Abstract
Acyl-lipid desaturases are enzymes that convert a C–C single bond into a C=C double bond in fatty acids that are esterified to membrane-bound glycerolipids. Four types of acyl-lipid desaturase, namely DesA, DesB, DesC, and DesD, acting at the Δ12, Δ15, Δ9, and Δ6 positions of fatty acids respectively, have been characterized in cyanobacteria. These enzymes are specific for fatty acids bound to the sn−1 position of glycerolipids. In the present study, we have cloned two putative genes for a Δ9 desaturase, designated desC1 and desC2, from Nostoc species. The desC1 gene is highly similar to the desC gene that encodes a Δ9 desaturase that acts on C18 fatty acids at the sn−1 position. Homologues of desC2 are found in genomes of cyanobacterial species in which Δ9-desaturated fatty acids are esterified to the sn−2 position. Heterologous expression of the desC2 gene in Synechocystis sp. PCC 6803, in which a saturated fatty acid is found at the sn−2 position, revealed that DesC2 could desaturate this fatty acid at the sn−2 position. These results suggest that the desC2 gene is a novel gene for a Δ9 acyl-lipid desaturase that acts on fatty acids esterified to the sn-2 position of glycerolipids.
Keywords: cyanobacteria, desaturase, DesC2, fatty acids, glycerolipids, Nostoc sp
Abbreviations: Des, acyl-lipid desaturase; DGDG, digalactosyl diacylglycerol; LB, Luria–Bertani; MGDG, monogalactosyl diacylglycerol; ORF, open reading frame; PG, phosphatidylglycerol; SQDG, sulphoquinovosyl diacylglycerol
INTRODUCTION
Cyanobacteria have been classified into four groups according to the positional distribution of fatty acids that are esterified to specific sn− positions in glycerolipids (for reviews, see [1–4]). Strains in Group 1 (Prochlorothrix hollandica, Synechococcus sp. PCC 6301, Synechococcus sp. PCC 7942, Synechococcus elongatus, Thermosynechococcus elongatus and Thermosynechococcus vulcanus) are characterized by the presence of mono-unsaturated fatty acids at both sn−1 and sn−2 positions. Strains in Group 2 (Anabaena variabilis, Anabaena sp. PCC 7120, Synechococcus sp. PCC 7002, Nostoc punctiforme, Nostoc sp. 36, Trichodesmium erythraeum and Gloeobacter violaceus) contain a tri-unsaturated C18 fatty acid (α-linolenic acid) at the sn−1 position and a mono-unsaturated C16 fatty acid, C16:1(9), at the sn−2 position. Strains in Group 3 (Spirulina platensis and Prochlorococcus marinus) have a tri-unsaturated C18 fatty acid (γ-linolenic acid) at the sn−1 position and a C16:0 (saturated C16 acid), at the sn−2 position. Strains in Group 4 (Synechocystis sp. PCC 6803) are similar to strains in Group 3 except that they have, in addition, tri-unsaturated C18 (α-linolenic acid) and tetra-unsaturated C18 fatty acids (C18:4) at the sn-1 position.
Fatty acid desaturases are enzymes that convert a C–C single bond into a C=C double bond in a fatty-acyl chain [5]. In particular, Des (acyl-lipid desaturases) proteins act on fatty acids that are esterified to the glycerol backbone of glycerolipids. Several acyl-lipid desaturases and their genes have been characterized in cyanobacteria [6] and plants [7–10]. In general, desaturases have strict specificity with respect to the position in the fatty acid at which a double bond is introduced, and to the sn-position of the glycerol moiety of glycerolipids to which fatty acids are esterified. Wada et al. [11] cloned the desA gene encoding DesA, which introduces a double bond at the Δ12 position of fatty acids at the sn-1 position, from Synechocystis sp. PCC 6803 (hereafter termed Synechocystis sp.). Subsequently, genes for various acyl-lipid desaturases were cloned from a variety of cyanobacteria, such as Synechocystis sp. [12–14], Synechocystis PCC 6714 [15], Synechococcus PCC 7002 [15,16], Synechococcus vulcanus [17], Spirulina platensis [18], Anabaena variabilis [14,15] and Anacystis nidulans (now reclassified as Synechococcus sp. PCC 6301; [19]).
Des proteins and their genes have been extensively studied in Synechocystis sp., which belongs to Group 4 cyanobacteria [3,20]. This cyanobacterium encodes four Des proteins, DesA, DesB, DesC and DesD, that introduce a double bond at the Δ12, Δ15 (ω3), Δ9 and Δ6 positions of the C18 fatty acid at the sn−1 position respectively [3,12–14,20–22]. In Spirulina platensis, a strain in Group 3, three genes for acyl-lipid desaturases named desA, desC and desD have been cloned [18]. In addition, three genes for acyl-lipid desaturases named desA, desB and desC have also been cloned from the cyanobacterial strains Anabaena variabilis [14,15] and Synechococcus sp. PCC7002, which are characterized in Group 2 [16,23]. The strict specificity of DesC from Synechocystis sp. with respect to the sn−1 position has been definitively established [14]. However, the specificity of DesC with respect to the sn−1 position is unclear in the case of acyl-lipid desaturases of cyanobacteria that belong to Groups 1 and 2. Cyanobacterial strains in Groups 1 and 2 introduce a double bond into fatty acids at both the sn−1 and the sn−2 position [4]. Therefore it is conceivable that DesC from these strains might be unspecific with respect to a particular sn− position or, alternatively, that two Δ9 desaturases might exist, each of which catalyses Δ9 desaturation at a specific position, either sn−1 or sn−2.
Earlier studies have clearly demonstrated that when mesophilic cyanobacteria that grow optimally at 35 °C are subjected to low-temperature stress, by a downward shift in growth temperature to 25 °C, they can overcome this stress by increasing the synthesis of polyunsaturated fatty acids. The synthesis of the polyunsaturated fatty acids in cyanobacteria is catalysed by fatty acyl-lipid desaturases, encoded for by the des genes (desA, desB, desC and desD) [3,22]. In Synechocystis sp. PCC 6803, cold stress induced the expression of desA, desB and desD [3,22]. Further, the importance of the des genes with respect to cold adaptation has been unequivocally demonstrated by the observations that cyanobacterial mutants defective in these genes are cold-sensitive and grow slower than the wild-type cells [3,22]. These studies clearly indicated that polyunsaturated fatty acids and fatty acyl-lipid desaturases are essential for the acclimation of cyanobacteria to low temperatures [3,22]. However, in contrast with mesophilic cyanobacteria, psychrotolerant cyanobacteria grow optimally at 25 °C and are also capable of growing at 10 °C, a temperature at which the mesophilic cyanobacteria barely grow. Therefore it would be of interest to identify and characterize the des genes to ascertain the expression patterns of these genes, which will establish whether they are crucial for low-temperature survival in psychrotolerant cyanobacteria that are already adapted to low-temperature survival and growth. As part of this long-term project, the present study was undertaken on Nostoc sp. strain SO-36, a psychrotolerant strain isolated from a lake in Antarctica.
In the present study, we cloned two desC homologous genes from Nostoc sp. strain SO-36 (hereafter termed Nostoc sp.), which belongs to Group 2, and demonstrated that one of these genes encodes a Δ9 desaturase that introduces double bonds in fatty acids that are bound to the sn−2 position of the glycerol moiety of membrane glycerolipids. We designated this gene desC2 and the other gene desC1.
EXPERIMENTAL
Bacterial strains and growth conditions
Nostoc sp. strain SO-36 was isolated from a water sample from an Antarctic lake and identified as Nostoc sp. on the basis of its filamentous morphology, binary fission and characteristic tri-chomes, which are neither branched nor tapered and are made up of cells of equal size. The strain grows at temperatures between 10 and 30 °C. The partial sequence of the gene for 16 S rRNA from this micro-organism was highly similar to that of Nostoc punctiforme and Nostoc commune, indicating that all three micro-organisms are closely related to one another (results not shown). Synechocystis sp. PCC 6803 was obtained originally from Dr J. G. K. Williams (DuPont de Nemours and Co., Wilmington, DE, U.S.A.). Nostoc sp. and Synechocystis sp. were grown at 25 °C in BG-11 medium [24] supplemented with 10 mM Hepes buffer, pH 8.0, in light from a tungsten lamp at 350 and 3500 lux respectively, with a constant supply of 1% CO2 in air.
Escherichia coli DH10B cells, which served as the host for cloning, were grown at 37 °C in LB (Luria–Bertani) medium that contained 1% (w/v) tryptone, 0.5% (w/v) yeast extract and 1% (w/v) sodium chloride. The final pH of the medium was 7.2.
Analysis of fatty acid composition and the positional distribution of fatty acids in MGDG (monogalactosyl diacylglycerol)
Total cell lipids were extracted as described by Bligh and Dyer [25] and fatty acids were analysed essentially as described by Sato and Murata [26]. Lipase from Rhizopus delemar (Seikagaku Kogyo, Tokyo, Japan), which specifically dissociates fatty acids at the sn−1 position of polar glycerolipids, was used to identify fatty acids esterified to the sn−1 and sn−2 positions of the glycerol moieties of glycerolipids [27]. In this study, the major lipid fraction MGDG was purified by TLC and then treated with lipase from R. delemar to liberate the fatty acids esterified to the sn−1 position of polar glycerolipids and to simultaneously generate the 2-monoacyl product. The fatty acid at the sn−2 position was determined by analysing the fatty acids in the lysolipid. In addition, the fatty acid at the sn−1 position was determined by comparing the fatty acid composition of undigested MGDG with the fatty acid composition of the 2-monoacyl product.
Cloning of the desC genes
The genome of Anabaena sp. PCC 7120 includes two putative desC homologous genes. The nucleotide sequence of one is more similar than the other to that of the desC gene from Synechocystis sp. The former gene is designated desC1 and the latter is desC2. Referring to the sequences of these genes, we designed primers for PCR: desC1F (5'-ACTCAAAGGGACTGTTTCTGGTGG-3') and desC1R (5'-TGAGTGAGTTAGTTAGTGCCA-3') for amplification of desC1; and desC2F (5'-CGCCAGCATCATGCTCACACCGAAG-3') and desC2R (5'-ATGGTTGTCTTGCTAAATCAGGCGC-3') for amplification of desC2. We extracted and purified the genomic DNA from Nostoc sp. as described by Williams [28] and used this for PCR amplification using the above primers, which produced 350 bp and 160 bp fragments with sequences that were identical with the partial sequences of the desC1 and desC2 genes from Anabaena sp. PCC 7120 respectively. These PCR products were used in the following screening step.
Two partial genomic libraries of Nostoc sp. were constructed. One was constructed by digestion of the genomic DNA of Nostoc sp. with EcoRI and HindIII for cloning of the desC1 gene. Electrophoresis on a 0.8% agarose gel yielded fragments of approx. 6 kb that were recovered from the agarose gel using a silica-gel-membrane column (Qiagen GmbH, Hilden, Germany). The other library was constructed by digestion with DraI for cloning of the desC2 gene, and electrophoresis on a 0.8% agarose gel yielded fragments of approx. 1.3 kb. The fragments were ligated to pBluescript II KS(+) (Stratagene, La Jolla, CA, U.S.A.) that had been digested with EcoRI and HindIII or with DraI (for cloning of the desC1 or the desC2 genes respectively) and dephosphorylated with shrimp alkaline phosphatase (Boehringer-Mannheim GmbH, Mannheim, Germany). The ligation mixture was then used to transform E. coli DH10B cells by electroporation. Transformed cells were selected on agar-solidified LB medium with 150 μg/ml ampicillin. Screening of the two libraries was performed using the products of PCR as probes, using the method described by Sambrook et al. [29], which under high-stringency conditions yielded a single positive clone (pC36C) with an ORF (open reading frame) of 822 bp that corresponded to desC1, and a single clone (pC36C2) with an ORF of 855 bp that corresponded to desC2. Sequencing confirmed that the ORFs corresponded to the full-length desC1 and desC2 genes.
Conjugal transfer of the desC2 gene into Synechocystis sp.
Plasmid pC36C2 was digested with ClaI and SmaI to release the desC2 gene from the vector. The desC2 gene was then cloned into the shuttle vector pVZ321 [30], which carries kanamycin- and chloramphenicol-resistance genes (the nucleotide sequence of the plasmid is available in GenBank® under accession number AF100175), to generate pVZC2. E. coli DH5α cells were then transformed with pVZC2 by heat shock at 42 °C for 90 s as described previously [22]. E. coli DH5α cells were also transformed with a control vector pVZ321. The resultant transformants of E. coli DH5α were isolated on plates of agar-solidified LB medium with 20 μg/ml chloramphenicol. The presence of the desC2 gene was confirmed by PCR, using the plasmid isolated from transformants as the template. The plasmid was then transferred from E. coli DH5α to Synechocystis sp. by tri-parental mating, using the method described previously [30]. E. coli DH5α cells transformed with pVZ321 or pVZC2 were used as donor strains to generate control and transformed strains of Synechocystis sp. respectively, with E. coli R591 cells as the helper strain. Donor and helper cells were grown to stationary phase, while the recipient cells (Synechocystis sp.) were grown to a turbidity (D730) of 0.5. Suspensions of donor, helper and recipient cells were mixed in the following proportions, 1:1:10 (by vol.; total volume 1.2 ml). The cells were collected by centrifugation at 1000 g for 1 min in a microcentrifuge tube, then rinsed with BG-11 medium. After re-suspension in 40 μl of BG-11 medium, the cells were transferred on to a sterile Durapore™ membrane filter (13 mm diameter, 22 μm pore size; Millipore, Billerica, MA, U.S.A.). Membranes were placed on plates of agar-solidified BG-11 and 5% (v/v) LB medium and incubated overnight at 34 °C in light at 900 lux. Each membrane was then placed in a micro-centrifuge tube, to which 200 μl of BG-11 medium was added. Mixing on a vortex mixer separated the Synechocystis and E. coli cells from the membrane. The washed membrane was removed and the released cells were collected by centrifugation at 1000 g for 5 min. The supernatant was discarded, the cells were washed with fresh BG-11 medium and then pelleted by centrifugation as described above. The cells were then spread on agar-solidified BG-11 medium that contained 50 μg/ml trimethoprim, 20 μg/ml chloramphenicol and 25 μg/ml kanamycin for isolation of pVZ321/PCC 6803 transformants and 50 μg/ml trimethoprim and 20 μg/ml chloramphenicol for isolation of pVZC2/PCC 6803 transformants. The plates were incubated in light at 350 lux at 34 °C. Resultant colonies were lifted on to fresh plates.
RESULTS AND DISCUSSION
The fatty acid composition and positional distribution of fatty acids in Nostoc sp. strain SO-36 indicate that it belongs to Group 2 cyanobacteria
Cyanobacteria contain four classes of lipids [6,27,31,32], which are MGDG, PG (phosphatidylglycerol), SQDG (sulphoquinovosyl diacylglycerol) and DGDG (digalactosyl diacylglycerol). MGDG accounts for more than 50% of the total lipids. In the present study, lipid analysis revealed that the major lipid classes in Nostoc sp. are MGDG, DGDG, PG, and SQDG and that MGDG accounts for 55% of the total lipids (results not shown).
Fatty acid analysis indicated that the predominant fatty acids in total lipids were C16:0, C16:1(9), C18:0, C18:1(9), C18:2(9,12) and C18:3(9,12,15), with their composition in MGDG shown in Table 1. The distribution of fatty acids at the sn-positions of the glycerol backbone of MGDG revealed that the C18 fatty acids were present predominantly at the sn−1 position, whereas the C16 fatty acids were present exclusively at the sn−2 position of the glycerol moiety (Table 1). Moreover, the C16 fatty acid bound to sn−2 was strongly desaturated. The presence of C18:3(9,12,15) and C16:1(9) at the sn−1 and the sn−2 positions respectively indicated that the Nostoc sp. belongs to Group 2 of cyanobacteria with respect to the distribution of fatty acids at specific sn-positions.
Table 1. Fatty acid composition of total lipids, in MGDG and at sn−1 and sn−2 positions of MGDG of Nostoc sp. (strain 36).
Results are means±S.D. of three experiments and are expressed as mol% of total fatty acids. T implies a trace amount that was less than 0.5 mol%. ND, not detected.
| Fatty acid (mol% of total) | ||||
|---|---|---|---|---|
| Lipid | Total lipids | MGDG | sn−1 of MGDG | sn−2 of MGDGTT |
| C16:0 | 12.0±0.3 | 6.0±0.6 | T | 5.0±0.3 |
| C16:1(9) | 30.0±0.4 | 41.0±0.7 | T | 41.0±0.5 |
| C18:0 | 4.0±0.1 | 1.2±0.2 | T | T |
| C18:1(9) | 17.0±0.3 | 18.0±0.4 | 15.0±0.3 | 3.0±0.2 |
| C18:1(11) | 1.0±0.3 | T | T | T |
| C18:2(9,12) | 29.0±0.5 | 27.0±0.5 | 27.0±0.5 | T |
| C18:3(9,12,15) | 7.0±0.3 | 7.0±0.3 | 7.0±0.2 | ND |
Characteristics of DesC1 and DesC2 of Nostoc sp.
To examine the details of the desaturation of fatty acids in Nostoc sp., we cloned two desC homologous genes from this organism and determined their nucleotide sequences (accession numbers AJ621244 for desC1 and AJ621247 for desC2 in the NCBI database). These two genes were found at separate loci of the Nostoc sp. genome. The desC1 gene encodes an ORF of 822 bp and its predicted product contains 274 amino acids. The desC2 gene encodes an ORF of 855 bp and its predicted product contains 285 amino acids. The predicted amino acid sequence of DesC1 is more similar than DesC2 to the predicted amino acid sequence of DesC of Synechocystis sp., with 77% and 65% similarity respectively. Since DesC from Synechocystis sp. catalyses desaturation exclusively at the sn−1 position [3,14,32], we predicted that DesC1 would desaturate fatty acids at the sn-1 position. Thus we postulated that DesC2 of Nostoc sp. would catalyse the desaturation of C16 saturated fatty acids at the sn-2 position.
Functional expression of DesC2 in Synechocystis sp. introduces Δ9 desaturation at the sn−2 position
To examine whether DesC2 acts on fatty acids at the sn−2 position, we transformed Synechocystis sp., which cannot desaturate fatty acids at the sn−2 position, with the desC2 gene from Nostoc sp. by tri-parental mating, as described in the Experimental section. The distribution of fatty acids at sn−1 and sn−2 positions of MGDG indicated that in Synechocystis sp. cells that had been transformed with the desC2 gene (pVZC2), there was a prominent increase in the level of C16:1 at the sn−2 position (Table 2). These results suggest, that when DesC2 is expressed in Synechocystis sp., it catalyses the desaturation of fatty acids at the sn−2 position. We also observed that the level of C16:0 at the sn−1 position decreased, even though it is normally only a minor constituent at this sn− position. This observation suggests that DesC2 might also act on fatty acids at the sn−1 position.
Table 2. Distribution of fatty acids at sn−1 and sn−2 positions of MGDG from Synechocystis sp. cells that had been transformed with either pVZ321 (control) or pVZC2 carrying the desC2 gene from Nostoc sp. (strain 36).
Results are means±S.D. from three independent experiments, and are expressed as mol% of total fatty acids. T implies a trace amount that corresponded to less than 0.5 mol%. ND, not detected.
| Fatty acid (mol %) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Vector | Position | C16:0 | C16:1(9) | C18:0 | C18:0(9) | C18:2(9,12) | C18:3(6,9,12) | C18:3(9,12,15) | C18:4(6,9,12,15) |
| pVZ321 | sn−1 | 3±0.2 | 2±0.3 | T | T | 5±0.3 | 29±0.4 | 0.7±0.2 | 9±0.3 |
| sn−2 | 49±0.4 | 0.5±0.2 | T | T | ND | T | ND | ND | |
| pVZC2 | sn−1 | T | 4.0±0.3 | T | 1.5±0.3 | 5.6±0.3 | 28.5±0.3 | 1.0±0.1 | 9.0±0.3 |
| sn−2 | 11.8±0.3 | 36.1±0.4 | T | 0.9±0.2 | T | ND | ND | ND | |
Homologues of DesC1 and DesC2 in cyanobacteria
The complete nucleotide sequences of the genomes of several strains of cyanobacteria (http://www.kazusa.or.jp/cyano/) indicate that each strain has one or two putative desC genes for Δ9 acyllipid desaturases that are homologous with the desC gene of Synechocystis sp. Genes homologous with the desC2 gene are found in genomes of cyanobateria that belong to Group 2, namely Anabaena sp. PCC 7120, Anabaena variabilis, and Nostoc punctiforme, and also in Thermosynechococcus elongatus, which belongs to Group 1. By contrast, there is apparently no desC2 gene in Spirulina platensis and Synechocystis sp., which belong to Group 3 and Group 4, respectively (Figure 1). Previous studies have demonstrated that the sn−2 position of the glycerol moiety of glycerolipids is associated with saturated and unsaturated fatty acids in strains in Groups 1 and 2, but is associated only with C16:0 in strains in Groups 3 and 4 [16,22]. The desC2 gene is present in all strains in Group 2 that have been examined and in one strain in Group 1, but it is not present in any strains that have been examined in Groups 3 and 4. These findings are consistent with the mode of desaturation of fatty acids at the sn−2 position. By contrast, the desC1 gene has been found in all strains examined in Groups 1–4 (Figure 1). Therefore, it is likely to be generally true that DesC2, encoded by the desC2 gene, acts on fatty acids at the sn−2 position, whereas DesC1, encoded by the desC1 gene, is specific to the sn−1 position.
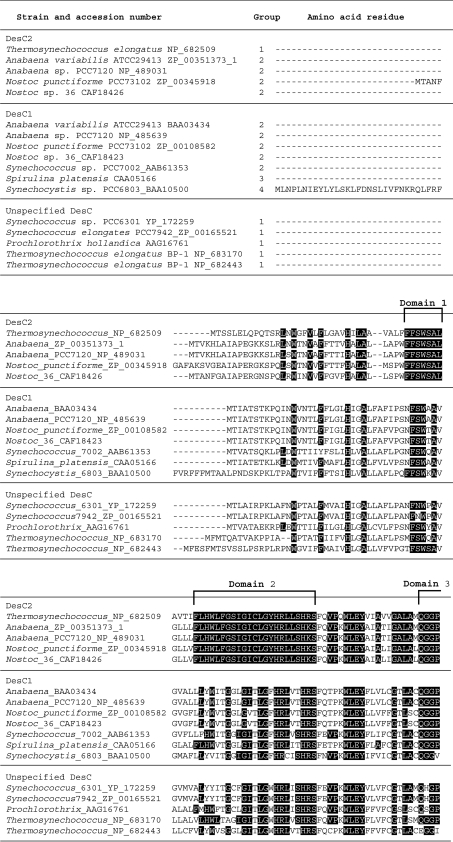
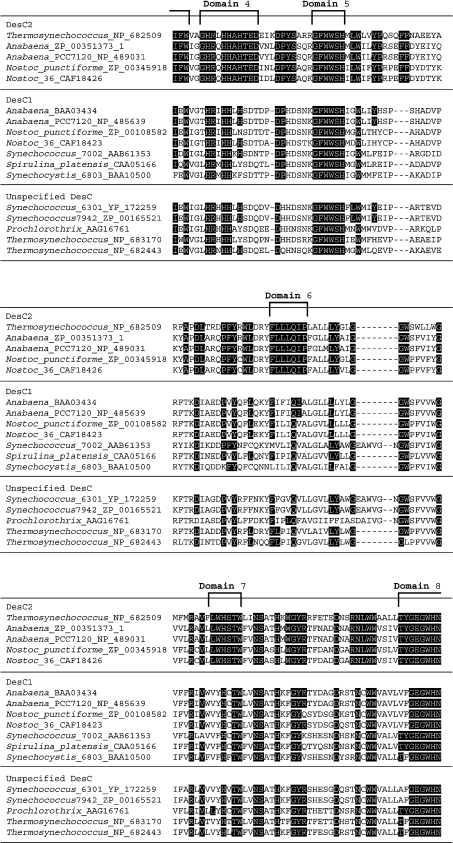
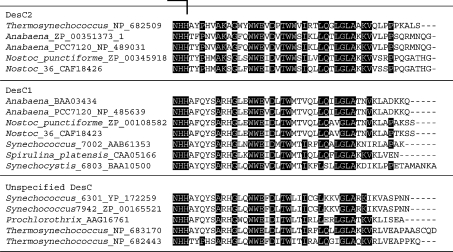
Figure 1. Alignment of the complete deduced amino acid sequences of DesC1 and DesC2 of cyanobacteria, grouped on the basis of the distribution of fatty acids at sn− positions.
Amino acid residues that are conserved in DesC2 are highlighted in black boxes.The eight domains that are conserved in DesC2 are indicated by square brackets. The deduced amino acid sequences were obtained from databases (GenBank®, EMBL and DDBJ) and the sequences were aligned using CLUSTAL W version 1.83 software. The accession number of each DesC sequence and the name of corresponding cyanobacterium are indicated on the left.
Three strains in Group 1, namely Synechococcus sp. PCC 6301, Synechococcus sp. PCC 7942, and Prochlorothrix hollandica, each contain a single gene homologous to desC. The MGDG in these cyanobacterial strains is esterified with C16:1 at the sn−2 position [3,4]. It seems likely that the DesC in these strains might be non-specific with respect to sn− position. We demonstrated that overexpression of the desC gene from Synechococcus sp. PCC 6301 in tobacco plants raised the level of C16:1(9) in MGDG at the expense of C16:0 at the sn−2 position [19]. Therefore it is possible that most cyanobacterial strains in Group 1 contain only one type of DesC, which acts on fatty acids at both the sn−1 and the sn−2 position.
Conserved domains in DesC2
All acyl-lipid desaturases contain three histidine clusters, whose structures are unique to individual classes of acyl-lipid desaturases and are related to the specificity of individual desaturases to the position of carbon atoms in fatty acids at which unsaturated bonds are introduced [4,20,33]. In a previous study, we demonstrated that the structures of the three histidine clusters in DesC can be represented as H-X4-H, W-X3-H-X2-H-H and H-X2-H-H (where X represents any amino acid) [14,15,33]. The deduced amino acid sequence of DesC2 includes histidine clusters with the same sequences (Figure 1).
Figure 1 shows an alignment of the deduced amino acid sequences of the DesC proteins from cyanobacteria for which the genome sequences are known and/or the distributions of fatty acids at sn− positions have been determined or predicted. There are eight domains with strongly conserved amino acid sequences in all the DesC2 proteins examined to date: domain 1 is partly conserved in DesC1; domain 2 is a large conserved domain and includes the first histidine cluster, this sequence is also partly conserved in DesC1; domain 3 is relatively strongly conserved in DesC1; domain 4 (includes one amino acid that is either glutamine or leucine) contains the second histidine cluster. This domain is poorly conserved in DesC1; domain 5 is fully conserved in DesC1. Only a single glutamine residue in domain 6 of DesC2 is conserved in DesC1; domain 7 is only partly conserved in DesC1, and; domain 8 (11 amino acids) contains the third histidine cluster and is well conserved in DesC1, with only one or two amino acids differences in the examined DesC1 proteins. Many amino acids that are not included in these eight domains are also conserved between both DesC1 and DesC2 proteins. It is likely that domains that are well conserved in both DesC1 and DesC2 proteins are responsible for the specificity of each enzyme with respect to the position of the double bond that is introduced into a fatty acid, and that domains that are conserved within the DesC1 and DesC2 proteins, but not between them, are responsible for the specificity with respect to the sn− position of the glycerol moiety of glycerolipids.
Phylogenetic relationships between DesC1 and DesC2 proteins
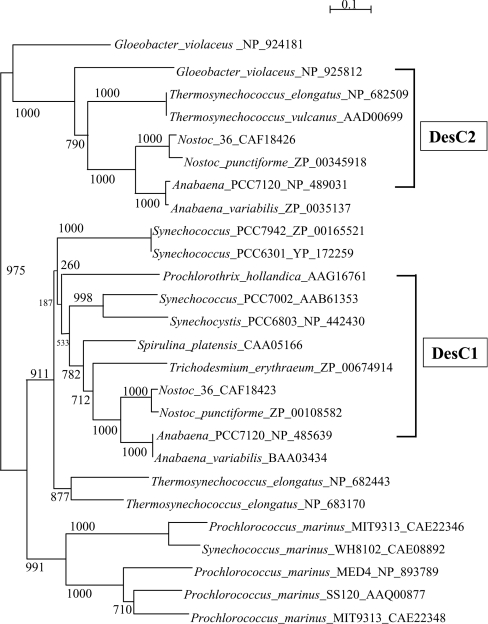
We constructed a phylogenetic tree using the deduced amino acid sequences of the DesC proteins available in databases. The tree indicates that DesC2 proteins from Nostoc sp., Nostoc punctiforme, Anabaena sp. PCC 7120, and Anabaena variabilis form a robust clade with bootstrap values of 100% (Figure 2). This clade is closely related to another formed by DesC2 proteins of Thermosynechococcus elongatus and Thermosynechococcus vulcanus. The tree indicates that two desaturases of G. violaceus are related to the DesC2 clade. However, G. violaceus seems to belong to Group 3 or 4 of cyanobacteria, even though its lipid and fatty acid composition is unusual [34,35]. DesC1 proteins from Anabaena variabilis, Anabaena sp. PCC 7120, Nostoc punctiforme, Nostoc sp. strain SO-36, Trichodesmium erythraeum, Spirulina platensis, Synechocystis sp. PCC 6803, and Synechococcus sp. PCC 7002 constitute a separate clade (Figure 2). DesC proteins from Prochlorothrix hollandica, Synechococcus sp. PCC 6301, and Synechococcus sp. PCC 7942 and two DesC proteins from Thermosynechococcus elongatus are related to the DesC1 clade. The genomic data also indicate the presence of the third robust clade. However, no fatty acid composition of any of the cyanobacteria whose DesC homologues are included in this clade has yet been determined. The analysis of fatty acids in the cyanobacteria in this clade should provide useful information to establish whether these enzymes are of the DesC1 or DesC2 type.
Figure 2. Phylogenetic tree determined on the basis of deduced amino acid sequences of DesC homologues in cyanobacteria.
The amino acids sequences corresponding to the desC genes in cyanobacteria were obtained from databases (GenBank, EMBL and DDBJ) and the sequences were aligned with CLUSTAL W version 1.83 (as shown in Figure 1). The phylogenetic tree was drawn with NJjplot (http://pbil.univ-lyon1.fr/software/njplot.html) using PHYLIP (phylogeny inference package) version 3.5c. The accession number of each desC homologue is indicated beside the name of the corresponding cyanobacterium. Bootstrap values (expressed relative to 1000 replications; [3]) are given at the respective nodes.
The results in Figures 1 and 2 suggest that strains in Group 2 might have both a desC1 and a desC2 gene, whereas strains in Groups 3 and 4 might only have a desC1 gene. Such a distribution of desC homologous genes would be consistent with the characterization of the fatty acids esterified to the sn-positions of glycerol moieties of glycerolipids, that is, strains in Groups 3 and 4 have only C16:0 at the sn−2 position whereas strains in Group 2 have C16:1(9) in addition to C16:0 at the sn−2 position. The desC homologous genes of strains in Group 1 are widely distributed on the phylogenetic tree (Figure 2). Thermosynechococcus elongatus has three desC homologous genes, which consist of one desC2 and two unspecified desC genes. By contrast, Synechococcus sp. PCC 7942, Synechococcus sp. PCC 6301 and P. hollandica have only one desC homologous gene, while Thermosynechococcus vulcanus has potentially only one desC homologous gene [17], which is included in the desC2 clade. The relationship between phylogenetic groupings and the specificity of DesC1 and DesC2 with respect to sn− positions in strains in Group 1 remains an open question.
Acknowledgments
This work was supported by a grant from the India–Japan Cooperative Science Programme of the Department of Science and Technology, Government of India, and the Japanese Society for the Promotion of Science to I. S., N. M. and S. S. and by the Programme for Cooperative Research on Stress Tolerance of Plants of the National Institute for Basic Biology, Japan. S. C. thanks the University Grants Commission, New Delhi, Government of India, for a Junior and a Senior Research Fellowship.
References
- 1.Kenyon C. N. Fatty acid composition of unicellular strains of blue-green algae. J. Bacteriol. 1972;109:827–834. doi: 10.1128/jb.109.2.827-834.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kenyon C. N., Rippka R., Stanier R. Y. Fatty acid composition and physiological properties of some filamentous blue–green algae. Arch. Mikrobiol. 1972;83:216–236. doi: 10.1007/BF00645123. [DOI] [PubMed] [Google Scholar]
- 3.Murata N., Wada H. Acyl lipid desaturases and their importance in the tolerance and acclimatization to cold of cyanobacteria. Biochem. J. 1995;308:1–8. doi: 10.1042/bj3080001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wada H., Murata N. Membrane lipids in cyanobacteria. In: Siegenthaler P. A., Murata N., editors. Lipids in Photosynthesis: Structure, Function and Genetics. Dordrecht: Kluwer Academic; 1998. pp. 65–81. [Google Scholar]
- 5.Shanklin J., Cahoon E. B. Desaturation and related modifications of fatty acids 1. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998;49:611–641. doi: 10.1146/annurev.arplant.49.1.611. [DOI] [PubMed] [Google Scholar]
- 6.Sakamoto T., Murata N. Regulation of desaturation of fatty acids and its role in tolerance to cold and salt stress. Curr. Opin. Microbiol. 2002;5:208–210. doi: 10.1016/s1369-5274(02)00306-5. [DOI] [PubMed] [Google Scholar]
- 7.Fukuchi-Mizutani M., Tasaka Y., Tanaka Y., Ashikari T., Kusumi T., Murata N. Characterization of δ9 acyl-lipid desaturase homologues from Arabidopsis thaliana. Plant Cell Physiol. 1998;39:247–253. doi: 10.1093/oxfordjournals.pcp.a029364. [DOI] [PubMed] [Google Scholar]
- 8.Harwood J. L. Recent advances in the biosynthesis of plant fatty acids. Biochim. Biophys. Acta. 1996;1301:7–56. doi: 10.1016/0005-2760(95)00242-1. [DOI] [PubMed] [Google Scholar]
- 9.Hitz W. D., Carlson T. J., Booth J. R., Jr, Kinney A. J., Stecca K. L., Yadav N. S. Cloning of a higher-plant plastid [omega]-6 fatty acid desaturase cDNA and its expression in a cyanobacterium. Plant Physiol. 1994;105:635–641. doi: 10.1104/pp.105.2.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kunst L., Browse J., Somerville C. A mutant of Arabidopsis deficient in desaturation of palmitic acid in leaf lipids. Plant Physiol. 1989;90:943–947. doi: 10.1104/pp.90.3.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wada H., Gombos Z., Murata N. Enhancement of chilling tolerance of a cyanobacterium by genetic manipulation of fatty acid desaturation. Nature (London) 1990;347:200–203. doi: 10.1038/347200a0. [DOI] [PubMed] [Google Scholar]
- 12.Reddy A. S., Nuccio M. L., Gross L. M., Thomas T. L. Isolation of Δ6 desaturase gene from the cyanobacterium Synechocystis sp. strain PCC6803 by gain-of-function expression in Anabaena sp. strain PCC7120. Plant Mol. Biol. 1993;22:293–300. doi: 10.1007/BF00014936. [DOI] [PubMed] [Google Scholar]
- 13.Sakamoto T., Los D. A., Higashi S., Wada H., Nishida I., Ohmori M., Murata N. Cloning of ω3 desaturase from cyanobacteria and its use in altering the degree of membrane-lipid unsaturation. Plant Mol. Biol. 1994;26:249–263. doi: 10.1007/BF00039536. [DOI] [PubMed] [Google Scholar]
- 14.Sakamoto T., Wada H., Nishida I., Ohmori M., Murata N. Δ9 acyl-lipid desaturases of cyanobacteria. Molecular cloning and substrate specificities in terms of fatty acids, sn-positions and polar head groups. J. Biol. Chem. 1994;269:25576–25580. [PubMed] [Google Scholar]
- 15.Sakamoto T., Wada H., Nishida I., Ohmori M., Murata N. Identification of conserved domains in the Δ12 desaturases of cyanobacteria. Plant Mol. Biol. 1994;24:643–650. doi: 10.1007/BF00023560. [DOI] [PubMed] [Google Scholar]
- 16.Sakamoto T., Bryant D. A. Temperature-regulated mRNA accumulation and stabilization for fatty acid desaturase genes in the cyanobacterium Synechococcus sp. strain PCC 7002. Mol. Microbiol. 1997;23:1281–1292. doi: 10.1046/j.1365-2958.1997.3071676.x. [DOI] [PubMed] [Google Scholar]
- 17.Kiseleva L. L., Serebriiskaya T. S., Horvath I., Vigh L., Lyukevich A. A., Los D. A. Expression of the gene for the delta 9 acyl-lipid desaturase in the thermophilic cyanobacterium. J. Mol. Microbiol. Biotechnol. 2000;2:331–338. [PubMed] [Google Scholar]
- 18.Deshnium P., Paithoonrangsarid K., Suphatrakul A., Meesapyodsuk D., Tanticharoen M., Cheevadhanarak S. Temperature-independent and -dependent expression of desaturase genes in filamentous cyanobacterium Spirulina platensis strain C1 (Arthrospira sp. PCC 9438) FEMS Microbiol. Lett. 2000;184:207–213. doi: 10.1111/j.1574-6968.2000.tb09015.x. [DOI] [PubMed] [Google Scholar]
- 19.Ishizaki-Nishizawa O., Fujii T., Azuma M., Sekiguchi K., Murata N., Ohtani T., Toguri T. Low-temperature resistance of higher plants is significantly enhanced by a nonspecific cyanobacterial desaturase. Nat. Biotechnol. 1996;14:1003–1006. doi: 10.1038/nbt0896-1003. [DOI] [PubMed] [Google Scholar]
- 20.Murata N., Wada H., Gombos Z. Modes of fatty-acid desaturation in cyanobacteria. Plant Cell Physiol. 1992;33:933–941. [Google Scholar]
- 21.Higashi S., Murata N. An in vivo study of substrate specificities of acyl-lipid desaturases and acyltransferases in lipid synthesis in Synechocystis PCC6803. Plant Physiol. 1993;102:1275–1278. doi: 10.1104/pp.102.4.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Los D. A., Ray M. K., Murata N. Differences in the control of the temperature-dependent expression of four genes for desaturases in Synechocystis sp. PCC 6803. Mol. Microbiol. 1997;25:1167–1175. doi: 10.1046/j.1365-2958.1997.5641912.x. [DOI] [PubMed] [Google Scholar]
- 23.Sakamoto T., Higashi S., Wada H., Murata N., Bryant D. A. Low-temperature-induced desaturation of fatty acids and expression of desaturase genes in the cyanobacterium Synechococcus sp. PCC 7002. FEMS Microbiol. Lett. 1997;152:313–320. doi: 10.1111/j.1574-6968.1997.tb10445.x. [DOI] [PubMed] [Google Scholar]
- 24.Stanier R. Y., Kunisawa R., Mandel M., Cohen-Bazire G. Purification and properties of unicellular blue-green algae (order Chroococcales) Bacteriol. Rev. 1971;35:171–205. doi: 10.1128/br.35.2.171-205.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bligh E. G., Dyer W. J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 26.Sato N., Murata N. Membrane lipids. Methods Enzymol. 1988;167:251–259. [Google Scholar]
- 27.Sato N., Murata N. Lipid biosynthesis in the blue-green alga, Anabaena variabilis. I. Lipid classes. Biochim. Biophys. Acta. 1982;710:271–278. [Google Scholar]
- 28.Williams J. G. K. Construction of specific mutations in photosystem II photosynthetic reaction center by genetic engineering methods in Synechocystis 6803. Methods Enzymol. 1988;167:766–778. [Google Scholar]
- 29.Sambrook J., Fritsch E. F., Maniatis T. 2nd edn. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 1989. Molecular Cloning: A Laboratory Manual. [Google Scholar]
- 30.Zinchenko V. V., Piven I. V., Melnik V. A., Shestakov S. V. Vectors for complementation analysis of cyanobacterial mutants. Russ. J. Genet. 1999;35:228–232. [Google Scholar]
- 31.Murata N. Low temperature effects on cyanobacterial membranes. J. Bioenerg. Biomembr. 1989;21:61–75. doi: 10.1007/BF00762212. [DOI] [PubMed] [Google Scholar]
- 32.Wada H., Murata N. Synechocystis sp. PCC6803 mutants defective in desaturation of fatty acids. Plant Cell Physiol. 1989;30:971–978. [Google Scholar]
- 33.Los D. A., Murata N. Structure and expression of fatty acid desaturases. Biochim. Biophys. Acta. 1998;1394:3–15. doi: 10.1016/s0005-2760(98)00091-5. [DOI] [PubMed] [Google Scholar]
- 34.Rippka R., Waterbury J., Cohen-Bazire G. A cyanobacterium which lacks thylakoids. Arch. Microbiol. 1974;100:419–436. [Google Scholar]
- 35.Selstam E., Campbell D. Membrane lipid composition of the unusual cyanobacterium Gloeobacter violaceus sp. PCC 7421, which lacks sulfoquinovosyl diacylglycerol. Arch. Microbiol. 1996;166:132–135. [Google Scholar]