Abstract
Chromosomal abnormalities involving telomeric associations (TAs) often precede replicative senescence and abnormal chromosome configurations. We report here that telomere cleavage following exposure to pro-apoptotic agents is an early event in apoptosis. Exposure of human and murine cancer cells to a variety of pro-apoptotic stimuli (staurosporine, thapsigargin, anti-Fas antibody, and cancer chemotherapeutic agents) resulted in telomere cleavage and aggregation, and finally their extrusion from the nuclei. Telomere loss was associated with arrest of cells in G2/M phase and preceded DNA fragmentation. Telomere erosion and subsequent large-scale chromatin cleavage were inhibited by overexpression of the anti-apoptotic protein, bcl-2, and two peptide caspase inhibitors (BACMK and zVADfmk), indicating that both events are regulated by caspase activation. The results demonstrate that telomere cleavage is an early chromatin alteration detected in various cancer cell lines leading to drug-induced apoptosis, and suggest that this event contributes to mitotic catastrophe and induction of cell death. Results also suggest that the decrease of telomeric-repeat binding factor 2 (TRF2) may be the earliest event in the ara-C-induced telomere shortening, induction of endoreduplication and chromosomal fragmentation leading to cell death.
Keywords: telomeric erosion, DNA fragmentation, caspase inhibitors, fluorescence in situ hybridization, telomeric-repeat binding factor (TRF)
Introduction
Telomeres, the short tandem DNA repeats of (T2AG3)n [1,2] localized to the distal ends of chromosomes, play a crucial role in chromosomal protection and replication [3–6]. In most normal somatic cells, telomeric DNA is lost at a rate of 50 to 200 bp per doubling due to replication-associated erosion [7–10]. Eventually telomere erosion leads to cell cycle arrest and senescence [8,9,11], and it has been suggested that telomere erosion may therefore dictate cellular life-span. However, cancer cells are capable of resynthesizing telomeric DNA through the activation/upregulation of an enzyme complex known as telomerase [5]. Some cells can exploit telomerase-dependent and telomerase-independent or alternative lengthening of telomeres (ALT) pathways to reverse telomeric DNA loss, leading to immortalization associated with cancer development [12–19].
Recent studies have shown that cancer chemotherapeutic agents (cisplatin, AZT), capable of binding to or being incorporated into telomeric repeats, cause telomere erosion in cancer cells [20,21]. Because these agents also induce apoptosis, we wondered whether telomere cleavage was linked to programmed cell death. Here, we demonstrate a rapid erosion of telomeres in different types of cancer cells exposed to pro-apoptotic stimuli including staurosporine, thapsigargin, anti-Fas antibody and the cancer chemotherapeutic agent 1-β-d-arabinofuranosylcytosine (ara-C). The telomere shortening by these agents was protected by treatment of cancer cells with two caspase inhibitors, suggesting that caspase-dependent telomere cleavage is an important event of apoptosis. Furthermore, our results suggest that a decreased level of telomere-repeat binding factor 2 (TRF2) may be the rate-limiting step of the ara-C-induced telomere shortening of cancer cells.
Materials and Methods
Cell Culture
We used a UVR-induced murine metastatic melanoma cell line K1735 clone X-21, a bcl-2 transfected cell line of clone X-21, and the nonmetastatic clone 23 of K1735 cells (gifts from Dr. I.J. Fidler). These tumor cells were grown in T-75 tissue culture flasks in RPMI 1640 medium supplemented with 10% fetal calf serum (Gibco BRL, Gaithersburg, MD) and incubated at 37°C in 5% CO2 and 95% air. A human prostate cancer cell line, PC-3M, was also used in anti-Fas antibody- and TNFα-induced apoptosis, determined by FACS analysis. Human and murine cancer cell lines were used to address the general nature of this phenomenon.
Treatment with ara-C
Exponentially growing murine melanoma cells were treated with ara-C (0.1 µg and 1.0 µg/ml) continuously for 72 hours. In another set of experiments, murine melanoma cells were treated with the above mentioned concentrations of ara-C for 24 hours, and later cultures were washed in prewarmed medium, and then incubated for an additional 48 hours in drug-free medium at 37°C. This protocol was followed for ara-C recovery experiments. Control cultures for both the continuous and recovery experiments were treated with distilled water, adenosine, and 2-deoxycytidine (0.1 µg and 1.0 µg/ml). To investigate the kinetics of telomeric erosion in relation to the subdiploid cell population, K1735 clone X-21 cells were treated continuously with ara-C (1.0 µg/ml) for 24, 48 or 72 hours. At each time point, the cell cultures (ara-C-treated and control) were harvested for cytologic preparations and for the FACS analyses as described later. To determine the frequency of cells with decreased telomeric signals and with blebbing, K1735 clone X-21 cells were treated with ara-C (0.1 µg to 5.0µg/ml) continuously for 24, 48 and 72 hours.
Pretreatment with Caspase Inhibitors
The X-21 cells were pretreated with 10 µM BACMK [bocaspartyl (benzyl) chloromethyl ketone] or 25 µM zVADfmk [benzyloxycarbonyl-val-ala-Asp(OMe)-fluoromethyl ketone] purchased from Enzyme Systems Products (Livermore, CA). After 30 minutes cells were treated with ara-C (1.0 µg/ml for 72 hours) as described previously.
Cytologic Preparations
The control and treated cells were exposed to Colcemid (0.04 µg/ml) for 25 minutes at 37°C and to hypotonic treatment (0.075 M KCl) for 20 minutes at room temperature. Cells were fixed in a methanol and acetic acid (3:1 by volume) mixture for 15 minutes, and washed three times in the fixative. The slides were air-dried and coded for the blind analysis [22]. Later the slides were decoded for the evaluation of results.
Several parameters of the mitotic catastrophe were evaluated including the frequency of mitotic index, frequencies of endoreduplication, tetraploidy, and chromosome aberrations (as evidenced by both chromosome- and chromatid-type breaks), as well as telomeric associations (TAs). Dead cells were also identified at the time of harvesting by the trypan blue exclusion test.
Fluorescence In Situ Hybridization (FISH) with Telomeric DNA Probe and its Quantitation
For FISH analysis, cytologic preparations of both experimental and control cells were hybridized with an all-human telomeric DNA probe according to the manufacturer's instructions (Oncor, Inc., Gaithersburg, MD) with slight modifications [23,24]. All coded FISH preparations were examined under a Nikon photomicroscope. A minimum of 200 interphase nuclei from each set of experiments were examined for determining the telomeric DNA signals and the frequency of cells with blebbing, which were photographed using a triple-band pass filter (Omega Optical, Inc., Brattleboro, VT). Metaphase cells were not scored because of severe rearrangements in some cell preparations.
The percentage of telomeric area in the interphase nuclei of FISH preparations was quantified using a software of Metaview Imaging System version 3.6a (Universal Imaging Co., Westchester, PA). From each sample, at least 50 interphase nuclei were quantified for determining the mean as well as median percentages of telomeric areas compared to the total nuclear areas. We routinely use this protocol for quantitation of the telomeric DNA of the interphase nuclei of FISH preparations [25].
Determination of Subdiploid Population by the FACS Analysis
Control and ara-C-treated cell cultures of X-21 and bcl-2-transfected X-21 were washed in 1 ml of cold PBS. Approximately 1x106 cells from each set were resuspended in 0.5 ml propidium iodide (PI) solution (50 µg/ml PI, 0.1% Triton X-100, and 0.1% sodium citrate in PBS). Cells were incubated at 4°C for 2 hours in the dark in the PI solution and then the fluorescence was read on Coulter Epics (R) XL (Beckman-Coulter, Brea, CA). The percentage of subdiploid cell populations was calculated using the multigraph program. The X-21 cells treated with caspase inhibitors and ara-C were also processed for the FACS analysis as described above.
Treatment with Other Pro-apoptotic Inducers
Murine melanoma K1735 clone X-21 cells were treated with 1 µM staurosporine for 24 hours. The human prostate cancer cell line PC-3M was treated separately with anti-Fas antibody CH-11 (0.1 µg/ml) (Kamiya Biomedical Co., Tukwila, WA) and tumor necrosis factor α (TNFα; 10 µg/ml) along with 10 µM cyclohexamide for 24 hours to induce apoptosis. Another clone, C-23, of the murine melanoma cell line K1735 was treated with 1 µM thapsigargin for 24 hours. After treatment, these cells were dislodged from the culture flasks, washed in the cold PBS and resuspended in 0.5 ml of PI solution. Later, the cells were incubated at 4°C in the dark for 2 hours, and then read for the percentage of subdiploid cell population. One set of the treated and control cell samples was processed for FACS analysis. The second set of cells was treated with a hypotonic solution (0.075 M KCl) for 20 minutes at room temperature, and then fixed in methanol and acetic acid (3:1 by volume) mixture. After three washes in the fixative, air-dried slides were made and processed for FISH preparations as described for the analysis of telomere signals.
Western Blot Analysis for TRF1 and TRF2
Control and ara-C-treated cells of two human prostate cancer lines, PC-3M and LNCaP C4-2, and the murine melanoma K1735 clone X-21 were processed for determining the levels of TRF1 and TRF2 proteins by Western blot analysis following the procedure described elsewhere [26]. Proteins of the whole cell extracts were resolved by SDS-PAGE, electroblotted, and then probed with TRF1 (C-19) or TRF2 (N-20) polyclonal antibodies (Santa Cruz Biotech., Santa Cruz, CA). After desired incubation with a secondary antibody, expected signals of TRF1 and TRF2 were detected by ECL reagent (Amersham Pharmacia Biotech, Inc., Piscataway, NJ).
Results and Discussion
To test the hypothesis that telomere erosion is linked to apoptosis, we treated a metastatic murine melanoma cell line, K1735 clone X-21, a nonmetastatic K1735 clone C-23 and a human prostate cancer cell line, PC-3M, with a variety of pro-apoptotic stimuli, and later assayed the cells for telomere erosion. We tested staurosporine, thapsigargin, anti-Fas-antibody (CH-11), TNFα, and cancer chemotherapeutic agents, such as ara-C, which act by diverse, independent biochemical mechanisms. Cells were given either continuous treatment with the drug for 72 hours, or they were pulse-treated for 24 hours and then incubated in normal medium for an additional 48 hours (recovery experiments). Telomere integrity was analyzed by conventional cytogenetics in metaphase spreads and by FISH analysis of interphase nuclei with a telomere-specific probe. No metaphases were present in cultures of the parental X-21 cell line treated continuously for 72 hours with 1.0 µg/ml of ara-C. In recovery experiments with X-21 cells, only 37% of metaphases were normal (Figure 1A), and 58% of spreads showed endoreduplication with moderate to severe chromosomal fragmentations (Figure 1B). Five percent of metaphases were tetraploid with severe chromosomal abnormalities (Figure 1C). These abnormalities included simple chromatid and isochromatid breaks, TAs, and ring configurations. In some metaphases, the chromosomal abnormalities and fragmentations were extensive and beyond quantitation. In control experiments, the chromosome morphology of 100% of metaphases was characteristic of X-21 cells, with no incidence of endoreduplication, TA, and tetraploidy. Telomere loss of the kind illustrated in Figure 2 was the apparent chromatin-associated alteration detected on FISH analysis in the treated human PC-3M cells (except ara-C), and murine melanoma clones X-21 and C-23 cells, regardless of the pro-apoptotic stimulus employed. In Table 1 is listed the percentage of nuclei showing decreased telomeric signals and blebbing in K1735 clone X-21 cells treated with different doses of ara-C (0.1 to 5.0 µg/ml) for 24, 48 and 72 hours. The treated cells showed loss of telomeric DNA as early as 24 hours. Maximum frequency of cells showing blebbing with telomeric DNA was observed after 72 hours of treatment in a dose-dependent manner. Kinetic analyses performed on X-21 cells demonstrated that telomere loss was associated with the arrest of cells in the G2/M phase of the cell cycle. Furthermore, the frequency of cells demonstrating the loss of telomeric DNA was higher than the frequency of subdiploid cells (Figure 3), indicating telomere loss as an early event. Interestingly, probe-reactive material (the bulk of the telomeric segments), emerging from the nucleus appears to be nucleoplasmic vacuoles (Figure 4) that were ultimately extruded into the culture medium (data not shown).
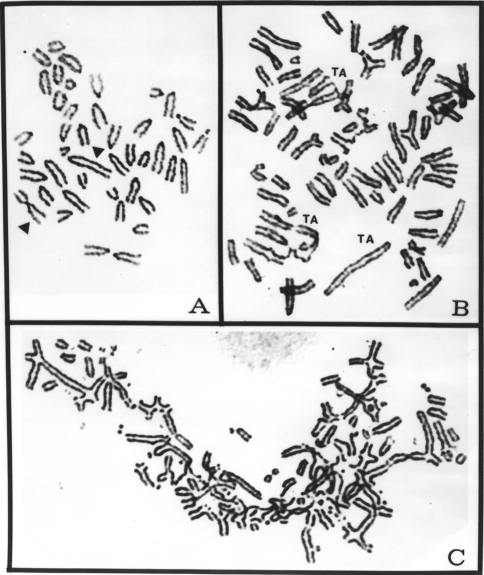
Figure 1.
Conventionally Giemsa-stained metaphases of the K1735 clone X-21 showing characteristic chromosome morphology with two markers (arrowheads) from an untreated control (A), an endoreduplicated metaphase spread with telomeric association (TA) from an ara-C-treated X-21 culture (B), and a severely damaged metaphase spread of X-21 treated for 24 hours with ara-C and then recovered for 48 hours in a drug-free culture medium. Note the presence of extensive chromosomal fragmentation and TAs.
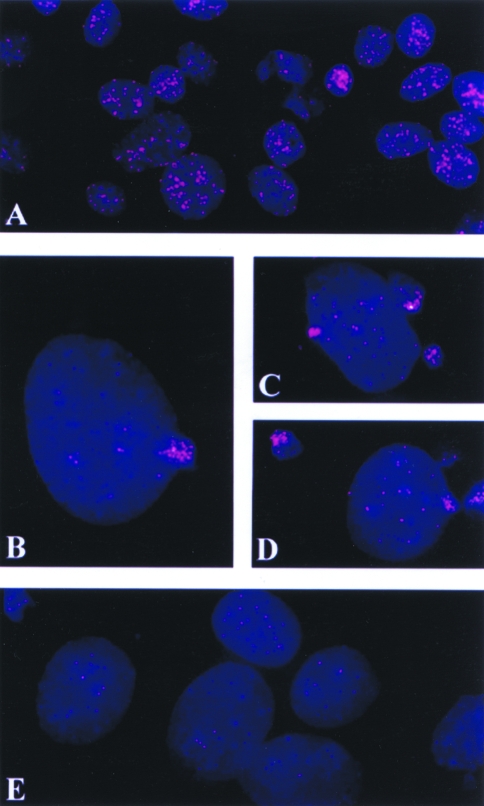
Figure 2.
FISH preparations of human prostate cancer cell line PC-3M and murine melanoma cell lines K1735 clone X-21 and clone 23 showing localization of telomeric DNA signals in interphase nuclei. PC-3M control showing normal telomeric signals (A), PC-3M cells treated with anti-Fas antibody (B) and PC-3M cells treated with TNFα (C), both for 24 hours and both showing reduced telomeric signals. K1735 clone X-21 untreated control cells showing normal amount of telomeric signals (D), and X-21 cells treated with staurosporine for 24 hours with reduced telomeric signals (E). K1735 clone-23 control interphase nuclei showing normal amount of telomeric signals (F), and thapsigargin treated (24 hours) clone-23 cells showing drastically reduced telomeric signals (G).
Table 1.
Percent Cells Showing Decreased Telomeric Signals and Frequency of Blebbing in K1735 Clone X-21 Cells Treated with ara-C.
| Dose (µg/ml) | Duration of Treatment (Hours) | ||
| 24 | 48 | 72 | |
| 0.1 | 28.3±6.5a* | 37.5±7.2a* | 51.4±10.5a* |
| 1.2±0.7b | 5.6±1.5b* | 17.7±4.0b* | |
| 0.5 | 30.5±6.0a** | 39.6±8.6a* | 48.6±9.3a* |
| 0.3±0.5b | 5.3±1.5b* | 20.6±2.5b** | |
| 1.0 | 35.4±6.2a** | 47.4±12.1a* | 57.3±7.3a** |
| 2.0±1.0b | 8.0±2.6b* | 39.0±7.0b** | |
| 2.0 | 41.3±4.5a** | 49.8±6.7a** | 53.8±8.1a** |
| 4.3±2.1b | 7.7±2.0b* | 56.7±5.6b* | |
| 5.0 | 43.7±7.0a** | 44.0±7.3a** | 62.4±4.7a** |
| 3.3±1.1b* | 18.6±3.5b** | 58.5±12.0b* | |
| Control | 4.4±0.7a | 4.4±0.7a | 4.4±0.7a |
| 0.0b | 0.0b | 0.0b | |
Percent cells showing reduced telomeric signals.
Percent cells showing blebbing of telomeric signals.
p<0.01;
p<0.001.
Figure 3.
Kinetic studies of K1735 clone X-21 cells treated with ara-C (1.0 µg/ml) for various time periods and then processed for FACS analyses of the subdiploid cell populations and measurements of the telomeric signal areas in pixels. The frequency of cells losing telomeres is more than the frequency of subdiploid cells.
Figure 4.
FISH preparations of interphase cells of the K1735 clone X-21 cells, treated and untreated with ara-C. Cells are stained with DAPI for DNA (blue), and the telomeric DNA labeled with rhodamine (red). Untreated control cells showing a large quantity of telomeric DNA present (A). Telomeric DNA in bundles being extruded from nuclei of X-21 cells treated with ara-C for 24 and 48 hours (B, C, and D). Large nuclei from ara-C-treated cells showing reduced telomeric signals (E). All microphotographs are made at the same magnification.
Members of the bcl-2 family regulate apoptosis in an evolutionary conserved fashion [27]. Although bcl-2 is expressed in mitochondria, a large fraction of the protein is also localized on the nuclear envelope, where it may regulate apoptosis-associated nuclear events [28–30]. Therefore, we compared the effects of pro-apoptotic stimuli on telomere fragmentation of the parental and bcl-2-transfected K1735 clone X-21 cells. Experiments confirmed that the bcl-2-transfected K1735 clone X-21 cells did not exhibit significantly higher TA frequency or tetraploidy with aberrations compared to the untreated parental cells (data not shown). Parental and bcl-2-transfected K1735 clone X-21 cells were treated with ara-C and analyzed by FISH. As shown in Figure 5A, the parental K1735 clone X-21 cells were more sensitive to ara-C-induced apoptosis than were the bcl-2-transfected K1735 clone X-21 cells. Consistent with these results, telomere fragmentation was completely suppressed in the bcl-2-transfected cells (Figure 5B). From these results we conclude that bcl-2 functions upstream of the factors that regulate telomere erosion of the murine K1735 clone X-21 cells.
Figure 5.
FACS analyses of the parental clone X-21 and bcl-2-transfected X-21 cells showing percentage of cells that were subdiploid in parental untreated control, ara-C-treated (1.0 µg/ml for 72 hours), bcl-2-transfected X-21 as control, bcl-2-transfected cells treated with ara-C (1.0 µg/ml for 72 hours), parental clone X-21 cells pretreated with BACMK and zVAD (two caspase inhibitors), for 30 minutes and then exposed to ara-C (1.0 µg/ml) for 72 hours. Note the presence of approximately 27% of cells showing apoptosis in X-21 cells treated with ara-C. However, there was no significant increase in the frequency of apoptotic cells in bcl-2-transfected cells treated with ara-C as compared to the control. Caspase inhibitor treatment before ara-C exposure protected X-21 cells by 12% to 13% (A). Telomeric area measurements in percentages of the parental X-21 cells treated with ara-C (1.0 µg/ml for 72 hours), bcl-2-transfected X-21 cells (control), bcl-2-transfected X-21 cells-treated with ara-C (1.0 µg/ml for 72 hours), BACMK- and zVAD-pretreated X-21 cells exposed to ara-C (1.0 µg/ml for 72 hours). The percent telomeric area in interphase nuclei of FISH preparations was quantitated as compared to the total nuclear areas and expressed as the mean value (B). These values are just the reverse of values shown in (A).
At the core of the apoptotic effector machinery are the caspases, a family of aspartate-specific cysteine proteases that cleave cytoplasmic and nuclear substrates [31]. Caspase activation is controlled in an evolutionary conserved fashion by members of the bcl-2 family [27]. A typical oligonucleosomal DNA fragmentation (“DNA laddering”) observed in apoptotic cells appears to be dependent on caspase activation in most (if not all) examples of apoptosis investigated to date, and endonuclease activation is probably initiated by caspase-mediated activation of DNA fragmentation factor (DFF) [32]. To directly determine the role of caspases in apoptosis-associated telomere fragmentation, we treated clone X-21 cells with pro-apoptotic stimuli in the absence or presence of two peptide-based, active site caspase antagonists (boc-Dcmk BACMK and zVADfmk), and analyzed telomere integrity and DNA fragmentation. The inhibitors partially prevented both events (Figure 5). These results are consistent with the data obtained with the bcl-2-transfected cells and indicate that telomere fragmentation is triggered (directly or indirectly) by caspase activation.
It is widely suspected that the function of DNA fragmentation in apoptosis is to eliminate host genetic material, which in many cases may contain viral or mutant genes [33]. In addition, inactivation of the genome is incompatible with host cell viability and may ensure cell suicide. Previous work has linked telomere erosion to replicative senescence, a form of differentiation that in a functional sense is equivalent to programmed cell death. Here we show that telomere cleavage is the earliest chromosomal alteration detected in apoptotic cells, thereby establishing a direct link between the two events. Importantly, we have observed (unpublished data) apoptosis-associated telomere erosion in cell lines (e.g., the human breast adenocarcinoma line MCF-7) that apparently do not exhibit typical chromatin fragmentation [34]. Telomere inactivation may, therefore, be the first line of defense to prevent inappropriate replication of dangerous host DNA sequences.
Although our results demonstrate that caspase activation is required for telomere erosion, precisely how caspases promote the response is not clear. It is possible that the same molecular mechanism mediates both telomere erosion and the more typical DNA “laddering,” which is associated with apoptosis. Recent work has identified a caspase substrate, termed DNA fragmentation factor-45 (DFF-45) or inhibitor of caspase-activated DNAse (ICAD), which acts as an inhibitor of an endogenous endonuclease in resting cells. On induction of apoptosis, DFF-45/ICAD is cleaved and inactivated, releasing the endonuclease and allowing it to trigger DNA fragmentation. It is, therefore, possible that this mechanism first leads to telomere erosion before it promotes large-scale chromatin cleavage.
Alternatively, other work has shown that several caspase substrates are nuclear proteins involved in DNA repair [35], and two of these proteins (DNA-PK and PARP) have also been implicated in telomere maintenance. Recently, Karlseder et al. [36] have shown that the lack of TRF2 protein in telomeres may result in the loss of the G-strand overhangs from telomere termini and may be responsible for fusion of chromosome ends or TAs. The TRF1 and TRF2 proteins play critical roles in the maintenance of telomere length [36–41]. TRF1 is a negative regulator of telomerase activity, thus the loss of TRF1 may enable telomerase to extend telomeric ends [41]. The main function of TRF2 is to bind and protect the 3′ telomeric end by folding back the single-stranded 5′-TTAGGG-3′ overhang into upstream telomeric duplex DNA forming a displacement loop (D-loop) with complimentary 3′-AATCCC-5′ sequences, hence protecting telomere ends from degradation and chromosome endoreduplication [36,42]. Furthermore, the loss of TRF2 may create G overhangs at the end of telomeres, which may produce DNA damage signals, hence stimulating cell cycle arrest and/or apoptosis [36]. Endoreduplication of chromosomes has been a consistent characteristic feature of apoptotic cells [12,13,43]. In the present studies, our results show telomere loss, cell cycle arrest in G2/M phase and extensive endoreduplication of chromosomes (a pro-apoptotic stage of cell death of K1735 clone X-21 cells after treatment with ara-C).
If the level of TRF2 is associated with telomere loss, endoreduplication, and cell death, then K1735 clone X-21 cells treated with ara-C could be expected to show decreased levels of TRF2. Thus, to establish a correlation between TRF2 levels, the loss of telomere length and endoreduplication of chromosomes, we treated K1735 clone X-21 cells with different concentrations of ara-C. For comparison, we used two human prostate cancer cell lines, PC-3M and C4-2. The PC-3M cells showed neither loss of telomere length nor endoreduplication of chromosomes, and were resistant to apoptosis after treatment with ara-C. The C4-2 cells exhibited characteristics similar to K1735 clone X-21 cells (data not shown). The level of TRF1 in the K1735 clone X-21 cells was drastically reduced (Figure 6); however, the level of TRF1 in PC-3M and C4-2 cells was unchanged after treatment with ara-C (Figure 6). The ara-C-induced level of TRF2 in PC-3M cells was unchanged, but it was significantly decreased in the C4-2 and the X-21 cells (Figure 6). The resistance to apoptosis, telomere erosion and TRF2 loss in the PC-3M cells could be attributed to the presence of mutated p53 in this cell line (our unpublished data). These results thus establish a correlation of TRF2 levels with telomere shortening, endoreduplication of chromosomes, and cell death of cancer cell lines after treatment with ara-C.
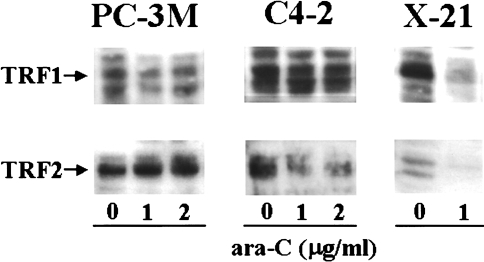
Figure 6.
Protein levels of TRF1 and TRF2 of prostate cancer cell lines PC-3M and C4-2 and mouse melanoma cell line K1735 clone X-21 after treatment with different concentrations of ara-C for 24 hours. Cellular lysates were processed for determining the levels of TRF1 and TRF2 by Western blotting. Photographs of the autoradiograms are representative of two independent experiments.
The mechanism by which the reduced levels of TRF2 in K1735 clone X-21 cells after treatment with ara-C may induce telomere shortening can be understood by its role in the maintenance of the D-loop structure of the telomere ends [36–41]. The loss of TRF2 may induce distortion of the D-loop conformation of the telomere, releasing the 3′ overhang of the single-stranded DNA. The exposed 3′ overhang DNA may become more susceptible for degradation and then shortening. The loss of TRF2 may also interfere with the formation of telomeric G-quadruplexes at the late S/G2 phase, during which yeast telomeres, at least, acquire long, single-stranded G tails. The late S/G2 phase is the most prominent DNA damage checkpoint in yeast. If the G tails are not taken up in an alternative DNA structure such as G quadruplexes, the chromosome replication is not signaled as complete and the DNA repair process could cleave off the G tails, bundle them up and bleb them out of the nucleus (Figure 4). From our results, it appears that TRF2 is the first target of ara-C treatment, followed by telomere shortening, endoreduplication of choromosomes, and finally the death of K1735 clone X-21 cells. Thus, decreased levels of TRF2 can be the initiating event of ara-C-induced death of some cancer cells. However, further studies are required to define the exact biochemical mechanisms involved in the process of drug-induced telomere end maintenance, telomere length, endoreduplication of chromosomes, and cell death of cancer cells.
Acknowledgements
We acknowledge Jessica Dolhonde and Karen Ramirez for expert technical assistance and Cynthia Furlong and Walter J. Pagel for their editorial comments.
Abbreviations
- TA
telomeric association
- TNF
tumor necrosis factor
- ara-C
1-β-D-arabinofuranosylcytosine
- FISH
fluorescence in situ hybridization
- DFF
DNA fragmentation factor
- FACS
fluorescence-activated cell-sorting
- TRF
telomeric-repeat binding factor
- ALT
alternative lengthening of telomeres
Footnotes
This work was supported in part by the Summerfield G. Robert Foundation of Dallas, Texas, the Institutional Prostate Cancer Research Program, NIH grant RRO 499901 (to S.P.) and CA 77721 (to S.N.), and a D.B. Lane Cancer Professorship awarded (to R.A.N.).
References
- 1.Moyzis RK, Buckingham JM, Cram LS, Dani M, Deaven LL, Jones MD, Meyne J, Ratliff RL, Wu JR. A highly conserved repetitive DNA sequence, (TTAGGG)n, present at the telomeres of human chromosomes. Proc Natl Acad Sci USA. 1988;85:6622–6626. doi: 10.1073/pnas.85.18.6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meyne J, Ratliff RL, Moyzis RK. Conservation of the human telomere sequence (TTAGGG)n among vertebrates. Proc Natl Acad Sci USA. 1989;86:7049–7053. doi: 10.1073/pnas.86.18.7049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blackburn EH. Structure and function of telomere. Nature. 1991;350:569–573. doi: 10.1038/350569a0. [DOI] [PubMed] [Google Scholar]
- 4.Greider CW. Mammalian telomere dynamics: healing, fragmentation, shortening and stabilization. Curr Opin Genet Dev. 1994;4:203–211. doi: 10.1016/s0959-437x(05)80046-2. [DOI] [PubMed] [Google Scholar]
- 5.Greider CW. Telomere length regulation. Annu Rev Biochem. 1996;65:337–365. doi: 10.1146/annurev.bi.65.070196.002005. [DOI] [PubMed] [Google Scholar]
- 6.Zakian VA. Telomeres: beginning to understand the end. Science. 1995;270:1601–1607. doi: 10.1126/science.270.5242.1601. [DOI] [PubMed] [Google Scholar]
- 7.Cook HJ, Smith BA. Variability eat the telomeres of the human X/Y pseudoautosomal region. Cold Spring Harbor Symp Quant Biol. 1986;L1:213–219. doi: 10.1101/sqb.1986.051.01.026. [DOI] [PubMed] [Google Scholar]
- 8.Allsopp RC, Vaziri H, Patterson C, Goldstein S, Younglai EV, Futcher AB, Greider CW, Harley CB. Telomere length predicts replicative capacity of human fibroblasts. Proc Natl Acad Sci USA. 1992;89:10114–10118. doi: 10.1073/pnas.89.21.10114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harley CB, Futcher AB, Greider CW. Telomeres shorten during aging of human fibroblasts. Nature. 1990;245:458–460. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- 10.Levy MZ, Allsopp RC, Futcher AB, Greider CW, Harley CB. Telomere end-replication problem and cell aging. J Mol Biol. 1992;225:951–960. doi: 10.1016/0022-2836(92)90096-3. [DOI] [PubMed] [Google Scholar]
- 11.Vaziri H, Dragowska W, Allsopp RC, Thomas TE, Harley CB, Lansdorp PM. Evidence for a mitotic clock in human hematopoietic stem cells: loss of telomeric DNA with age. Proc Natl Acad Sci USA. 1994;91:9857–9860. doi: 10.1073/pnas.91.21.9857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pathak S, Risin S, Brown NM, Berry K. Telomeric association of chromosomes is an early manifestation of programmed cell death. Int J Oncol. 1994;4:323–328. doi: 10.3892/ijo.4.2.323. [DOI] [PubMed] [Google Scholar]
- 13.Pathak S, Dave BJ, Gagos SH. Chromosome alterations in cancer development and apoptosis. In Vivo. 1994;8:843–850. [PubMed] [Google Scholar]
- 14.Counter CM, Hirte HW, Bacchetti S, Harley CB. Telomerase activity in human ovarian carcinoma. Proc Natl Acad Sci USA. 1994;91:2900–2904. doi: 10.1073/pnas.91.8.2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Lang T. Activation of telomerase in a human tumor. Proc Natl Acad Sci USA. 1994;91:2882–2885. doi: 10.1073/pnas.91.8.2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shay JW, Tomlinson G, Piatyszek MA, Gollahon LS. Spontaneous in vitro immortalization of breast epithelial cells from a patient with Li-Fraumeni syndrome. Mol Cell Biol. 1995;15:425–432. doi: 10.1128/mcb.15.1.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bryan TM, Englezou A, Dalla-Pozza L. Evidence for an alternative mechanism for maintaining telomere length in human tumors and tumor-derived cell lines. Nat Med. 1997;3:1271–1274. doi: 10.1038/nm1197-1271. [DOI] [PubMed] [Google Scholar]
- 18.Rhyu MS. Telomeres, telomerase, and immortality. J Natl Cancer Inst. 1995;87:884–894. doi: 10.1093/jnci/87.12.884. [DOI] [PubMed] [Google Scholar]
- 19.Zakian VA. Life and cancer without telomerase. Cell. 1997;91:1–3. doi: 10.1016/s0092-8674(01)80001-5. [DOI] [PubMed] [Google Scholar]
- 20.Multani AS, Furlong C, Pathak S. Reduction of telomeric signals in murine melanoma and human breast cancer cell lines treated with 3′-azido-3′-deoxythymidine (AZT) Int J Oncol. 1998;13:923–925. doi: 10.3892/ijo.13.5.923. [DOI] [PubMed] [Google Scholar]
- 21.Ishibashi T, Lippard SJ. Telomere loss in cells treated with cisplatin. Proc Natl Acad Sci USA. 1998;95:4219–4223. doi: 10.1073/pnas.95.8.4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pathak S. Chromosome banding techniques. J Reprod Med. 1976;17:25–28. [PubMed] [Google Scholar]
- 23.Multani AS, Hopwood VL, Pathak S. A modified fluorescence in situ hybridization (FISH) technique. Anticancer Res. 1996;16:3435–3438. [PubMed] [Google Scholar]
- 24.Ozen M, Imam SA, Datar RH, Multani AS, Narayanan R, Chung LWK, von Eschenbach AC, Pathak S. Telomeric DNA: marker for human prostate cancer development. The Prostate. 1998;36:264–271. doi: 10.1002/(sici)1097-0045(19980901)36:4<264::aid-pros8>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 25.Multani AS, Ozen M, Sen S, Mandal AK, Price JE, Fan D, Radinsky R, Ali-Osman F, von Eschenbach AC, Fidler IJ, Pathak S. Amplification of telomeric DNA directly correlates with metastatic potential of human and murine cancers of various histological origin. Int J Oncol. 1999;15:423–429. doi: 10.3892/ijo.15.3.423. [DOI] [PubMed] [Google Scholar]
- 26.Narayan S, Jaiswal AS. Activation of adenomatous polyposis coli (APC) gene expression by the DNA-alkylating agent N-methyl-N′-nitro-N-nitrosoguanidine requires p53. J Biol Chem. 1997;272:30619–30622. doi: 10.1074/jbc.272.49.30619. [DOI] [PubMed] [Google Scholar]
- 27.Adams JM, Cory SA. The BCL-2 protein family: arbiters of cell survival. Science. 1998;281:1322–1325. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- 28.Reed JC. Bcl-2 and the regulation of programmed cell death. J Cell Biol. 1994;124:1–6. doi: 10.1083/jcb.124.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fernandez A, Fosdick LJ, Martin MC, Diaz C, McDonnell TJ, Ananthaswamy HN, McConkey DJ. Differential regulation of endogenous endonuclease activation in isolated murine fibroblast nuclei by ras and bcl-2. Oncogene. 1995;10:769–774. [PubMed] [Google Scholar]
- 30.Marin MC, Fernandez A, Bick RJ, Brisbay S, Buja M, Snuggs M, McConkey DJ, von Eschenbach AC, Keating MJ, McDonnell TJ. Apoptosis suppression by Bcl-2 is correlated with the regulation of nuclear and cytosolic Ca2+ Oncogene. 1996;12:2259–2266. [PubMed] [Google Scholar]
- 31.Thornberry NA, Lazebnik Y. Caspases: enemies within. Science. 1998;281:1312–1316. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- 32.Liu X, Zou H, Slaughter C, Wang X. DFF, a heterodimeric protein that functions downstream of caspase-3 to trigger DNA fragmentation during apoptosis. Cell. 1997;89:175–184. doi: 10.1016/s0092-8674(00)80197-x. [DOI] [PubMed] [Google Scholar]
- 33.Martz E, Howell DM. CTL: virus control cells first and cytolytic cells second? Immunol Today. 1989;10:79–86. doi: 10.1016/0167-5699(89)90231-4. [DOI] [PubMed] [Google Scholar]
- 34.Oberhammer F, Wilson JW, Dive C, Morris ID, Hickman JA, Wakeling AE, Walker PR, Sikorska M. Apoptotic death in epithelial cells: cleavage of DNA to 300 and/or 50 kb fragments prior to or in the absence of internucleosomal fragmentation. EMBO J. 1993;12:3679–3684. doi: 10.1002/j.1460-2075.1993.tb06042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Casciola-Rosen L, Nicholson DW, Chong T, Rowan KR, Thornberry NA, Miller DK, Rosen A. Apopain/CPP32 cleaves proteins that are essential for cellular repair: a fundamental principal of apoptotic death. J Exp Med. 1996;183:1957–1964. doi: 10.1084/jem.183.5.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karlseder J, Broccoli D, Dai Y, Hardy S, de Lange T. p53-and ATM-dependent apoptosis induced by telomeres lacking TRF2. Science. 1999;283:1321–1325. doi: 10.1126/science.283.5406.1321. [DOI] [PubMed] [Google Scholar]
- 37.Chong L, van Steensel B, Broccoli D, Erdjument-Bromage H, Hanish J, Tempst P, de Lange T. A human telomeric protein. Science. 1995;270:1663–1667. doi: 10.1126/science.270.5242.1663. [DOI] [PubMed] [Google Scholar]
- 38.Bilaud T, Brun C, Ancelin K, Koering CE, Laroche T, Gilson E. Telomeric localization of TRF2, a novel human telobox protein. Nat Genet. 1997;17:236–239. doi: 10.1038/ng1097-236. [DOI] [PubMed] [Google Scholar]
- 39.Broccoli D, Smogorzewska A, Chong L, de Lange T. Human telomeres contain two distinct Myb-related proteins, TRF1 and TRF2. Nat Genet. 1997;17:231–235. doi: 10.1038/ng1097-231. [DOI] [PubMed] [Google Scholar]
- 40.Griffith JD, Comeau L, Rosenfield S, Stansel RM, Bianchi A, Moss Heidi, de Lange T. Mammalian telomeres end in a large duplex loop. Cell. 1999;97:503–514. doi: 10.1016/s0092-8674(00)80760-6. [DOI] [PubMed] [Google Scholar]
- 41.van Steensel B, de Lange T. Control of telomere length by the human telomeric protein TRF1. Nature. 1997;385:740–743. doi: 10.1038/385740a0. [DOI] [PubMed] [Google Scholar]
- 42.Greider CW. Telomeres do D-loop-T-loop. Cell. 1999;97:419–422. doi: 10.1016/s0092-8674(00)80750-3. [DOI] [PubMed] [Google Scholar]
- 43.Pathak S, Multani AS, Amoss MS. Telomere, telomerase and malignant melanomas in human and domestic animals. Arch Zootec. 1996;45:141–149. [Google Scholar]