Abstract
We examined the tumorigenic and metastatic potentials of three human non-small cell lung cancer (NSCLC) cell lines. PC-14, A549 or Lu-99 cell lines suspended in Matrigel-containing phosphate-buffered saline were orthotopically implanted into the lungs of nude mice. The formation of a solitary tumor nodule in the lung was observed after the implantation of all cell lines. Intrapulmonary implantation of PC-14 or Lu-99 cells resulted in spontaneous distant metastases. In contrast, A549 cells caused multiple intrapulmonary metastases to the right and left lobes of the lung without producing visible lymphatic metastasis. We also investigated the expression of matrix metalloproteinases (MMPs), urokinase-type plasminogen activator (u-PA), u-PA receptor (u-PAR) and c-MET in these cell lines in vitro and in vivo. Reverse transcription polymerase chain reaction (RT-PCR) analysis showed that the expression of MMP-2 and membrane-type 1 MMP (MT1-MMP) was elevated in PC-14 as compared with the other two cell lines. In contrast, stronger expression of c-MET was observed in A549 than in PC-14 or Lu-99. These results indicate that differential patterns of metastasis of lung cancer might be associated with differential expression of metastasis-associated molecules. Our orthotopic implantation models display clinical features resembling those of NSCLC, and may provide a useful basis for lung cancer research.
Keywords: orthotopic tumor growth; animal model; distant metastasis, matrix metalloproteinases; c-MET
Introduction
Despite the advances in diagnostic techniques for the early detection of lung cancer and the significant improvement in surgical procedures, the prognosis of patients with metastasizing lung cancer is generally poor, even in the early stages of cancer, as compared with other malignant neoplasms [1]. Lymphatic metastasis is one of the most critical factors for the prognosis of lung cancer patients [2]. According to the new TNM revision [3], the 5-year survival rate of patients with N2 lung cancer is only about 20%, even if the patients undergo curative resection [4]. Therefore, more potent therapeutic approaches are needed to improve the prognosis of these patients.
Lung cancer is basically classified into two major groups, namely, non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC), which have different biological features. Currently, NSCLC is the major group, accounting for about three quarters of all lung cancer histologies. The development of suitable animal models for NSCLC diseases that display clinical features of lung cancer is necessary for analysis of the mechanism of metastatic modalities or of therapeutical approaches. Although several investigators have reported experimental models of human lung cancer, these models have included some drawbacks, such as ectopic implantation and complicated procedures [5–7]. Recently, we established a useful model for the formation of a solitary pulmonary nodule and spontaneous lymph node metastasis of murine Lewis lung carcinoma [8].
In addition, abundant clinical and experimental findings indicate that matrix-degrading enzymes such as matrix metalloproteinases (MMPs) or urokinase-type plasminogen activator (u-PA)/urokinase-type plasminogen activator receptor (u-PAR) systems play an important role in tumor invasion and metastasis [9,10]. Significant correlation between the overexpression of some proteinases and prognosis of lung cancer patients who have metastatic disease has been reported [11,12]. The development and analysis of experimental models of metastatic lung cancer may contribute to a better understanding of the metastatic properties of lung cancer.
In the present study, we investigated the tumorigenic and metastatic potentials of three human NSCLC cell lines using our previously developed orthotopic implantation method, and analyzed the involvement of metastasis-associated molecules.
Materials and Methods
Animals
Six-week-old male KSN mice were purchased from Japan SLC, Inc. (Sizuoka, Japan). The animals were maintained under laminar air flow conditions in the Laboratory for Animal Experiments, Institute of Natural Medicine, Toyama Medical and Pharmaceutical University. This study was conducted in accordance with the standards established by the Guidelines for the Care and Use of Laboratory Animals of Toyama Medical and Pharmaceutical University.
Cell Lines and Cultures
PC-14 (human lung adenocarcinoma) was kindly provided by Dr. N. Saijo (National Cancer Research Institute, Japan). A549 (human lung adenocarcinoma) and Lu-99 (human lung large cell carcinoma) were obtained from the Japanese Cancer Research Resources Bank (Tokyo, Japan). These cell lines were maintained as monolayer cultures in RPMI-1640 supplemented with 10% fetal bovine serum (FBS) and l-glutamine (M.A. Bioproducts, Walkersville, MD, USA) in an atmosphere containing 5% CO2/air at 37°C.
Intrapulmonary Implantation Procedure
Orthotopic implantation of human NSCLC cells into the lung was performed as described before [8]. Briefly, PC-14, Lu-99 and A549 cells (5x107/ml) were suspended in phosphate-buffered saline (calcium- and magnesium-free) containing 1 mg/ml of Matrigel (Collaborative Biomedical Products, MA). The left chests of anesthetized mice were incised and 20-µl aliquots of cell suspension (1x106 cells, 20 µg Matrigel) were injected into the lung parenchyma through the intercostal space (approximately 3 mm depth). The skin incision was closed with Autoclips (Becton Dickinson Co., MD, USA). The whole implantation process was performed within approximately 50 seconds per mouse, and the operative mortality was less than 5%.
Macroscopic Findings of Orthotopic and Metastatic Tumor Formation
In the first set of experiments using PC-14 cells, mice were sacrificed on days 14 (n = 2), 35 (n = 3) and 56 (n = 3) after the implantation, and tumor formation was macroscopically observed. Orthotopic tumor growth was assessed by tumor volume [13], calculated using the following formula: Tumor volume (mm3)=length x (width)2/2. In other experiments, the mice which have been injected with PC-14 (n = 8), A549 (n = 4) or Lu-99 (n = 5) were sacrificed on day 35, 56 or 56, respectively. The formation of tumors at the implantation site and the metastatic features of the three cell lines were investigated. After the macroscopic evaluation, PC-14 tumor sections derived from primary and metastatic tumors were subjected to histopathological analysis.
Histopathological Analysis
The sections of PC-14 prepared from the primary tumor nodule, axillary lymph nodes and lumbar vertebra were fixed with formalin and embedded in paraffin. Three-micrometer tissue sections were prepared and stained with hematoxylin and eosin.
Reverse Transcription Polymerase Chain Reaction (RT-PCR) for MMPs, u-PA, u-PAR and c-MET mRNA Expression
RT-PCR was performed as described previously [14]. Briefly, in vitro, the three lung cancer cell lines were seeded into six-well plates (2x105/well) and incubated for 48 h. In vivo, orthotopic tumors were obtained from the mice on day 35 after the implantation. Total RNAs of the three types of cultured cells and tumors were isolated using Isogen (Nippon Gene, Inc., Tokyo, Japan) and reverse-transcribed to produce complementary DNA using a First Strand cDNA Synthesis Kit (Life Science, Inc., St. Petersburg, FL). PCR was performed by denaturation (94°C for 30 seconds), annealing (appropriate temperatures for 1 minute) and extension (72°C for 1.5 minutes) using a TAKARA Ex Taq PCR Kit (Takara Shuzo Co., Ltd., Kyoto, Japan). The sequences of PCR primers are shown in Table 1. The PCR products were electrophoresed on 1.5% agarose gels and detected by ethidium bromide staining.
Table 1.
The Sequences of PCR Primers.
| Gene | Sense | Anti-sense | Reference |
| MT1-MMP | 5′-CCCTATGCCTACATCCGTGA-3′ | 5′-TCCATCCATCACTTGGTTAT-3′ | [39] |
| MMP-1 | 5′-GAGCAGATGTGGACCAT-3′ | 5′-ACCGGACTTCATCTCTGTCG-3′ | [40] |
| MMP-2 | 5′-CCACGTGACAAGCCCATGGGGCCCC-3′ | 5′-GGAGCCTAGCCAGTCGGATTTGATG-3′ | [41] |
| MMP-3 | 5′-GCAGAAGTTCCTTGGATTGG-3′ | 5′-TATCATCTTGAGACAGGCGG-3′ | [40] |
| MMP-7 | 5′-AACAATTGTCTCTGGACGGC-3′ | 5′-ATGGAGTGGAGGAACAGTGC-3′ | [40] |
| MMP-9 | 5′-AACGCTATGGTTACACTCGG-3′ | 5′-AACTGGATGACGATGTCTGC-3′ | [40] |
| u-PA | 5′-AGAATTCACCACCATCGAGA-3′ | 5′-ATCAGCTTCACAACAGTCAT-3′ | [42] |
| u-PAR | 5′-TTACCTCGAATGCATTTCCT-3′ | 5′-TTGCACAGCCTCTTACCATA-3′ | [43] |
| C-MET | 5′-CAGTGGCATGTCAACATCG-3′ | 5′-AGGATACTGCACTTGTCGGC-3′ | [14] |
| GAPDH | 5′-TGAAGGTCGGAGTCAACGGATTTGGT-3′ | 5′-CATGTGGGCCATGAGGTCCACCAC-3′ | [14] |
Gelatin and Fibrin Zymographies
Gelatin and fibrin zymographies were performed as described previously [15]. Conditioned media of three cell lines were prepared by culturing the cells for 24 h in 0.1% BSA-containing DMEM/F-12. The conditioned media were collected, centrifuged to remove debris, and stored at -20°C until use. The conditioned media were mixed with a non-reducing sample buffer and electrophoresed on gelatin (Iwaki Scitech, Tokyo, Japan; 0.1%)- or plasminogen-rich fibrinogen (Nakalai Tesque Inc., Kyoto, Japan; 0.54 mg/ml)/thrombin (Sigma Chemical Co., St. Louis, MO, USA; 10 NIH U/ml)-containing gels. The electrophoresed gels were washed and incubated with reaction buffer at 37°C for 24 or 48 hours. The gels were then stained with 0.1% Coomassie brilliant blue solution and destained until the lytic bands were clearly visible. Gelatinolytic and fibrinolytic activities were quantified using a Master Scan Gel Analysis System (Scanalytics, Billerica, MA).
Cell Invasion Assay
Tumor cell invasion through reconstituted Matrigel was assayed using previously described methods with some modifications [16]. Transwell chambers (Costar, Cambridge, MA) with polycarbonate filters (Nucleopore, Pleasanton, CA; 8-µm pores) were coated with fibronectin (Iwaki Glass Co. Ltd., Tokyo, Japan; 1 µg/40 µl) and Matrigel (Collaborative Research Inc., Bedford, MA; 5 µg/10 µl) on the lower and upper surface of the filters, respectively. The filters were washed extensively with phosphate-buffered saline. NSCLC cells (1x105/chamber) were added to the upper compartment of the chamber and incubated in the presence or absence of recombinant human hepatocyte growth factor (HGF; Collaborative Biomedical Products) in both the upper and lower compartments. After 6 hours of incubation at 37°C, the filters were fixed and the number of cells that had invaded the lower surface was determined by crystal violet staining followed by measuring the absorbance at 590 nm.
Statistical Analysis
The statistical significance of differences between the groups was determined by applying Student's two-tailed t-test.
Results
Orthotopic Implantation of PC-14 cells in KSN Mice and Histopathological Analysis
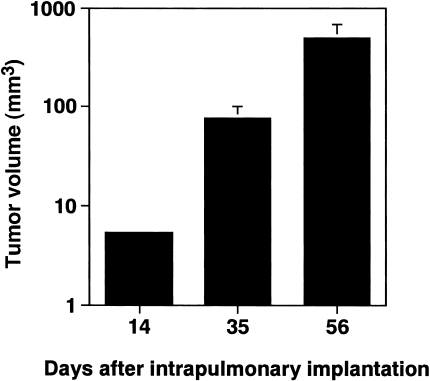
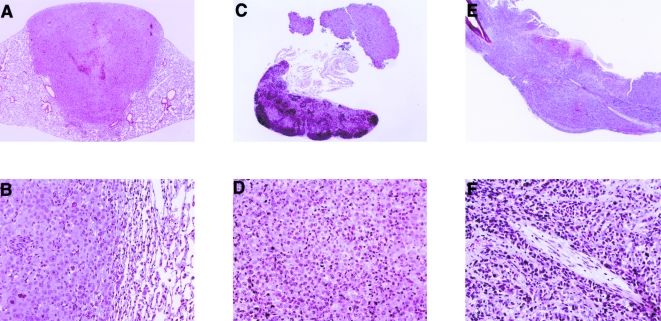
To investigate the characteristics of the present experimental model for lung cancer, PC-14 cells were implanted into the lungs of KSN athymic nude mice according to our previously described method [8]. The mice were dissected on day 14, 35 or 56 after the implantation. Orthotopic implantation of PC-14 cells resulted in the formation of a solitary tumor nodule at the injection site on day 14. The primary tumor volume increased in a time-dependent manner (Figure 1), and was 5.30±0.39, 75.56±23.55 and 497.13±181.91 mm3 on day 14, 35 and 56 after the implantation, respectively. Similar to our previous findings with murine lung cancer [8], multiple mediastinal lymph node metastases were also observed (Figure 2). In addition, swelling at the axillary lymph nodes and lumbar vertebrae was seen in the mice with a primary tumor nodule on day 35 after the implantation (Figure 2). The histopathological study indicated that the swelling of axillary lymph nodes and lumbar vertebrae was caused by the metastasis of PC-14 cells (Figure 3).
Figure 1.
Orthotopic tumor growth of PC-14 cells in the lungs of athymic nude mice. PC-14 cells (1x106 cells/0.02 ml) suspended in 1 mg/ml of Matrigel were implanted into the left lungs of KSN nude mice. The mice were sacrificed on day 14, 35 or 56 after the implantation, and the volume of the primary tumor was determined according to the following formula: Tumor volume (mm3)=lengthx (width)2/2.
Figure 2.
Macroscopic findings of primary tumor and lymphatic metastases of PC-14 cells. Autopsy of the mice was performed on day 35 after orthotopic implantation of PC-14 cells. Arrows indicate the tumor at the inoculation site (P), and metastases to mediastinal lymph nodes (M) and axillary lymph nodes (A).
Figure 3.
Histopathological findings of the pulmonary tumor nodule and metastases. Histopathological sections were prepared from a pulmonary tumor nodule (A, B), axillary lymph nodes (C, D) and lumbar vertebrae (E, F) on day 35 after the intrapulmonary implantation of PC-14 cells and stained with hematoxylin and eosin. (A, C, E) Original magnification, x5. (B, D, F) Original magnification, x50.
Differences of Tumorigenic and Metastatic Properties of PC-14, A549 and Lu-99 Cells After Orthotopic Implantation
We next examined the tumorigenic and metastatic potentials of two other NSCLC cell lines (A549 and Lu-99 cells) in comparison with those of PC-14 cells. The results are summarized in Table 2. The formation of a solitary tumor nodule at the primary site was observed in most animals implanted with tumor cells. PC-14 tumors at the implanted site grew faster than A549 and Lu-99 tumors (Figure 4), and approximately 30% of PC-14-bearing mice were dead by day 56 (data not shown). Therefore, the incidences of tumor formation and metastasis were assessed on days 35, 56 and 56 after the intrapulmonary implantation of PC-14, A549 and Lu-99 cells, respectively. All PC-14-bearing mice produced metastasis to mediastinal and axillary lymph nodes, and some of them showed metastasis to lumbar vertebrae. In the case of Lu-99 tumors, a metastatic pattern similar to that of PC-14 tumors was observed. Interestingly, orthotopic implantation of A549 cells caused intrapulmonary metastasis to the right and left lobes of the lung in all implanted mice (Figure 4), but few lymph node metastases were macroscopically observed.
Table 2.
Primary Tumor Growth and Metastatic Features after Orthotopic Implantation of Human NSCLC Cell Lines in KSN Mice.
| Tumor | Days after implantation | Number of animals | Primary tumor | Tumor volume (mm3, Mean±SD) | Metastasis | |||
| Intrapulmonary | MLN | ALN | Others* | |||||
| PC-14 | 35 | 8 | 7/8 | 70.76±61.93 | - | 7/8 | 7/8 | LV, HLN, CW |
| A549 | 56 | 4 | 4/4 | 56.06±24.84 | 4/4(28.3±16.9)† | - | - | - |
| Lu-99 | 56 | 5 | 4/5 | 49.33±33.40 | - | 4/5 | 3/5 | LV, HLN, CW |
PC-14, Lu-99 and A549 cells were implanted into the lung parenchyma (1x106 cells/0.02 ml with 20 µg of Matrigel). Mice were sacrificed on the indicated days after tumor implantation, and the primary tumor volumes and the incidences of primary tumor and metastasis formation were determined as described in Materials and Methods section.
Abbreviations represent the different metastatic sites: MLN, mediastinal lymph node; ALN, axillary lymph node; LV, lumbar vertebrae; HLN, hilus lymph node of the lung; CW, chest wall.
Numbers in parentheses represent the mean number of tumor nodules on the lung surface±SD.
Figure 4.
Macroscopic observation of primary and metastatic tumor nodules in the lungs of athymic nude mice after orthotopic implantation of PC-14, A549 and Lu-99 cells. PC-14, A549 or Lu-99 cells were implanted into the lungs of KSN mice as described in Figure 1. Autopsy was performed on day 56 after the implantation and the primary (open arrowheads) and metastatic (closed arrowheads) tumor nodules of each cell line had been observed.
The In Vitro and In Vivo Expression Patterns of the mRNAs for MMPs, u-PA, and u-PAR in NSCLC Cells
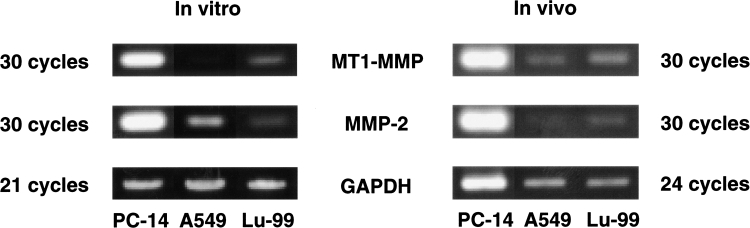
Since MMPs, u-PA and u-PAR are known to play important roles in tumor invasion and metastasis [9,10], the mRNA expression of these molecules in in vitro cultured cells and in vivo tumor tissues of NSCLC cell lines was examined by RT-PCR (Figure 5). Expression of mRNAs for MT1-MMP and MMP-2 in PC-14 cells was strong (Figure 6) and that in Lu-99 cells was moderate. In contrast, A549 cells showed weak expression of mRNAs for MT1-MMP and MMP-2, and strong expression of mRNA for MMP-7. The expression of mRNAs for u-PA and u-PAR was seen in all three cell lines. The expression patterns of these mRNAs in the in vivo tumor tissues was almost the same as those in the in vitro cultured cells.
Figure 5.
The expression patterns of mRNAs for MMPs, u-PA and u-PAR in PC-14, A549 and Lu-99. PCR was performed by using specific primers for the MMPs, u-PA and u-PAR. The mRNA-derived PCR products for MT1-MMP (550 bp), MMP-1 (646 bp), MMP-2 (480 bp), MMP-3 (566 bp), MMP-7 (920 bp), MMP-9 (361 bp), u-PA (474 bp) and u-PAR (455 bp) were amplified for 30, 30, 30, 33, 30, 36, 30 and 30 cycles for in vitro cultured cells, or for 30, 30, 30, 30, 24, 39, 27 and 30 cycles for in vivo tumor tissues, respectively. The PCR products were separated on 1.5% agarose gels. The intensity of the RT-PCR products for each of the MMPs, u-PA and u-PAR was determined by densitometric analysis and normalized by the intensity of the PCR product derived from GAPDH mRNA. PC-14 (closed columns); A549 (hatched columns); Lu-99 (open columns).
Figure 6.
Comparison of MT1-MMP and MMP-2 mRNA expression in PC-14, A549 and Lu-99. RT-PCR products for MT1-MMP (550 bp), MMP-2 (480 bp) and GAPDH (983 bp) were amplified for 30, 30 and 21 cycles for in vitro cultured cells, or for 30, 30, and 24 cycles for in vivo tumor tissues, respectively. The amplified products were separated on 1.5% agarose gels.
Gelatinolytic and Fibrinolytic Activities in the Conditioned Media of NSCLC Cells
Gelatinolytic and fibrinolytic activities in the conditioned media of NSCLC cells were assessed as parameters for the invasiveness of tumor cells. Potent enzymatic activities of MMP-2 (72 kDa) and u-PA (approximately 55 kDa) were detected in the conditioned media of PC-14 cells (Figure 7), consistent with their mRNA expression assessed by RT-PCR. The conditioned media of Lu-99 cells showed gelatinolytic activity of MMP-9 (92 kDa) and moderate fibrinolytic activity of u-PA. Slight gelatinolytic activity of MMP-2 and MMP-9 and moderate fibrinolytic activity of u-PA were detected in the conditioned media of A549 cells. However, no activity of MMP-7 in the conditioned media of A549 cells was detected in our assay system.
Figure 7.
Comparison of the production of MMPs and u-PA from PC-14, A549 and Lu-99 cells. The conditioned media from the cells were subjected to electrophoresis in gelatin- or fibrinogen-containing SDS polyacrylamide gels. After electrophoresis, the gels were incubated and stained with Coomassie brilliant blue. (A) The locations of gelatinolytic (left panel) or fibrinolytic (right panel) enzymes were detected as clear bands. (B) The gelatinolytic and fibrinolytic activities were densitometrically quantified using a Master Scan Gel Analysis System. PC-14 (closed columns); A549 (hatched columns); Lu-99 (open columns).
Expression of mRNA for c-MET and the Invasiveness of NSCLC Cells in Response to HGF
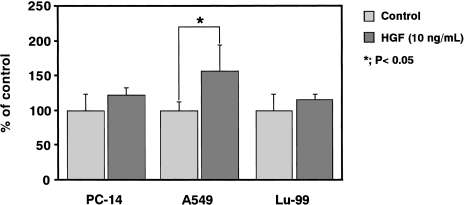
We also examined the expression of c-MET, which is a receptor for HGF and one of the factors responsible for tumor migration and invasion [17]. As shown in Figure 8, marked expression of c-MET mRNA was found in both in vitro cultured A549 cells and in vivo A549 tumor tissues, as compared with PC-14 and Lu-99. Matrigel invasion assays were performed to assess the effect of HGF on the invasiveness of NSCLC cells. Treatment with HGF (10 ng/ml) enhanced the invasion of all the NSCLC cell lines, especially of A549 cells (Figure 9). The increase of invasiveness in PC-14, A549 and Lu-99 cells was 1.2-, 1.6- and 1.2-fold, respectively.
Figure 8.
Expression of c-MET mRNA in PC-14, A549 and Lu-99. RT-PCR products for c-MET (722 bp) were amplified for 30 cycles and separated on 1.5% agarose gels. (A) Detection of PCR product for c-MET mRNA in in vitro cultured cells (left panel) or in vivo tumor tissues (right panel). (B) The intensity of c-MET expression in the cell lines was compared as described in Figure 5. PC-14 (closed columns); A549 (hatched columns); Lu-99 (open columns).
Figure 9.
Effect of HGF on the invasive ability of PC-14, A549 and Lu-99 cells. PC-14, A549 or Lu-99 cells (1x105/chamber) were seeded onto filters precoated with 5 µg of Matrigel on the upper surface and 1 µg of fibronectin on the lower surface in Transwell chambers in the presence or absence of HGF (10 ng/ml). After 6 hours of incubation, the number of cells that had invaded to the lower surface was determined by crystal violet staining and colorimetric assessment by measuring the absorbance at 590 nm. *P < .05, as compared with control by Student's two-tailed t-test.
Discussion
Several studies have shown that spontaneous metastasis was caused by orthotopic implantation of some human cancer cell lines, including colon, kidney, and pancreas cancer, into relevant organs of nude mice [18–21]. Although some investigators have reported models for human lung cancer produced by orthotopic implantation, these models have some drawbacks [6,22,23]. In the present study, we developed an experimental model for human lung cancer, which caused tumor growth in the lung and spontaneous metastasis, using our previously established technique [8]. Intrapulmonary injection of the human NSCLC cells resulted in the formation of a primary tumor nodule and spontaneous distant metastasis in athymic nude mice. The growth of PC-14 tumors at the implantation site progressed more rapidly than that of A549 and Lu-99 tumors. Macroscopic and histopathological studies indicated that PC-14 tumors metastasized to the mediastinal and axillary lymph nodes and lumbar vertebrae by day 35 after the implantation (Figures 2 and 3, Table 2). In some cases, metastases to the hilus lymph nodes of the lung and chest wall were also observed (data not shown). However, few intrapulmonary metastases were macroscopically visible in these mice. Similar results were obtained after the orthotopic implantation of Lu-99 cells (Table 2). On the other hand, A549 cells showed intrapulmonary metastases to the right and left lobes of the lung in all implanted mice, but no visible lymph node metastases were detected. Thus, the three types of NSCLC cells exhibited different metastatic potentials in vivo.
Degradation of the extracellular matrix by tumor cells is a crucial step in the process of tumor invasion. Among the matrix-degrading enzymes, MMPs and the u-PA/u-PAR system are considered to play very important roles in the invasive spread [9,10] and many clinicopathological investigations of MMPs, u-PA and u-PAR have been performed [25–28]. MT1-MMP [29] can activate pro-MMP-2 to active MMP-2 [30–33] and can also degrade some extracellular matrix components [34]. It has been reported that there was a significant correlation between activation of MMP-2 and expression of MT1-MMP in lung cancer patients with lymphatic metastasis [27]. RT-PCR analysis and gelatin zymography indicated that PC-14 cells, which show marked lymphatic metastasis, strongly expressed MT1-MMP and MMP-2 mRNAs, and appeared to produce MMP-2 protein (Figures 5–7). Lu-99 cells also expressed moderate amounts of mRNAs for both MMPs (Figure 5). Similar expression patterns of the MMPs were observed in in vivo tumor tissues of PC-14 and Lu-99. Thus, the co-expression of these MMPs might play an important role in lymphatic metastasis of lung cancer. PC-14 and Lu-99 also showed expression of mRNAs for u-PA and u-PAR (in in vitro cultured cells and in vivo tumor tissues) and marked fibrinolytic activity (Figures 5 and 7). Lu-99 cells also showed the gelatinolytic activity of MMP-9 (Figure 7), which has been reported to be activated by the u-PA/u-PAR-plasmin system [24]. Therefore, these molecules may also contribute to the complex interactions that control the invasiveness of lung cancer cells and that may result in the development of lymphatic and distant metastases.
On the other hand, no marked expression of mRNAs for MMPs, except for MMP-2 and MMP-7, was observed in A549. In addition, few gelatinolytic activities were detected in the conditioned media of A549 cells, although moderate fibrinolytic activity was detected (Figure 7). Therefore, the intrapulmonary metastasis of A549 cells may depend predominantly on some other factor(s). Kochhar and Lyer [17] have shown that HGF markedly enhanced the motility and invasiveness of A549 cells. HGF stimulates the cell migration or invasion of various epithelial cell lines through the cell surface receptor encoded by the c-met proto-oncogene [35]. As shown in Figure 8, A549 exhibited stronger expression of c-MET mRNA than PC-14 and Lu-99. The responsiveness of A549 invasion to HGF was parallel to the marked expression of c-MET mRNA. (Figure 9). Several investigators have shown that poor prognosis of lung cancer patients was correlated with high expression of HGF [36,37]. Significant correlation between intraorgan metastasis and c-MET expression has been reported in the case of hepatocellular carcinoma [38], although such correlation was not found in the case of lung cancer. These findings suggest that the HGF/c-MET signal may be one of the factors responsible for intraorgan migration and metastasis.
Conclusion
We demonstrated that orthotopic implantation of three human NSCLC cell lines into the lungs of athymic nude mice caused a solitary tumor nodule in the lung and subsequent spontaneous metastasis. Furthermore, the different metastatic patterns of NSCLC may be partly related to the expression profiles of metastasis-related factors such as MMPs, u-PA/u-PAR and HGF receptor.
Acknowledgements
We thank Dr. M. Kawaguchi, Second Department of Histopathology, Toyama Medical and Pharmaceutical University, for pathological determination of specimens and Ms. Yukiko Shimizu for assistance.
Abbreviations
- NSCLC
non-small cell lung cancer
- SCLC
small cell lung cancer
- RT-PCR
reverse transcription polymerase chain reaction
- MMP
matrix metalloproteinase
- u-PA
urokinase-type plasminogen activator
- u-PAR
urokinase-type plasminogen activator receptor
- HGF
hepatocyte growth factor
Footnotes
This work was supported, in part, by Grants-in-Aid for Cancer Research from the Japanese Ministry of Education, Science, Sports and Culture (no. 09254101).
References
- 1.Ginsberg RJ, Vokes EE, Raben A. Non-small cell lung cancer: Section 2. Cancer of the lung. Cancer: Principles and Practice of Oncology. (5th edn) 1997;1:858–911. Chap. 30. [Google Scholar]
- 2.Naruke T, Goya T, Tsuchiya R, Suemasu K. The importance of surgery to non-small cell carcinoma of lung with mediastinal lymph node metastasis. Ann Thorac Surg. 1988;46:603–610. doi: 10.1016/s0003-4975(10)64717-0. [DOI] [PubMed] [Google Scholar]
- 3.Mountain CF. Revisions in the international system for staging lung cancer. Chest. 1997;111:1710–1717. doi: 10.1378/chest.111.6.1710. [DOI] [PubMed] [Google Scholar]
- 4.Vansteenkiste JF, De Leyn PR, Deneffe GJ, Stalpaert G, Nackaerts KL, Lerut TE, Demedts MG. Survival and prognostic factors in resected N2 non-small cell lung cancer: a study of 140 cases. Leuven lung cancer group. Ann Thorac Surg. 1997;63:1441–1450. doi: 10.1016/s0003-4975(97)00314-7. [DOI] [PubMed] [Google Scholar]
- 5.Yano S, Sone S. Novel metastasis model of human lung cancer cells representing different histological types in SCID mice depleted of NK cells. Jpn J Can Chem. 1997;24:489–494. [PubMed] [Google Scholar]
- 6.Mclemore TL, Mark CL, Penny CB, Marybelle G, Michael CA, Betty JA, Robert HS, Mark EB, Charles CL, Walter CH, Robert HB, James BM, Donald LF, Joseph CE, Joseph GM, Michael RB. Novel intrapulmonary model for orthotopic propagation of human lung cancers in athymic nude mice. Cancer Res. 1987;47:5132–5140. [PubMed] [Google Scholar]
- 7.Howard RB, Chu H, Zeligman BE, Marcell T, Bunn PA, Mclemore TL, Mulvin DW, Cowen ME, Johnston MR. Irradiated nude rat model for orthotopic human lung cancers. Cancer Res. 1991;51:3274–3280. [PubMed] [Google Scholar]
- 8.Doki Y, Murakami K, Yamaura T, Sugiyama S, Misaki T, Saiki I. Mediastinal lymph node metastasis model by orthotopic intrapulmonary implantation of Lewis lung carcinoma cells in mice. Br J Cancer. 1999;79:1121–1126. doi: 10.1038/sj.bjc.6690178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stetler-Stevenson WG, Liotta LA, Kleiner DJ. Extracellular matrix 6: role of matrix metalloproteinases in tumor invasion and metastasis. FASEB J. 1993;7:1434–1441. doi: 10.1096/fasebj.7.15.8262328. [DOI] [PubMed] [Google Scholar]
- 10.Andreasen PA, Kjoller L, Christensen L, Duffy MJ. The urokinase-type plasminogen activator system in cancer metastasis: a review. Int J Cancer. 1997;72:1–22. doi: 10.1002/(sici)1097-0215(19970703)72:1<1::aid-ijc1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 11.Kodate M, Kasai T, Hashimoto H, Yasumoto K, Iwata Y, Manabe H. Expression of matrix metalloproteinase (gelatinase) in T1 adenocarcinoma of the lung. Pathol Int. 1997;47:461–469. doi: 10.1111/j.1440-1827.1997.tb04525.x. [DOI] [PubMed] [Google Scholar]
- 12.Volm M, Mattern J, Koomagi R. Relationship of urokinase and urokinase receptor in non-small cell lung cancer to proliferation, angiogenesis, metastasis and patient survival. Oncol Rep. 1999;6:611–615. doi: 10.3892/or.6.3.611. [DOI] [PubMed] [Google Scholar]
- 13.Jefferson MF, Pendleton N, Faragher EB, Dixon GR, Myskow MW, Horan MA. ’Tumour volume’ as a predictor of survival after resection of non-small cell lung cancer (NSCLC) Br J Cancer. 1996;74:456–459. doi: 10.1038/bjc.1996.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murakami K, Matsuura T, Sano M, Hashimoto A, Yonekura K, Sakukawa R, Yamada Y, Saiki I. 4-[3,5-Bis (trimethylsilyl) benzamido] benzoic acid (TAC-101) inhibits the intrahepatic spread of hepatocellular carcinoma and prolongs the lifespan of tumorbearing animals. Clin Exp Metastasis. 1998;16:633–643. doi: 10.1023/a:1006567229929. [DOI] [PubMed] [Google Scholar]
- 15.Ikeda T, Murakami K, Hayakawa Y, Fujii H, Ohkoshi M, Saiki I. Anti-invasive activity of synthetic serine protease inhibitors and its combined effect with a matrix metalloproteinase inhibitor. Anticancer Res. 1998;18:4259–4266. [PubMed] [Google Scholar]
- 16.Saito K, Oku T, Ata N, Miyashiro H, Hattori M, Saiki I. A modified and convenient method for assessing tumor cell invasion and migration and its application to screening for inhibitor. Biol Pharm Bull. 1997;20:345–348. doi: 10.1248/bpb.20.345. [DOI] [PubMed] [Google Scholar]
- 17.Kochhar KS, Lyer AP. Hepatocyte growth factor induces activation of Nck and phospholipase C-gamma in lung carcinoma cells. Cancer Lett. 1996;104:163–169. doi: 10.1016/0304-3835(96)04244-9. [DOI] [PubMed] [Google Scholar]
- 18.Morikawa K, Walker SM, Jessup JM, Fidler IJ. In vivo selection of highly metastatic cells from surgical specimens of different primary human colon carcinomas implanted into nude mice. Cancer Res. 1988;48:1943–1948. [PubMed] [Google Scholar]
- 19.Naito S, Von Eschenbach AC, Giavazzi R, Fidler IJ. Growth and metastasis of tumor cells isolated from a human renal cell carcinoma implanted into different organs of nude mice. Cancer Res. 1986;46:4109–4115. [PubMed] [Google Scholar]
- 20.Tan MH, Chu TM. Characterization of the tumorigenic and metastatic properties of a human pancreatic tumor cell line (AsPC-1) implanted orthotopically into nude mice. Tumor Biol. 1985;6:89–98. [PubMed] [Google Scholar]
- 21.Mohammad RM, Al-Katib A, Pettit GR, Vaitkevicius VK, Joshi U, Adsay V, Majumdar APN, Sarkar FH. An orthotopic model of human pancreatic cancer in severe combined immunodeficient mice: potential application for preclinical studies. Clin Cancer Res. 1998;4:887–894. [PubMed] [Google Scholar]
- 22.Mclemore TL, Eggleston JC, Shoemaker RH, Abbott BJ, Bohlman ME, Liu MC, Fine DL, Mayo JG, Boyd MR. Comparison of intrapulmonary, percutaneous intrathoracic, and subcutaneous model for the propagation of human pulmonary and non-pulmonary cancer cell lines in athymic nude mice. Cancer Res. 1988;48:2880–2886. [PubMed] [Google Scholar]
- 23.Wang X, Fu X, Hoffman RM. A new patient-like metastatic model of human lung cancer constructed orthotopically with intact tissue via thoracotomy in immunodeficient mice. Int J Cancer. 1992;51:992–995. doi: 10.1002/ijc.2910510626. [DOI] [PubMed] [Google Scholar]
- 24.Mazzieri R, Masiero L, Zanetta L, Monea S, Onisto M, Garbisa S, Mignatti P. Control of type IV collagenase activity by components of the urokinase-plasmin system: a regulatory mechanism with cell-bound reactants. EMBO J. 1997;16:2319–2332. doi: 10.1093/emboj/16.9.2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hayashi T, Fleming MV, Stetler-Stevenson WG, Liotta LA, Moss J, Ferrans VJ, Travis WD. Immunohistochemical study of matrix metalloproteinases (MMPs) and their tissue inhibitors (TIMPs) in pulmonary lymphangioleiomyomatosis (LAM) Hum Pathol. 1997;28:1071–1078. doi: 10.1016/s0046-8177(97)90061-7. [DOI] [PubMed] [Google Scholar]
- 26.Kawano N, Osawa H, Ito T, Nagashima Y, Hirahara F, Inayama Y, Nakatani Y, Kimura S, Kitajima H, Koshikawa N, Miyazaki K, Kitamura H. Expression of gelatinase A, tissue inhibitor of metalloproteinases-2, matrilysin, and trypsin (ogen) in lung neoplasms: an immunohistochemical study. Hum Pathol. 1997;28:613–622. doi: 10.1016/s0046-8177(97)90085-x. [DOI] [PubMed] [Google Scholar]
- 27.Tokuraku M, Sato H, Murakami S, Okada Y, Watanabe Y, Seiki M. Activation of the precursor of gelatinase A/72 kDa type IV collagenase/MMP-2 in lung carcinomas correlates with the expression of membrane-type matrix metalloproteinase (MT-MMP) and with lymph node metastasis. Int J Cancer. 1995;64:355–359. doi: 10.1002/ijc.2910640513. [DOI] [PubMed] [Google Scholar]
- 28.Anderson IC, Sugarbaker DJ, Ganju RK, Tsarwhas DG, Richards WG, Sunday M, Kobzik L, Shipp MA. Stromelysin-3 is overexpressed by stromal elements in primary non-small cell lung cancers and regulated by retinoic acid in pulmonary fibroblasts. Cancer Res. 1995;55:4120–4126. [PubMed] [Google Scholar]
- 29.Sato H, Takino T, Okada Y, Cao J, Shinagawa A, Yamamoto E, Seiki M. A matrix metalloproteinase expressed on the surface of invasive tumour cells. Nature. 1994;370:61–65. doi: 10.1038/370061a0. [DOI] [PubMed] [Google Scholar]
- 30.Will H, Atkinson SJ, Butler GS, Smith B, Murphy G. The soluble catalytic domain of membrane type 1 matrix metalloproteinase cleaves the propeptide of progelatinase A and initiates autoproteolytic activation. Regulation by TIMP-2 and TIMP-3. J Biol Chem. 1996;271:17119–17123. doi: 10.1074/jbc.271.29.17119. [DOI] [PubMed] [Google Scholar]
- 31.Lichte A, Kolkenbrock H, Tschesche H. The recombinant catalytic domain of membrane-type matrix metalloproteinase-1 (MT1-MMP) induces activation of progelatinase A and progelatinase A complexed with TIMP-2. FEBS Lett. 1996;397:277–282. doi: 10.1016/s0014-5793(96)01206-9. [DOI] [PubMed] [Google Scholar]
- 32.Butler GS, Butler MJ, Atkinson SJ, Will H, Tamura T, Van Westrum SS, Crabbe T, Clements J, D'Ortho MP, Murphy G. The TIMP-2 membrane type 1 metalloproteinase ‘receptor’ regulates the concentration and efficient activation of progelatinase A. A kinetic study. J Biol Chem. 1998;273:871–880. doi: 10.1074/jbc.273.2.871. [DOI] [PubMed] [Google Scholar]
- 33.Kinoshita T, Sato H, Okada A, Ohuchi E, Imai K, Okada Y, Seiki M. TIMP-2 promotes activation of progelatinase A by membrane-type 1 matrix metalloproteinase immobilized on agarose beads. J Biol Chem. 1998;273:16098–16103. doi: 10.1074/jbc.273.26.16098. [DOI] [PubMed] [Google Scholar]
- 34.Ohuchi E, Imai K, Fujii Y, Sato H, Seiki M, Okada Y. Membrane type 1 matrix metalloproteinase digests intestinal collagens and other extracellular matrix macromolecules. J Biol Chem. 1997;272:2446–2451. doi: 10.1074/jbc.272.4.2446. [DOI] [PubMed] [Google Scholar]
- 35.Naldini L, Weidner KM, Vigna E, Gaudino G, Bardelli A, Ponzetto C, Narsimhan RP, Hartmann G, Zarnegar R, Michalopoulos GK, Birchmeier W, Comoglio PM. Scatter factor and hepatocyte growth factor are indistinguishable ligands for the MET receptor. EMBO J. 1991;10:2867–2878. doi: 10.1002/j.1460-2075.1991.tb07836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ichimura E, Maeshima A, Nakajima T, Nakamura T. Expression of c-met/HGF receptor in human non-small cell lung carcinomas in vitro and in vivo and its prognostic significance. Jpn J Cancer Res. 1996;87:1063–1069. doi: 10.1111/j.1349-7006.1996.tb03111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Siegfried JM, Weissfeld LA, Singh-Kaw P, Weyant RJ, Testa JR, Landreneau RJ. Association of immunoreactive hepatocyte growth factor with poor survival in resectable non-small cell lung cancer. Cancer Res. 1997;57:433–439. [PubMed] [Google Scholar]
- 38.Ueki T, Fujimoto J, Suzuki T, Yamamoto H, Okamoto E. Expression of hepatocyte growth factor and its receptor c-met proto-oncogene in hepatocellular carcinoma. Hepatology. 1997;25:862–866. doi: 10.1002/hep.510250413. [DOI] [PubMed] [Google Scholar]
- 39.Yamamoto M, Mohanam S, Sawaya R, Fuller GN, Seiki M, Sato H, Gokaslan ZL, Liotta LA, Nicolson GL, Rao JS. Differential expression of membrane-type matrix metalloproteinase and its correlation with gelatinase A activation in human malignant brain tumors in vivo and in vitro. Cancer Res. 1996;56:384–392. [PubMed] [Google Scholar]
- 40.Murakami K, Sakukawa R, Ikeda T, Matsuura T, Hasumura S, Nagamori S, Yamada Y, Saiki I. Invasiveness of hepatocellular carcinoma cell lines: contribution of membrane-type 1 matrix metalloproteinase. Neoplasia. 1999;4:1–7. doi: 10.1038/sj.neo.7900046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Onisto M, Garbisa S, Caenazzo C, Freda MP, Di Francesco C, Nitti D, Liotta LA, Stetler-Stevenson WG. Reverse transcription polymerase chain reaction phenotyping of metalloproteinases and inhibitors involved in tumor matrix invasion. Diagn Mol Pathol. 1995;2:74–80. [PubMed] [Google Scholar]
- 42.Riccio A, Grimaldi G, Verde P, Sebastio G, Boast S, Blasi F. The human urokinase-plasminogen activator gene and its promoter. Nucleic Acids Res. 1995;13:2759–2771. doi: 10.1093/nar/13.8.2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Min HY, Semnani R, Mizukami IF, Watt K, Todd RF, Liu DY. cDNA for Mo3, a monocyte activation antigen, encodes the human receptor for urokinase plasminogen activator. J Immunol. 1992;148:3636–3642. [PubMed] [Google Scholar]