including nucleocapsids, enveloped virions, and cell membrane-enclosed virions (24). The distance the virus particle must traverse from nucleus to cell surface is relatively small, so the requirement for specialized cytoplasmic motors and cytoskeletal elements is limited (32). For example, treatment of cultured retinal pigment epithelial cells with the microtubule-destabilizing drug nocodazole failed to block completely the transport of HSV from the cell surface to the nucleus. Moreover, transfer by cell fusion or cell lysis frequently occurs.
However, maturation and delivery of HSV in neurons present additional challenges. The neuron is highly polarized, with an axon extending long distances. Transport of all cell products within the axon requires polarized microtubules and actin filaments as well as large motor protein complexes, such as myosins, kinesins, and dyneins. In the case of HSV transport, there is also a physical limit to the size of viral particles that can enter and travel within some cell axons. For example, immature axons and many retinal ganglion cell axons in vitro have diameters that are about 200 nm, and thus they are too thin to permit transport of fully enveloped virions (2, 25). This limitation requires the transport of some sort of subvirion particles. The same limitation does not apply to transport in mature rodent retinal axons (27). These axons have an average diameter of about 0.75 μm, and only a few have a diameter of less than 0.25 μm.
Mature axons do not contain endogenous protein-synthesizing machinery (12). Axonal transport provides the major means by which the axon is supplied with new endogenous proteins (12; see, however, reference 11). In light of the fact that viral infections of neurons result in alterations in the regulation of normal protein synthesis in host cells, we tested the possibility that infection might also disrupt the normal segregation of viral transcriptional machinery within the host cell axon. We found no evidence of gD mRNA in the axons of HSV-infected retinal ganglion cells from animals that had been treated with valacyclovir. Thus, the viral glycoprotein that is found within the retinal ganglion cell axons is manufactured in the retina and subsequently targeted to the axon. Moreover, different proteins are transported at different times and possibly by different mechanisms.
Experimental support for the concept of targeted axonal transport of separate virion subassemblies has come from several laboratories (23-25, 30). Holland et al. originally suggested that HSV envelope proteins were identified in the axons of immature neurons in culture several hours before capsid and tegument proteins could be identified in the processes (15). Tomishima and Enquist confirmed this and highlighted the role of the pseudorabies virus Us9 gene product in the separate delivery of membrane proteins to the axon compartment of cultured neurons (30).
Direct comparisons of the features of HSV subassembly transport in vivo with those features identified in neurons in vitro are problematic. Nevertheless, the present study provides in vivo evidence that supports the subassembly hypothesis. Only gD was identified in the ON1 and ON2 in Western blots 48 h after infection, despite evidence that both gD and VP5 were expressed in infected retina by 2 days after infection. Although gD accumulated most clearly in the nerve segments near the neuron cell bodies, there was also evidence of gD in the optic chiasm and optic tract segments at 2 days. This suggested a rapid transport of at least a fraction of the expressed gD in the axon.
Furthermore, the apparent decline in the concentration of gD with distance along the pathway early in the infection (Fig. 5A, days 2 and 3) points to delivery of gD to an axonal specific site, as well as targeting to the synaptic ending. This buildup along the axon may be a function of how the gD is transported or retained. One possible mechanism involves piggybacking of viral envelope components onto neuronal cytoplasmic transport packets destined to deliver endogenous proteins, such as NgCAM (a neuron-glia cell adhesion molecule) or glutamate decarboxylase 65 to axon nodal and or synaptic terminals (3, 17). This possibility can be examined with immunoelectron microscopy and coimmunoprecipitation assays in the future. Future studies will also include examination of additional envelope glycoproteins to determine whether the pattern of delivery of other components of the envelope of the virus coincides with that of gD.
The capsid component was first identified in the proximal axon segment in one experiment at 3 days after infection and in the three additional experiments only after 4 days. Among other factors, this delay may be due to delayed transcription, posttranslational modifications, and/or loading onto microtubules in the axon hillock region for anterograde transport (13). A more complicated synthesis and assembly of capsid proteins and DNA has been suggested by Miranda-Saksena et al., who found that VP5 entered the cultured axon several hours after viral envelope proteins (23). We saw no gradient in VP5 immunostaining with distance along the optic pathway, suggesting that the capsid parcel does not accumulate along the axon length. Recent evidence indicates that the nucleocapsid VP5 and associated tegument proteins colocalize with one of the subunits of kinesin, a cytoplasmic motor (4). The identification of the specific viral proteins that link to the cytoplasmic motor enzymes may provide important clues for identification of the endogenous host cell proteins (and sequences) that are necessary for axonal transport. The delay in capsid protein accumulation in retinal axons also suggests that there is a window of several days for initiation of effective antiviral treatment with valacyclovir.
Smith et al. noted that viral transmembrane proteins and capsids accumulate in the growth cones of immature sensory neurons in vitro (28). They proposed that the failure of virions to exit the growth cones may have something to do with the absence of neighboring cells to trigger egress. The isolation procedure for these kinds of in vitro experiments requires the loss of glial cells. Obviously, investigation of their role requires their presence. It will be of interest to examine the valacyclovir-treated animals further to see if the adjacent glial cells have viral DNA or capsids in their cytoplasm.
In conclusion, we propose that there must be at least two specific mechanisms for subvirion axonal transport. One mechanism underlies the translocation of the viral capsid and DNA, possibly with a complement of tegument proteins. The second mechanism for transport and delivery of the viral envelope proteins may depend on movement of the host cell axonal membrane components. There may be additional mechanisms to regulate the complex processes of assembly and release along the axon and at the nerve terminals. Finding a way to block these processes will be of significant clinical relevance.
Acknowledgments
This work was supported by Public Health Service grants P01 HL-24136 (to S.S.S.) and EY-08773 and P30 EY02162 (to J.H.L.) (Department of Ophthalmology, UCSF) from the National Eye Institute and by funds from That Man May See, Inc., and Fight for Sight and an REAC grant from UCSF.
We thank Gary Cohen and Roselyn Eisenberg for gifts of monoclonal antibodies and antisera. We also thank Amy DeIpolyi, John Whitehead, and Ammon Corl for their help and Amanda Codd, Peter Ohara, and Russell Snyder for their helpful comments.
REFERENCES
- 1.Bassell, G. J., H. Zhang, A. L. Byrd, A. M. Femino, R. H. Singer, K. L. Taneja, L. M. Lifshitz, I. M. Herman, and K. S. Kosik. 1998. Sorting of b-actin mRNA and protein to neurites and growth cones in culture. J. Neurosci. 18:251-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Black, J. A., S. G. Waxman, B. R. Ransom, and M. D. Feliciano. 1986. A quantitative study of developing axons and glia following altered gliogenesis in rat optic nerve. Brain Res. 380:122-135. [DOI] [PubMed] [Google Scholar]
- 3.Burack, M. A., M. A. Silverman, and G. Banker. 2000. The role of selective transport in neuronal protein sorting. Neuron 26:465-472. [DOI] [PubMed] [Google Scholar]
- 4.Diefenbach, R. J., M. Miranda-Saksena, E. Diefenbach, D. H. Holland, R. A. Boadle, P. J. Armati, and A. L. Cunningham. 2002. Herpes simplex virus tegument protein US11 interacts with conventional kinesin heavy chain. J. Virol. 76:3282-3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dingwell, K. S., C. R. Brunetti, R. L. Hendricks, Q. Tang, M. Tang, A. J. Rainbow, and D. C. Johnson. 1994. Herpes simplex virus glycoproteins E and I facilitate cell-to-cell spread in vivo and across junctions of cultured cells. J. Virol. 68:834-845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eng, H., K. Lund, and R. Campenot. 1999. Synthesis of β-tubulin, actin, and other proteins in axons of sympathetic neurons in compartmented cultures. J. Neurosci. 19:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Enquist, L. W., P. J. Husak, B. W. Banfield, and G. A. Smith. 1998. Infection and spread of alphaherpesviruses in the nervous system. Adv. Virus Res. 51:237-347. [DOI] [PubMed] [Google Scholar]
- 8.Field, H. J., D. Tewari, D. Sutton, and A. M. Thackray. 1995. Comparison of efficacies of famciclovir and valacyclovir against herpes simplex virus type 1 in a murine immunosuppression model. Antimicrob. Agents Chemother. 39:1114-1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Field, H. J., and A. M. Thackray. 1995. The effects of delayed-onset chemotherapy using famcyclovir or valaciclovir in a murine immunosuppression model for herpes simplex virus. Antiviral Chem. Chemother. 6:210-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garner, J. A., and J. H. LaVail. 1999. Differential anterograde transport of HSV type 1 viral strains in the murine optic pathway. J. Neurovirol. 5:140-150. [DOI] [PubMed] [Google Scholar]
- 11.Giuditta, A., B. B. Kaplan, J. van Minnen, J. Alvarez, and E. Koenig. 2002. Axonal and presynaptic protein synthesis: new insights into the biology of the neuron. Trends Neurosci. 25:400-404. [DOI] [PubMed] [Google Scholar]
- 12.Grafstein, B. 1995. Axonal transport: function and mechanisms, p. 185-199. In S. G. Waxman, J. D. Kocsis, and P. K. Stys (ed.), The axon. Oxford University Press, New York, N.Y.
- 13.Hammerschlag, R., A. R. Dravid, and A. Y. Chiu. 1975. Mechanism of axonal transport: a proposed role for calcium ions. Science 188:273-275. [DOI] [PubMed] [Google Scholar]
- 14.Hendrickson, A. E., N. Wagoner, and W. M. Cowan. 1972. An autoradiographic and electron microscopic study of retino-hypothalamic connections. Z. Zellforsch. 135:1-26. [DOI] [PubMed] [Google Scholar]
- 15.Holland, D. J., M. Miranda-Saksena, R. A. Boadle, P. Armati, and A. L. Cunningham. 1999. Anterograde transport of herpes simplex virus proteins in axons of peripheral human fetal neurons: an immunoelectron microscopy study. J. Virol. 73:8503-8511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson, D. C., M. C. Frame, M. Ligas, A. M. Cross, and N. D. Stow. 1988. Herpes simplex virus immunoglobulin G Fc receptor activity depends on a complex of two viral glycoproteins, gE and gI. J. Virol. 62:1347-1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kanaani, J., A. E. El-Husseini, A. Aguilera-Morena, J. M. Diacovo, D. S. Bredt, and S. Baekkeskov. 2002. A combination of three distinct trafficking signals mediates axonal targeting and presynaptic clustering of GAD65. J. Cell Biol. 158:1229-1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.LaVail, J., R. Nixon, and R. Sidman. 1978. Genetic control of retinal ganglion cell projection. J. Comp. Neurol. 182:399-422. [DOI] [PubMed] [Google Scholar]
- 19.LaVail, J. H., A. N. Tauscher, and P. T. Ohara. 2000. Two potential mechanisms for the delivery of HSV from infected trigeminal ganglion cells bodies to the cornea. J. Neurovirol. 6:69. [Google Scholar]
- 20.LaVail, J. H., K. S. Topp, J. A. Garner, and P. A. Giblin. 1997. Factors that contribute to the efficiency of transneuronal spread of herpes simplex virus. J. Neurosci. Res. 49:485-496. [PubMed] [Google Scholar]
- 21.LeBlanc, R. A., L. Pesnicak, M. Godleski, and S. E. Straus. 1999. The comparative effects of famciclovir and valacyclovir on herpes simplex virus type 1 infection. J. Infect. Dis. 180:594-595. [DOI] [PubMed] [Google Scholar]
- 22.Lumb, W. V. 1963. Small animal anesthesia. Lea and Febiger, Philadelphia, Pa.
- 23.Miranda-Saksena, M., P. Armati, R. A. Boadle, D. J. Holland, and A. L. Cunningham. 2000. Anterograde transport of herpes simplex virus type 1 in cultured, dissociated human and rat dorsal root ganglion neurons. J. Virol. 74:1827-1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ohara, P. T., M. S. Chin, and J. H. LaVail. 2000. The spread of herpes simplex virus type 1 from trigeminal neurons to murine cornea: an immunoelectron microscopy study. J. Virol. 74:4776-4786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Penfold, M. E. T., P. Armati, and A. L. Cunningham. 1994. Axonal transport of herpes simplex virions to epidermal cells: evidence for a specialized mode of virus transport and assembly. Proc. Natl. Acad. Sci. USA 91:6529-6533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Preston, C. M. 2000. Repression of viral transcription during herpes simplex virus latency. J. Gen. Virol. 81:1-19. [DOI] [PubMed] [Google Scholar]
- 27.Reese, B. E. 1987. The distribution of axons according to diameter in the optic nerve and optic tract of the rat. Neuroscience 22:1015-1024. [DOI] [PubMed] [Google Scholar]
- 28.Smith, G. A., S. P. Gross, and L. W. Enquist. 2001. Herpesviruses use bidirectional fast-axonal transport to spread in sensory neurons. Proc. Natl. Acad. Sci. USA 98:3466-3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Strom, R. C., and R. W. Williams. 1998. Cell production and cell death in the generation of variation in neuron number. J. Neurosci. 18:9948-9953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tomishima, M. J., and L. W. Enquist. 2001. A conserved α-herpesvirus protein necessary for axonal localization of viral membrane proteins. J. Cell Biol. 154:741-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tomishima, M. J., and L. W. Enquist. 2002. In vivo egress of an alphaherpesvirus from axons. J. Virol. 76:8310-8317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Topp, K. S., K. Bisla, N. D. Saks, and J. H. LaVail. 1996. Centripetal transport of herpes simplex virus in human retinal pigment epithelial cells in vitro. Neuroscience 71:1133-1144. [DOI] [PubMed] [Google Scholar]