Abstract
Ras-transformed intestinal epithelial cells are resistant to the growth inhibitory actions of TGFβ and have a marked decrease in expression of the TGFβ type II receptor (TGFβRII). Rat intestinal epithelial cells (RIE) were stably transfected with activated Ras, Sos and Raf constructs and tested for expression of TGFβRII and sensitivity to growth inhibition by TGFβ. The parental RIE line and the RIE-Raf cells were non-transformed in morphology and were sensitive to TGFβ (70–90% inhibited). In contrast, the RIE-Ras and RIE-Sos lines were transformed, resistant to TGFβ and expressed 5- to 10-fold decreased levels of the TGFβRII mRNA and protein. Cyclin D1 protein expression was repressed by TGFβ treatment in parental RIE and RIE-Raf cells, whereas levels of cyclin D1 in RIE-Ras and RIE-Sos cells remained unchanged. Treatment of RIE-Ras cells with 25 µM farnesyl transferase inhibitor, FTI L739,749, for 48 hours restored expression of TGFβRII to levels equivalent to control cells. In addition, treatment of RIE-Ras cells for 48 hours with PD-98059, a specific MAPKK inhibitor, also increased expression of TGFβRII to control levels. Collectively these results suggest that downregulation of TGFβRII and loss of sensitivity to growth inhibition by TGFβ in Ras-transformed intestinal epithelial cells is not mediated exclusively by the conventional Ras/Raf/MAPKK/MAPK pathway. However, activation of MAPK, perhaps by an alternate Ras effector pathway, appears to be necessary for Ras-mediated downregulation of TGFβRII.
Keywords: Ras, colorectal carcinoma, TGFβ, Raf, Intestinal epithelium, TGFβRII
Introduction
The transforming growth factor β (TGFβ) family of ligands and receptors are key regulators of normal epithelial cell homeostasis. The most prominent biologic activity of TGFβ in epithelial cells is growth inhibition, although a number of other diverse activities such as induction of differentiation, regulation of cell migration and induction of extracellular matrix synthesis have been described [1,2]. The three mammalian TGFβ isoforms, TGFβ1, TGFβ2 and TGFβ3, share 70–75% sequence identity and a nearly equivalent spectrum of biologic activity. These proteins are secreted as inactive precursors and are activated by cleavage of a precursor sequence to form the mature 25-kDa homodimer which is capable of binding to the TGFβ receptor. Three classes of high-affinity cell surface TGFβ receptors have been identified by cross-linking analyses and by molecular cloning [3–5]. These are TGFβRI (53 kDa), TGFβRII (75 kDa) and TGFβRIII (≈300 kDa) [6–8]. Each of these three receptors binds each of the three TGFβ ligands, albeit with modest differences in affinity [9].
TGFβ signaling occurs through a heterotetrameric complex involving TGFβRI and TGFβRII [10–12]. Binding occurs with the extracellular domain of TGFβRII which possesses a constitutively active cytoplasmic serine/threonine kinase, whereas TGFβRI only binds TGFβ in the presence of the TGFβRII. Formation of the heteromeric complex results in transphosphorylation of TGFβRI and activation of the cytoplasmic serine/threonine kinase. This kinase transiently phosphorylates and activates a family of unique intracellular signaling molecules, designated Smads, which translocate to the nucleus and regulate gene expression [4].
It has been hypothesized that disruption of the TGFβ pathway may result in loss of normal growth restraint and favor cellular transformation. Indeed, TGFβ resistance occurs in a wide variety of human neoplasms [13]. Although loss of TGFβ tumor suppressor activity has been detected at multiple points along the ligand-receptor-signaling axis, alterations in the expression of TGFβRII are of particular interest in colon carcinoma cells [14,15]. In transformed colon lines, microsatellite instability due to a defect in DNA base-base mismatch repair genes characteristically results in a frame shift mutation in TGFβRII, leading to production of a nonfunctional, truncated receptor and loss of TGFβ responsiveness [15]. Such mutations in TGFβRII occur in nearly all patients with microsatellite instability, including those with hereditary nonpolyposis colon cancer (HNPCC), and account for approximately 15% of colorectal tumors. In sporadic human colon cancers resistant to TGFβ, genomic mutations in TGFβRII occur infrequently (6–10% of sporadic tumors) and primarily in the context of genomic instability [16–18].
The aforementioned data indicate that unrecognized mutations at other points in the TGFβ pathway or that epigenetic events involving TGFβRII are operative in many colon tumors. Mutational disruption of the key signal proteins Madr2 and DPC4 (smad4) occurs in 4% and 16% of colorectal carcinomas respectively [19,20]. Also, decreased expression of TGFβRII is found in intestinal adenomas from min mice harboring loss of APC tumor suppressor gene function [21]. An apparent epigenetic event resulting in decreased TGFβRII expression has been described in intestinal epithelial cells transformed by an activated Ras oncogene [22,23]. The Ras gene product is a 21 kDa, membrane-associated, guanine nucleotide-binding signaling protein which is mutationally activated in about 50% of colorectal carcinomas [24,25]. A number of parallel and interacting intracellular signaling pathways are activated by Ras, the end result of which is regulation of gene expression. Previous studies have found that nontransformed jejunal epithelial cells transfected with the H-Ras oncogene become resistant to growth inhibition by TGFβ [22,23]. This occurs in the context of reduced expression of TGFβRII mRNA and protein [22,23,26,27]. Data suggest that downregulation of TGFβRII occurs transcriptionally [27]. Similar observations have been made in other cell lines [28–30]; however, downregulation of TGFβRII is not a universal observation in Ras transformed cells [31].
In the present study, the relationship between Ras overexpression, TGFβRII expression and TGFβ sensitivity in intestinal epithelial cells is further examined by using cell lines transfected with activated Sos and Raf, signaling proteins functioning immediately before and after Ras activation in the “conventional” Ras signaling pathway [24]. The results show that a Ras-effector pathway operating independent of Raf serine/threonine kinase activation, but dependent on MAPK activity, is involved in downregulation of TGFβRII and induction of TGFβ resistance.
Materials and Methods
Cell Lines and Reagents
RIE-1 rat intestinal epithelial cells [32] were obtained from Ken Brown (Cambridge, UK) and were maintained in DIMEM supplemented with 5% fetal calf serum. RIE-Ras cells were kindly supplied by Dr. Robert Coffey (Vanderbilt University) and were stably transfected with pSV2-H-ras (12 V) containing human sequences encoding the transforming H-Ras (12 V) protein [33]. The RIE-Raf and RIE-Sos lines were kindly provided by Dr. Channing Der (University of North Carolina). RIE-Raf cells are stably transfected with pZIP-Δraf22W, a c-Raf-1 mutant activated by NH2-terminal truncation [34]. Δraf22W is transforming in fibroblasts, but not epithelial cells [35]. The RIE-Sos line stably overexpresses the constitutively activated, membrane-targeted Sos1 protein (Sos-CAAX). It has been previously determined that Sos-CAAX causes transformation of NIH3T3 cells by chronic activation of endogenous Ras. RIE-Sos cells exhibit the same growth and morphologic characteristics observed with Ras-transformed cells (personal communication, Sean Oldham, University of North Carolina). For each transfected cell line, multiple G418-resistant clones (>50) were pooled for use in subsequent studies. TGFβ1 was obtained from R&D Systems, Minneapolis, MN.
RNA Isolation and Northern Blotting
Total cellular RNA was extracted with an SDS-based lysis buffer, subjected to proteinase K digestion and the poly(A) fraction was isolated by oligo-dT selection as previously described [36]. Poly(A) RNA was separated by 1.2% agarose gel electrophoresis and transferred to nylon membranes by Northern blotting [37]. cDNA probes were labeled by random primer extension using Redivue [32P]-dCTP and the Rediprime DNA labeling system, both from Amersham Life Sciences, Arlington Heights, IL. The TGFβRII probe is a 343-bp EcoRI/PstI fragment of the murine TGFβRII sequence encoding a portion of the 5′ coding region and the extracellular domain. The VEGF probe is a 448-bp PstI/SacII fragment of rat VEGF which detects all splice variants. A 700-bp BamHI/PstI fragment of the cyclophilin gene (1B15) was used as a constitutive probe. Hybridizations and posthybridization washes were performed as described previously [38].
Immunoprecipitation and Western Blotting
Cell monolayers in 100-mm culture dishes were solubilized in lysis buffer (20 mM Tris-HCl, pH 7.4, 120 mM NaCl, 100 mM NaF, 200 µM Na3V04, 4 mM PMSF, 10 µg/ml leupeptin, 10 µg/ml aprotinin, 0.5% NP-40, and 2 mM benzamidine) for 30 minutes at 4°C. After centrifugation at 12,000g for 15 minutes, the supernatant was incubated overnight with a rabbit polyclonal anti-TGFβRII antibody (sc#400, Santa Cruz Biotechnology). Immunoprecipitates were incubated with protein A-agarose for 1.5 hours and then washed repeatedly in phosphate-buffered saline containing 0.05% NP-40. The immune complexes were eluted in SDS sample buffer (62.5 mM Tris-HCl, pH 6.8, 10% glycerol, 2% SDS, 1% β-mercaptoethanol, 0.005% bromophenol blue) for 5 minutes at 95°C, resolved by 10% SDS-PAGE and transferred onto PVDF membranes in 25 mM Tris, 192 mM glycine, 20% methanol buffer at 30 V overnight. Membranes were then blocked, incubated with TGFβRII antibody (1:1000 dilution) followed by horseradish peroxidase-conjugated goat anti-rabbit IgG (Vector Labs, Burlingame, CA). The ECL Plus detection system (Amersham) was used to detect the antigen-antibody complexes. For cyclin D1 immunoblotting, total cell lysates were resolved by 10% SDS-PAGE, transferred onto PVDF at 15 V for 0.7 hour and incubated with rabbit anti-human cyclin D1 polyclonal antibody (UBI, Lake Placid, NY). Immunodetection was performed as described above.
Cell Proliferation Assays
[3H]-thymidine incorporation assays were carried out in 24-well tissue culture plates as previously described [39]. Cells were seeded at a density of 20,000 cells/well, allowed to attach for at least 24 hours, then treated as described in the figure legends. A [3H]-thymidine pulse (1 µCi/well) (NEN, Boston, MA) was provided between the 18th and 21st hour of treatment. Radioactivity incorporated into trichloroacetic acid insoluble material was determined by scintillation counting and results are presented as the mean±SEM for triplicate or quadruplicate measurements. Each experiment was repeated at least three times. For cell-counting experiments, cells were treated with TGFβ1 (5 ng/ml) or vehicle (4 mM HCL, 0.1% BSA) for 48 hours. Cells were trypsinized and counted using a hemacytometer. Results are presented as the mean±SD of at least three determinations.
Cross-linking
Cross-linking was performed as previously described [40]. Briefly, cells were washed twice in KRH (50 mM HEPES pH 7.5, 130 mM NaCl, 5 mM MgS04, 1.3 mM CaCl2 and 5 mM KCl) and preincubated with binding buffer (KRH with 0.5% w/v BSA) for 30 minutes at 37°C. The radioligand [125I]-TGFβ1 (NEN-Dupont) was added to a final concentration of 50 pM in the presence or absence of 500 pM TGFβ1 and the incubation continued for an additional 2 hours at 4°C. The ligand solution was removed, the cells washed four times with cold KRH and 0.1 mg/ml disuccinylsuberate (Pierce Chemical Company, Rockford, IL) in KRH was added for 15 minutes at 4°C. Cells were incubated in cold lysis buffer (1% Triton X-100, 10 mM Tris pH 7.4, and 1 mM EDTA) for 30 minutes and the resulting supernatant was collected without scraping. Equal volumes of protein were added per gel lane within each experiment and ranged between 55 and 90 µg. Samples were resolved on 6% polyacrylamide gels, stained with fresh Coomassie stain, destained in an excess of 5% methanol and 7% acetic acid and dried in BioDesign Gel Wrap. The resulting banding patterns were examined by autoradiography and Phosphorimager analysis.
Results
Cell Morphology and TGFβ Sensitivity
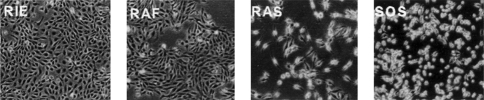
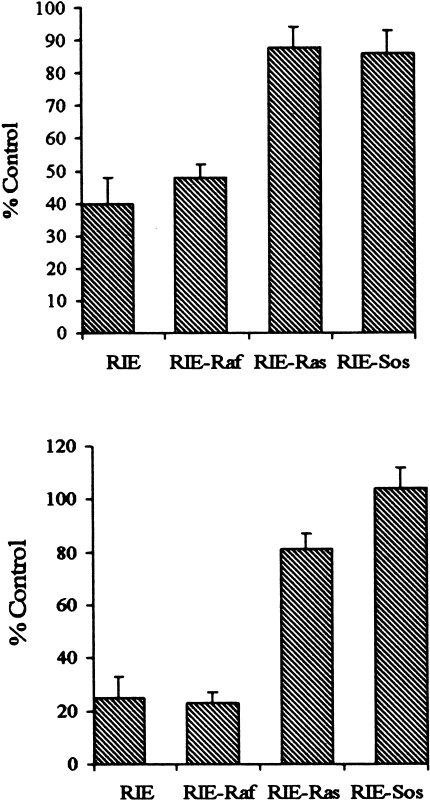
Striking differences in the morphology of the RIE transfectants are observed. The parental RIE and RIE-Raf cells are large and epithelioid with abundant cytoplasm, whereas the RIE-Sos and RIE-Ras cells are smaller, spindle-shaped cells with scant cytoplasm and small, retractile nuclei (Figure 1). Thymidine incorporation assays and cell counting were used to determine TGFβ sensitivity. Rapidly growing, subconfluent parental RIE, RIE-Sos, RIE-Ras and RIE-Raf cells were treated with varying concentrations of TGFβ1. Cell proliferation was determined by a [3H]-thymidine pulse 18 to 21 hours after treatment (Figure 2A) as well as cell counting 48 hours after treatment (Figure 2B). Parental RIE cells and RIE-Raf transfectants are more sensitive than the RIE-Ras and RIE-Sos lines. For example, TGFβ1 inhibits thymidine incorporation and growth of parental RIE and RIE-Raf cells 60–80% at a concentration of 5 to 10 ng/ml, whereas RIE-Ras and RIE-Sos cells are inhibited from 10 to 20%. These data indicate the lines with a transformed morphology are relatively resistant to the growth inhibitory action of TGFβ while the nontransformed lines are sensitive.
Figure 1.
Morphology of parental and transformed RIE-1 cells. Cell lines were obtained as described in Materials and Methods. RIE: parental cells, RAF: RIE-Δraf22w, RAS: RIE-Ras(12V), SOS: RIE-Sos. Magnification, x200.
Figure 2.
(A) TGFβ sensitivity measured by [3H]-thymidine incorporation in parental and transformed RIE-1 cells. Subconfluent, rapidly growing cells were treated with 10 ng/ml TGFβ1 for 18 hours. Thymidine incorporation was measured as described in Materials and Methods. Results are expressed as a percentage of thymidine uptake in cells treated with vehicle alone. Individual data points are the mean of quadruplicate determinations. Similar results were obtained in three additional experiments. (B) TGFβ sensitivity measured by cell counting. Subconfluent, rapidly growing cells were treated with 10 ng/ml TGFβ for 48 hours. Cells were trypsinized and counted using a hemacytometer. Results are expressed as a percentage of cell numbers in cells treated with vehicle alone. Individual data points are the mean of quadruplicate determinations. Similar results were obtained in three additional experiments.
TGFβRII mRNA and Protein Expression
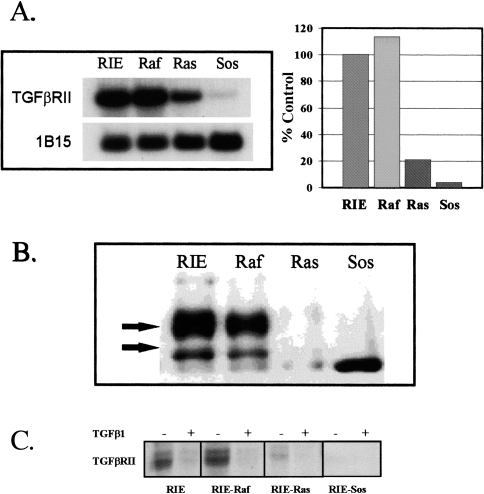
Decreased expression of TGFβRII occurs in most Ras-transformed lines including the RIE-Ras line [22,23,26–30]. Extension of this observation to additional RIE cell lines overexpressing immediate upstream (Sos) and downstream (Raf) components of the Ras/MAPK pathway are shown in Figure 3. The TGFβ-sensitive lines with a nontransformed morphology (parental RIE and RIE-Raf) express 5- to 10-fold more TGFβRII mRNA than the resistant lines with a transformed morphology (RIE-Sos and RIE-Ras) (Figure 3B). Western analysis using immunoprecipitated TGFβRII indicates that TGFβRII protein expression is also decreased in RIE-Ras and RIE-Sos cells (Figure 3B). The Western blots show two TGFβRII protein products, reflecting differential processing as previously described [41,42]. The nature of the prominent low molecular weight signal detected in the RIE-Sos is uncertain, but was observed consistently. In experiments not shown, inclusion of blocking peptide in a separate experiment successfully competed away all signals shown in Figure 3B, including the low molecular weight signal in the RIE-Sos cells. Cross-linking with [125I]-TGFβ1 was also used to determine the relative expression of cell-surface associated TGFβRII (3C). These analyses confirmed observations made using Northern and Western blotting.
Figure 3.
(A) TGFβRII mRNA expression in parental and transformed RIE-1 cells. Poly(A) RNA was isolated from subconfluent monolayers. Four micrograms of RNA were loaded per lane. Northern blots were prepared and probed with radiolabeled cDNA, as described in Materials and Methods. 1B15 was used as a constitutive probe to control for loading. This experiment was repeated at least four times. Results are expressed as a percentage of mRNA expression in parental RIE-1 cells, as determined by densitometry. (B) TGFβRII protein levels in parental and transformed RIE-1 cells. Protein lysates were prepared from subconfluent cells. Immunoprecipitations and Western blotting were done as described in Materials and Methods. The antibody is a rabbit polyclonal anti-TGFβRII antibody. The arrows indicate the two primary processed forms of TGFβRII [37,38]. All signals were competed away by pre-incubation with a blocking peptide (not shown). (C) Cross-linking to surface TGFβRII. [125I]-TGFβ1 (NEN-Dupont) was added to a final concentration of 50 pM in the presence (+) or absence (-) of 500 pM TGFβ1 and the incubation continued for an additional two hours at 4°C. The ligand solution was removed, the cells washed four times with cold KRH and 0.1 mg/ml disuccinylsuberate in KRH was added for 15 minutes at 4°C. Autoradiograms were prepared as described in Materials and Methods.
Regulation of Gene Expression in TGFβ-Sensitive and TGFβ-Resistant Lines
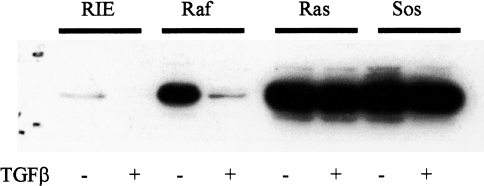
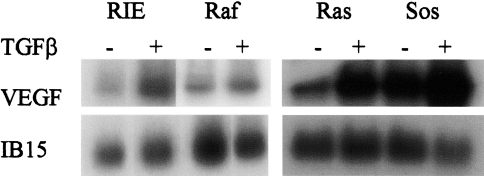
Expression of TGFβ-responsive genes relevant to cellular proliferation (cyclin D1) as well as genes relevant to nonproliferative actions of TGFβ (vascular endothelial growth factor, VEGF) were examined in the RIE lines. Previous studies have established that growth factor stimulation of quiescent epithelial cells results in rapid induction of cyclin D expression and subsequent cell proliferation, whereas growth factor withdrawal results in decreased cyclin D protein levels and G0/G1 growth arrest [43]. Inhibition of cyclin D1 expression is recognized as an integral component of TGFβ-mediated growth inhibition in intestinal epithelial cells [44]. As expected, cyclin D1 protein levels were markedly decreased 12 hours after treatment of subconfluent, rapidly growing parental RIE and RIE-Raf cells with TGFβ. Although the cyclin D1 level in RIE-Raf cells exceeded the level in parental RIE cells, substantial downregulation occurred with TGFβ treatment (Figure 4). In contrast, the elevated levels observed in the RIE-Ras and RIE-Sos lines remained increased following TGFβ treatment. Thus, the decreased expression of TGFβRII in Ras and Sos-overexpressing cells is associate with resistance to TGFβ growth inhibition, and is reflected, in part at least, by persistence of elevated levels of cyclin D1. Expression of VEGF following treatment with TGFβ was also examined in the RIE lines. Increased expression of VEGF by TGFβ contributes to the angiogenic response induced by TGFβ [45]. Figure 5 depicts the degree to which TGFβ induces VEGF expression in logarithmically growing RIE cells. Ras and Sos overexpressing cells had increased basal levels of VEGF expression, but all cell lines responded to TGFβ treatment with a further induction of VEGF expression. This response was most prominent in the Ras-transformed (10-fold) and Sos-transformed (five-fold) RIE lines, indicating preservation of signaling by a nonmitogenic pathway, despite a functionally significant reduction of TGFβ signaling by pathways relevant to growth regulation.
Figure 4.
Regulation of cyclin D1 expression by TGFβ1 in parental and transformed RIE-1 cells. Rapidly growing, subconfluent cells were treated with 5 ng/ml TGFβ1 (+) or vehicle (-) for 12 hours and total cellular lysates were prepared. Western blotting was performed as described in Materials and Methods.
Figure 5.
Induction of VEGF mRNA by TGFβ1. Cell lines were treated with 5 ng/ml TGFβ1 for 18 hours. Poly(A) RNA was isolated and Northern blots prepared as described in Materials and Methods. The membranes were probed with cDNA complementary to VEGF and 1B15. Autoradiograms were scanned and quantitated by laser densitometry. Similar results were obtained in two additional experiments.
Inhibition of Ras Activity by Farnesyl Transferase Inhibitor (FTI) Restores TGFβRII
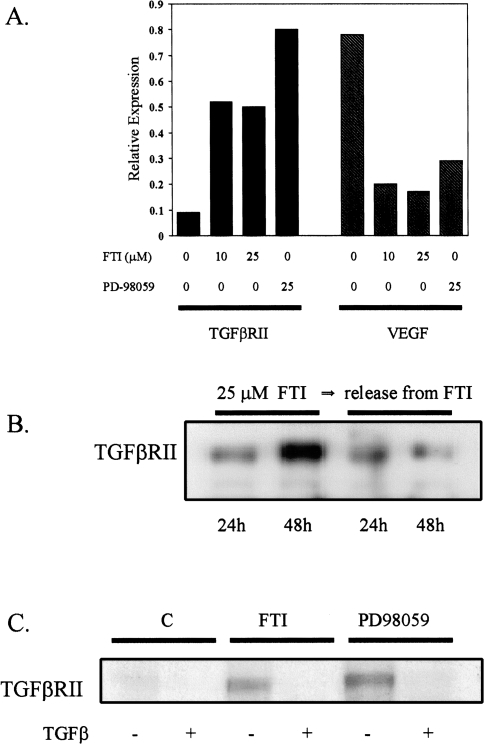
Cytosolic Ras is partitioned to the cell membrane by a series of posttranslational modifications, including addition of a 15-carbon isoprenyl (farnesyl) group to a sulfhydryl moiety in the C-terminal CAAX sequence, a reaction catalyzed by farnesyl transferase. Farnesylation and membrane localization of Ras to the cytosolic face of the cell membrane is required for Ras activity [24,46,47]. Thus, inhibition of farnesylation by FTI suppresses Ras function. In the present series of experiments, addition of the FTI L739,749 to Ras transformed RIE-1 cells was used to determine the specificity and reversibility of Ras-mediated downregulation of TGFβRII. Treatment with FTI at the concentrations used in these experiments are sufficient to inhibit H-Ras processing and transformation in RIE-1 cells (Nywanna Sizemore et al., personal communication). Rapidly growing, subconfluent RIE-Ras cells were treated with 10 and 25 µM L739,749 for 48 hours. As shown in Figure 6A, a nearly five-fold induction of TGFβRII mRNA expression was noted at both concentrations of FTI. This induction principally occurred between the 24th and 48th hours of treatment because initial experiments showed little increase after the shorter interval (not shown). Thus, the suppression of TGFβRII expression by Ras overexpression is directly related to the activation of Ras at the cytosolic face of the cell membrane and is reversible with persistent inhibition of farnesyltransferase activity. The specificity of the FTI effect on TGFβRII was also examined by determination of its effect on VEGF, a known Ras-regulated gene [48,49]. As expected, L739,749 markedly downregulates VEGF expression in Ras transformed RIE cells. Figure 6B shows the effect of farnesyltransferase inhibition on Ras-mediated downregulation of TGFβRII is reversible within 24 hours of L739,749 withdrawal.
Figure 6.
Effect of FTI and PD-98059 on TGFβRII and VEGF expression in RIE-Ras cells. (A) RIE-Ras cells were treated for 48 hours with the concentration of FTI (L739,749) and PD-98059 shown. Poly(A) RNA was isolated and Northern blots were prepared. Membranes were probed with 32P cDNA complementary to TGFβRII, VEGF and 1B15. Autoradiograms were scanned and quantitated by laser densitometry. The signals in each lane were normalized to the respective level of 1B15 expression and plotted relative to expression in untreated RIE-Ras cells. Both compounds are suspended in DMSO, which has no apparent effect on expression of either mRNA species. This experiment was repeated twice. Similar results were seen in RIE-Sos cells. Solid bars: TGFβRII expression; hatched bars VEGF expression. (B) RIE-Ras cells were treated with 25 µM FTI (L739,749) for 24 and 48 hours as shown and protein isolates were subjected to Western analysis as described in Materials and Methods. In separate experiments, the cells were treated with FTI for 48 hours followed by removal (R) of the inhibitor for 24 or 48 hours as shown. (C) Confluent RIE-Ras monolayers were treated with vehicle, 25 µM L739,749 or 25 µM PD-98059 for 48 hours. Crosslinking was performed as described in Materials and Methods. Unlabeled TGFβ1 was included in assays (+) to control for specificity of binding. Similar results were observed in RIE-Sos cells.
Inhibition of MAPKK also Upregulates TGFβRII Expression
Raf is the first signaling component downstream of Ras in the MARK cascade, yet, as we have shown, RIE cells overexpressing Raf are biologically and phenotypically more similar to parental RIE cells than Ras-overexpressing cells. These observations suggest that an alternate Ras effector system is involved in the downregulation of TGFβRII and associated TGFβ-resistance and altered morphology. Put in a different way, Ras-mediated downregulation of TGFβRII and emergence of TGFβ resistance appears to occur, at least in part, by Raf-independent pathways. If a Ras effector pathway exclusively independent from the traditional Ras/Raf/MAPKK/MAPK pathway is responsible for downregulation of TGFβRII, inhibition of the MAPK pathway using the specific MAPKK inhibitor PD-98059 [50] should not affect TGFβRII levels. Treatment with 25 µM PD-98059 for 24 hours resulted in a modest increase in TGFβRII mRNA levels to approximately 1.5-fold above control in all lines tested (not shown). Subsequent studies showed a eight-fold increase in RIE-Ras cells treated for 48 hours (Figure 6A). This degree of TGFβRII induction approximates levels of expression in the TGFβ-sensitive parental RIE lines. Cross-linking analysis confirmed that induction of TGFβRII by L739,749 and PD-98059 resulted in induction of cell-surface receptor biologically capable of binding [125I]-TGFβ1 (Figure 6C).
Discussion
TGFβ is a potent inhibitor of cellular proliferation in epithelial cells, including those of the intestinal tract. Both TGFβ and TGFβ receptors are expressed in the normal intestinal epithelium and perform a vital role in the tightly regulated balance of proliferation and differentiation along the intestinal crypt-villus axis [39,51–53]. It has long been hypothesized that loss of normal TGFβ-mediated growth inhibition may result in unregulated growth of the intestinal epithelium and contribute to colorectal carcinogenesis. Indeed, mammalian TGFβ receptors are now considered tumor suppressor gene products. Inactivating mutations in the type II TGFβ receptor occur in more than 90% of colorectal tumors that exhibit microsatellite instability, including persons with HNPCC [14,15]. Loss of a functional TGFβRII in HNPCC results in resistance to TGFβ-mediated growth inhibition and unregulated colon epithelial cell growth [15].
Our laboratory recently reported decreased levels of TGFβRII and resistance to TGFβ in Ras-transformed intestinal epithelial cells [23], a finding confirmed in related studies by other investigators [22]. This Ras-related epigenetic event involving the TGFβRII results in an molecular defect analogous to that described in HNPCC. Activating p21 Ras mutations are observed in approximately 50% of colorectal tumors and are believed to occur relatively early in the sequence of cumulative molecular defects leading to colorectal neoplasia [54]. Thus, Ras-mediated effects on TGFβ sensitivity may be a major contributor to colorectal carcinogenesis.
The mechanism by which sustained Ras activation results in reduced TGFβRII expression is not clear. Zhao and Buick [27] found that dexamethasone-inducible expression of Ras under the control of a MMTV promoter reduced TGFβRII mRNA levels at least in part by reducing rates of RNA transcription in IEC-18 cells. In these studies [27], the half-life of the TGFβRII mRNA in Ras-expressing cells was not different from control cells and the investigators found a conversion from TGFβRII to TGFβRI expression in response to Ras induction by dexamethasone. In our studies, a similar conversion was not observed in RIE cells stably transformed by Ras (data not shown).
In the present study, a role for the conventional Raf/MAPKK/MAPK pathway in regulation of TGFβRII was investigated by exploring TGFβ sensitivity and receptor levels in Ras-transformed RIE cells, as well as RIE cells stably transfected with activated signaling proteins functioning immediately before (Sos) and after (Raf) Ras. Sos mediates activation of p21 Ras by linking it to receptor tyrosine kinase activation [24]. The RIE-Sos transfectants are characterized by normal levels of endogenous (wild-type) Ras; however, Ras in RIE-Sos cells is predominantly membrane-associated in the activated GTP-bound state, resulting in transformation. TGFβRII expression and TGFβ-sensitivity in RIE-Sos clones is similar in all respects to the activated, mutant Ras-overexpressing transfectants. In contrast, RIE-Raf clones exhibited biologic behavior more similar to the parental RIE line than the RIE-Ras or RIE-Sos clones, despite the fact that Raf activation is the immediate downstream consequence of Ras activation. Prior work indicates equivalent Raf kinase activity in the RIE-Ras and RIE-Raf cells used in our experiments, thus validating the significance of our observations [33]. In conjunction with results using FTI L739,749, our work suggests alternate Ras effector pathways are involved in reversible regulation of TGFβRII expression. In related studies, signaling through a Raf-independent Ras effector pathway has also been found to mediate Ras-induced cellular transformation in RIE-1 cells [33].
Inasmuch as inhibition of MAPKK by the pharmacological agent PD-98059 restores TGFβRII levels in RIE-Ras cells, our data collectively indicate that diverse Ras effector pathways may act in concert to downregulate TGFβRII. The level of Raf kinase activity in the RIE-Ras and RIE-Raf lines used in our study is similar, further reinforcing our conclusions. Indeed, accumulating evidence supports a great deal of complexity in Ras signal transduction and biologic responses, such as transformation, occur as the integrated result of signaling by multiple Ras effectors. It is attractive to postulate that the Rho family of small GTP-binding signaling proteins are candidate effectors with which the conventional Raf/MAPKK/MAPK may interact to downregulate TGFβRII [24]. Substantial data exist in support of Rho proteins as Raf-independent Ras effectors and in some cell systems, Rho function is necessary for full oncogenic transformation by Ras [55–58]. It has been suggested that Rho may cooperate with Raf to cause transformation and recent data suggest that Rho proteins cooperate with Raf to activate p42MAPK and p44MAPK, [59]. These data are congruous with our own observation that Ras-mediated downregulation of TGFβRII is not exclusively dependent on Raf activation and requires MAPK activation. Full delineation of the interacting pathways involved in Ras-mediated downregulation of TGFβRII may permit design of rational strategies for inhibition of the pathways and restoration of TGFβRII and possibly growth sensitivity.
We found that Ras and Sos overexpressing cells had markedly increased basal levels of VEGF expression, an observation that has been previously reported [48,49], but the RIE-Ras and RIE-Sos clones responded to TGFβ treatment with a further induction of VEGF expression, despite resistance to the growth inhibitory actions of TGFβ. This response was most prominent in the Ras- and Sos-transformed RIE lines, indicating preservation of signaling by a nonmitogenic pathway, despite a functionally significant reduction of TGFβ signaling by mitogenic pathways. Similar observations have been reported in other cell systems [27,60,61] and it has been suggested that signaling related to nonmitogenic actions of TGFβ may occur primarily thru the TGFβRI. The precise mechanism by which this occurs is uncertain. These results indicate a dual adverse effect of Ras-transformation on RIE-1 cell biology, including loss of an autocrine growth inhibitory pathway and stimulation of angiogenic molecules which may further favor tumor growth in vivo, the end result of which is an enhanced potential for tumorigenesis.
Recent data in lung and mammary epithelial cells suggest that oncogenic Ras may interfere with TGFβ signaling by phosphorylation of Smad2 and Smad3 in the polylinker region resulting in inhibition of Smad complex translocation into the nucleus [62]. TGFβRII levels were not reported in this study. These results suggest both receptor and post-receptor mechanisms may be involved in TGFβ resistance in Ras-transformed intestinal epithelial cells.
Acknowledgements
This study was supported by NIH grants DK49637 (JAB), Veterans Association Merit Review (RHC), DK52334 and CA69457 (RDB), HL52922 (JVB) and Cancer Center Support grant CA68485.
References
- 1.Massague J, Attisano A, Wrana JL. The TGFβ family and its composite receptors. Trends Cell Biol. 1994;4:172–178. doi: 10.1016/0962-8924(94)90202-x. [DOI] [PubMed] [Google Scholar]
- 2.Kingsley DM. The TGFβ superfamily: new members, new receptors, and new genetic tests of function in different organisms. Genes Dev. 1994;8:133–146. doi: 10.1101/gad.8.2.133. [DOI] [PubMed] [Google Scholar]
- 3.Lin HY, Lodish HL. Receptors for the TGFβ superfamily: multiple polypeptides and serine/threonine kinases. Trends Cell Biol. 1993;3:14–19. doi: 10.1016/0962-8924(93)90195-7. [DOI] [PubMed] [Google Scholar]
- 4.Attisano L, Wrana JL. Signal transduction by members of the transforming growth factor-β superfamily. Cytokine Growth Factor Rev. 1996;7:327–339. doi: 10.1016/s1359-6101(96)00042-1. [DOI] [PubMed] [Google Scholar]
- 5.Lin HY, Moustakas A. TGFβ receptors. Structure and function. Cell Mol Biol. 1994;40:337–349. [PubMed] [Google Scholar]
- 6.Attisano L, Garcamo J, Ventura F, Weis FMB, Massague J, Wrana JL. Identification of human activin and TGFβ type I receptors that form heteromeric kinase complexes with type II receptors. Cell. 1993;75:671–680. doi: 10.1016/0092-8674(93)90488-c. [DOI] [PubMed] [Google Scholar]
- 7.Lin HY, Wang X-F, Ng-Eaton E, Weinberg RA, Lodish HF. Expression cloning of the TGFβ type II receptor, a functional transmembrane serine/threonine kinase. Cell. 1992;68:775–785. doi: 10.1016/0092-8674(92)90152-3. [DOI] [PubMed] [Google Scholar]
- 8.Lopez-Gasillas F, Cheifez S, Doody J, Andres JL, Lane WS, Massague J. Structure and expression of the membrane proteoglycan betaglycan, a component of the TGFβ receptor system. Cell. 1991;64:785–795. doi: 10.1016/0092-8674(91)90073-8. [DOI] [PubMed] [Google Scholar]
- 9.Graycar JL, Miller DA, Arrick BA, Lyons RM, Moses HL, Derynck R. Human transforming growth factor β3, Recombinant expression, purification, and biological activities in comparision with transforming growth factors β1 and β2. Mol Endocrinol. 1992;3:1977–1986. doi: 10.1210/mend-3-12-1977. [DOI] [PubMed] [Google Scholar]
- 10.Laiho M, Weiss FMB, Boyd FT, Ignotz RA, Massague J. Responsiveness to transforming growth factor β restored by genetic complementation between cells defective in TGFβ receptors I and II. J Biol Chem. 1991;266:9108–9112. [PubMed] [Google Scholar]
- 11.Geiser AG, Burmester JK, Webbink R, Roberts AB, Sporn MB. Inhibition of growth by transforming growth factor β following fusion of two nonresponsive human carcinoma cell lines, Implication of the type II receptor in growth inhibitory responses. J Biol Chem. 1992;267:2588–2593. [PubMed] [Google Scholar]
- 12.Okadome T, Yamashita H, Franzen P, Moren A, Heldin C-H, Miyazono K. Distinct roles of the intracellular domains of transforming growth factor β type I and type II receptors in signal transduction. J Biol Chem. 1994;269:30753–30756. [PubMed] [Google Scholar]
- 13.Filmus J, Kerbel RS. Development of resistance mechanisms to the growth-inhibitory effects of transforming growth factor-β during tumor progression. Curr Opin Oncol. 1993;5:123–129. [PubMed] [Google Scholar]
- 14.Brattain MG, Markowitz SD, Wilson JKV. The type II transforming growth factor-β receptor as a tumor-suppressor gene. Curr Opin Oncol. 1996;8:49–53. doi: 10.1097/00001622-199601000-00009. [DOI] [PubMed] [Google Scholar]
- 15.Markowitz S, Wang J, Myeroff L, Parsons R, Sun L, Lutterbaugh J, Fao R, Zborowska E, Kinzler K, Vogelstein B, Brattain M, Willson J. Inactivation of the type II TGFβ receptor in colon cancer cells with microsatellite instability. Science. 1995;268:1336–1338. doi: 10.1126/science.7761852. [DOI] [PubMed] [Google Scholar]
- 16.Akiyama Y, Iwanaga R, Ishikawa T, Sakamoto K, Nishi N, Nihei N, Iwama T, Saitoh K, Yuasa Y. Mutations of the transforming growth factor-β type II receptor gene are strongly related to sporadic proximal colon carcinomas with microsatellite instability. Cancer. 1996;78:2478–2484. doi: 10.1002/(sici)1097-0142(19961215)78:12<2478::aid-cncr5>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 17.Takenoshita S, Tani M, Nagashima M, Hagiwara K, Bennett WP, Yokota J, Harris CO. Mutation analysis of coding sequences of the entire transforming growth factor beta type II receptor gene in sporadic human colon cancer using genomic DNA and intron primers. Oncogene. 1997;14:1255–1258. doi: 10.1038/sj.onc.1200938. [DOI] [PubMed] [Google Scholar]
- 18.Vincent F, Nagashima M, Takenoshita S, Khan MA, Gemma A, Hagiwara K, Bennett WP. Mutation analysis of the transforming growth factor-β type II receptor in human cell lines resistant to growth inhibition by transforming growth factor-β. Oncogene. 1997;15:117–122. doi: 10.1038/sj.onc.1201166. [DOI] [PubMed] [Google Scholar]
- 19.Eppert K, Scherer SW, Ozcelik H, Pirone R, Hoodless P, Kim H, Tsui LG, Bapat B, Gallinger S, Andrulis IL, Thomson GH, Wrana JL, Attisano L. MADR2 maps to 18q21 and encodes a TGFβ-regulated MAD-related protein that is functionally mutated in colorectal carcinoma. Cell. 1996;86:543–552. doi: 10.1016/s0092-8674(00)80128-2. [DOI] [PubMed] [Google Scholar]
- 20.Takagi Y, Kohmura H, Futamura M, Kida H, Tanemura H, Simokawa K, Saji S. Somatic alterations of the DPC4 gene in human colorectal cancers in vivo. Gastroenterology. 1996;111:1369–1372. doi: 10.1053/gast.1996.v111.pm8898652. [DOI] [PubMed] [Google Scholar]
- 21.Zhang T, Nanney LB, Peeler MO, Williams CS, Lamps L, Heppner KJ, DuBois RN, Beauchamp RD. Decreased transforming growth factor beta type II receptor expression in intestinal adenomas from Min/+ mice is associated with increased cyclin D1 and cyclin-dependent kinase 4 expression. Cancer Res. 1997;57:1638–1643. [PubMed] [Google Scholar]
- 22.Filmus J, Zhao J, Buick RN. Overexpression of H-ras oncogene induces resistance to the growth inhibitory action of transforming growth factor beta-1 and alters the number and type of TGFβ receptors in rat intestinal epithelial cell clones. Oncogene. 1992;7:521–526. [PubMed] [Google Scholar]
- 23.Winesett MP, Ramsey GW, Barnard JB. Type II TGFβ receptor expression in intestinal cell lines in the intestinal tract. Carcinogenesis. 1996;17:989–995. doi: 10.1093/carcin/17.5.989. [DOI] [PubMed] [Google Scholar]
- 24.Khosravi-Far R, Campbell S, Rossman KL, Der CJ. Increasing complexity of Ras signal transduction, involvement of Rho family proteins. Adv Cancer Res. 1998;72:57–107. doi: 10.1016/s0065-230x(08)60700-9. [DOI] [PubMed] [Google Scholar]
- 25.Bos JL, Fearon ER, Hamilton SR, Verlaan-de Vries M, van Boom JH, van der Eb AJ, Vogelstein B. Prevalence of ras gene mutations in human colorectal cancers. Nature. 1987;327:293–297. doi: 10.1038/327293a0. [DOI] [PubMed] [Google Scholar]
- 26.Zhao J, Buick RN. Relationship of levels and kinetics of H-ras expression to transformed phenotype and loss of TGF-β1-mediated growth regulation in intestinal epithelial cells. Exp Cell Res. 1993;204:82–87. doi: 10.1006/excr.1993.1011. [DOI] [PubMed] [Google Scholar]
- 27.Zhao J, Buick RN. Regulation of transforming growth factor β receptors in H-ras oncogene-transformed rat intestinal epithelial cells. Cancer Res. 1995;55:6181–6188. [PubMed] [Google Scholar]
- 28.Wakefield LM, Smith DM, Masui T, Harris CC, Sporn MB. Distribution and modulation of the cellular receptor for transforming growth factor β. J Cell Biol. 1987;105:965–975. doi: 10.1083/jcb.105.2.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Houck KA, Michalopoulos GK, Strom SC. Introduction of a Ha-ras oncogene into rat liver epithelial cells and parenchymal hepatocytes confers resistance to the growth inhibitory effects of TGFβ. Oncogene. 1989;4:19–25. [PubMed] [Google Scholar]
- 30.Coppa A, Mincione G, Lazzereschi D, Ranieri A, Turco A, Lucignano B, Scarpa S, Ragano-Caracciolo M, Colletta G. Restored expression of transforming growth factor β type II receptor in k-ras-transformed thyroid cells, TGFβ-resistant, reverts their malignant phenotype. J Cell Physiol. 1997;172:200–208. doi: 10.1002/(SICI)1097-4652(199708)172:2<200::AID-JCP7>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 31.Longstreet M, Miller B, Howe PH. Loss of transforming growth factor β1-induced growth arrest and p34cdc2 regulation in ras-transfected epithelial cells. Oncogene. 1992;5:1549–1556. [PubMed] [Google Scholar]
- 32.Blay J, Brown KD. Epidermal growth factor promotes the chemotactic migration of cultured rat intestinal epithelial cells. J Cell Physiol. 1985;124:107–112. doi: 10.1002/jcp.1041240117. [DOI] [PubMed] [Google Scholar]
- 33.Gangarosa LM, Sizemore N, Graves-Deal R, Oldham SM, Der GJ, Coffey RJ. A Raf-independent epidermal growth factor receptor autocrine loop is necessary for Ras transformation of rat intestinal epithelial cells. J Biol Chem. 1997;272:18926–18931. doi: 10.1074/jbc.272.30.18926. [DOI] [PubMed] [Google Scholar]
- 34.Stanton VP, Nichols DW, Laudano AP, Cooper GM. Definition of the human raf amino-terminal regulatory region by deletion mutagenesis. Mol Cell Biol. 1989;9:639–647. doi: 10.1128/mcb.9.2.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oldham SM, Clark GJ, Gangarosa LM, Coffey RJ, Jr, Der CJ. Activation of the Raf-1/MAP kinase cascade is not sufficient for Ras transformation of RIE-1 epithelial cells. Proc Natl Acad Sci USA. 1996;93:6924–6928. doi: 10.1073/pnas.93.14.6924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dobner PR, Kawaski ES, Yu LY, Bancroft FC. Thyroid or glucocorticoid hormone induces pre-growth hormone mRNA and its probable nuclear precursor in rat pituitary cells. Proc Natl Acad Sci USA. 1981;78:2230–2234. doi: 10.1073/pnas.78.4.2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schwab M, Alitalo K, Varmus HE, Bishop JM. A cellular oncogene (c-Ki-ras) is amplified, overexpressed and located within karyotypic abnormalities in mouse adrenocortical tumor cells. Nature (London) 1983;303:497–501. doi: 10.1038/303497a0. [DOI] [PubMed] [Google Scholar]
- 38.Thomas PS. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci USA. 1980;77:5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barnard JA, Beauchamp RD, Coffey RJ, Moses HL. Regulation of intestinal epithelial cell growth by transforming growth factor type β. Proc Natl Acad Sci USA. 1989;86:1578–1582. doi: 10.1073/pnas.86.5.1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barnett JV, Moustakas A, Lin W, Fang X-F, Lin HY, Galper JB, Maas RL. Cloning and developmental expression of the chick type II and type III TGFβ receptors. Dev Dyn. 1994;199:12–27. doi: 10.1002/aja.1001990103. [DOI] [PubMed] [Google Scholar]
- 41.Koli KM, Arteaga CL. Processing of the transforming growth factor β type I and II receptors. J Biol Chem. 1997;272:6423–6427. doi: 10.1074/jbc.272.10.6423. [DOI] [PubMed] [Google Scholar]
- 42.Wells RG, Yankelev H, Lin HY, Lodish HF. Biosynthesis of the type I and type II TGF-β receptors. J Biol Chem. 1997;272:11444–11451. doi: 10.1074/jbc.272.17.11444. [DOI] [PubMed] [Google Scholar]
- 43.Sherr CJ. D-type cyclins. TIBS. 1995;20:187–190. doi: 10.1016/s0968-0004(00)89005-2. [DOI] [PubMed] [Google Scholar]
- 44.Ko TC, Sheng HM, Reisman D, Thompson EA, Beauchamp RD. Transforming growth factor beta inhibits cyclin D1 expression in intestinal epithelial cells. Oncogene. 1995;10:177–184.. [PubMed] [Google Scholar]
- 45.Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1997;18:4–25. doi: 10.1210/edrv.18.1.0287. [DOI] [PubMed] [Google Scholar]
- 46.Reiss Y, Stradley SJ, Gierash LM, Brown MS, Goldstein JL. Sequence requirement for peptide recognition by rat brain p21ras protein farnesyltransferase. Proc Natl Acad Sci USA. 1991;88:732–736. doi: 10.1073/pnas.88.3.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cox AS, Der CJ. Farnesyltransferase inhibitors and cancer treatment, targeting simply Ras? Blochem Blophys Acta. 1997;133:F51–F71. doi: 10.1016/s0304-419x(97)00011-5. [DOI] [PubMed] [Google Scholar]
- 48.Rak J, Mitsuhashi Y, Bayko L, Filmus J, Shirasawa S, Sasazuki T, Kerbel RS. Mutant ras oncogenes upregulate VEGF/VPF expression, implications for induction and inhibition of tumor angiogenesis. Cancer Res. 1995;55:4575–4580. [PubMed] [Google Scholar]
- 49.Grugel S, Finkenzeller G, Weindel K, Barleon B, Marme D. Both v-Ha-Ras and v-Raf stimulate expression of the vascular endothelial growth factor in NIH 3T3 cells. J Biol Chem. 1995;270:25915–25919. doi: 10.1074/jbc.270.43.25915. [DOI] [PubMed] [Google Scholar]
- 50.Alessi DR, Cuenda A, Cohen P, Dudley DT, Saltiel AR. PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J Biol Chem. 1995;270:27489–27494. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- 51.Kurokowa M, Lynch K, Podolsky DK. Effects of growth factors on an intestinal epithelial cell line, transforming growth factor β inhibits proliferation and stimulates differentiation. Blochem Blophys Res Commun. 1987;142:775–782. doi: 10.1016/0006-291x(87)91481-1. [DOI] [PubMed] [Google Scholar]
- 52.Koyama S, Podolsky DK. Differential expression of transforming growth factors α and β in rat intestinal epithelial cells. J Clin Invest. 1989;63:1768–1773. doi: 10.1172/JCI114080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barnard JA, Warwick GJ, Gold LI. Localization of transforming growth factor β isoforms in the normal murine small intestine and colon. Gastroenterology. 1993;105:67–73. doi: 10.1016/0016-5085(93)90011-z. [DOI] [PubMed] [Google Scholar]
- 54.Fearon ER. Molecular genetic studies of the adenoma-carcinoma sequence. Adv Med. 1994;39:123–147. [PubMed] [Google Scholar]
- 55.Westwick JK, Lambert QT, Clark GJ, Symons M, Van Aelst L, Pestell RG, Der CJ. Rac regulation of transformation, gene expression, and actin organization by multiple PAK-independent pathways. Mol Cell Biol. 1997;17:1324–1335. doi: 10.1128/mcb.17.3.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Prendergast GC, Khosravi-Far CR, Solski H, Kurzawa H, Lebowitz PF, Der CJ. Critical role of RhoB in cell transformation by oncogenic Ras. Oncogene. 1995;10:2289–2296. [PubMed] [Google Scholar]
- 57.Qiu RG, Chen J, McCormick F, Symons M. A role for Rho in Ras transformation. Proc Natl Acad Sci USA. 1995;92:11781–11795. doi: 10.1073/pnas.92.25.11781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Qiu R-G, Chen J, Kirn D, McCormick F, Symons M. An essential role for Rac in Ras transformation. Nature. 1995;372:457–459. doi: 10.1038/374457a0. [DOI] [PubMed] [Google Scholar]
- 59.Frost JA, Steen H, Shapiro P, Lewis T, Ahn N, Shaw P, Cobb MH. Cross-cascade activation of ERKs and ternary complex factors by Rho family proteins. EMBO J. 1997;16:6426–6438. doi: 10.1093/emboj/16.21.6426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen R-H, Ebner R, Derynck R. Inactivation of the type II receptor reveals two receptor pathways for the diverse TGFβ activities. Science. 1993;250:1335–1338. doi: 10.1126/science.8388126. [DOI] [PubMed] [Google Scholar]
- 61.Miettinen PJ, Ebner R, Lopez AR, Derynck R. TGFβ induced transdifferentiation of mammary epithelial cells to mesenchymal cells, involvement of type I receptors. J Cell Biol. 1994;27:2021–2036. doi: 10.1083/jcb.127.6.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kretzschmar M, Doody J, Timokhina I, Massague J. A mechanism of repression of TGFβ/Smad signaling by oncogenic Ras. Genes Dev. 1999;13:804–816. doi: 10.1101/gad.13.7.804. [DOI] [PMC free article] [PubMed] [Google Scholar]