Abstract
Recombinant adeno-associated viruses (rAAVs) have attracted considerable interest as gene delivery systems because they show long-term expression in vivo and transduce numerous cell types. Limitations to successful gene transduction from rAAVs have prompted investigations of a variety of treatments to enhance transgene expression from rAAV vectors. Tyrphostin-1, an epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor, dramatically enhances rAAV transgene expression. Elegant studies have demonstrated that a single-strand D-sequence-binding protein (ssDBP) is phosphorylated by EGFR and binds to the D sequence element in the AAV terminal repeat (TR). Binding of the Tyr-phosphorylated ssDBP prevents conversion of single-stranded vector DNA to a double-strand conformation. We observed dramatic increases in transgene expression in lung epithelial cells (IB3) with tyrphostin treatment. Gel shift analysis of ssDBP revealed that its DNA binding characteristics were unchanged after tyrphostin treatment or adenovirus infection. Tyrphostin stimulated rAAV transgene expression to a greater extent than adenovirus coinfection. Southern hybridizations revealed that the vector DNA remained in the single-strand conformation in tyrphostin-treated cells but double-stranded replicative form monomer DNA was most abundant in adenovirus-infected cells. Northern analyses revealed that tyrphostin treatment enhanced mRNA accumulation more than in adenovirus-infected cultures even though replicative form DNA was undetectable. Analysis of the JNK, ERK, and p38K mitogen-activated protein kinase pathways revealed that tyrphostin treatment stimulated the activity of JNK and p38K. Our data suggest that tyrphostin-induced alteration of stress response pathways results in dramatic enhancement of transcription on linear vector DNA templates in the IB3 cell line. These results expand the downstream targets of the EGFR in regulating rAAV transduction.
Gene delivery vectors developed from recombinant adeno-associated viruses (rAAVs) have shown successful gene transduction in a variety of cell types in culture and tissues in animal models. Successful transduction has been demonstrated in muscle (37, 47), lungs (10, 19), the central nervous system (23), the retina (4), hematopoietic cells (18), liver (36), skin (22), and heart (24). The success of preclinical investigations has led to several ongoing and planned human gene therapy trials.
In spite of the vector's wide tropism, there are limitations to successful transduction. In the initial step of virus uptake, the virion binds to coreceptors. For adeno-associated virus type 2 (AAV2), heparin sulfate proteoglycan is believed to be the primary receptor, whereas 2,3-linked sialic acid is the primary receptor for AAV5 (44, 45). The fibroblast growth factor receptor and αVβ5 integrin have been reported to function as coreceptors for AAV2 (29, 40). A lack of heparin sulfate proteoglycan or its localization to a less accessible cell location such as the basement membrane in airway epithelium limits AAV2 vector transduction (13, 49). After internalization, the virion translocates to the nucleus. Some cell types do not effectively move the virion to the nucleus, resulting in diminished transgene expression (21). A component of the internalized virus is shunted to proteosomes, resulting in vector degradation (11). Upon entry into the nucleus, the virus is uncoated, and the single-stranded DNA is converted to a transcriptionally competent double-stranded conformation.
A variety of physical, chemical, and biological agents have been used to stimulate rAAV transgene expression. Inhibition of proteosome activity results in increased transgene expression, presumably due to more efficient vector delivery to the nucleus (11). Treatment of cells with DNA-damaging agents such as UV light and gamma irradiation has been shown to stimulate expression by several hundred-fold (3, 32). UV irradiation increases the accumulation of double-stranded circular vector DNA (33).
Coinfection of the target cell with adenovirus stimulates transgene expression by several mechanisms. Expression of adenovirus E4 orf 6 results in conversion of single-stranded to double-stranded vector DNA with concomitant increases in transgene expression (15, 16, 33). Expression of the adenovirus VA gene also stimulates AAV gene expression (45). One of the strongest stimulators of vector delivery and expression is inhibition of the epidermal growth factor receptor (EGFR) via tyrphostins (26). EGFR phosphorylates a single-stranded D-sequence-binding protein (ssDBP), also identified as FKBP52, on Tyr residues (27). This phosphorylation alters the protein's interaction with the D sequence in the AAV terminal repeat (TR). When the interaction is altered, second-strand DNA synthesis is enabled, resulting in subsequent expression of the genes carried by the vector. Analysis of extracts from tissues that are refractory to AAV2 transduction, such as the lung, reveal that there is a greater ratio of phosphorylated to dephosphorylated ssDBP than observed in tissues that are more readily transduced (28).
We began a study of tyrphostin's extraordinary ability to stimulate vector transduction to determine if other factors involved in transgene expression are also affected by inhibition of the EGFR. Our results indicate that tyrphostin treatment of IB3 airway epithelial cells has a profound stimulatory effect on rAAV-mediated transgene expression. Curiously, tyrphostin treatment did not increase single- to double-stranded conversion of vector DNA, and the D-sequence-binding properties of the ssDBP were not altered in IB3 cells. Further analysis revealed that the stress-activated protein kinase pathway (JNK and SAPK) and the p38 kinase pathway were stimulated by tyrphostin. Stimulation of these pathways is known to increase transgene expression from reporter plasmids driven by the cytomegalovirus (CMV) early promoter (7). Thus, cellular stress responses induced by tyrphostin may explain the dramatic increase in transgene expression from the CMV promoter-driven rAAV vector. Our data suggest that a tyrphostin-induced alteration of stress response pathways results in dramatic enhancement of transcription on linear vector DNA templates in the IB3 cell line.
MATERIALS AND METHODS
Tyrphostin-1 and cell lines.
A storage solution of tyrphostin-1 (Sigma), an epidermal growth factor receptor inhibitor, was prepared by dissolving in dimethyl sulfoxide (DMSO) to a final concentration of 50 mM and stored at −20°C in tubes wrapped in foil. Care should be taken to protect tyrphostin from light and to minimize repeated freeze-thaw cycles. Exposure to light will result in less stimulation of transgene expression. The working stock solution of 500 μM tyrphostin was prepared in culture medium by first diluting the storage solution 1:5 in ethanol and then adding culture medium so that the final concentrations of DMSO and ethanol were 1 and 5%, respectively.
IB3 cells are undifferentiated simian virus 40 T antigen-transformed human lung epithelial cells isolated from a cystic fibrosis patient (50). IB3 cells were maintained on LHC-8 medium with glutamine (Biofluids) supplemented with antibiotics and 5% fetal bovine serum. 293 cells were used for packaging rAAV. KB, HeLa, and 293 cells were maintained on minimal essential medium supplemented with glutamine, antibiotics, and 10% fetal bovine serum. IB3 cells were grown to confluence and shifted to medium lacking fetal bovine serum for 2 to 4 days prior to transduction with vectors.
Recombinant AAV vector production and purification.
The vAVCMVluc (derived from pAVCMVluc) vector contains the cytomegalovirus early promoter attached to the firefly luciferase gene, followed by a simian virus 40 poly(A) signal. The vAVgfp vector (derived from pTRUF5) contains the CMV promoter driving the green fluorescent protein gene (gfp) followed by a simian virus 40 poly(A) signal. Downstream of the gfp expression cassette is the herpes simplex virus thymidine kinase promoter driving a neo gene, followed by the bovine growth hormone gene poly(A) signal. Both vectors were produced by the previously described adenovirus-free, two-plasmid transfection system (8).
The vector was purified by the single-step gravity flow (heparin) column purification strategy of Auricchio et al. (5). The physical particle number of recombinant vector stocks was determined by quantitative real-time PCR analysis of rAAV genomes; this procedure gave more reproducible results than quantitative DNA slot blot analysis and required less sample for analysis. Primers for real-time PCR analysis were designed to amplify a 119-bp fragment of the CMV promoter. The CMV forward primer (5′-CATATATGGAGTTCCGCGTTACATAA-3′) and CMV reverse primer (5′-CCCTATTGGCGTTACTATGGGA-3′) were designed to have an annealing temperature of 60°C. The CMV Taqman probe (5′-CCTGGCTGACCGCCCAACGAC-3′) was designed to have an annealing temperature of 70°C and was purchased as a dual-labeled probe with a 5′ 6-FAM fluorophore and a 3′ BlackHole Quencher-1 quencher (Integrated DNA Technologies). Real-time PCR was performed with a GeneAmp 5700 sequence detection system. The cycling consisted of 10 min at 50°C, 10 min at 95°C, and 40 PCR cycles of 1 min at 60°C and 20 s at 95°C. Quantitation of vector genomes was done with the GeneAmp 5700 sequence detection system software.
Gel shift analysis of ssDBP.
Cells were grown and treated for each experiment as described in Discussion. Briefly, IB3 cells were grown to confluence and placed on serum-free medium for 2 days. KB and HeLa cells were grown to 80% confluence in six-well plates prior to treatment and harvesting. Harvesting of cells was accomplished by washing the cells in phosphate-buffered saline and scraping in 1.0 ml of phosphate-buffered saline. The cells were pelleted by low-speed centrifugation and stored at −70°C until lysis. Whole-cell extracts (WCE) were prepared as described previously (30). Cell pellets were lysed in electrophoretic mobility shift assay (EMSA) buffer (50 mM Tris, 150 mM CaCl2, 0.5% Nonidet P40, 20 mM EDTA, 0.1% Tween 20, 1.0 mM phenylmethylsulfonyl fluoride, 1.0 mM leupeptin, and 1.0 mM pepstatin, pH 8.0). Upon lysis, the extracts were frozen and thawed three times. Cellular debris was pelleted by low-speed centrifugation, and the supernatants (WCE) were saved. The protein concentration of each WCE was measured with the detergent competent protein assay as described by the manufacturer (Bio-Rad). WCE were adjusted to a final concentration of 5.0 μg/μl by dilution in EMSA buffer.
Gel shift analysis was performed with WCE and substrate DNA consisting of a 20-nucleotide oligomer derived from the D-minus sequence (5′-AGGACCCCCTAGTGATGGAG-3′) of the AAV2 TRs. Ten picomoles of D-minus sequence was end labeled for 30 min at 37°C with 50 μCi of [γ-32P]ATP with T4 polynucleotide kinase (Promega). For gel shift analysis, 10 μg (2 μl) of WCE was incubated for 20 min at room temperature with 10,000 cpm of radiolabeled D-minus sequence, 4 μl of 5× binding buffer (50 mM HEPES-NaOH, 40 mM MgCl2, 200 mM KCl, 40% glycerol, and 1 mM dithiothreitol, pH 7.9), and 1.0 μg of poly(dI:dC) brought to a final volume of 20 μl with water. Nondenaturing 4% Tris-borate (6) 15-cm acrylamide gels were run for 1 h at 100 V prior to loading. Gels were loaded and electrophoresed at 100 V for 2.5 h. The gels were dried and exposed to Kodak Biomax X-ray film.
Luciferase and protein assays.
Cell lysates of transduced or transfected cultures were prepared by washing cells with phosphate-buffered saline containing 5 mM MgCl2 and lysed by adding 250 μl of 1× reporter lysis buffer (Promega) to each well of a 48-well plate. The entire plate was frozen at −70°C and thawed at room temperature while shaking. The freeze-thaw cycle was repeated two more times, and cellular debris was pelleted and discarded after low-speed centrifugation. Then 20 μl of lysate was added to luminometer tubes (Sarstedt no. 55.476 tubes), and luminometric quantitation was performed with a Perkin Elmer Lumat LB 9501 luminometer.
The detergent competent protein assay was also performed on the lysates as per the manufacturer's protocol (Bio-Rad) to determine total protein in the lysates. Optical density measurements were taken at 750 nm with a microplate reader. Protein concentrations in the samples were determined by comparing optical density measurements of the samples to those of a series of bovine serum albumin standards.
Nucleus/cytoplasm fractionation of transduced IB3 cells.
One to three days after transduction, cells were harvested by scraping and pelleted by low-speed centrifugation. Pellets were fractionated into nuclear and cytoplasmic fractions by a procedure modified from that of Sperinde and Nugent (39). Cell pellets were resuspended in 200 μl of ice-cold HEPES-NP-40 buffer (10 mM HEPES, 10 mM KCl, 0.1 mM EDTA, 0.1 mM EGTA, and 0.625% Nonidet P40, pH 7.4) by vortexing for 10 s and incubating on ice for 10 min. The mixture was centrifuged at 10,000 × g for 10 s at 4°C, and the supernatant (cytoplasmic fraction) was removed and saved. The nuclear pellets were washed two more times with HEPES-NP-40 buffer, and the centrifugation was repeated after each washing step. Finally, the nuclear pellet was redissolved in 200 μl of HEPES-NP-40.
In order to test for the efficiency of fractionation of the cells, the cytoplasmic and nuclear fractions were assayed for acid phosphatase activity by a procedure modified from that of Connolly et al. (9). Acid phosphatase is a lysosomal enzyme and is found exclusively in the cytoplasm of cells. The assay was performed by adding 400 μl of 2× substrate 20 mM p-nitrophenylphosphate (104 phosphatase substrate; Sigma Chemical Co.), 400 μl of 2× acid phosphatase buffer (0.4 M sodium acetate, pH 5.5), and 20 μl of either the nuclear or cytoplasmic fraction to a microcentrifuge tube and incubating at 37°C for 2 h. The samples were vortexed and centrifuged briefly, and 200 μl was added in triplicate to a 96-well plate. The optical density of each sample was measured at 410 nm. For all fractionations, less than 1% of total phosphatase activity was found in the nuclear fraction (data not shown).
Southern and Northern hybridization analyses.
IB3 cells were grown to confluence in six-well plates and placed on serum-free medium for 2 days. The cultures were treated with serum-free medium, 500 μM tyrphostin, or adenovirus (multiplicity of infection of 5) for 1 h and washed with serum-free medium. The cultures were then infected with 500 rAAV2 (vAVCMVluc) particles per cell (2.5 × 109 total particles) for 2 h in serum-free medium. The inoculum was removed, and fresh medium was placed on the cultures and incubated for 3, 24, and 48 h. The cultures were harvested and fractionated into nuclear and cytoplasmic fractions as described above.
Luciferase assays were performed on equal aliquots of the cytoplasmic fraction. An equal volume of 2× capsid digestion buffer (100 mM Tris-HCl [pH 8], 2 mM EDTA, 1% sodium dodecyl sulfate [SDS], 0.4 mg of proteinase K per ml) was added to the remaining portions of the nuclear and cytoplasmic fractions and incubated at 37°C overnight. The following day, the samples were extracted with phenol-chloroform and concentrated by ethanol precipitation. The replication control samples were prepared by transfection of 293 cells with the pAVCMVluc vector plasmid and the pSH5 plasmid (8). Total DNA was isolated from these cells after 24 h as described above and digested with DpnI at 37°C for 3 h to remove input pAVCMVluc and pSH5 plasmids. Agarose gel electrophoresis and Southern hybridizations were performed with a radiolabeled CMV DNA fragment from pAVCMVluc as described previously (6).
IB3 cells were grown to confluence in 10-cm plates and placed on serum-free medium for 2 days. Untreated, tyrphostin-treated, or adenovirus-infected (multiplicity of infection of 5) cells were transduced with 100 particles of vAVgfp per cell. Cells were harvested by scraping at 24, 48, and 72 h. Total cellular RNA was isolated with Trizol reagent according to the manufacturer's instructions (Invitrogen). Then 20 μg of total RNA was electrophoresed on a 1% agarose-formaldehyde gel. The RNA was transferred to nitrocellulose filter paper. The RNA was then hybridized to a radiolabeled DNA probe from the gfp gene as described previously (6). The amount of radioactive signal on the blot was determined with a Molecular Dynamics phosphoimager.
Transfection studies of IB3 cells.
The pAVCMVluc plasmid was linearized by digestion with PvuII or PvuI. PvuII produces a full-length rAAV2 monomer. PvuI cuts the pAVCMVluc plasmid twice, generating two linear DNA species, one of which has the linear double-stranded rAAV2 genome flanked by plasmid DNA and the other of which consists of a fragment of the plasmid backbone. The PvuII-digested products were separated from undigested plasmid DNA on a 1% agarose electrophoresis gel and purified on gel extraction columns (Qiagen). Similarly, the rAAV2-containing fragment from PvuI digestion of pAVCMVluc was separated and purified as described above. Uncut pAVCMVluc plasmid DNA was run on a 1% agarose gel, and a relaxed and a supercoiled fraction were separated and purified as described above. IB3 cells were grown to confluence in 48-well plates and placed on serum-free medium for 2 days. Cells were Lipofectamine transfected (according to the manufacturer's instructions) with approximately 100 ng of each form of DNA described above and incubated for 24 h. Cells were washed, and appropriate cells were treated with serum-free medium, 500 μM tryphostin-1, or adenovirus (multiplicity of infection of 5) for 1 h, washed again, and incubated for 24 h. Cells were harvested, and the luciferase assay and detergent competent protein assay were performed.
Immunoblot analysis of IB3 cells.
IB3 cells were grown to confluence in six-well plates and placed on serum-free medium for 2 days. The cultures were treated with a variety of agents. Cultures were treated for 2 h with: the vehicle control for tyrphostin (1% DMSO and 5% ethanol); 500 μM tyrphostin; adenovirus (multiplicity of infection of 5); the vehicle control for PD98059 and anisomycin (1% DMSO); 50 μM PD98059 (Upstate); 300 μM anisomycin (Sigma); or combinations of virus and reagent. Cultures were also treated with 200 ng of epidermal growth factor (EGF) (Sigma) for 30 min. Cultures were washed with serum-free medium and harvested either immediately or 24 h later. A set of cultures were treated with 100 μJ/m2 of UV light and harvested 1 h or 24 h later. Cultures were harvested and lysed by scraping into 150 μl of sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer (6). Protein samples were separated by electrophoresis on SDS-10% PAGE gels. After electrophoresis, the proteins were electrophoretically transferred to a nitrocellulose membrane. Immunodetection of phosphorylated and total ERK1/2, JNK1/2, and p38 was performed with antibodies against the phosphorylated and total protein (Cell Signaling) as per the manufacturer's protocol. The proteins were detected with an enhanced chemiluminescence detection kit (ECL; Amersham) as per the manufacturer's protocol.
RESULTS
Luciferase expression in vAVCMVluc-transduced, tyrphostin-treated IB3 cells.
Previous studies have demonstrated that tyrphostin treatment of several different cell types results in a dramatic stimulation of rAAV transgene expression (26). To determine if tyrphostin affects transgene expression in lung cells, we used the IB3 cell line. IB3 cells are simian virus 40 T antigen-transformed airway epithelial cells originally derived from a cystic fibrosis patient (50). To more closely approximate the relatively low proliferation level of airway epithelia, the cells were grown to confluence and then incubated in serum-free medium for 2 to 4 days. The use of quiescent cells also enables an analysis of the effects of tyrphostin on vector transgene expression without the stimulating effects of proliferating cells on transgene expression. Cells prepared in this manner were quiescent, as judged by a lack of [3H]thymidine incorporation (results not shown).
To determine the maximum level of tyrphostin for use with IB3 cells, quiescent cells were treated with 250, 500, and 750 μM tryphostin-1 for 2 h and then infected with 100 particles of rAAV2 (vAVCMVluc) per cell. The cells were washed with serum-free medium and incubated for 48 h. The cultures were harvested by scraping, and luciferase and detergent compatible protein assays were performed. Maximal stimulation of transduction was realized at a concentration of 500 μM (Fig. 1A). This result for IB3 cells is comparable to that obtained with other cell lines (26). To determine the optimal time of treatment with tyrphostin, we added it 2 h before, at the same time as, and 2 h after rAAV2 infection. We routinely observed the highest stimulation of transgene expression when tyrphostin was added 2 h after treatment with the vector (Fig. 1B). Others have shown that induction of DNA damage, presumably subsequent repair, leads to an increase in rAAV2 transgene expression (3). We observed no detectable DNA synthesis, as measured by [3H]thymidine incorporation upon tyrphostin treatment of quiescent cells, suggesting that DNA synthesis was not induced by the treatment (results not shown).
FIG. 1.
Luciferase expression from vAVCMVluc-transduced, tyrphostin-treated IB3 cells. (A) IB3 cells were grown to confluence and placed on serum-free medium for 2 days. Cells were treated with the indicated concentrations of tyrphostin for 2 h. The tyrphostin-containing medium was replaced with normal medium, and the cells were infected with 100 particles per cell of vector for 2 h. The vector was replaced with normal medium and incubated for 48 h. Cells were harvested, and protein and luciferase assays were performed on cell extracts. Luciferase activity is expressed as relative light units (RLU) per microgram of total protein. Error bars represent the standard deviation of the mean of triplicate assays. (B) IB3 cells were made quiescent as described in the text and treated with 250 μM tyrphostin for 2 h at 2 h prior to infection (−2 h), at the same time as infection (0 h), or 2 h after infection (+2 h). The cells were harvested 48 h from the zero time point, and luciferase expression was assessed as described above.
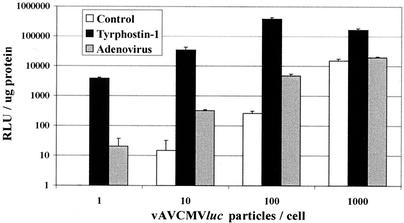
To determine if the amount of rAAV affects the level of tyrphostin stimulation, quiescent IB3 cells were transduced with increasing amounts of vAVCMVluc and 500 μM tyrphostin at 2 h after addition of the vector. Twenty-four hours after transduction, the cultures were harvested and analyzed for luciferase expression. For comparison, separate cultures were transduced with rAAV and adenovirus. Increasing amounts of vector resulted in increased gene expression in tyrphostin-treated cells up to 100 particles per cell. Over a 1,000-fold stimulation with tyrphostin (compared to the control cells) was realized up to the 100 particle-per-cell level (Fig. 2). However, at 1,000 particles per cell, the tyrphostin stimulation effect dropped to slightly over 10-fold. The diminished tyrphostin-mediated stimulation at greater than 100 particles suggests that a limiting factor that is depleted at higher doses may be required for transduction.
FIG. 2.
Luciferase expression from IB3 cells transduced with increasing amounts of vAVCMVluc. Quiescent IB3 cells were infected with the indicated amounts of vAVCMVluc for 2 h and left untreated (control), treated with 500 μM tyrphostin for 2 h, or infected with adenovirus at a multiplicity of infection of 5. Twenty-four hours later, the cultures were harvested, and protein and luciferase assays were performed. Results are reported as relative light units (RLU) per microgram of protein. Error bars represent the standard deviation of the mean of triplicate assays.
Temporal levels of transgene expression in the presence of adenovirus and tyrphostin.
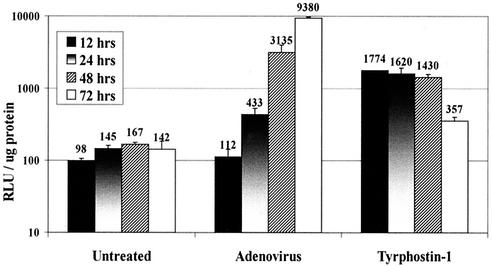
rAAV vector expression increases with time of incubation in cell culture and in animal models. The increase is believed to be due to the accumulation of transcriptionally competent, double-stranded vector DNA with time. To determine the kinetics of tyrphostin stimulation, cells were left untreated, infected with adenovirus, or treated with tryphostin-1 for 2 h and then transduced with 100 particles of vAVCMVluc per cell. Transduced cultures were incubated for the indicated times and harvested, and luciferase activity was determined (Fig. 3). In the untreated cultures, there was a gradual increase in transduction that peaked at 48 h. Luciferase activity increased with time in the adenovirus-infected cultures up to the 72-h time point. Tyrphostin-treated cultures realized maximum expression at 12 h, which then diminished at each succeeding time point. These results suggest that tyrphostin rapidly stimulates transgene expression compared to adenovirus-infected cultures. These results also suggest that there should be more double-stranded, transcriptionally competent vector DNA in the tyrphostin-treated cultures than in the adenovirus-infected and untreated cells. Alternatively, tyrphostin may enhance vector transduction by additional means.
FIG. 3.
Time course of luciferase expression from IB3 cells transduced with vAVCMVluc and treated with tyrphostin or coinfected with adenovirus. Quiescent IB3 cells were left untreated or treated with 250 μM tyrphostin for 2 h. Cells were washed to remove the tyrphostin and infected with 100 particles per cell of vAVCMVluc for 2 h. A parallel set of cultures were coinfected with the vector and adenovirus at a multiplicity of infection of 5. The viruses were removed, and the cultures were incubated in normal medium. The cultures were harvested at the indicated times, and protein and luciferase assays were performed. Results are reported as relative light units (RLU) per microgram of protein. Error bars represent the standard deviation of the mean of triplicate assays.
Tyrphostin does not alter the D-sequence-binding characteristics of ssDBP from IB3 cells.
The ssDBP is phosphorylated by the EGFR on Tyr residues (30). Phosphorylation of ssDBP can be detected in EMSAs with radiolabeled single-stranded AAV D sequence (26, 30). Inhibition of EGFR by tyrphostins results in alteration of the ssDBP-D sequence complex in EMSAs (26). Several studies have shown that the level of Tyr phosphorylation of the ssDBP is inversely proportional to the level of rAAV transgene expression (28, 30). This is believed to be reflective of the amount of single- to double-stranded vector DNA conversion.
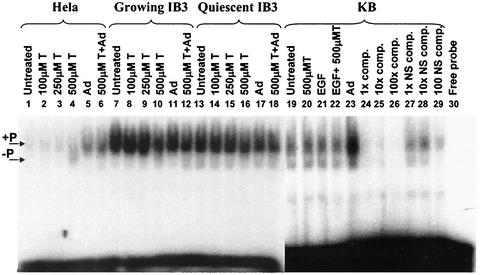
To determine if the ssDBP is similarly affected in IB3 cells, whole-cell extracts were prepared from actively growing HeLa, KB, and IB3 cells and quiescent IB3 cells that were left untreated or treated with different amounts of tyrphostin. Equal amounts of protein were then used to shift an end-labeled D-sequence oligomer. The banding pattern of the shifted fragments observed in Fig. 4 was very similar to that observed by others, in which the top band is the Tyr-phosphorylated form of the ssDBP (26, 28, 30). The predominant ssDBP-D sequence complex in all three cell types corresponded to the Tyr-phosphorylated form of the protein (Fig. 4, lanes 1, 7, 13, and 19). The only change in the ratio of phosphorylated to dephosphorylated ssDBP was observed in HeLa cell extracts from cultures treated with 500 μM tyrphostin (lane 4). Alteration of the ssDBP binding pattern was not observed in IB3 cells regardless of the treatment (Fig. 4, lanes 7 to 18). The specificity of the D sequence binding was demonstrated with extracts of KB cells in which increasing amounts of unlabeled D sequence oligonucleotide successfully competed for binding to the labeled probe (Fig. 4, lanes 24 to 26). Increasing amounts of a nonspecific oligonucleotide did not efficiently compete for ssDBP binding (Fig. 4, lanes 27 to 29). These results suggest that in IB3 cells, tyrphostin treatment or adenovirus infection does not alter the D-sequence-binding characteristics of the ssDBP. Thus, tyrphostin stimulation of rAAV transgene expression in IB3 cells probably occurs by a different mechanism from that observed in other cell lines.
FIG. 4.
D-sequence EMSA analyses of HeLa, IB3, and KB cell extracts. Quiescent IB3 cells and exponentially growing HeLa, IB3, and KB cells were treated with increasing amounts of tyrphostin (T) for 2 h, and then the cells were harvested and WCE were prepared as described in Materials and Methods. Alternatively, cells were infected with adenovirus (Ad) for 36 h prior to preparation of the WCE. For adenovirus-infected tyrphostin-treated cells, the tyrphostin was added to the cultures for 2 h after the 36-h adenovirus infection. KB cells were also treated with 1 μg of EGF per ml for 24 h prior to WCE preparation. Equal amounts of protein were used to shift a radioactively end-labeled, single-stranded D-sequence oligonucleotide. KB cell extracts were also incubated with a 1-, 10-, and 100-fold excess of unlabeled competitor (comp.) D sequence or a nonspecific (NS) competitor oligonucleotide. The locations of the Tyr-phosphorylated ssDBP/DNA complex is indicated as +P. The Tyr-dephosphorylated form is indicated as −P on the left side of the figure.
Analysis of vector DNA from vAVCMVluc-transduced, tyrphostin-treated IB3 cells.
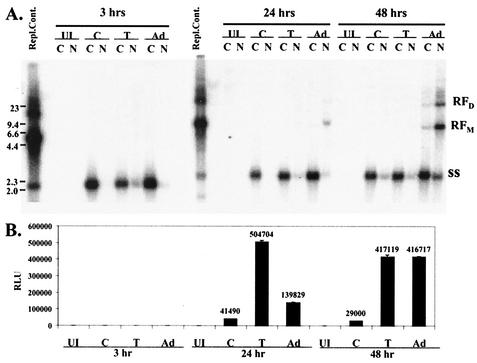
To examine the conformation of vector DNA, IB3 cells were transduced with 500 particles per cell of vAVCMVluc and treated with tyrphostin, infected with adenovirus, or left untreated. The cultures were harvested at 3, 24, and 48 h and separated into nuclear and cytoplasmic fractions. Total DNA was isolated, separated by agarose gel electrophoresis, and analyzed by hybridization with a radiolabeled probe. At 3 h, all of the detectable vector DNA was in the single-stranded conformation (Fig. 5A). There was a slight increase in vector DNA in the nucleus of the tyrphostin-treated cultures; however, this phenomenon did not occur in subsequent analyses (data not shown).
FIG. 5.
Southern hybridization analyses of RF vector DNA. Quiescent IB3 cells were left uninfected and untreated (lanes UI), infected with 500 particles per cell of vAVCMVluc (lanes C), infected with vAVCMVluc and treated with 500 μM tyrphostin (lanes T), or infected with vAVCMVluc and adenovirus at a multiplicity of infection of 5 (lanes Ad). The cultures were harvested at 3, 24, or 48 h and separated into cytoplasmic (C) and nuclear (N) fractions. (A) Total DNA was isolated from equal proportions of cytoplasmic and nuclear fractions and analyzed by agarose gel electrophoresis and hybridization to a radiolabeled CMV sequence probe. The replication control (Repl.Cont.) was obtained from 293 cells cotransfected with the pAVCMVluc and pSH5 (8) plasmids, infected with adenovirus, and harvested 24 h after transfection. The locations of the monomer (RFM) and dimer (RFD) RFs and single-stranded DNAs (ss) are indicated on the right. The positions of DNA size markers are shown on the left (in kilobase pairs). (B) Luciferase assays were performed on aliquots of cytoplasmic fractions from each of the indicated treatments and time points. RLU, relative light units.
The cytoplasmic fractions from the transductions were also assessed for luciferase activity (Fig. 5B). At 3 h there was no detectable luciferase activity in any of the samples. At 24 h, replicative form monomer (RFm) DNA was only observed in the nuclei of adenovirus-infected cells. However, luciferase activity was substantially higher in the extracts from tyrphostin-treated cells. The amount of RF vector DNA increased in the adenovirus-infected cultures by 48 h but was not detected in either the control or tyrphostin-treated transductions. Luciferase activity at 48 h was comparable between the tyrphostin-treated and adenovirus-infected cultures. These results suggest that there were no dramatic changes in nuclear accumulation of vector DNA in tyrphostin-treated cultures compared to adenovirus-infected or untreated cells. Adenovirus infection induced single-stranded to double-stranded vector DNA conversion more efficiently than in tyrphostin-treated or untreated cells. However, there was more luciferase activity in tyrphostin-treated cells at 24 h in spite of undetectable RF DNA. Thus, the RF DNA in the tyrphostin-treated cells would seem to be more transcriptionally active than that found in the adenovirus-infected cells.
Tyrphostin treatment results in higher levels of mRNA from vAVgfp.
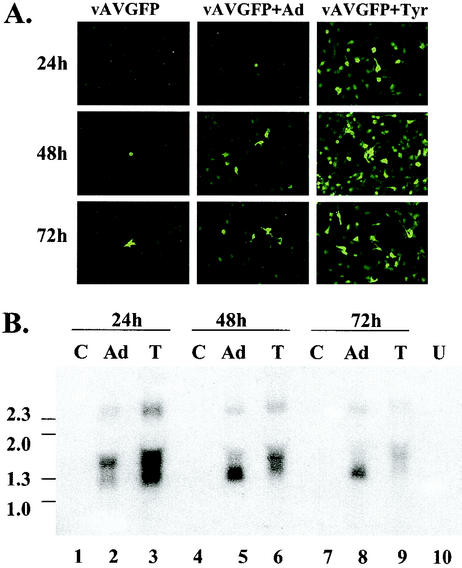
To determine if an increase in mRNA transcription could explain the dramatic increase in transgene expression in the apparent absence of detectable double-stranded RF vector DNA, Northern analyses were performed. Quiescent IB3 cells were left untreated, treated with tyrphostin, or infected with adenovirus and transduced with 100 particles of vAVgfp per cell. Expression of the gfp gene was monitored by fluorescence microscopy (Fig. 6A). The tyrphostin-treated cultures showed substantially more gfp expression than the adenovirus-infected cultures at 24 h. Unlike the luciferase results shown in Fig. 3 and 5, gfp expression in the tyrphostin-treated cultures remained greater than the level of expression in the adenovirus-infected cultures at 48 and 72 h. This difference in expression may be a function of differences in protein stability between the luciferase and green fluorescent proteins.
FIG. 6.
Northern analysis of mRNA from vAVgfp-transduced IB3 cells. Quiescent IB3 cells were left untreated, infected with adenovirus type 5 (Ad) at a multiplicity of infection of 10, or treated with tyrphostin and transduced with 100 particles per cell of vAVgfp. (A) At 24, 48, and 72 h later, expression was assessed by fluorescence microscopy prior to RNA isolation. (B) Northern analysis of RNA isolated from vector-transduced cells that were left untreated (lanes C), adenovirus-infected at a multiplicity of infection of 5 (lanes Ad), and treated with 500 μM tyrphostin (lanes T). A sample of RNA from untransduced cells (lanes U) was included. The locations of single-stranded DNA size markers (in kilobases) are shown on the left of the image.
Total RNA was prepared at 24, 48, and 72 h after transduction and analyzed by Northern hybridization. At 24 h there was approximately threefold more gfp mRNA (Fig. 6B, lane 3) in the tyrphostin-treated culture compared to the adenovirus-infected cells (lane 2). At 48 h, the mRNA level in tyrphostin-treated cells diminished (Fig. 6B, lane 6) and mRNA from adenovirus-infected cells increased (Fig. 6B, lane 5). At 72 h more gfp mRNA remained in the adenovirus-infected cells (Fig. 6B, lane 8) compared to the tyrphostin-treated cultures (Fig. 6B, lane 9). The vAVgfp vector contains a CMV promoter attached to the gfp gene, followed by the simian virus 40 poly(A) signal and the herpes simplex virus thymidine kinase transcription promoter attached to a neo gene and bovine growth hormone poly(A) signal (51). The largest mRNA species of approximately 2.3 kb is likely due to readthrough of the polymerase through a poly(A) signal at the end of the gfp cassette, which then terminates at the poly(A) signal for the adjacent neo gene. The two smaller mRNA species likely terminate at the poly(A) signal downstream of the gfp cassette and may be the result of alternative splicing or perhaps differential transcription initiation. These results demonstrate that tyrphostin treatment results in a significant increase in mRNA levels in the absence of detectable RF vector DNA. Thus, tyrphostin stimulates transcription from the CMV promoter or increases the stability of the mRNA.
Tyrphostin-mediated stimulation of gene expression from linearized plasmid DNA.
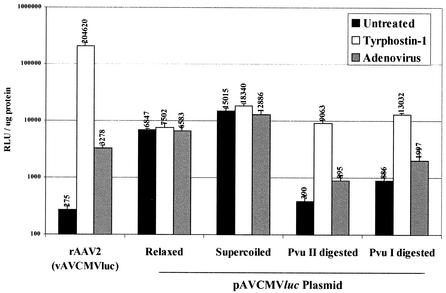
The results from the Northern and Southern analyses shown above suggested that tyrphostin stimulates rAAV transduction of IB3 cells by mechanisms other than increasing single- to double-stranded conversion of vector DNA. Several attempts to demonstrate tyrphostin stimulation of luciferase expression in DNA transfections with pAVCMVluc failed to demonstrate any effect of the inhibitor on luciferase activity (data not shown). We hypothesized that the supercoiled plasmid used in transfection does not accurately emulate the linear vector DNA molecule. To determine if a linear form of the vector is affected by tyrphostin treatment, we treated the pAVCMVluc plasmid with either PvuI or PvuII. PvuI cuts once, in the backbone of the plasmid, and PvuII cuts at the ends of the AAV TR elements in the plasmid, releasing the vector DNA from the plasmid. The linearized, supercoiled, and relaxed circular plasmid conformations were purified after separation by gel electrophoresis. The purified DNAs were then transfected onto IB3 cells, and 24 h later the cells were treated with tyrphostin or infected with adenovirus. The cultures were harvested 24 h later, and luciferase activity was determined.
As expected, tyrphostin and adenovirus infection stimulated vAVCMVluc transduction (Fig. 7). Neither agent improved gene expression from relaxed or supercoiled plasmid transfection compared to untreated cultures. However, tyrphostin, and to a lesser extent adenovirus, stimulated gene expression from both linearized plasmid DNAs. Supercoiled plasmid DNA is well known to be more efficiently transfected into cells in culture and gave the highest level of activity here. However, since we did not determine nuclear plasmid copy number, we cannot definitively determine which DNA conformation was transfected most efficiently under these conditions. These results, combined with the Northern analyses, suggest that when the vector DNA is in a linear conformation, tyrphostin stimulates transcription from the CMV promoter. If tyrphostin increased mRNA stability, there would be no difference in the level of expression between the supercoiled and linear transfected DNAs.
FIG. 7.
Tyrphostin-mediated stimulation of gene expression from vAVCMVluc and linear plasmids. Quiescent IB3 cells were transfected with 100 ng of pAVCMVluc plasmid DNA that had been isolated after agarose gel electrophoresis separation of supercoiled, relaxed, and PvuI- or PvuII-digested DNA. At 24 h later, the DNA-transfected cultures were treated with 500 μM tyrphostin as described in Materials and Methods. Parallel cultures were left untreated, transduced with 50 particles per cell of vAVCMVluc and treated with tyrphostin, or coinfected with 50 particles per cell of vAVCMVluc and adenovirus (multiplicity of infection of 5). At 24 h later, the cultures were harvested, and luciferase assays were performed. The results of triplicate assays are shown. Error bars represent the standard deviation of the mean. RLU, relative light units.
Analysis of role of MAPK pathways in rAAV2-mediated transgene expression in tyrphostin-treated IB3 cells.
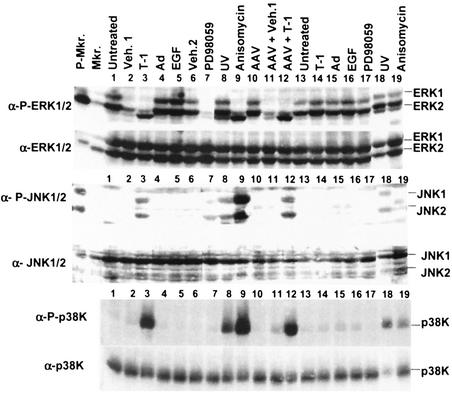
The EGFR is a strong activator of the ERK1/2 mitogen-activated protein kinase (MAPK) pathway (31), and tyrphostin is a potent inhibitor of the EGFR tyrosine kinase (20). We hypothesized that tyrphostin enhances rAAV2-mediated transgene expression in IB3 cells by inhibiting one of the major downstream targets of EGFR, the ERK1/2 MAPK pathway. To determine if tyrphostin treatment of IB3 cells could decrease phosphorylation (and therefore activation) of the ERK1/2 MAPK pathway, we used immunoblot analysis of extracts from cells treated with tyrphostin and a variety of agents that may affect the ERK1/2 pathway.
Quiescent IB3 cells were treated with agents as indicated in Fig. 8 and harvested immediately after treatment or 24 h later. Immunoblot analysis for phosphorylated and total ERK1/2 was performed. Surprisingly, the basal phosphorylation of ERK1/2 in untreated cells on serum-free medium was higher than expected (Fig. 8, lanes 1 and 13). Treatment with tyrphostin dramatically decreased the amount of phosphorylated ERK1/2 in cells harvested immediately after treatment (Fig. 8, lane 3); however, the phosphorylation status of ERK1/2 returned to nearly normal levels 24 h later (Fig. 8, lane 14). The vehicle for tyrphostin (1% DMSO and 5% ethanol) also decreased the amount of phosphorylated ERK1/2 (Fig. 8, lane 2) but to a lesser extent than that observed from tyrphostin treatment. Thus, tyrphostin blocks signal transduction through the ERK pathway. EGF treatment (Fig. 8, lane 5) and perhaps adenovirus infection (lane 4) led to a marginal increase in ERK1/2 phosphorylation. PD98059, an inhibitor of MEK1/2 (2), caused a decrease in phosphorylation of ERK1/2 in cells harvested immediately after treatment (Fig. 8, lane 7), but the phosphorylation status of ERK1/2 returned to normal levels 24 h later (Fig. 8, lane 17). UV irradiation (Fig. 8, lane 8) and AAV infection (Fig. 8, lane 10) did not affect ERK1/2 phosphorylation. Anisomycin, a potent agonist of MAP kinases JNK/SAPK and p38K (14), led to a decrease in ERK1/2 phosphorylation (Fig. 8, lane 9), but that activity was restored to normal levels within 24 h (Fig. 8, lane 19). These results indicate that treatment of IB3 cells with anisomycin, PD98059, tyrphostin, or the tyrphostin vehicle alone led to a decrease in the level of phosphorylated ERK1/2.
FIG. 8.
Immunoblot analyses of signal transduction pathways. Quiescent IB3 cells were treated as indicated at the top of the figure. One set of cultures were harvested immediately after treatment (lanes 1 to 12). A parallel set were harvested 24 h later (lanes 13 to 19). Equal amounts of protein were loaded in each lane and subjected to SDS-PAGE and subsequent immunoblot analyses. The blots were first probed with antibodies to the phosphorylated form (α-P) of the proteins (anti-phosphorylated-ERK1/2, anti-phosphorylated-JNK1/2, and anti-phosphorylated-p38K). The blots were then stripped of the first antibody and reprobed with antibodies that detect the total protein (anti-ERK1/2, anti-JNK1/2, and anti-p38K). Veh.1 refers to the vehicle in which tyrphostin (T-1) was dissolved. Veh.2 refers to the vehicle in which PD98059 was dissolved. AAV and adenovirus (Ad) were used at multiplicities of infection of 1,000 and 5, respectively, in these experiments. Lanes containing phosphorylated (P-Mkr) and nonphosphorylated (Mkr) ERK and JNK marker proteins are indicated.
The transfection data showing tyrphostin stimulation of gene expression from linearized plasmid DNA suggest that the EGFR inhibitor's mode of action may be similar to a cellular stress response. To determine if stress-activated protein kinase (JNK/SAPK) is activated by tyrphostin, IB3 cell extracts were analyzed by immunoblots. With the same set of treatment conditions described above, immunoblots were performed with antibodies against phosphorylated JNK1/2 and p38K and total JNK1/2 and p38K (Fig. 8). The responses of the two different types of MAP kinases were similar. IB3 cells treated with tyrphostin (Fig. 8, lanes 3 and 12) showed activation of JNK1/2 and p38K immediately after treatment. Known inducers of the JNK1/2 pathway, UV irradiation (Fig. 8, lanes 8) and anisomycin (lane 9), also showed activation of JNK1/2 and p38K immediately after treatment. Activation of JNK1/2 and p38K persisted for 24 h after UV treatment (Fig. 8, lane 18). The p38K remained phosphorylated in the anisomycin-treated cells up to 24 h later (Fig. 8, lane 19). These results indicate that tyrphostin treatment of IB3 cells resulted in a transient stimulation of the p38K and JNK pathways.
Stimulation of rAAV transduction by inducers of the JNK1/2 and p38K pathways.
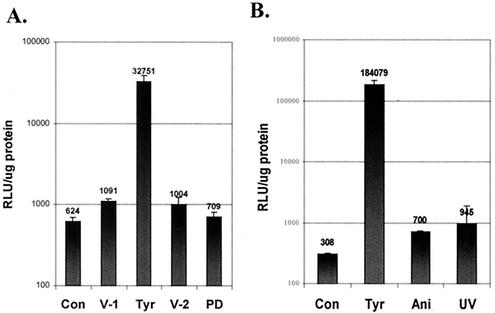
UV irradiation of cells in culture is a known stimulator of rAAV transduction (3). To determine if PD98059 or anisomycin treatment stimulates vector transduction, IB3 cells were transduced with vAVCMVluc, treated with these agents, and harvested 24 h later. Luciferase assays revealed that PD98059 did not affect transduction efficiency over control cells or cells treated with the vehicles for tyrphostin or the MEK inhibitor (Fig. 9A). Therefore the ERK pathway alone is not responsible for the stimulation in vector transduction. In comparison, anisomycin treatment and UV irradiation induced modest increases in vector transduction in IB3 cells (Fig. 9B). These results suggest that agents that stimulate the JNK1/2 and p38K pathways result in increased transduction from the vAVCMVluc vector. However, inhibition of phosphorylation by PD98059 of ERK1/2, a downstream target of the EGFR, is not sufficient for increased transduction.
FIG. 9.
Stimulation of transgene expression in tyrphostin-, PD98059-, anisomycin-, and UV-treated IB3 cells. Quiescent IB3 cells were transduced with 50 particles per cell of vAVCMVluc. (A) The cultures were then left untreated (Con) or treated for 2 h with the vehicle for tyrphostin (V-1), 500 μM tyrphostin (Tyr), the vehicle for PD98059 (V-2), or PD98059 (PD). (B) Alternatively, cultures were left untreated (Con) or treated for 2 h with 500 μM tyrphostin (Tyr) or anisomycin (Ani) or irradiated with UV light (UV). At 48 h later, the cultures were harvested and assayed for luciferase activity. The results of triplicate assays are shown. Error bars represent the standard deviation of the mean. RLU, relative light units.
DISCUSSION
The limitations of rAAV vector transduction prompted the investigation of a variety of agents for their ability to enhance gene transduction. One of the most potent stimulators of transduction is the EGFR tyrosine kinase inhibitor tyrphostin (26). The proposed mechanism of stimulation of transduction from tyrphostin treatment is induction of second-strand DNA synthesis on the single-stranded vector genome. DNA synthesis is enabled by alteration of ssDBP binding to the D element in the viral terminal repeat (26, 27). Tyrphostin treatment of cells results in inhibition of Tyr phosphorylation on the ssDBP, which in turn affects the protein's interaction with the AAV TR (30). The altered affinity enables single-stranded to double-stranded conversion of the vector genome. The studies presented here extend the investigations into the mechanisms of how tyrphostin stimulates recombinant AAV2 vector-mediated gene transduction.
With an undifferentiated airway epithelial cell line and transduction with an AAV luciferase vector, we demonstrated up to a 1,000-fold increase in transgene expression when cells were treated with tyrphostin and transduced with a low multiplicity of infection of vector. This observation is consistent with earlier studies in other cell lines (26, 30). An inconsistency between our results and those of others is the lack of an effect on ssDBP interactions with the AAV TR D sequence. Although we saw an alteration in ssDBP interactions with its cognate binding site in tyrphostin-treated HeLa cells, we did not observe similar effects on ssDBP interactions from IB3 cells. None of the following treatments resulted in alteration of the shifted bands in the EMSA: adenovirus coinfection, EGF treatment, or the use of extracts from exponentially growing IB3 cells. In the adenovirus-coinfected cells, ssDBP binding was not affected, yet RF DNA was produced, suggesting that a detectable alteration in D-sequence binding is not predictive of RF formation or transgene expression. The reason for this discrepancy may be due to the airway epithelial origin of the cell line or perhaps the simian virus 40 T antigen used to originally establish the cell line (50). These results suggest that the mechanism of tyrphostin-mediated transgene expression may be unrelated to the ssDBP in the IB3 cell line.
Among other agents known to stimulate rAAV transduction, adenovirus coinfection has also been shown to stimulate single- to double-stranded DNA conversion of the vector genome (15, 16). Adenovirus coinfection stimulated vector transduction in IB3 cells. However, comparison of the levels of adenovirus- and tyrphostin-mediated stimulation from 12 to 72 h after transduction indicated that the two agents probably enhance transgene expression by different mechanisms. Tyrphostin stimulation of transgene expression peaked at 12 h and diminished thereafter. Adenovirus stimulation was at its lowest at 12 h and increased to its highest level at the 72-h time point. The accumulation of RFM vector DNA in the adenovirus-coinfected cultures and corresponding increases in gene expression are consistent with the model of adenovirus-mediated single- to double-stranded DNA conversion leading to higher transgene expression. However, the higher level of luciferase activity at 24 h in the complete absence of RFM DNA in the tyrphostin-treated cells strongly suggests that in IB3 cells tyrphostin stimulates transgene expression by a mechanism other than single- to double-stranded vector DNA conversion.
The absence of accumulation of RF DNA in the tyrphostin-treated cultures and the abundance of RF in the adenovirus-coinfected cultures raises several possible hypotheses regarding rAAV transduction. It is possible that only a very low level of RF DNA is required for active transcription and that this level is attained in the tyrphostin-treated cells. Therefore, a similar low level of RF DNA in the adenovirus-infected cultures would be sufficient for transcription to occur, and the majority of the RF DNAs are replication products that are transcriptionally inactive. Another possible explanation for our results is that second-strand synthesis on the single-stranded vector DNA extends past the 3′ end of the CMV promoter so that transcription initiates from the double-stranded promoter region, and this partially double-stranded vector DNA would then be activated by tyrphostin. In this scenario, the partially double-stranded vector DNA would be indistinguishable from input vector in the agarose gel. Although the tyrphostin stimulation is significant, the mechanism of stimulation remains to be fully defined.
Northern analyses revealed that vector from tyrphostin-treated cells yielded approximately threefold more mRNA than from adenovirus-coinfected cultures. This suggests that although much more RF DNA is found in adenovirus-infected than in tyrphostin-treated cultures, the tyrphostin treatment results in a more transcriptionally active template. This interpretation is further supported by the results of the DNA transfection assays. Several attempts to determine if tyrphostin stimulated gene expression from the CMV promoter in the vector by transfection assays with supercoiled plasmid were unsuccessful. However, when we transfected plasmid DNA that had been linearized, we observed tyrphostin-mediated stimulation of transgene expression. Our rationale for this approach was that the linearized plasmid DNA closely approximates the linear conformation of RFM vector DNA in the vector-transduced cell.
The requirement for free DNA ends suggests that tyrphostin's effects may be part of a stress or DNA damage response. The presence of free DNA ends on plasmid or RFM vector DNA would be analogous to the presence of double-strand breaks in chromosomal DNA. Free double-stranded ends are binding sites for the DNA end-binding Ku protein, which in turn would recruit double-stranded DNA-activated protein kinase (DNA-PK), which are part of the damage response resulting in nonhomologous end-joining repair mechanisms (25). How the cell handles free DNA ends on AAV vectors affects the episomal conformation of vector DNA. The conformation of AAV vector DNA is affected by DNA-PK status, with circular genomes persisting in DNA-PKcs+ mice, which contain the enzyme catalytic subunit, and linear genomes persisting in DNA-PKcs− mice (38). In cell culture, UV treatment followed by vector transduction results in the formation of circular vector genomes, whereas adenovirus infection or expression of adenovirus E4 orf 6 results in a greater abundance of linear vector genomes (12). There is some evidence that DNA-PK also affects the regulation of cellular mRNA transcription (35, 43), but a direct effect of the kinase on AAV gene expression has not been demonstrated.
The EGFR is a strong activator of the ERK1/2 MAPK pathway (31), and tyrphostin is a potent inhibitor of the EGFR (20). It is not uncommon for one or more of the MAPK pathways to play an integral role in the infectious process of a virus, such as adenovirus (1, 34, 42). We hypothesized that tyrphostin stimulates rAAV2-mediated transgene expression by inhibiting the ERK1/2 MAPK pathway. Tyrphostin and the MEK1/2 inhibitor PD98059 decreased phosphorylation, and hence inactivation, of ERK1/2 in IB3 cells. However, inhibition of ERK1/2 phosphorylation by treatment of cells with PD98059 did not enhance rAAV2-mediated transgene expression. Because inhibition of ERK1/2 phosphorylation alone was insufficient to enhance rAAV2-mediated transgene expression, we concluded that tyrphostin must have other effects on IB3 cells, perhaps including effects on other MAPK pathways.
Others have alluded to the possibility that tyrphostin exerts pleiotropic effects with respect to rAAV transduction (26). Analysis of the p38K and JNK/SAPK pathways revealed that tyrphostin, UV irradiation, and anisomycin stimulated these pathways. Tyrphostin not only affects the EGFR tyrosine kinase pathway but induces a cellular stress response as well. Tyrphostin and UV irradiation stimulated the JNK/SAPK and p38K pathways to comparable levels, and anisomycin was the strongest stimulator of these pathways. UV irradiation and anisomycin were also found to stimulate rAAV transgene expression two- to threefold in IB3 cells, but in the same experiment tyrphostin stimulated expression over 600-fold. Thus, activation of the stress response stimulates rAAV transgene expression. Tyrphostin's dramatic effects on transgene expression indicate that other, unknown effects exerted by the EGFR inhibitor may significantly stimulate expression.
Our investigations have extended the observation of others that tyrphostin dramatically increases rAAV transgene expression. At least for IB3 cells, the mechanism appears to be independent of phosphorylation of the ssDBP. This interpretation is supported by the results from the Southern hybridization studies, in which there was no detectable increase in RFM DNA in response to tyrphostin, whereas there was a demonstrable increase in RFM and replicative form dimer in adenovirus-infected cells. We believe that the double-stranded RF vector DNA in the tyrphostin-treated cells is more transcriptionally active than the vector DNA in the adenovirus-infected cells. Others have demonstrated that transcription from the CMV promoter can be upregulated by cellular stressors (7). Stressors that activate the JNK/SAPK and p38 MAPK pathways have been shown to dramatically increase expression from the CMV promoter (7). Our transfection studies with linearized plasmid DNA are consistent with the hypothesis that tyrphostin, as a cellular stressor, dramatically increases expression from the CMV promoter. These studies indicate that multiple pathways may be modulated in attempts to increase rAAV vector transduction in airway epithelial cells.
Acknowledgments
We thank Vivian Kalman-Maltese for excellent technical assistance.
This work was supported in part by a grant from the National Institutes of Health (GM64765).
REFERENCES
- 1.Alcorn, M. J., J. L. Booth, K. M. Coggeshall, and J. P. Metcalf. 2001. Adenovirus type 7 induces interleukin-8 production via activation of extracellular regulated kinase 1/2. J. Virol. 75:6540-6549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alessi, D. R., A. Cuenda, P. Cohen, D. T. Dudley, and A. R. Saltiel. 1995. PD098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J. Biol. Chem. 270:27489-27494. [DOI] [PubMed] [Google Scholar]
- 3.Alexander, I. E., D. W. Russell, and A. D. Miller. 1994. DNA-damaging agents greatly increase the transduction of nondividing cells by adeno-associated virus vectors. J. Virol. 68:8282-8287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ali, R. R. 1996. Gene transfer into the mouse retina mediated by an adeno-associated viral vector. Hum. Mol. Genet. 5:591-594. [DOI] [PubMed] [Google Scholar]
- 5.Auricchio, A., M. Hildinger, E. O'Connor, G. P. Gao, and J. M. Wilson. 2001. Isolation of highly infectious and pure adeno-associated virus type 2 vectors with a single-step gravity-flow column. Hum. Gene Ther. 12:71-76. [DOI] [PubMed] [Google Scholar]
- 6.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.). 1989. Current protocols in molecular biology, vol. 2. John Wiley and Sons Inc., New York, N.Y.
- 7.Bruening, W., B. Giasson, W. Mushynski, and H. D. Durham. 1998. Activation of stress-activated MAP protein kinases up-regulates expression of transgenes driven by the cytomegalovirus immediate/early promoter. Nucleic Acids Res. 26:486-489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collaco, R. F., X. Cao, and J. P. Trempe. 1999. A helper virus-free packaging system for recombinant adeno-associated virus vectors. Gene 238:397-405. [DOI] [PubMed] [Google Scholar]
- 9.Connolly, D. T., M. B. Knight, N. K. Harakas, A. J. Wittwer, and J. Feder. 1986. Determination of the number of endothelial cells in culture with an acid phosphatase assay. Anal. Biochem. 152:136-140. [DOI] [PubMed] [Google Scholar]
- 10.Conrad, C. K., S. S. Allen, S. A. Afione, T. C. Reynolds, S. E. Beck, M. Fee-Maki, X. Barrazza-Ortiz, R. Adams, A. F. B., B. J. Carter, W. B. Guggino, and T. R. Flotte. 1996. Safety of single-dose administration of an adeno-associated virus (AAV)-CFTR vector in the primate lung. Gene Ther. 3:658-668. [PubMed] [Google Scholar]
- 11.Douar, A. M., K. Poulard, D. Stockholm, and O. Danos. 2001. Intracellular trafficking of adeno-associated virus vectors: routing to the late endosomal compartment and proteasome degradation. J. Virol. 75:1824-1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duan, D., P. Sharma, L. Dudus, Y. Zhang, S. Sanlioglu, Z. Yan, Y. Yue, Y. Ye, R. Lester, J. Yang, K. J. Fisher, and J. F. Engelhardt. 1999. Formation of adeno-associated virus circular genomes is differentially regulated by adenovirus E4 ORF6 and E2a gene expression. J. Virol. 73:161-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duan, D., Y. Yue, Z. Yan, P. B. McCray, Jr., and J. F. Engelhardt. 1998. Polarity influences the efficiency of recombinant adenoassociated virus infection in differentiated airway epithelia. Hum. Gene Ther. 9:2761-2776. [DOI] [PubMed] [Google Scholar]
- 14.Faris, M., N. Kokot, K. Latinis, S. Kasibhatla, D. R. Green, G. A. Koretzky, and A. Nel. 1998. The c-Jun N-terminal kinase cascade plays a role in stress-induced apoptosis in Jurkat cells by upregulating Fas ligand expression. J. Immunol. 160:134-144. [PubMed] [Google Scholar]
- 15.Ferrari, F. K., T. Samulski, T. Shenk, and R. J. Samulski. 1996. Second-strand synthesis is a rate-limiting step for efficient transduction by recombinant adeno-associated virus vectors. J. Virol. 70:3227-3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fisher, K. J., G.-P. Gao, M. D. Weitzman, R. DeMatteo, J. F. Burda, and J. M. Wilson. 1996. Transduction with recombinant adeno-associated virus for gene therapy is limited by leading-strand synthesis. J. Virol. 70:520-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fisher, K. J., K. Jooss, J. Alston, Y. Yang, S. E. Haecker, K. High, R. Pathak, S. E. Raper, and J. M. Wilson. 1997. Recombinant adeno-associated virus for muscle directed gene therapy. Nat. Med. 3:306-312. [DOI] [PubMed] [Google Scholar]
- 18.Fisher-Adams, G., K. K. Wong, Jr., G. Podsakoff, S. J. Forman, and S. Chatterjee. 1996. Integration of adeno-associated virus vectors in CD34+ human hematopoietic progenitor cells after transduction. Blood 88:492-504. [PubMed] [Google Scholar]
- 19.Flotte, T. R., S. A. Afione, C. Conrad, S. A. McGrath, R. Solow, H. Oka, P. L. Zeitlin, W. B. Guggino, and B. J. Carter. 1993. Stable in vivo expression of the cystic fibrosis transmembrane conductance regulator with an adeno-associated virus vector. Proc. Natl. Acad. Sci. USA 90:10613-10617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gazit, A., P. Yaish, C. Gilon, and A. Levitzki. 1989. Tyrphostins I: synthesis and biological activity of protein tyrosine kinase inhibitors. J. Med. Chem. 32:2344-2352. [DOI] [PubMed] [Google Scholar]
- 21.Hansen, J., K. Qing, H. J. Kwon, C. Mah, and A. Srivastava. 2000. Impaired intracellular trafficking of adeno-associated virus type 2 vectors limits efficient transduction of murine fibroblasts. J. Virol. 74:992-996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hengge, U. R., and A. Mirmohammadsadegh. 2000. Adeno-associated virus expresses transgenes in hair follicles and epidermis. Mol. Ther. 2:188-194. [DOI] [PubMed] [Google Scholar]
- 23.Kaplitt, M. G., P. Leone, R. J. Samulski, X. Xiao, D. W. Pfaff, K. L. O'Malley, and M. J. During. 1994. Long-term gene expression and phenotypic correction with adeno-associated virus vectors in the mammalian brain. Nat. Genet. 8:148-154. [DOI] [PubMed] [Google Scholar]
- 24.Kaplitt, M. G., X. Xiao, R. J. Samulski, J. Li, K. Ojamaa, I. L. Klein, H. Makimura, M. J. Kaplitt, R. K. Strumpf, and E. B. Diethrich. 1996. Long-term gene transfer in porcine myocardium after coronary infusion of an adeno-associated virus vector. Ann. Thorac. Surg. 62:1669-1676. [DOI] [PubMed] [Google Scholar]
- 25.Khanna, K. K., and S. P. Jackson. 2001. DNA double-strand breaks: signalling, repair and the cancer connection. Nat. Genet. 27:247-254. [DOI] [PubMed] [Google Scholar]
- 26.Mah, C., K. Qing, B. Khuntirat, S. Ponnazhagan, X. S. Wang, D. M. Kube, M. C. Yoder, and A. Srivastava. 1998. Adeno-associated virus type 2-mediated gene transfer: role of epidermal growth factor receptor protein tyrosine kinase in transgene expression. J. Virol. 72:9835-9843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qing, K., J. Hansen, K. A. Weigel-Kelley, M. Tan, S. Zhou, and A. Srivastava. 2001. Adeno-associated virus type 2-mediated gene transfer: role of cellular FKBP52 protein in transgene expression. J. Virol. 75:8968-8976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qing, K., B. Khuntirat, C. Mah, D. M. Kube, X. S. Wang, S. Ponnazhagan, S. Zhou, V. J. Dwarki, M. C. Yoder, and A. Srivastava. 1998. Adeno-associated virus type 2-mediated gene transfer: correlation of tyrosine phosphorylation of the cellular single-stranded D sequence-binding protein with transgene expression in human cells in vitro and murine tissues in vivo. J. Virol. 72:1593-1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qing, K., C. Mah, J. Hansen, S. Zhou, V. Dwarki, and A. Srivastava. 1999. Human fibroblast growth factor receptor 1 is a coreceptor for infection by adeno-associated virus 2. Nat. Med. 5:71-77. [DOI] [PubMed] [Google Scholar]
- 30.Qing, K., X. S. Wang, D. M. Kube, S. Ponnazhagan, A. Bajpai, and A. Srivastava. 1997. Role of tyrosine phosphorylation of a cellular protein in adeno-associated virus 2-mediated transgene expression. Proc. Natl. Acad. Sci. USA 94:10879-10884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Riese, D. J., and D. F. Stern. 1998. Specificity within the EGF family/ErbB receptor family signalling network. Bioessays 20:41-48. [DOI] [PubMed] [Google Scholar]
- 32.Russell, D. W., I. E. Alexander, and A. D. Miller. 1995. DNA synthesis and topoisomerase inhibitors increase transduction by adeno-associated virus vectors. Proc. Natl. Acad. Sci. USA 92:5719-5723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sanlioglu, S., D. Duan, and J. F. Engelhardt. 1999. Two independent molecular pathways for recombinant adeno-associated virus genome conversion occur after UV-C and E4orf6 augmentation of transduction. Hum. Gene Ther. 10:591-602. [DOI] [PubMed] [Google Scholar]
- 34.See, R. H., and Y. Shi. 1998. Adenovirus E1B 19,000-molecular-weight protein activates c-Jun N-terminal kinase and c-Jun-mediated transcription. Mol. Cell. Biol. 18:4012-4022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sheppard, H. M., and X. Liu. 2000. Transcription by RNA polymerase II in DNA-PK deficient scid mouse cells. Biochim. Biophys. Acta 1493:41-47. [DOI] [PubMed] [Google Scholar]
- 36.Snyder, R. O., C. H. Miao, G. A. Patijn, S. K. Spratt, O. Danos, D. Nagy, A. M. Gown, B. Winther, L. Meuse, L. K. Cohen, A. R. Thompson, and M. A. Kay. 1997. Persistent and therapeutic concentrations of human factor IX in mice after hepatic gene transfer of recombinant AAV vectors. Nat. Genet. 16:270-276. [DOI] [PubMed] [Google Scholar]
- 37.Snyder, R. O., S. K. Spratt, C. Lagarde, D. Bohl, B. Kaspar, B. Sloan, L. K. Cohen, and O. Danos. 1997. Efficient and stable adeno-associated virus-mediated transduction in the skeletal muscle of adult immunocompetent mice. Hum. Gene Ther. 8:1891-1900. [DOI] [PubMed] [Google Scholar]
- 38.Song, S., P. J. Laipis, K. I. Berns, and T. R. Flotte. 2001. Effect of DNA-dependent protein kinase on the molecular fate of the rAAV2 genome in skeletal muscle. Proc. Natl. Acad. Sci. USA 98:4084-4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spirende, V., and M. A. Nugent. 1998. Heparan sulfate proteoglycans control intracellular processing of bFGF in vascular smooth muscle cells. Biochemistry 37:13153-13164. [DOI] [PubMed] [Google Scholar]
- 40.Summerford, C., J. S. Bartlett, and R. J. Samulski. 1999. AlphaVbeta5 integrin: a coreceptor for adeno-associated virus type 2 infection. Nat. Med. 5:78-82. [DOI] [PubMed] [Google Scholar]
- 41.Summerford, C., and R. J. Samulski. 1998. Membrane-associated heparan sulfate proteoglycan is a receptor for adeno-associated virus type 2 virions. J. Virol. 72:1438-1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tibbles, L. A., J. C. Spurrell, G. P. Bowen, Q. Liu, M. Lam, A. K. Zaiss, S. M. Robbins, M. D. Hollenberg, T. J. Wickham, and D. A. Muruve. 2002. Activation of p38 and ERK signalling during adenovirus vector cell entry leads to expression of the C-X-C chemokine IP-10. J. Virol. 76:1559-1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tuteja, R., and N. Tuteja. 2000. Ku autoantigen: a multifunctional DNA-binding protein. Crit. Rev. Biochem. Mol. Biol. 35:1-33. [DOI] [PubMed] [Google Scholar]
- 44.Walters, R. W., S. M. Yi, S. Keshavjee, K. E. Brown, M. J. Welsh, J. A. Chiorini, and J. Zabner. 2001. Binding of adeno-associated virus type 5 to 2,3-linked sialic acid is required for gene transfer. J. Biol. Chem. 276:20610-20616. [DOI] [PubMed] [Google Scholar]
- 45.West, M. H., J. P. Trempe, J. D. Tratschin, and B. J. Carter. 1987. Gene expression in adeno-associated virus vectors: the effects of chimeric mRNA structure, helper virus, and adenovirus VA1 RNA. Virology 160:38-47. [DOI] [PubMed] [Google Scholar]
- 46.Xiao, X., J. Li, T. J. McCown, and R. J. Samulski. 1997. Gene transfer by adeno-associated virus vectors into the central nervous system. Exp. Neurol. 144:113-124. [DOI] [PubMed] [Google Scholar]
- 47.Xiao, X., J. Li, and R. J. Samulski. 1996. Efficient long-term gene transfer into muscle tissue of immunocompetent mice by adeno-associated virus vector. J. Virol. 70:8098-8108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yan, Z., R. Zak, G. W. Luxton, T. C. Ritchie, U. Bantel-Schaal, and J. F. Engelhardt. 2002. Ubiquitination of both adeno-associated virus type 2 and 5 capsid proteins affects the transduction efficiency of recombinant vectors. J. Virol. 76:2043-2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zabner, J., M. Seiler, R. Walters, R. M. Kotin, W. Fulgeras, B. L. Davidson, and J. A. Chiorini. 2000. Adeno-associated virus type 5 (AAV5) but not AAV2 binds to the apical surfaces of airway epithelia and facilitates gene transfer. J. Virol. 74:3852-3858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zeitlin, P. L., L. Lu, J. Rhim, G. Cutting, G. Stetten, K. A. Kieffer, R. Craig, and W. B. Guggino. 1991. A cystic fibrosis bronchial epithelial cell line: immortalization by adeno-simian virus 40 infection. Am. J. Respir. Cell. Mol. Biol. 4:313-319. [DOI] [PubMed] [Google Scholar]
- 51.Zolotukhin, S., M. Potter, W. W. Hauswirth, J. Guy, and N. Muzyczka. 1996. A humanized green fluorescent protein cDNA adapted for high-level expression in mammalian cells. J. Virol. 70:4646-4654. [DOI] [PMC free article] [PubMed] [Google Scholar]