Abstract
The prolonged use of nonsteroidal anti-inflammatory drugs (NSAIDs) has been shown to exert a chemopreventive effect in esophageal and other gastrointestinal tumors. The precise mechanism by which this occurs, however, is unknown. While the inhibition of COX-2 as a potential explanation for this chemopreventive effect has gained a great deal of support, there also exists evidence supporting the presence of cyclooxygenase-independent pathways through which NSAIDs may exert their effects. In this study, immunohistochemical analysis of 29 Barrett's epithelial samples and 60 esophageal adenocarcinomas demonstrated abundant expression of the COX-2 protein in Barrett's epithelium, but marked heterogeneity of expression in esophageal adenocarcinomas. The three esophageal adenocarcinoma cell lines, Flo-1, Bic-1, and Seg-1, also demonstrated varying expression patterns for COX-1 and COX-2. Indomethacin induced apoptosis in all three cell lines, however, in both a time- and dose-dependent manner. In Flo-1 cells, which expressed almost undetectable levels of COX-1 and COX-2, and in Seg-1, which expressed significant levels of COX-1 and COX-2, indomethacin caused upregulation of the pro-apoptotic protein Bax. The upregulation of Bax was accompanied by the translocation of mitochondrial cytochrome c to the cytoplasm, and activation of caspase 9. Pre-treatment of both cell lines with the specific caspase 9 inhibitor, z-LEHD-FMK, as well as the broad-spectrum caspase inhibitor, z-VAD-FMK, blocked the effect of indomethacin-induced apoptosis. These data demonstrate that induction of apoptosis by indomethacin in esophageal adenocarcinoma cells is associated with the upregulation of Bax expression and mitochondrial cytochrome c translocation, and does not correlate with the expression of COX-2. This may have important implications for identifying new therapeutic targets in this deadly disease.
Keywords: esophageal adenocarcinoma, Barrett's esophagus, NSAIDs, chemoprevention, apoptosis
Introduction
In the United States, the incidence of esophageal adenocarcinoma has been rising at an alarming rate. While the incidence for squamous cell carcinomas of the esophagus has decreased slightly, the incidence of esophageal adenocarcinoma has increased over 350% in the past three decades [1]. The lack of a serosal covering on the esophagus and rich submucosal lymphatic plexus contribute to a high number of patients with advanced disease at initial diagnosis, and low 5-year survival rates of approximately 10% to 20% [1]. This highlights the importance of early detection and chemoprevention as strategies for minimizing the morbidity and mortality of this disease.
Over the past decade, there has been a wealth of epidemiological evidence associating the prolonged use of nonsteroidal anti-inflammatory drugs (NSAIDs) with a decreased incidence of various cancers, particularly tumors of colonic, rectal, gastric, and esophageal origin [2,3]. The epidemiological evidence is in accordance with various experimental animal models where chemically and genetically induced colonic tumor growth is inhibited by NSAIDs such as aspirin, sulindac, and indomethacin [4,5]. The mechanism by which NSAIDs exert this chemopreventive effect, however, remains unclear. The most well-known target of this class of drugs is the enzyme, cyclooxygenase, which catalyzes the metabolism of arachidonic acid to the various biologically active prostaglandins [6]. Since the discovery of the two isoforms of cyclooxygenase (the constitutive COX-1 and the inducible COX-2) [7], numerous studies have demonstrated the upregulation of COX-2 expression in various cancers, including esophageal, gastric, colorectal, pancreatic, prostatic, and lung tumors [8–12]. The selective inhibition of COX-2 has become a potentially attractive strategy for chemoprevention due to the avoidance of side effects associated with COX-1 inhibition, such as gastric mucosal erosion and ulceration [13]. COX-2 inhibition as an anticancer target is supported by experiments in the ApcΔ716 mouse model, where genetic and pharmacological inhibition of COX-2 led to a decrease in the number and size of intestinal polyps [14]. In vitro, overexpression of COX-2 in rat intestinal epithelial cells resulted in inhibition of apoptosis and increased cellular adhesion to extracellular matrix proteins, traits favoring carcinogenesis [15]. It is thus hypothesized that the chemopreventive effects of NSAIDs may be due to their ability to inhibit the COX-2 isoform.
There exists evidence, however, that suggests the presence of biological pathways other than prostaglandin inhibition through which NSAIDs may also exert their chemopreventive effects. The NSAID, sulindac, has been shown to decrease the number of colorectal polyps and the risk for colon cancer in experimental animal models and in patients with familial adenomatous polyposis [16–18]. Sulindac is metabolized to sulfide and sulfone forms. While the sulfide derivative has potent inhibitory effects on cyclooxygenase and well-known anti-inflammatory properties, the sulfone derivative lacks the ability to inhibit cyclooxygenase [19]. Both sulindac and the sulindac sulfide metabolite have been shown to inhibit cellular proliferation and induce apoptosis in colonic adenocarcinoma cells in vitro [20]. Piazza et al., [19] have shown, however, that the sulfone derivative has the same effect in vitro without possessing the ability to inhibit the cyclooxygenase enzyme. In the rodent-azoxymethane model of colon carcinogenesis, sulindac sulfone has been shown to have the same antineoplastic effects as sulindac sulfide and piroxicam, without affecting tissue prostaglandin synthesis [21]. It has also been shown in several models that the addition of exogenous prostaglandin fails to prevent or reverse the effects seen with NSAID treatment [22,23]. Additionally, the doses of NSAIDs used in many in vitro models to elicit observed effects far exceed the concentrations required to inhibit the cyclooxygenase enzymes [24], again indicating the presence of pathways outside the cyclooxygenase mediated production of prostaglandins through which NSAIDs may exert chemopreventive effects.
Several studies have demonstrated the ability of NSAIDs and the newer selective COX-2 inhibitors to induce apoptotic cell death [19,25,26]. Apoptosis, or programmed cell death, is the result of the activation of a series of cysteine proteases, termed caspases, leading to characteristic morphological changes such as cell shrinkage and rounding, nuclear condensation and DNA fragmentation, and ultimately cellular dissolution with no associated inflammatory response [27]. The chemopreventive effects of NSAIDs may be due to their ability to induce apoptotic cell death.
The aims of this study were three-fold. First, the expression of COX-2 in Barrett's epithelium and esophageal adenocarcinoma was characterized. Next, three different esophageal adenocarcinoma cell lines, with varying expression profiles for COX-1 and COX-2, were used to examine the effects of indomethacin on apoptotic cell death in vitro. Indomethacin induced apoptosis in all three adenocarcinoma cell lines in a time and dose-dependent manner, despite varying levels of expression of the cyclooxygenase isoforms within the cells. Finally, we examined the specific pathways of caspase activation and patterns of Bcl-2 class protein expression after treatment with indomethacin in order to identify potential targets within the cellular apoptotic machinery that may have roles in the antitumor effects of NSAIDs.
Materials and Methods
Tissue Specimen Procurement
Informed consent was obtained from patients undergoing esophagectomy for cancer at the University of Michigan Medical Center from 1991 to 1998. Specimens from patients receiving preoperative chemotherapy or radiotherapy were excluded from this study. Samples of normal esophageal squamous mucosa, esophageal adenocarcinoma and, when present, Barrett's metaplasia were transported to the laboratory in DME on ice. A portion of each sample was then immediately frozen in ornithine carbamoyltransferase (OCT) compound with isopentane cooled to the temperature of liquid nitrogen for cryostat sectioning. The remaining portion of the specimens was frozen in liquid nitrogen and stored at -70°C.
Immunohistochemical Staining and Analysis
Immunohistochemistry was performed on 5 µm cryostat tissue sections as previously described [28]. The tissue sections were incubated with COX-2 polyclonal antibody (Cayman Chemical, Ann Arbor, MI) at a dilution of 1:1000 in phosphate-buffered saline (PBS)-1% BSA overnight at 4°C. A section of tissue on each slide was incubated with PBS-1% BSA without primary antibody to identify potential nonspecific immunoreactivity. Staining patterns were classified as either homogenous or heterogeneous. The intensity of immunohistochemical staining was subjectively graded on a scale from 1 to 3, with a score of 3 representing the most intense staining. Examination and classification of staining patterns by light microscopy were done independently by three observers.
Cell Culture and Drug Treatments
The human esophageal adenocarcinoma cell lines, Flo-1, Bic-1, and Seg-1, were utilized for in vitro studies [29]. Cells were cultured in DME supplemented with 10% FBS and a 1% solution of penicillin, streptomycin, and fungizone (Gibco BRL, Life Technologies, Gaithersburg, MD).
For drug treatments, a stock solution of indomethacin (Sigma-Aldrich, St. Louis, MO) in DMSO was added directly to culture media. The final concentration of DMSO in media did not exceed 1%. As negative controls, cells not receiving drug treatment and treatment with vehicle alone (DMSO) were examined.
In experiments using the selective caspase 9 inhibitor, z-LEHD-FMK (Biovision Research Products, Palo Alto, CA), as well as the nonselective inhibitor, z-VAD-FMK (Biovision), cells were treated for 12 hours with inhibitor alone, prior to the administration of indomethacin. Indomethacin (100 µM) was then added directly to culture media. Fresh peptide inhibitor was subsequently added to culture media at 12-hour intervals according to manufacturer's recommendations for the duration of the experiment. For trypan exclusion assays, non-adherent and adherent cells were collected and stained with a 0.5% solution of trypan blue in PBS for 3 minutes. Cells were then counted immediately with a hemocytometer.
Morphological Assay for Apoptosis
Following treatment with indomethacin (200 µM) for 48 hours, apoptotic morphology was assessed by propidium iodide staining as previously described [30].
DNA Fragmentation Assay
Flo-1, Seg-1, and Bic-1 cells were treated with 200 µM indomethacin for 48 hours. Cells were then trypsinized and harvested. After centrifugation at 2000g for 15 minutes, cells were washed once in ice-cold PBS. The cell pellet was then lysed using a buffer containing 10 mM Tris-HCl (pH 7.4), 5 mM EDTA, and 1% Triton X-100 for 20 minutes on ice. The lysate containing the fragmented DNA was then centrifuged at 11,000g for 20 minutes. The supernatant was treated with RNase (50 µg/µl) at 37°C for 1 hour, followed by treatment with proteinase K (0.1 mg/ml) at 37°C for an additional hour. The DNA was extracted using phenol-chloroform and precipitated in 70% ethanol overnight at -20°C. The samples were then separated on a 1.2% agarose gel with 0.5 µg/ml of ethidium bromide and visualized under UV light.
Isolation of RNA and Protein
RNA was isolated from tissue samples and cell lines using the Trizol reagent (Gibco BRL) according to manufacturer's specifications. Whole cell protein was extracted from tissue samples and cell lines with NP-40 lysis buffer (0.2% Igepal, 100 mM Tris-HCl at pH 8.0, 200 mM NaCl, 0.01% SDS).
Reverse Transcription Polymerase Chain Reaction (RT-PCR)
Total RNA from cell and tissue samples were treated with DNase I (Promega, Madison, WI) prior to performing reverse transcription. Two micrograms of total RNA was reverse-transcribed using reverse transcriptase (Gibco BRL) and primed with both (dT)18 and random hexamers in a total of 40 µl of reaction volume. RT-PCR was performed with 2 µl of cDNA using the following primers: COX-1 sense, 5′-CAAGACGGCCACACTGAAGAA-3′; COX-1 antisense, 5′-TGTCAACCCCAACACTCACCA-3′ (252 bp); COX-2 sense, 5′-CTGTTGCGGAGAAAGGAGTCAT-3′; COX-2 antisense, 5′-ACTTTCAGCATTTTGGCATCTTG-3′ (224 bp). As an internal control, the housekeeping gene, GAPDH, was co-amplified in all reactions. The forward primers of the control and test fragments were end-labeled with 32P-γ-[ATP] (NEN Life Science Products, Boston, MA) using T4 polynucleotide kinase (New England Biolabs, Beverly, MA). PCR was conducted with incorporation of a 50 ng template in 25 µl of total reaction volume using Taq polymerase (Promega). The PCR conditions included an initial denaturing step at 94°C for 2 minutes followed by 35 cycles of 94°C for 45 seconds, 60°C for 45 seconds, and 72°C for 1 minute using the PTC100 thermal cycler (M&J Research, Watertown, MA). The PCR products were then resolved on 8% denaturing polyacrylamide gels. After vacuum drying, the gels were exposed to PhosphorImage screens (Molecular Dynamics, Sunnyvale, CA) and visualized using Image-Quant software (Molecular Dynamics).
Western Blot Analysis
Western blot analysis was performed as previously described [29]. Anti-Bax rabbit polyclonal antibody (Santa Cruz Biotechnologies, Palo Alto, CA) at a 1:10,000 dilution, anti-caspase 9 rabbit polyclonal antibody (Cayman) at a 1:1000 dilution, and anti-Bcl-2 mouse mAb (Santa Cruz Biotechnologies) at a 1:1000 dilution were used for protein detection.
Cytosolic Cytochrome c Assay
After treatment with indomethacin (100 µM) for 30 hours, cytosolic extracts from Flo-1 and Seg-1 cells were prepared as previously described [31], and further centrifuged at 100,000g for 30 minutes at 40°C. Proteins from the resulting supernatant were separated on 15% SDS-PAGE gels and transferred to nitrocellulose paper. Cytochrome c protein was detected using anti-cytochrome c mAb (Pharmingen, Franklin Lakes, NJ) and immunoreactivity detected using enhanced chemiluminescence.
Results
Immunohistochemical Staining for COX-2 in Barrett's Mucosa and Esophageal Adenocarcinomas
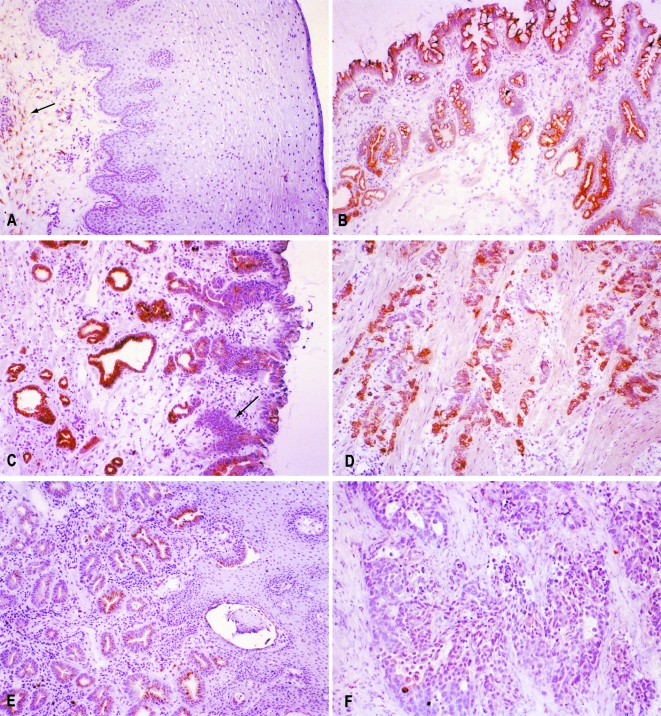
Tables 1 and 2 summarize the results of immunohistochemical analysis for COX-2 in Barrett's epithelium and esophageal adenocarcinomas. In 29 cases of Barrett's epithelium, 100% of Barrett's without dysplasia (n = 12) and 94% of Barrett's with dysplasia (n = 17) demonstrated positive staining for COX-2. For Barrett's epithelial samples, staining was further classified by the morphological location of protein expression. Both groups, Barrett's epithelium with dysplasia and without dysplasia, showed the majority of staining in the epithelium and glandular regions of the lamina propria. Barrett's with dysplasia demonstrated increased staining in the muscularis and submucosa (65% and 18%, respectively) when compared to the group without dysplasia (17% and 0%). Of the 60 adenocarcinoma samples examined, the majority demonstrated a heterogeneous staining pattern, with 42% staining at grade 3 (most intense), 28% at grade 2, and 12% at grade 1. Fifteen percent demonstrated no staining for COX-2. Less than 2% of tumors demonstrated a homogenous staining pattern for COX-2 at any intensity. While COX-2 expression was not seen in the normal squamous epithelial layers of the esophagus, certain samples demonstrated positive staining within inflammatory and smooth muscle cells of the stroma (Figure 1). Limited evaluation for COX-1 expression demonstrated some positive staining within the basal layers of the normal squamous epithelium, but negative staining within esophageal adenocarcinomas (data not shown).
Table 1.
Summary of Cyclooxygenase-2 Protein Expression in Barrett's Epithelium*.
| Number of cases | (%) Positive staining | Morphological staining pattern | ||||
| Epithelium | Lamina propria | Muscularis | Submucosa | |||
| Barrett's epithelium | 12 | 12 (100%) | 11 (92%) | 10 (83%) | 2 (17%) | 0 |
| Barrett's epithelium with dysplasia | 17 | 16 (94%) | 16 (94%) | 14 (82%) | 11 (65%) | 3 (18%) |
Positive immunohistochemical staining for COX-2 protein in Barrett's epithelium with and without dysplasia was classified according to the morphologic location of protein expression.
Table 2.
Histological and Anatomic Classification of Cyclooxygenase-2 Protein Expression in Esophageal Adenocarcinomas.
| Tissue/location | Cases | Nonstaining | Staining pattern* | |||||
| Heterogeneous | Homogenous | |||||||
| 1 | 2 | 3 | 1 | 2 | 3 | |||
| Adenocarcinoma | ||||||||
| Upper | 0 (0%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Mid | 3 (5%) | 0 | 0 | 1 | 1 | 1 | 0 | 0 |
| Lower | 34 (57%) | 4 | 3 | 12 | 15 | 0 | 0 | 0 |
| Cardia | 19 (32%) | 4 | 3 | 4 | 7 | 0 | 1 | 0 |
| Lower+cardia | 4 (7%) | 1 | 1 | 0 | 2 | 0 | 0 | 0 |
| Total | 60 (100%) | 9 (15%) | 7 (12%) | 17 (28%) | 25 (42%) | 1 (<2%) | 1 (<2%) | 0 (0%) |
Staining patterns were classified as either homogenous or heterogeneous. Within each category, staining intensity was graded on a subjective scale from 1 to 3, with 3 representing the most intense staining. The table shows the number of positive staining samples for each staining category of 60 esophageal adenocarcinomas, arranged by anatomic location of the tumors.
Figure 1.
Immunohistochemical analysis of COX-2 protein expression. (A) While the normal squamous esophageal epithelium did not demonstrate expression for COX-2, there was expression seen in cellular components of the stroma in some specimens (arrow). (B) Barrett's metaplasia demonstrating expression of COX-2 within the epithelial and glandular regions of the lamina propria. (C) Expression was also seen in Barrett's epithelium with dysplasia, but strongly dysplastic areas (arrow) appeared to show less immunoreactivity for COX-2 when compared to nondysplastic areas. (D) A highly invasive esophageal adenocarcinoma demonstrating abundant COX-2 protein expression in tumor cells and smooth muscle cells. (E) An esophageal adenocarcinoma demonstrating heterogenous expression of COX-2 within tumor cells. (F) A COX-2-negative adenocarcinoma.
Expression of COX-1 and COX-2 mRNA in Esophageal Adenocarcinomas and Paired Normal Esophageal Squamous Mucosa
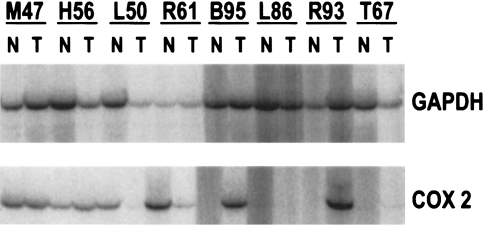
RT-PCR was used to assess the expression of COX-2 in esophageal adenocarcinomas and squamous epithelium from the same patients. The heterogeneity of COX-2 protein expression seen by immunohistochemical staining was also reflected in mRNA expression (Figure 2). Of eight esophageal adenocarcinomas and their paired normal tissues, three sample pairs demonstrated COX-2 mRNA expression in both the normal and tumor specimens. One patient's specimens showed abundant COX-2 expression in the normal tissue, but only low level expression in the tumor. Two of the eight sample pairs demonstrated COX-2 mRNA in the tumor, but not in the corresponding normal tissue. In two patients, COX-2 mRNA was either absent or present only in very low amounts in both the normal and cancerous tissues. The finding of COX-2 mRNA in the normal esophagus may be explained, in part, by the immunohistochemical detection of COX-2 protein in the stromal elements of some squamous samples (Figure 1A). While COX-1 mRNA expression was detected in the normal squamous mucosa, levels were very low to absent in esophageal adenocarcinoma samples, in accordance with immunohistochemical findings (data not shown).
Figure 2.
RT-PCR for COX-2 demonstrating the variability in COX-2 expression in different patient samples. Three samples demonstrate COX-2 expression both in the normal esophagus and tumor, while two samples show expression only within the tumor. One sample (L50) demonstrates abundant COX-2 expression in the normal epithelium, but low level expression in the tumor sample. Two patient specimens show either very low level or absent expression in both the normal and tumorous tissues. GAPDH was co-amplified as an internal control and is shown in the upper panels.
Expression of COX-1 and COX-2 mRNA in Esophageal Adenocarcinoma Cell Lines, Bic-1, Flo-1, and Seg-1
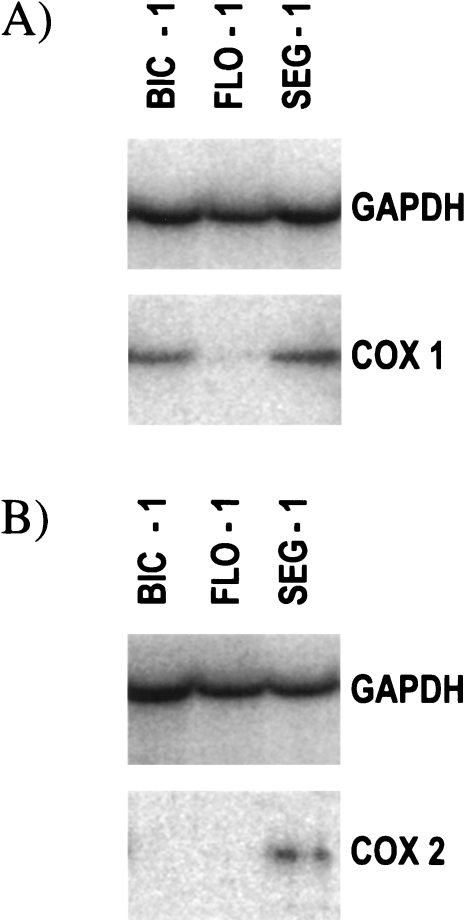
The esophageal adenocarcinoma cell lines, Bic-1, Flo-1, and Seg-1, were examined by RT-PCR for expression of COX-1 and COX-2. COX-1 mRNA expression was detected in all three cell lines. However, only trace levels were found in Flo-1 (Figure 3A).
Figure 3.
RT-PCR analysis of (A) COX-1 and (B) COX-2 expression in the human esophageal adenocarcinoma cell lines, Bic-1, Flo-1, and Seg-1. GAPDH was co-amplified as an internal control and is shown in the upper panels.
Seg-1 demonstrated significant expression of COX-2 mRNA. In contrast, COX-2 expression was almost undetectable in Flo-1, and absent in Bic-1 (Figure 3B). The adenocarcinoma cell lines thus showed varying expression profiles for the cyclooxygenase isoforms. Seg-1 showed significant expression of both COX-1 and COX-2; Bic-1 demonstrated expression of COX-1 but not COX-2; Flo-1 demonstrated only trace amounts of COX-1 and almost undetectable levels of COX-2 mRNA.
Effect of Indomethacin on Apoptosis in Esophageal Adenocarcinoma Cell Lines
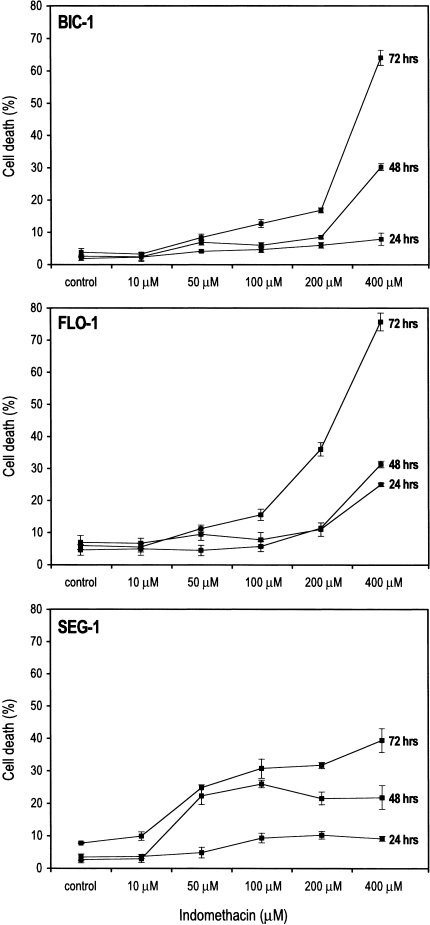
The effect of indomethacin on cell death was examined in the cell lines, Bic-1, Flo-1, and Seg-1. Indomethacin was able to induce cell death in a time- and dose-dependent manner in all three cell lines as assessed by trypan blue exclusion (Figure 4). The sensitivities of the three cell lines to indomethacin-induced cell death varied. However, in all three cell lines, indomethacin caused cellular death at concentrations greater than that required to inhibit either cyclooxygenase isoform, based on the IC50 values for indomethacin (IC50 for COX-1 = 0.28 µM, IC50 for COX-2 = 1.68 µM). No significant effect on cell death was observed at 10 µM, a concentration sufficient to inhibit both COX-1 and COX-2, implying the involvement of mechanisms other than cyclooxygenase inhibition in this effect.
Figure 4.
Time and dose-response curves of Bic-1, Flo-1, and Seg-1 to indomethacin. Cells were treated with increasing doses of indomethacin for 24, 48, and 72 hours. At the indicated time points, all cells were harvested and cell viability assessed by a trypan blue exclusion assay. Graphs represent the percentage of nonviable cells of total cells harvested at the indicated time and dose points.
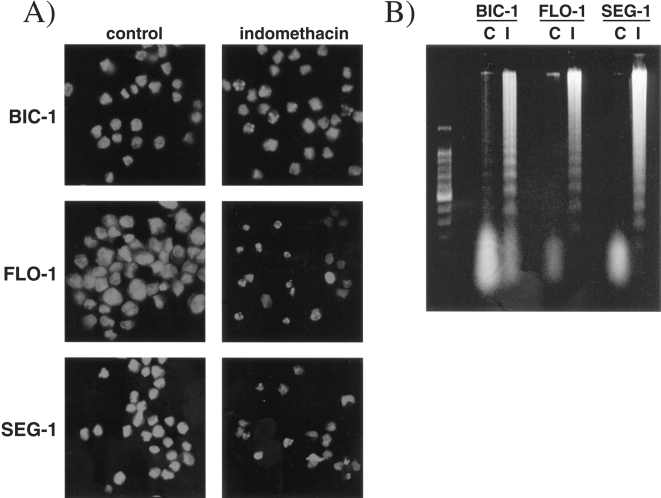
While trypan exclusion allows for the identification of nonviable cells, it does not discriminate the etiology of cell death (i.e. apoptosis versus necrosis). To determine if cell death induced by indomethacin was due to apoptosis, cells were assessed for DNA fragmentation and morphological changes characteristic of apoptotic cell death. Following staining with propidium iodide, cells treated with 200 µM indomethacin for 48 hours demonstrated characteristic apoptotic changes such as nuclear condensation and fragmentation, as well as cell shrinkage (Figure 5A). These morphological changes corresponded with loss of adherence to cell culture dishes. Internucleosomal DNA fragmentation was observed in the three cell lines after treatment with 200 µM indomethacin for 48 hours (Figure 5B). Thus, the finding of cell death by trypan blue correlated with characteristic morphological changes of apoptosis and DNA fragmentation.
Figure 5.
(A) Propidium iodide staining of Bic-1, Flo-1, and Seg-1 cells after treatment with indomethacin (200 µM) for 48 hours. Cells treated with indomethacin (right panels) demonstrate characteristic features of apoptotic death including cellular shrinkage, nuclear condensation and fragmentation. (B) Cell lines treated with 200 µM indomethacin for 48 hours demonstrate evidence of internucleosomal DNA fragmentation when compared to untreated cells C. A low-molecular weight DNA marker is pictured in the far left lane.
Mechanism of Indomethacin-Induced Apoptosis Involves Upregulation of Bax, Translocation of Mitochondrial Cytochrome c, and Activation of Caspase 9
The mechanism of indomethacin-induced apoptosis was examined in the adenocarcinoma cell lines, Flo-1 and Seg-1. The differences in these two cell lines provided us with a useful in vitro model for determining whether indomethacin's effect was dependent on cyclooxygenase expression. Seg-1 was the only cell line among the three that constitutively expressed significant levels of COX-2. Flo-1 was chosen because it expressed almost undetectable levels of both COX-1 and COX-2 (Figure 3).
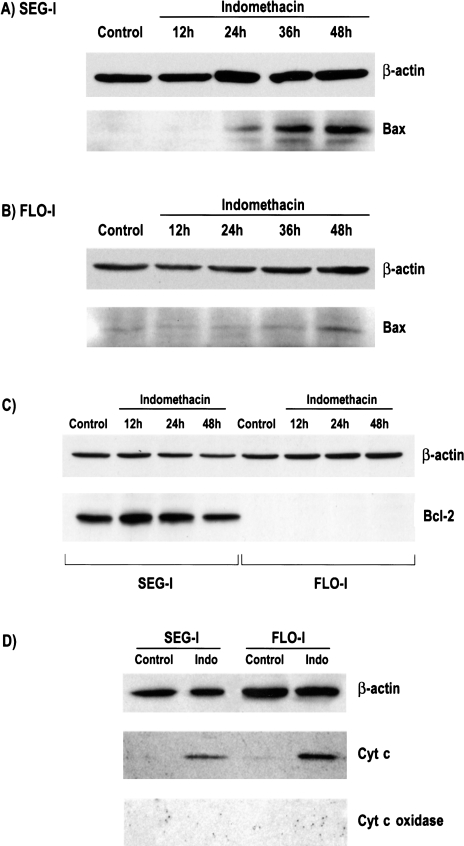
Expression of two Bcl class proteins, Bcl-2 and Bax, were examined before and after treatment with indomethacin. In untreated and indomethacin-treated Flo-1 cells, no Bcl-2 protein expression was detected by Western blot analysis. Untreated Seg-1 cells showed expression of Bcl-2. After treatment with indomethacin, levels appeared to increase slightly at 12 hours, but were not significantly changed from control at 24 and 48 hours (Figure 6C).
Figure 6.
Western blot analysis of Bax, Bcl-2, and cytosolic cytochrome c in Seg-1 and Flo-1 cells after treatment with indomethacin (100 µM). Bax upregulation is seen in both (A) Seg-1 and Flo-1 cells after treatment with indomethacin. (C) Flo-1 cells did not express Bcl-2 protein, and this was not altered by treatment with indomethacin. Bcl-2 was expressed by Seg-1 cells. After 12 hours of treatment, the level of Bcl-2 expression did appear to increase slightly, but levels after 24 and 48 hours of treatment were not significantly different from untreated cells. (D) Western blot analysis of cytosolic extracts demonstrating the translocation of cytochrome c to the cytosol after treatment with 100 µM for 30 hours. As a control for mechanical disruption of mitochondria during harvesting, the presence of cytochrome c oxidase was not detected. β-actin is shown in the upper panels.
Expression of the pro-apoptotic protein Bax was also evaluated in both cell lines by Western blot analysis. In both Flo-1 and Seg-1, treatment with 100 µM indomethacin led to upregulation of Bax by 48 hours. Upregulation was seen as early as 24 hours in Seg-1 cells. While Flo-1 cells appeared to express higher levels of Bax at baseline, Bax upregulation was markedly less pronounced when compared with Seg-1 (Figure 6A and B). As seen in Figure 4, Seg-1 cells demonstrate a greater sensitivity to indomethacin-induced apoptosis. An interesting observation is that this seems to correlate with the enhanced upregulation of Bax in these cells, which express COX-2. One component of Bax's pro-apoptotic effect lies in its ability to induce the release of mitochondrial cytochrome c into the cytoplasm. To determine the functional consequence of Bax upregulation, translocation of mitochondrial cytochrome c was evaluated. Although Bax upregulation was more evident in Seg-1 cells, the upregulation of Bax in both cell lines was accompanied by the release of mitochondrial cytochrome c (Figure 6D). To verify that the observed cytoplasmic cytochrome c was not due to mechanical disruption of the mitochondria, simultaneous analysis was done for cytochrome c oxidase, which was not detected in the cytosolic extracts of any samples.
Effect of the Caspase 9 Inhibitor, z-LEHD-FMK, on Indomethacin-Induced Apoptosis in Esophageal Adenocarcinoma Cell Lines
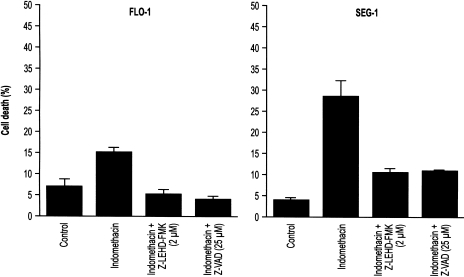
The release of mitochondrial cytochrome c is a pivotal event in the caspase 9-mediated apoptotic pathway. Cytochrome c within the cytoplasm subsequently binds to APAF-1, which in turn associates with and activates caspase 9. To verify the involvement of caspase 9 in indomethacin-induced apoptosis, cells were pre-treated with z-LEHD-FMK, a specific caspase 9 inhibitor. Pre-treatment with z-LEHD-FMK blocked indomethacin-induced apoptosis in both Flo-1 and Seg-1 (Figure 7). Pre-treatment with z-VAD-FMK, a broad caspase family inhibitor, also attenuated the effects of indomethacin on apoptotic cell death. Cells treated with z-LEHD-FMK and z-VAD-FMK alone demonstrated no change in the rate of cell death. These results support the involvement of caspases, specifically a Bax-mediated caspase 9 pathway, in the apoptotic effect of this drug.
Figure 7.
Treatment of Flo-1 and Seg-1 cells with the selective caspase 9 inhibitor, Z-LEHD-FMK, and the broad-spectrum caspase inhibitor, z-VAD-FMK. Cells were pretreated with the caspase inhibitors for 12 hours prior to treatment with indomethacin for 60 hours. Viability was assessed by trypan exclusion. To test if the inhibitors alone exerted any cytotoxic effect, cells treated only with the caspase inhibitors were also examined and showed no difference in viability from untreated cells (data not shown). The graphs show nonviable cells expressed as a percentage of the total cell population harvested.
Discussion
Deregulation of apoptosis or normal programmed cell death is one well-recognized component of carcinogenesis [32]. Damage to cellular DNA is an important stimulus for a cell to undergo apoptosis. In this setting, failure of this process to occur can allow a cell sustaining mutational events to undergo uncontrolled proliferation, leading to tumor formation. Studies have demonstrated the ability of several NSAIDs to induce apoptosis in cancer cells in vitro [19,25]. The hypothesis that NSAID-induced apoptosis occurs by inhibition of COX-2 is lent support by studies that have demonstrated upregulation of the COX-2 protein within tumors, and by the chemopreventive effect observed with selective COX-2 inhibition in animal models of carcinogenesis [33,34]. However, it has also been shown that NSAIDs such as salicylic acid and the sulfone derivative of sulindac, which do not possess any anti-cyclooxygenase activity, also induce apoptosis in vitro as well as inhibiting tumor growth in vivo [19,21]. Elder et al., [35] have shown that the selective COX-2 inhibitor, NS-398, induced apoptotic cell death in two different human colorectal carcinoma cell lines regardless of COX-2 expression. Such data question the importance of COX-2 as the sole mediator of the antineoplastic effects of NSAIDs.
Our characterization of COX-2 expression demonstrated abundant protein levels in Barrett's epithelium. Morphologically, staining was strongest along the epithelial surface and in the glandular structures of the lamina propria. In Barrett's mucosa with dysplasia, while staining was observed along the epithelial surface, there appeared to be decreased levels of staining within the areas of dysplasia as compared to the nondysplastic mucosa (Figure 1C). In addition, a higher percentage of Barrett's mucosa with dysplasia demonstrated positive staining for COX-2 within the smooth muscle of the esophagus (65%, n = 17) when compared with nondysplastic Barrett's mucosa (17%, n = 12). The significance of increased staining in the smooth muscle of dysplastic Barrett's in unclear to us at the present time. Analysis of esophageal adenocarcinomas demonstrated expression of COX-2 protein in 82% of tumors. Forty percent of these tumors stained at low- and mid-range intensities of 1 or 2. The pattern of staining in nearly all tumors was markedly heterogenous, with many tumor cells in a section demonstrating no staining for COX-2. In addition, RT-PCR analysis showed that in four of eight patients, COX-2 mRNA expression could be detected in both the tumor sample and the paired normal esophageal squamous mucosa, with one patient having higher levels of COX-2 mRNA in the normal squamous epithelium versus the tumor sample. These results differ from those previously reported by Zimmermann et al., [11]. The finding of COX-2 mRNA in the normal epithelium of some patients may be explained by immunohistochemical localization of COX-2 protein to inflammatory cells and smooth muscle within the stroma of some sections, and thus, may represent the degree of inflammation present in histologically normal squamous tissue. Shattuck-Brandt et al., [36] have previously reported increased COX-2 mRNA and protein in noncancerous inflamed colonic tissue of IL-10(-/-) mice, where COX-2 protein was localized to inflammatory cells and subepithelial myofibroblasts. Our results suggest that the temporal sequence of COX-2 expression in esophageal carcinogenesis may be expression early during the development of Barrett's epithelium, but then diminished expression in dysplasia and then carcinoma. The decreased expression of COX-2 in areas of low- and high-grade dysplasia, as demonstrated by our immunohistochemical analysis, lends support to the notion that COX-2 may not be the only critical target in the chemopreventive effect of NSAIDs.
The three esophageal adenocarcinoma cell lines used in this study provided us with a valuable model for studying the dependency of indomethacin's effects on cyclooxygenase expression. Despite differences in expression of COX-1 and COX-2, indomethacin was able to induce apoptosis in all three cell lines. Also, the dose ranges at which apoptosis was observed were greater than the doses required to inhibit cyclooxygenase activity, implying the involvement of other pathways. The differences in drug delivery in vitro and in vivo are well established, making it difficult to correlate effective in vivo dosages based on results seen in vitro. Epidemiological evidence, however, also demonstrates that the chemopreventive effect of NSAIDs is dose-dependent, with increasing antitumor effects seen at doses beyond the anti-inflammatory range [2]. The anticancer effect of these drugs, thus, may not be limited to the inhibition of COX-2.
Our data support the hypothesis that a component of the chemopreventive effect of NSAIDs, such as indomethacin, in esophageal adenocarcinomas may be induced through the induction of apoptosis. The ability of indomethacin to induce apoptosis independently of COX-2 expression prompted us to investigate specific apoptotic effectors involved in the cell death of Seg-1, which was the only cell line to constitutively express COX-2, and Flo-1, which did not express significant levels of either cyclooxygenase isoform. The Bcl-2 family of proteins is a well-known regulator of the apoptotic response. Both Bcl-2 and Bcl-XL have been shown to have anti-apoptotic effects on the cell. This may occur through their ability to sequester APAF-1, and by inhibition of pro-apoptotic mitochondrial changes such as loss of the transmembrane potential and the release of cytochrome c [37,38]. Neither control or indomethacin-treated Flo-1 cells expressed Bcl-2. Constitutive levels of Bcl-2 in Seg-1 cells increased slightly after treatment with indomethacin at 12 hours, but returned to baseline by 24 hours (Figure 6C). Interestingly, the behavior of control Flo-1 and Seg-1 cells in culture seemed to correlate with the expression of Bcl-2. Flo-1 cells, which do not express the protein, demonstrate a higher basal rate of apoptosis than Seg-1.
Bax is a member of this class of proteins which has been shown to have pro-apoptotic effects on the cell [37]. By acting on VDAC, a mitochondrial membrane channel, Bax causes translocation of mitochondrial cytochrome c. Once in the cytoplasm, cytochrome c binds to APAF-1, with subsequent cleavage of procaspase 9 to the active form [39]. The initiator caspase 9 then activates downstream effector caspases, such as caspase 3, to bring about apoptotic death. Bax has been shown to be functional in apoptosis induced by certain chemotherapeutic agents and gamma radiation [37]. In this study, indomethacin caused increased expression of Bax in both Flo-1 and Seg-1 cells. The degree of upregulation was more pronounced in Seg-1 cells, and this finding correlated with an increased sensitivity of these cells to indomethacin-induced apoptosis. Interestingly, while the expression of COX-2 was not required for indomethacin-induced apoptosis (as seen in Flo-1 and Bic-1 cells), the expression of COX-2 in Seg-1 cells correlated with enhanced upregulation of Bax and an increased sensitivity of Seg-1 cells to drug treatment, when compared to Flo-1 cells. To verify the functional consequence of Bax upregulation, we also demonstrate that Bax upregulation was accompanied by the release of mitochondrial cytochrome c into the cytoplasm. The ability of both a caspase 9-specific peptide inhibitor and a broad-spectrum caspase inhibitor to attenuate this affect provides further support for the involvement of this pathway. To our knowledge, this is one of the first studies to demonstrate that indomethacin-induced apoptosis in esophageal adenocarcinoma cells is associated with increased expression of the pro-apoptotic protein Bax, mitochondrial cytochrome c translocation, and caspase 9 activation. These data provide evidence that the critical target in NSAID-induced apoptosis in esophageal cancer cells may not be limited to the inhibition of the cyclooxygenase enzyme, but may also be the ability of such agents to alter expression of critical Bcl-2 class proteins. This may have important implications for identifying new targets, such as initiator caspases and Bcl-2 family regulatory proteins, in the development of chemopreventive and chemotherapeutic strategies against this disease.
Acknowledgements
We thank Neeju Ravikant for technical assistance and Alane Koki for helpful discussions. We also thank Amy Pace for assistance in preparing the figures.
Footnotes
This study was funded, in part, by the National Institutes of Health grant CA71606, and National Institutes of Health training grant CA009672-08 (to S. Aggarwal).
References
- 1.Blot WJ, McLaughlin JK. The changing epidemiology of esophageal cancer. Sem Oncol. 1999;26:2–8. [PubMed] [Google Scholar]
- 2.Thun MJ, Namboodiri MM, Heath CW., Jr Aspirin use and reduced risk of fatal colon cancer. N Engl J Med. 1991;325:1593–1596. doi: 10.1056/NEJM199112053252301. [DOI] [PubMed] [Google Scholar]
- 3.Thun MJ. NSAID use and decreased risk of gastrointestinal cancers. Gastroenterol Clin North Am. 1996;25:333–348. doi: 10.1016/s0889-8553(05)70250-8. [DOI] [PubMed] [Google Scholar]
- 4.Narisawa T, Sato M, Tani M, Kudo T, Takahashi T, Goto A. Inhibition of development of methylnitrosourea-induced rat colon tumors by indomethacin treatment. Cancer Res. 1981;41:1954–1957. [PubMed] [Google Scholar]
- 5.Barnes CJ, Lee M. Chemoprevention of spontaneous intestinal adenomas in the adenomatous polyposis coli Min mouse model with aspirin. Gastoenterology. 1998;114:873–877. doi: 10.1016/s0016-5085(98)70305-1. [DOI] [PubMed] [Google Scholar]
- 6.Williams CS, Mann M, DuBois RN. The role of cyclooxygenases in inflammation, cancer, and development. Oncogene. 1999;18:7908–7916. doi: 10.1038/sj.onc.1203286. [DOI] [PubMed] [Google Scholar]
- 7.Simmons DL, Levy DB, Yannoni Y, Erikson RL. Identification of a phorbol ester-repressible v-src-inducible gene. Proc Natl Acad Sci U S A. 1989;86:1178–1182. doi: 10.1073/pnas.86.4.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sano H, Kawahito Y, Wilder RL, Hashiramoto A, Mukai S, Asai K, Kimura S, Kato H, Kondo M, Hla T. Expression of cyclooxygenase-1 and -2 in human colorectal cancer. Cancer Res. 1995;55:3785–3789. [PubMed] [Google Scholar]
- 9.Yip-Schneider MT, Barnard DS, Billings SD, Cheng L, Heilman DK, Lin A, Marshall SJ, Crowell PL, Marshall MS, Sweeney CJ. Cyclooxygenase-2 expression in human pancreatic adenocarcinomas. Carcinogenesis. 2000;21:139–146. doi: 10.1093/carcin/21.2.139. [DOI] [PubMed] [Google Scholar]
- 10.Gupta S, Srivastava M, Ahmad N, Bostiwick DG, Mukhtar H. Overexpression of cyclooxygenase-2 in human prostate adenocarcinoma. Prostate. 2000;42:73–78. doi: 10.1002/(sici)1097-0045(20000101)42:1<73::aid-pros9>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 11.Zimmermann KC, Sarbia M, Weber AA, Borchard F, Gabbert HE, Schror K. Cyclooxygenase-2 expression in human esophageal carcinoma. Cancer Res. 1999;59:198–204. [PubMed] [Google Scholar]
- 12.Wolff H, Saukkonen K, Anttila S, Karjalainen A, Vainio H, Ristimaki A. Expression of cyclooxygenase-2 in human lung carcinoma. Cancer Res. 1998;58:4997–5001. [PubMed] [Google Scholar]
- 13.Mitchell JA, Evans TW. Cyclooxygenase-2 as a therapeutic target. Inflammation Res. 1998;47:S88–S92. doi: 10.1007/s000110050287. [DOI] [PubMed] [Google Scholar]
- 14.Oshima M, Dinchuk E, Kargman SL, Oshima H, Hancock B, Kwong E, Trzaskos JM, Evans JF, Taketo MM. Suppression of intestinal polyposis in ApcΔ716 knockout mice by inhibition of cyclooxygenase-2 (COX-2) Cell. 1996;87:803–809. doi: 10.1016/s0092-8674(00)81988-1. [DOI] [PubMed] [Google Scholar]
- 15.Tsujii M, DuBois RN. Alterations in cellular adhesion and apoptosis in epithelial cells overexpressing prostaglandin endoperoxide synthase 2. Cell. 1995;83:493–501. doi: 10.1016/0092-8674(95)90127-2. [DOI] [PubMed] [Google Scholar]
- 16.Beazer-Barclay Y, Levy DB, Moser AR, Dove WF, Hamilton SR, Vogelstein B, Kinzler KW. Sulindac suppresses tumor-igenesis in the Min mouse. Carcinogenesis. 1996;17:1757–1760. doi: 10.1093/carcin/17.8.1757. [DOI] [PubMed] [Google Scholar]
- 17.Giardiello FM, Hamilton SR, Krush AJ, Piantadosi S, Hylind LM, Celano P, Booker SV, Robinson CR, Offerhaus GJ. Treatment of colonic and rectal adenomas with sulindac in familial adenomatous polyposis. N Engl J Med. 1993;328:1313–1316. doi: 10.1056/NEJM199305063281805. [DOI] [PubMed] [Google Scholar]
- 18.Rao CV, Rivenson A, Simi B, Zang E, Keloff G, Steele V, Reddy BS. Chemoprevention of colon carcinogenesis by sulindac, a nonsteroidal anti-inflammatory agent. Cancer Res. 1995;55:1464–1472. [PubMed] [Google Scholar]
- 19.Piazza GA, Rahm AL, Krutzsch M, Sperl G, Paranka NS, Gross PH, Brendel K, Burt RW, Alberts DS, Pamukcu R, Ahnen DJ. Antineoplastic drugs sulindac sulfide and sulfone inhibit cell growth by inducing apoptosis. Cancer Res. 1995;55:3110–3116. [PubMed] [Google Scholar]
- 20.Shiff SJ, Qiao L, Tsai LL, Rigas B. Sulindac sulfide, an aspirin-like compound, inhibits proliferation, causes cell cycle quiescence, and induces apoptosis in HT-29 colon adenocarcinoma cells. J Clin Invest. 1995;96:491–503. doi: 10.1172/JCI118060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Piazza GA, Alberts DS, Hixson LJ, Paranka NS, Li H, Finn T, Boger C, Guillen JM, Brendel K, Gross PH, et al. Sulindac sulfone inhibits azoxymethane-induced colon carcinogenesis in rats without reducing prostaglandin levels. Cancer Res. 1997;57:2909–2915. [PubMed] [Google Scholar]
- 22.Narisawa T, Hermanek P, Habs M, Schmahl D. Reduction of carcinogenicity of N-nitrosomethylurea by indomethacin and failure of resuming effect of prostaglandin E2 (PGE2) against indomethacin. J Cancer Res Clin Oncol. 1984;108:239–242. doi: 10.1007/BF00402475. [DOI] [PubMed] [Google Scholar]
- 23.Piazza GA, Rahm AK, Finn TS, Fryer BH, Li H, Stoumen AL, Pamukcu R, Ahnen DJ. Apoptosis primarily accounts for the growth-inhibitory properties of sulindac metabolites and involves a mechanism that is independent of cyclooxygenase inhibition, cell cycle arrest, and p53 induction. Cancer Res. 1997;57:2452–2459. [PubMed] [Google Scholar]
- 24.De Mello MC, Bayer BM, Beavon MA. Evidence that prostaglandins do not have a role in the cytostatic action of anti-inflammatory drugs. Biochem Pharmacol. 1980;29:311–318. doi: 10.1016/0006-2952(80)90506-7. [DOI] [PubMed] [Google Scholar]
- 25.Lu X, Xie W, Reed D, Bradshaw WS, Simmons DL. Nonsteroidal anti-inflammatory drugs cause apoptosis and induce cyclooxygenases in chicken embryo fibroblasts. Proc Natl Acad Sci U S A. 1995;92:7961–7965. doi: 10.1073/pnas.92.17.7961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Masferrer JL, Isakson PC, Seibert K. Cyclooxygenase-2 inhibitors: a new class of anti-inflammatory agents that spare the gastrointestinal tract. Gastroenterol Clin North Am. 1996;25:363–372. doi: 10.1016/s0889-8553(05)70252-1. [DOI] [PubMed] [Google Scholar]
- 27.Nunez G, Benedict MA, Hu Y, Inohara N. Caspases: the proteases of the apoptotic pathway. Oncogene. 1998;17:3237–3245. doi: 10.1038/sj.onc.1202581. [DOI] [PubMed] [Google Scholar]
- 28.Nambu Y, Hughes SJ, Rehemtulla A, Hamstra D, Orringer MB, Beer DG. Lack of cell surface Fas/APO-1 expression in pulmonary adenocarcinomas. J Clin Invest. 1998;101:1102–1110. doi: 10.1172/JCI1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hughes SJ, Nambu Y, Soldes OS, Hamstra D, Rehemtulla A, Iannettoni MD, Orringer MB, Beer DG. Fas/APO-1 is not translocated to the cell membrane in esophageal adenocarcinoma. Cancer Res. 1997;57:5571–5578. [PubMed] [Google Scholar]
- 30.Rehemtulla A, Hamilton CA, Chinnaiyan AM, Dixit V. Ultraviolet radiation-induced apoptosis is mediated by activation of CD-95 (Fas/APO-1) J Biol Chem. 1997;272:25783–25786. doi: 10.1074/jbc.272.41.25783. [DOI] [PubMed] [Google Scholar]
- 31.Finucane DM, Bossy-Wetzel E, Waterhouse NJ, Cotter TG, Green DR. Bax-induced caspase activation and apoptosis via cytochrome c release from mitochondria is inhibitable by Bcl-XL. J Biol Chem. 1999;274:2225–2233. doi: 10.1074/jbc.274.4.2225. [DOI] [PubMed] [Google Scholar]
- 32.Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456–1462. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- 33.Reddy BS, Rao CV, Seibert K. Evaluation of cyclooxygenase-2 inhibitor for potential chemopreventive properties in colon carcinogenesis. Cancer Res. 1996;56:4566–4569. [PubMed] [Google Scholar]
- 34.Kawamori T, Rao CV, Seibert K, Reddy BS. Chemopreventive activity of celecoxib, a specific cyclooxygenase-2 inhibitor, against colon carcinogenesis. Cancer Res. 1998;58:409–412. [PubMed] [Google Scholar]
- 35.Elder DJ, Halton D, Hague A, Paraskeva C. Induction of apoptotic cell death in human colorectal carcinoma cell lines by a cyclooxygenase-2 (COX-2)-selective nonsteroidal anti-inflammatory drug: independence from COX-2 protein expression. Clin Cancer Res. 1997;3:1679–1683. [PubMed] [Google Scholar]
- 36.Shattuck-Brandt RL, Varilek GW, Radhika A, Yang F, Washington MK, DuBois RN. Cyclooxygenase-2 expression is increased in the stroma of colon carcinomas from IL-10(-/-) mice. Gastroenterology. 2000;118:337–345. doi: 10.1016/s0016-5085(00)70216-2. [DOI] [PubMed] [Google Scholar]
- 37.Adams JM, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science. 1998;281:1322–1326. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- 38.Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 39.Nomura M, Shimizu S, Ito T, Narita M, Matsuda H, Tsujimoto Y. Apoptotic cytosol facilitates Bax translocation to mitochondria that involves cytosolic factor regulated by Bcl-2. Cancer Res. 1999;59:5542–5548. [PubMed] [Google Scholar]