Abstract
TRPM2 (transient receptor potential melastatin 2) is a Ca2+-permeable cation channel gated by ADPR (ADP-ribose) from the cytosolic side. To test whether endogenous concentrations of intracellular ADPR are sufficient for TRPM2 gating in neutrophil granulocytes, we devised an HPLC method to determine ADPR contents in HClO4 cell extracts. The reversed-phase ion-pair HPLC method with an Mg2+-containing isocratic eluent allows baseline resolution of one ADPR peak. Intracellular ADPR concentrations were approx. 5 μM in granulocytes and not significantly altered by stimulation with the chemoattractant peptide fMLP (N-formylmethionyl-leucylphenylalanine). We furthermore determined intracellular concentrations of cADPR (cyclic ADPR) with a cyclase assay involving enzymatic conversion of cADPR into NAD+ and fluorimetric determination of NAD+. Intracellular cADPR concentrations were approx. 0.2 μM and not altered by fMLP. In patch–clamp experiments, ADPR (0.1–100 μM) was dialysed into granulocytes to analyse its effects on whole-cell currents characteristic for TRPM2, in the presence of a low (<10 nM) or a high (1 μM) intracellular Ca2+ concentration. TRPM2 currents were significantly larger at high than at low [Ca2+] (e.g. −225±27.1 versus −7±2.0 pA/pF at 5 μM ADPR), but no currents at all were observed in the absence of ADPR (ADPR concentration ≤0.3 μM). cADPR (0.1, 0.3 and 10 μM) was without effect even in the presence of subthreshold ADPR (0.1 μM). We conclude that ADPR enables an effective regulation of TRPM2 by cytosolic Ca2+. Thus ADPR and Ca2+ in concert behave as a messenger system for agonist-induced influx of Ca2+ through TRPM2 in granulocytes.
Keywords: cyclic ADP-ribose, HPLC, N-formylmethionyl-leucylphenylalanine (fMLP), neutrophil granulocyte, patch–clamp, transient receptor potential melastatin 2 (TRPM2) calcium influx
Abbreviations: ADPR, ADP-ribose; cADPR, cyclic ADPR; CD38, cluster of differentiation 38; fMLP, N-formylmethionyl-leucylphenylalanine; HEK-293 cell, human embryonic kidney cell; MWCO, molecular-mass cutoff; NUD, Nudix hydrolase; NMDG, N-methyl-D-glucamine; PARP, poly(ADPR) polymerase; TBAHS, tetrabutylammonium dihydrogensulphate; TRPM2, transient receptor potential melastatin 2
INTRODUCTION
TRPM2 (transient receptor potential melastatin 2) is a non-selective cation channel expressed in a wide range of human tissues, including the brain, bone marrow, spleen and pancreas [1–3]. We have previously shown its expression in neutrophil granulocytes with reverse transcriptase–PCR analysis [4]. Moreover, we have provided functional evidence for its expression by demonstrating channels in neutrophils [4] that exhibit the same characteristic biophysical properties of channels found when TRPM2 is heterologously expressed in HEK-293 cells (human embryonic kidney cells) [5]. Thus TRPM2 is so far the only Ca2+-permeable channel in neutrophils identified by its functional properties that can be attributed to a particular channel protein.
Gating of TRPM2 channels can be induced by ADPR (ADP-ribose) [5]. Further activators of TRPM2 that either alone, or in co-operation with ADPR, or through the formation of ADPR may lead to the opening of the channel include cADPR (cyclic ADPR) [6], NAD+ [7,8], H2O2 [8,9] and Ca2+ ions [10]. For gating by ADPR, the NUDT9 (where NUD is Nudix hydrolase) homology region of TRPM2 is decisive. Most likely, binding of ADPR to a defined part within the homology region is involved [11]. The NUDT9 homology region is localized within the cytosolic C-terminal tail of TRPM2. Thus TRPM2 may be activated by an endogenous intracellular ligand, raising the question of whether ADPR has a role as a second messenger that regulates cation influx through TRPM2.
However, actual intracellular concentrations of ADPR are not known in granulocytes. When we started our study, no analytical method for the determination of ADPR levels had been reported. Furthermore, metabolic pathways through which the formation of ADPR may be induced are poorly understood, particularly with respect to their regulation. One possible source of ADPR in many cells are ADPR polymers, formed in the nucleus by PARPs [poly(ADPR) polymerases]. The polymers may be degraded to ADPR by glycohydrolases. PARP activation can be elicited by many factors, such as radiation, oxidative stress or some toxic substances. There have been many enzymes described with a PARP domain, numbered from PARP-1 to PARP-18 [12]. Most of the PARP activity is attributed to PARP-1. Strikingly, neutrophil granulocytes are within the few cells that do not express PARP-1 [13]. Therefore activation of TRPM2 in neutrophils by ADPR derived from ADPR polymers seems unlikely. As an alternative pathway for ADPR production, the membrane-associated protein CD38 (cluster of differentiation 38) may be considered in neutrophil granulocytes. This is an enzyme with glycohydrolase activity that degrades NAD+ and related compounds [14]. In the past, major relevance of CD38 has been attributed to its generation of cADPR [15]. cADPR is an established second messenger that mediates Ca2+ release from intracellular stores by action on the ryanodine receptor, a Ca2+ release channel in the membrane of calcium stores [16]. Moreover, cADPR at concentrations of 10 μM has been reported to potentiate the effects of ADPR [6]. However, cADPR contributes only marginally to the CD38 products. Within the products of CD38-catalysed metabolism of NAD+, the overwhelming majority (96%) is comprised of ADPR [17]. Thus CD38 may be viewed as an ADPR synthase. Yet it has to be taken into account that CD38 is an ectoenzyme [18] that catalyses the conversion of extracellular NAD+ into extracellular ADPR. At present, it has not been clarified whether neutrophil CD38 may provide intracellular ADPR formation as well or whether effective transport systems exist that shuffle the substrate and the product of CD38 through the plasma membrane.
The aim of the present study was the determination of the concentrations of ADPR and of cADPR in neutrophil granulocytes, to answer the question of whether these concentrations are sufficient for TRPM2 gating. Both nucleotides, in the determined physiological concentrations, were then dialysed into neutrophils and the currents through TRPM2 were monitored. Furthermore, we tested whether stimulation with the chemoattractant peptide fMLP (N-formylmethionyl-leucylphenylalanine), known to induce Ca2+ influx [19], led to increases in the concentrations of the nucleotides. We report the modification of an HPLC-based method, originally developed for the analysis of intracellular nucleotides [20], for ADPR determination, whereas cADPR was analysed with the cyclase assay developed by Graeff and Lee [21]. The levels of ADPR, but not of cADPR, were in the range that allows intracellular Ca2+ an effective regulation of TRPM2 channels in co-operation with ADPR. Since intracellular Ca2+ concentrations are raised by fMLP-induced release from stores, ADPR and Ca2+ in concert behave as a messenger system for the induction of cation influx through TRPM2.
EXPERIMENTAL
Materials
Nucleotide standards, nucleotide pyrophosphatase from Crotalus adamentus venom, alkaline phosphatase, NADase (an enzyme that degrades NAD+) from Neurospora crassa, ADP-ribosylcyclase, nicotinamide, alcohol dehydrogenase, resazurin, diaphorase and fMLP were from Sigma (Taufkirchen, Germany), and FMN and tri-n-octylamine were from Fluka (Sigma–Aldrich, Steinheim, Germany). 1,1,2-Trichlorotrifluoroethane was from Riedel-de Haën (Seelze, Germany). HClO4, methanol (LiChrosolv) and TBAHS (tetrabutylammonium dihydrogensulphate) were from Merck (Darmstadt, Germany).
Neutrophil isolation
Neutrophil granulocytes were prepared from peripheral blood of healthy volunteers as described in [4].
Cell extracts
Neutrophils (5–7×107) were stored in test tubes (15 ml; Greiner, Frickenhausen, Germany) at room temperature (21–23 °C) for up to 30 min and then kept at 37 °C for 2 or 5 min. During this time, some aliquots of the cell suspensions were stimulated with fMLP (1 μM). Thereafter, the tubes were placed in an ice–salt bath for 3 min prior to centrifugation (1000 g, 4 °C and 2 min). Pellets were resuspended in ice-cold 0.4 M HClO4, followed by three freeze–thaw cycles (liquid nitrogen/37 °C) and short ultrasonic disruption. Cell debris was removed by centrifugation at 550 g for 5 min at 4 °C. For ADPR determinations, supernatants were divided into two identical halves, to one of which an ADPR standard was added (spiking). Extracts were titrated to pH 5–6 with potassium carbonate. Precipitated potassium chlorate was removed by centrifugation (10000 g, 4 °C and 10 min). Finally, the supernatants were filtered through disposable 0.2 μm filters (Spin X; Corning Costar, Wiesbaden, Germany). For cADPR determinations, HClO4 was removed by mixing 1 vol. of the supernatants with a solution containing 3 vol. of 1,1,2-trichlorotrifluoroethane and 1 vol. of tri-n-octylamine and vortex-mixing the mixture for 1 min. Following a centrifugation step for 10 min at 1500 g, the probes were maintained on ice until the aqueous phase easily separated from the organic phase. The aqueous layer containing the cADPR was removed and adjusted to a pH of 7–8 with an identical volume of sodium phosphate (20 mM, pH 8).
Enzymatic degradation of ADPR
Neutralized extracts or ADPR standards were incubated with nucleotide pyrophosphatase (1.8 units/ml) in the presence of MgCl2 (2 mM) for 30 min at 37 °C. The enzyme was removed by centrifugation of the samples through Centrisart I filter devices [10 kDa MWCO (molecular-mass cutoff); Sartorius, Göttingen, Germany].
HPLC analysis
Neutralized neutrophil extracts were analysed by a reversed-phase HPLC method modified from a procedure developed for the analysis of purine nucleotides in tissue extracts [20,22]. A LaChrom Elite HPLC system (Hitachi, Tokyo, Japan) was used with an LiChrospher RP-8 column (250 mm×4 mm, 5 μm particle size; Merck). A volume of 20–40 μl of the samples (corresponding to the extract of 2–4×106 cells) or standards were injected with an autosampler (LaChrom Elite L-2200; Hitachi) that kept the samples at 4 °C. An isocratic eluent was used containing methanol (6%, w/v) and 130 mM KH2PO4, 3 mM TBAHS and 1 mM MgSO4 (pH 5.9). The flow rate was 1.0 ml/min. Absorbance was measured at 270 nm with a UV-absorbance detector (LaChrom Elite L-2400; Hitachi). Data were processed by the EZChrom Elite data acquisition system (the software of the acquisition system was from Scientific Software, Pleasonton, CA, U.S.A.). The recovery of the extraction was calculated by comparing an ADPR standard with spiked and non-spiked samples.
cADPR assay
cADPR concentrations were determined in standards and in cell extracts with the method described by Graeff and Lee [21], based on the conversion of cADPR into NAD+ with commercially available ADP-ribosyl-cyclase and the fluorimetric determination of the NAD+ concentration with the resazurin/resorufin indicator reaction. In short, 100 μl of the cell extracts was incubated overnight at room temperature in an enzyme mixture containing (final concentrations): nucleotide pyrophosphatase (0.44 unit/ml), alkaline phosphatase (12.5 units/ml), NADase (0.0625 units/ml), MgCl2 (2.5 mM) and sodium phosphate (20 mM, pH 8). Enzymes were removed by filtration with Vivaspin 500 filters (10 kDa MWCO; Vivascience, Hannover, Germany) and samples were recovered in the filtrate after centrifugation at 3000 g for 30 min at 4 °C. Conversion of cADPR into NAD+ was initiated by incubating 100 μl of the probes with 50 μl of a reagent for 15 min at room temperature. The reagent contained ADP-ribosyl-cyclase (0.3 μg/ml), nicotinamide (30 mM) and sodium phosphate (100 mM, pH 8). Afterwards, 100 μl of the cycling reagent was added, containing ethanol (2%, v/v), alcohol dehydrogenase (100 μg/ml), resazurin (20 μM), diaphorase (10 μg/ml), FMN (10 μM), nicotinamide (10 mM), BSA (100 μg/ml) and sodium phosphate (100 mM, pH 8). The samples were then put into glass tubes (0.3 ml; CS Chromatographie Service, Langerwehe, Germany) and the increase in the resorufin fluorescence (with excitation at 544 nm and emission at 590 nm) was monitored for 65 min in a fluorescence spectrophotometer (Cary Eclipse; Varian, Darmstadt, Germany). The cADPR concentration of the cell extracts was calculated from the fluorescence increase over time in comparison with that of cADPR standards. As negative controls, probes without cyclase addition were used.
cADPR purification
Since cADPR stock solutions contained approx 20% ADPR as contamination, ADPR was removed by incubation for 30 min at room temperature with nucleotide pyrophosphatase as described in the Experimental section for extracts.
Electrophysiology
Human granulocytes were studied with the patch–clamp technique in the whole-cell mode, using an EPC 9 amplifier equipped with a personal computer with Pulse 8.5 and X Chart software (HEKA, Lamprecht, Germany). Pipettes were made of borosilicate glass; the pipette tips were coated with dental wax (Moyco, Philadelphia, U.S.A.) to reduce thermic noise for the resolution of single-channel events in the whole-cell mode. The bath solution was as described in [4], with Na+ as main cation sometimes replaced by 150 mM NMDG (N-methyl-D-glucamine). The pipette solution contained 145 mM caesium glutamate, 8 mM NaCl, 2 mM MgCl2 and 10 mM Hepes (pH 7.2) (CsOH) and the Ca2+ concentration was adjusted to either <10 nM (10 mM Cs-EGTA) or 1 μM (0.886 mM CaCl2 and 1 mM Cs-EGTA). Ca2+ concentrations were calculated using the MAXC program (http://www.stanford.edu/~cpatton/maxc.html). The weakly Ca2+-buffered pipette solution contained 50 μM Cs-EGTA. ADPR was added from a stock solution to final concentrations of 0.1–100 μM. cADPR was added from a freshly prepared stock solution to final nominal concentrations of either 10, 0.3 or 0.1 μM. Neutrophils were held at −60 mV and current–voltage (I–V) relations were obtained from voltage ramps (from −90 to +60 mV over 400 ms). These experiments were carried out at room temperature.
RESULTS
To verify whether the chosen HPLC system enabled quantitative determinations of ADPR concentrations, the column was loaded with standards containing the following nucleotides in various mixtures: ATP, ADP, AMP, adenosine, GTP, cADPR, NAD+, nicotinamide and ADPR. These compounds were readily separated, resulting in defined peaks in the chromatograms that allowed the identification of each of them by their individual retention times (see Supplementary Figure S1 at http://www.BiochemJ.org/bj/398/bj3980225add.htm). The area of the peak attributed to ADPR was linearly correlated with the ADPR content in the sample. The detection limit was in the range of 1 pmol of ADPR.
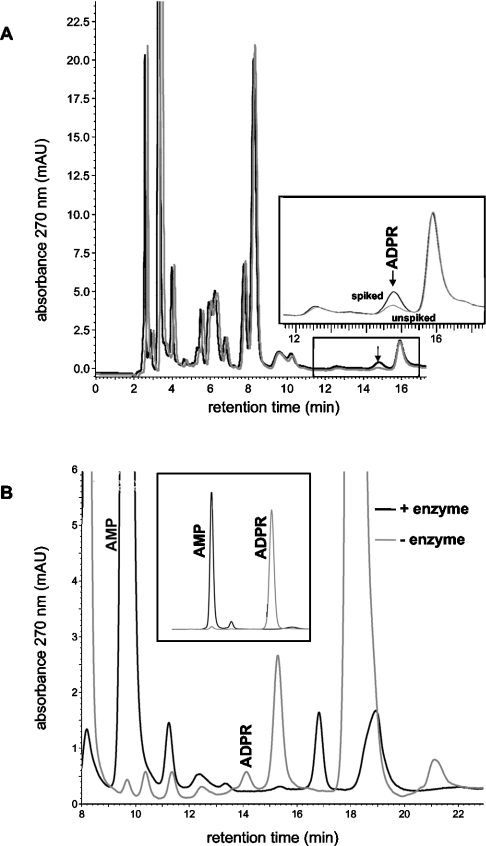
Chromatograms of neutralized HClO4 extracts from neutrophil granulocytes displayed several more peaks than the standards, many of them not well separated (Figure 1A). Importantly, however, there was one single baseline-resolved peak with the same retention time as that of ADPR standards. To test for the specificity of this peak attributed to ADPR, we spiked the extracts with ADPR, i.e. we added ADPR to the extracts prior to neutralization with potassium carbonate, thereby increasing their ADPR content by a defined amount. Only 50% of the volume of each extract was spiked, whereas the same volume of water was added to the other half. The two chromatograms of representative twin samples (spiked and not spiked) from neutrophils are compared in Figure 1(A). Spiking resulted in the specific enlargement of the ADPR peak. From a comparison of the peak area of the unspiked sample with that of an ADPR standard, the amount of ADPR in the unspiked sample after neutralization was calculated as 7.6 pmol. The comparison with the peak area of the spiked sample allows us to calculate a recovery rate during the sample preparation of 98%. The same recovery rate was found when 5-fold higher amounts of ADPR were used for spiking. As a further test for the specificity of the ADPR peak, we incubated standards as well as twin samples of cell extracts with pyrophosphatase that converts ADPR into AMP and ribose-5-phosphate. Figure 1(B) shows that pyrophosphatase incubation completely removed the ADPR peak, in standards and extracts alike. At the same time, the AMP peak was considerably enlarged, indicating that ADPR as well as other nucleotides were degraded to AMP.
Figure 1. Chromatograms of samples prepared by HClO4 extraction from neutrophil granulocytes.
(A) One extract (black) was spiked with ADPR (13 pmol). ADPR in extracts displayed the same retention time as ADPR in standards (shown in the Supplementary Figure S1 at http://www.BiochemJ.org/bj/398/bj3980225add.htm, displaying an HPLC analysis of a mixture of several standard nucleotides). The ADPR content in the non-spiked extract (grey chromatogram) was calculated as 7.6 pmol. The inset shows an enlarged section of the chromatogram that contained the ADPR peak. (B) Effects of pyrophosphatase incubation on the ADPR peak in extracts (and in standards, see inset). Note that the AMP peak is considerably enlarged by pyrophosphatase treatment, indicating that ADPR is not an exclusive substrate of the enzyme. The minor differences in the retention time in both panels are due to different columns (although of the same type) and different equilibration times with eluent (described in the Experimental section) (1 versus 2 days) in the chromatographies.
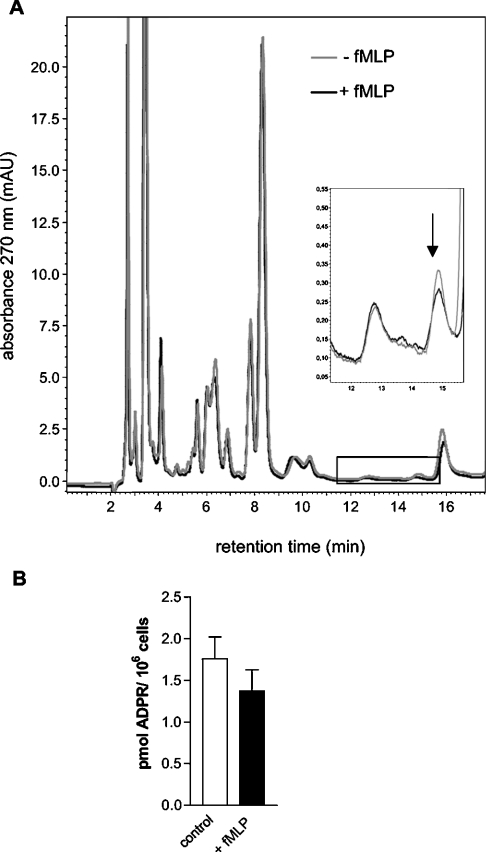
When neutrophil granulocytes were stimulated with fMLP (1 μM, 2 min) prior to acidic precipitation, the ADPR concentrations in the extracts were not significantly (paired t test) changed (Figure 2; n=6 experiments with extracts from stimulated versus control cells). There was a tendency for a small reduction of ADPR contents by fMLP (Figure 2B), in the range of the resolution of the method. Similar results were obtained after a stimulation of 5 min (results not shown). For comparison, we performed experiments on HEK-293 cells stimulated with H2O2 (10 mM, 10 min, n=3). This treatment diminished the ADPR contents by approx. 60% and strikingly altered several other peaks, noticeably those attributable to ATP, ADP and to further unidentified compounds (results not shown).
Figure 2. ADPR content in neutrophil granulocytes at basal conditions or after stimulation with fMLP.
(A) Chromatograms from two extracts of granulocytes from one donor. Cells were stimulated with fMLP (1 μM for 2 min; black chromatogram) or were not stimulated (control, grey chromatogram). (B) Summary of the fMLP effects on the ADPR content. Results shown (means±S.E.M.) are from six granulocyte preparations from different donors in which 50% of the cells were stimulated with fMLP and 50% were used as unstimulated controls.
The HPLC data so far define the amounts of ADPR present in a given number of neutrophil granulocytes. When these values are to be used for a calculation of intracellular ADPR concentrations, some uncertainties exist, most notably in the cell size and the ADPR distribution space within the cells. The cell volume of neutrophil granulocytes has been estimated as 350 fl [23]. We derived similar values from the determination of the cytocrit of granulocyte suspensions. When this volume is assumed along with a homogeneous intracellular ADPR distribution, the concentration of ADPR is 5.0±1.8 μM (mean±S.E.M.) in unstimulated neutrophil granulocytes and 3.9±1.8 μM after fMLP stimulation. We estimate that these values have an uncertainty factor of 0.5–6, i.e. the actual concentrations may be in the range 2–30 μM.
To test for the functional significance of these ADPR concentrations for TRPM2 gating in neutrophil granulocytes, we performed patch–clamp experiments in which ADPR was infused into granulocytes through the patch pipette. We considered that intracellular Ca2+ is a cofactor of ADPR-dependent TRPM2 gating [10]. However, this finding has only been obtained with heterologously expressed TRPM2 channels, which does not necessarily indicate that the same holds true in granulocytes. Therefore the experiments were performed with two different Ca2+ concentrations in the pipette solution, either a very low one achieved by addition of EGTA (10 mM) to a nominally Ca2+-free solution (Ca2+ concentration <10 nM), or a concentration corresponding to that in stimulated cells, i.e. approx. 1 μM, accomplished by a mixture of EGTA and CaCl2 (see the Experimental section).
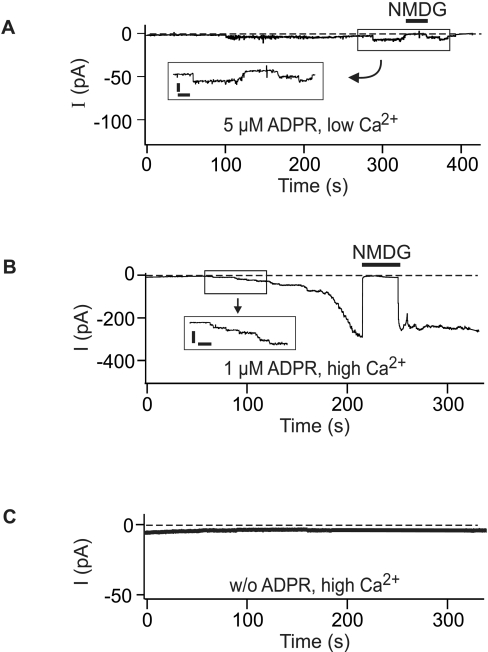
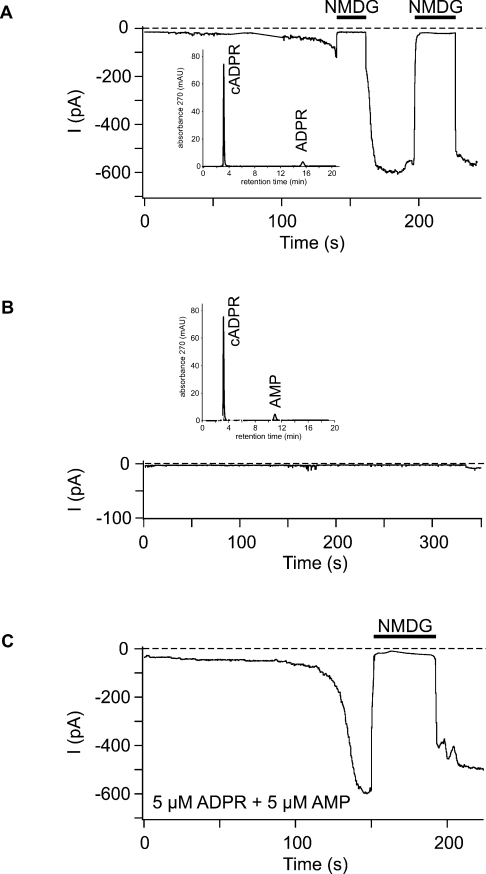
When ADPR (5 μM) was infused into granulocytes in the presence of low Ca2+, the opening of a few channels was induced that could be resolved within the total whole-cell currents (Figure 3A). The extremely long (several seconds) open times and the single-channel current amplitudes (consistent with the reported conductance of 50–70 pS) are characteristic for TRPM2 channels [5] and provide strong evidence that ADPR gates TRPM2 channels in neutrophil granulocytes. Sizeable whole-cell currents, rather than only some single-channel openings, were induced in granulocytes when ADPR (even at 1 μM) was infused along with Ca2+ (Figure 3B). No single-channel events can be resolved within large whole-cell currents. However, since the exchange of the cytosolic solutes with that of the pipette fluid is a time-dependent process, the currents typically developed gradually before a plateau was reached. During the initial phase of the current development, the consecutive opening of single channels was evident, again with the characteristic properties of TRPM2 (Figure 3B). Whole-cell currents were blocked by the large cation NMDG (Figure 3) reported to be mostly impermeant to TRPM2 [9].
Figure 3. Whole-cell currents in neutrophil granulocytes induced by low ADPR concentrations and the effects of intracellular Ca2+.
The pipette solution contained ADPR (5 or 1 μM) in the experiments shown in (A, B) and was free of ADPR in (C). The intracellular (pipette) Ca2+ concentration was low (<10 nM) in (A) and high (1 μM) in (B, C). The holding potential was −60 mV. The Na+ in the bath was replaced by the impermeable cation NMDG during time periods indicated by horizontal bars. The enlarged current tracings in the insets of (A, B) (scale bars, 5 pA and 10 s respectively) show single-channel openings resolved at low whole-cell current amplitudes.
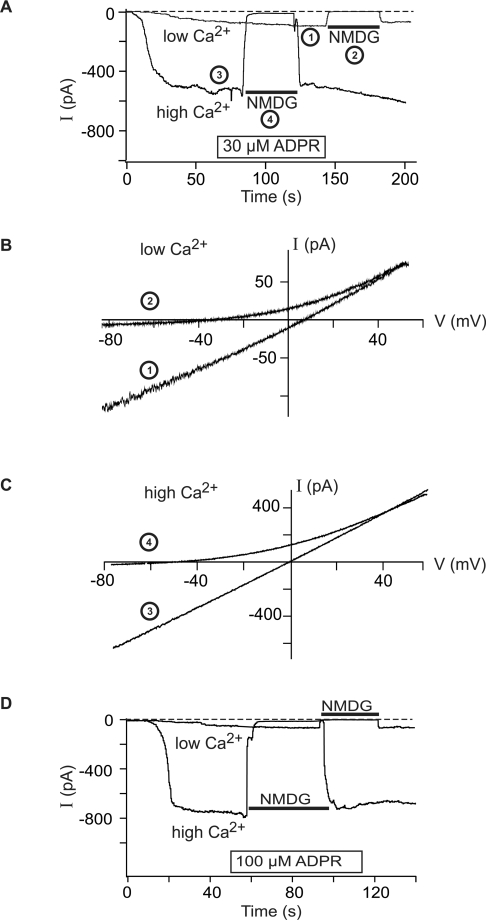
Experiments with higher ADPR concentrations (30 or 100 μM) demonstrated again a critical role of Ca2+. Although ADPR evoked whole-cell currents in the absence of Ca2+, the currents were strikingly larger in amplitude in the presence of Ca2+ (1 μM) in the pipette solution (Figure 4). The I–V relation of the currents, with either Na+ or NMDG+ as main extracellular cation, was characteristic for TRPM2 currents [4,5,9].
Figure 4. Whole-cell currents in neutrophil granulocytes induced by high ADPR concentrations and the effects of intracellular Ca2+.
The pipette solution contained 30 μM ADPR (A–C) or 100 μM ADPR (D) in the presence of either a low (<10 nM) or high (1 μM) Ca2+ concentration. The Na+ in the bath was replaced by the impermeable cation NMDG during time periods indicated by horizontal bars in (A, D). Current–voltage relations in low Ca2+ (B) and in high Ca2+ (C) were recorded with voltage ramps at time points indicated with numbers in (A). Note the different scaling of the ordinate in (B, C).
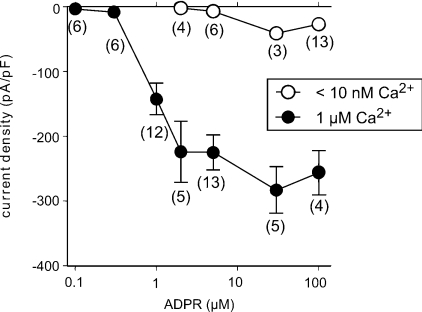
Importantly, gating of TRPM2 channels was not evoked by Ca2+ alone. When a pipette solution was used that contained the high Ca2+ concentration but no ADPR, no single-channel events and no increases in the whole-cell currents were observed (Figure 3C). Thus the presence of ADPR is essential for the effects of Ca2+ on TRPM2. The dependence of currents in granulocytes on the concentration of ADPR, in the presence of either a high or a low intracellular Ca2+ concentration, is summarized in Figure 5. A minimal ADPR concentration of 1 μM was required to induce currents in the presence of high Ca2+. At higher ADPR concentrations, a saturation of the effects was observed. Over the whole ADPR concentration range studied, considerably and significantly lower currents were found for low than for high [Ca2+].
Figure 5. Dependence of current densities on the concentration of ADPR, in the presence of either a high or a low [Ca2+].
Current densities (mean±S.E.M.) at low [Ca2+] (open circles) were significantly (Mann–Whitney U test) lower than that at high [Ca2+] (closed circles) for each ADPR concentration. The number of experiments is indicated for each experimental condition.
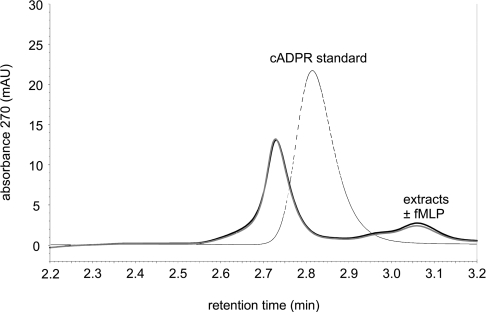
A modulator of ADPR-induced TRPM2 activation may be cADPR, implicated to be a second messenger regulated by receptor-dependent stimulation in other cells [24]. The cADPR concentration reported to enhance TRPM2 currents was 10 μM. Under our HPLC conditions, cADPR produces a peak at approx. 2.8 min retention time. For the range of this retention time, Figure 6 shows an expanded view of two chromatograms of cell extracts, one from fMLP-stimulated cells and one from resting cells, along with a chromatogram of a cADPR standard. The two extracts produced almost identical chromatograms (in the retention time range shown) but no peak at the expected retention time of cADPR. We cannot exclude that one of the neighbouring peaks may represent cADPR; in this case, an fMLP-induced concentration increase could be ruled out. More likely, cADPR is not present intracellularly in sufficient concentrations to be resolved with our HPLC conditions. Therefore we resorted to the enzymatic cADPR assay based on the conversion of cADPR into NAD+ and the fluorimetric determination of NAD+ [21] to assess intracellular cADPR concentrations. With standards nominally containing 0–10 nM cADPR, a calibration curve was obtained in which the cADPR concentration was linearly related to the increase in resorfin fluorescence (correlation coefficient r=0.92, see Supplementary Figure 2 at http://www.BiochemJ.org/bj/398/bj3980225add.htm). The cADPR concentrations found in extracts were between 1 and 9 nM (n=6). From the number of cells in each extract and the estimated cytosolic volume, the intracellular concentration was calculated to be 221±84 nM. Aliquots of the same granulocyte preparations were stimulated with fMLP (1 μM for 3 min), resulting in an intracellular concentration of 152±93 nM (n=6). When cADPR was added to cell extracts in amounts corresponding to an increase in the intracellular concentration by 50 nM, a concentration increase in the expected range was found in extracts from unstimulated cells as well as from fMLP-stimulated cells. Since we discovered later (see below) that the standards may have contained, to a large extent, ADPR rather than cADPR, the actual intracellular cADPR concentrations are likely to be approx. 25% lower.
Figure 6. Expanded view of HPLC chromatograms of extracts and a standard around the cADPR peak.
The standard (dotted line) contained nominally 16 μM cADPR. The two almost identical chromatograms were from two extracts from cells from one donor. One extract was from cells stimulated with fMLP (1 μM for 2 min; black) and the other one was from unstimulated cells (grey).
As in the case of ADPR, we wished to examine whether cADPR in physiological concentrations had effects on whole-cell currents. First, we repeated the experiments performed by Kolisek et al. [6] in TRPM2-transfected HEK-293 cells in granulocytes, i.e. we used a weakly Ca2+-buffered pipette solution with 10 μM cADPR. In contrast with Kolisek et al. [6], we observed large currents (−200.8±10.6 pA/pF, n=3) as if ADPR rather than cADPR was infused. Similar results were obtained with cADPR (10 μM) in our standard high Ca2+ pipette solution (Figure 7A). This prompted us to test for the purity of our cADPR solutions with an HPLC analysis (Figure 7A, inset) that demonstrated considerable contamination with ADPR that may be conservatively estimated to be at least 25%, even when cADPR solutions are freshly prepared from cADPR salts. These results were confirmed in all of several cADPR batches tested. Therefore the currents induced in our experiments with nominally 10 μM cADPR are explained by contaminating ADPR. To analyse possible effects of cADPR, cADPR stock solutions were incubated with a nucleotide pyrophosphatase, resulting in the complete conversion of ADPR into AMP (Figure 7B, inset). It was confirmed that pyrophosphatase-treated cADPR stock remained free of ADPR for several days after freezing. With pipette solutions prepared from ADPR-free cADPR, no currents were induced in granulocytes (n=4, results not shown), not even when ADPR was added in a subthreshold concentration (0.1 μM; n=4; Figure 7B). Since AMP has been reported as an inhibitor of TRPM2 currents [6], although only at high concentrations (0.5 mM), we furthermore tested AMP (5 μM, which slightly exceeds the concentrations used in the experiments with ADPR-free cADPR) in the presence of ADPR (5 μM), which did not noticeably alter ADPR-induced TRPM2 currents in granulocytes (n=4; Figure 7C). Finally, we tested cADPR at physiological concentrations. When cADPR was infused in concentrations of 0.1 μM (n=7) or 0.3 μM (n=6), along with ADPR at a subthreshold concentration (i.e. 0.1 μM), no current was induced in any experiment.
Figure 7. Effect of cADPR on whole-cell currents in neutrophil granulocytes in the presence of 1 μM intracellular Ca2+.
(A) The pipette solution that contained nominally 10 μM cADPR was prepared from a cADPR stock solution considerably contaminated with ADPR, as shown with HPLC (inset). Inward currents developing during infusion of contaminated cADPR were blocked by NMDG present in the bath during time periods indicated by horizontal bars. (B) In contrast with (A), the pipette solution was prepared from a cADPR stock solution incubated with nucleotide pyrophosphatase (2.7 units/ml, 20 min, 37 °C), resulting in the complete conversion of ADPR into AMP (inset). ADPR was then added to the pipette solution at a subthreshold concentration (0.1 μM). The absence of currents under these conditions was confirmed in three further experiments. (C) To check for the effects of AMP, AMP (5 μM) was added to a pipette solution containing ADPR (5 μM). AMP did not prevent or noticeably alter ADPR-induced currents in three further experiments.
DISCUSSION
In the present study, we provide measurements of intracellular ADPR concentrations, an activator of the Ca2+-permeable cation channel TRPM2, in neutrophil granulocytes. As the main finding, we report that the ADPR concentrations are in the range that allows intracellular Ca2+ an effective regulation of TRPM2 gating. Thus, even though ADPR concentrations were not increased by stimulation of the cells, and Ca2+ by itself is inactive on TRPM2, ADPR and Ca2+ in combination can act as a messenger system. This messenger system is capable of inducing Ca2+ influx through TRPM2 channels in response to stimuli like fMLP that lead to release of Ca2+ from intracellular stores. In contrast, no evidence was obtained for the regulation of TRPM2 by cADPR.
Measurements of ADPR became possible after we modified a reversed-phase ion-pair HPLC method devised for the measurements of intracellular purine nucleotides [20]. An isocratic eluent was used that offers the advantage that small concentration changes can be more precisely assessed than with a gradient elution. The separation of ADPR from ATP and ADP became possible due to the addition of Mg2+ as an additional counterion in the eluent [22]. The method provides the baseline resolution of an ADPR peak, not only in standards but also in extracts from granulocytes. A similar HPLC method, not known to us at the start of our study, was recently reported [25] and used to analyse ADPR concentrations in Jurkat cells [26]. In our method, the preparation of the samples from HClO4 extracts involves fewer and less time-consuming steps; the almost complete recovery rate indicates that no ADPR is lost during the preparation. The experiments with nucleotide pyrophosphatase provide further proof for the specificity of the ADPR peak, even though other nucleotides are certainly substrates for this enzyme.
From the ADPR contents measured in the cell extracts, a range of likely ADPR concentrations may be deduced from the consideration of several uncertainties. A relatively safe assumption is the cell size of 350 fl, meaning that the average intracellular concentration is approx. 5 μM. However, the distribution space of ADPR within the cell is largely unknown. Thus the cytosolic concentration facing TRPM2 could be several times larger. Fortunately, our results indicate that no matter whether the actual ADPR concentration may be 2 or 30 μM, the relevance of ADPR remains the same. This is because over this concentration range (and beyond), the concentration–effect curve of ADPR on TRPM2 currents reveals a uniform requirement for Ca2+ of ADPR-dependent TRPM2 gating (see Figure 5). In the absence of Ca2+, ADPR induced a few channel openings but no sizeable whole-cell currents. However, almost maximal TRPM2 currents were evoked in the presence of 1 μM Ca2+ when ADPR was infused at concentrations as low as 1 μM. Further experiments demonstrate the essential role of ADPR for TRPM2 currents. Infusion of an ADPR-free solution prohibited any currents. This is explained by a rapid dilution of endogenous ADPR when the cell is dialysed with the pipette fluid in the whole-cell configuration [27]. In this context, it is noteworthy that a pipette solution with 0.1 μM ADPR was without effect on TRPM2 even in the presence of 1 μM Ca2+, demonstrating that dilution down to and below this level occurs in patch–clamp experiments without ADPR.
We did not obtain a full concentration–effect curve for Ca2+ but confined the analysis to two concentrations, one extremely small and the other one in the range of peak intracellular concentrations after stimulation with fMLP. In between, accurate adjustment of Ca2+ concentrations with EGTA as chelator is problematic due to the equilibrium constant of EGTA/Ca2+. Attempts to buffer Ca2+ in the concentration range of resting or mildly stimulated cells may result in severe errors deriving from small inaccuracies of the calculated EGTA and CaCl2 amounts, aggravated by small calcium contaminations of other salts and not completely quantifiable effects of other ions. Moreover, the buffering is weak in this Ca2+ concentration range. It is expected that other Ca2+ concentrations may exert intermediate effects in comparison with those observed with the two extreme concentrations, but an exact quantification cannot be provided at present.
On the basis of our measurements of physiological ADPR levels, the co-operativeness of ADPR and Ca2+ and the previous well-established findings that fMLP induced increases in the cytosolic Ca2+ concentration due to release of Ca2+ from intracellular stores [19], we propose that ADPR in neutrophil granulocytes renders TRPM2 a Ca2+-dependent Ca2+ entry pathway that is activated in consequence of a phosphoinositide response. In this model, there is no need for a stimulated ADPR formation because Ca2+ acts at basal ADPR concentrations. These basal concentrations, however, are essential because Ca2+ has no effect on TRPM2 on its own. Ca2+ influx through TRPM2 is a self-enhancing process, in line with the fact that fMLP leads to an irreversible activation of neutrophil granulocytes. Taken together, ADPR and Ca2+ in concert mediate the effects of extracellular ligands like fMLP. Thus ADPR and Ca2+ as a pair behave like a second messenger system, where relevant increases is one player (Ca2+) become effective in the presence of the other one (ADPR).
These considerations of the ADPR/Ca2+ messenger system do not include the possibility of ADPR concentration increases confined to small areas around the cell membrane and, especially, TRPM2 channels. One prominent enzyme that could be responsible for the formation of ADPR in neutrophil granulocytes is CD38 that exhibits the paradoxical property of being an ectoenzyme [18]. At present, one can only hypothesize whether CD38 in the plasma membrane has a role as a source of intracellular ADPR, which would necessitate the transport of NAD+ (the substrate) to the outside and of ADPR (the product) to the inside [14]. However, if such transport systems exist, spatially increased ADPR concentrations would appear likely. The experimental demonstration of localized ADPR peaks would increase our knowledge of the second messenger role of the ADPR/Ca2+ system but would probably not dramatically diminish the importance of Ca2+, because ADPR even at concentrations considerably higher than the average physiological ones is far more effective in the presence than in the absence of Ca2+.
Other intracellular compounds may contribute to the Ca2+-modulated activation of TRPM2 by ADPR. Most notably, cADPR and NAD+ may act as enhancers and AMP as inhibitor [6]. However, the required concentrations of cADPR were quite high. Kolisek et al. [6] used up to 1 mM cADPR, when the compound stimulated TRPM2 on its own, whereas a potentiation of ADPR-induced currents was reported for 10 μM cADPR. Already our HPLC analysis of cell extracts allows us to exclude intracellular concentrations of 10 μM ADPR. This was confirmed with the cycling assay that revealed a concentration of approx. 0.2 μM. Our patch–clamp experiments in granulocytes with 10 μM cADPR were complicated by ADPR contamination of the commercially available cADPR. After enzymatic removal of ADPR, no effect of cADPR could be confirmed, not even in the presence of subthreshold ADPR concentrations.
Stimulation with fMLP did not lead to an increase in intracellular cADPR concentrations; there was rather a tendency towards a decrease. However, since cADPR levels were at the detection limit of the method, small and possibly spatially limited concentration increases cannot be excluded. Such increases could indirectly be relevant for TRPM2 if cADPR induced Ca2+ release from stores in granulocytes. Interestingly, 8-bromo-cADPR, a membrane-permeant antagonist of cADPR, inhibited Ca2+ influx in response to fMLP [28]. These findings would be in line with Ca2+ release and subsequent Ca2+-induced Ca2+ influx as well as with direct activation of Ca2+ entry pathways. On the basis of our experiments, a direct effect of cADPR on TRPM2 channels in neutrophil granulocytes appears unlikely.
In conclusion, our study provides evidence for a physiological role of TRPM2 gating by ADPR in neutrophil granulocytes. The role in these cells is different from that proposed for Jurkat cells [26] that display slight increases in their ADPR content in response to concanavalin A, interpreted as a second messenger role of ADPR. In granulocytes, the regulatory function of ADPR depends on its co-operation with Ca2+. A second messenger role can be attributed only to ADPR and Ca2+ in concert. In effect, basal levels of ADPR enable activation by Ca2+ of TRPM2, which will induce further increases in the cytosolic Ca2+ concentration due to Ca2+ influx. This mechanism may be associated with the irreversible phase of neutrophil activation. Future studies in other cells may address the question of whether a decrease in ADPR concentrations is used to terminate Ca2+ influx through TRPM2.
Online Data
Acknowledgments
We thank Ilinca Ionescu for excellent technical assistance. This work was supported by grants from the Deutsche Forschungsgemeinschaft (Project B5 of SFB542).
References
- 1.Nagamine K., Kudoh J., Minoshima S., Kawasaki K., Asakawa S., Ito F., Shimizu N. Molecular cloning of a novel putative Ca2+ channel protein (TRPC7) highly expressed in brain. Genomics. 1998;54:124–131. doi: 10.1006/geno.1998.5551. [DOI] [PubMed] [Google Scholar]
- 2.Inamura K., Sano Y., Mochizuki S., Yokoi H., Miyake A., Nozawa K., Kitada C., Matsushime H., Furuichi K. Response to ADP-ribose by activation of TRPM2 in the CRI-G1 insulinoma cell line. J. Membr. Biol. 2003;191:201–207. doi: 10.1007/s00232-002-1057-x. [DOI] [PubMed] [Google Scholar]
- 3.Uemura T., Kudoh J., Noda S., Kanba S., Shimizu N. Characterization of human and mouse TRPM2 genes: identification of a novel N-terminal truncated protein specifically expressed in human striatum. Biochem. Biophys. Res. Commun. 2005;328:1232–1243. doi: 10.1016/j.bbrc.2005.01.086. [DOI] [PubMed] [Google Scholar]
- 4.Heiner I., Eisfeld J., Halaszovich C. R., Wehage E., Jüngling E., Zitt C., Lückhoff A. Expression profile of the transient receptor potential (TRP) family in neutrophil granulocytes: evidence for currents through long TRP channel 2 induced by ADP-ribose and NAD. Biochem. J. 2003;371:1045–1053. doi: 10.1042/BJ20021975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perraud A. L., Fleig A., Dunn C. A., Bagley L. A., Launay P., Schmitz C., Stokes A. J., Zhu Q., Bessman M. J., Penner R., et al. ADP-ribose gating of the calcium-permeable LTRPC2 channel revealed by Nudix motif homology. Nature (London) 2001;411:595–599. doi: 10.1038/35079100. [DOI] [PubMed] [Google Scholar]
- 6.Kolisek M., Beck A., Fleig A., Penner R. Cyclic ADP-ribose and hydrogen peroxide synergize with ADP-ribose in the activation of TRPM2 channels. Mol. Cell. 2005;18:61–69. doi: 10.1016/j.molcel.2005.02.033. [DOI] [PubMed] [Google Scholar]
- 7.Sano Y., Inamura K., Miyake A., Mochizuki S., Yokoi H., Matsushime H., Furuichi K. Immunocyte Ca2+ influx system mediated by LTRPC2. Science. 2001;293:1327–1330. doi: 10.1126/science.1062473. [DOI] [PubMed] [Google Scholar]
- 8.Hara Y., Wakamori M., Ishii M., Maeno E., Nishida M., Yoshida T., Yamada H., Shimizu S., Mori E., Kudoh J., et al. LTRPC2 Ca2+-permeable channel activated by changes in redox status confers susceptibility to cell death. Mol. Cell. 2002;9:163–173. doi: 10.1016/s1097-2765(01)00438-5. [DOI] [PubMed] [Google Scholar]
- 9.Wehage E., Eisfeld J., Heiner I., Jüngling E., Zitt C., Lückhoff A. Activation of the cation channel long transient receptor potential channel 2 (LTRPC2) by hydrogen peroxide: a splice variant reveals a mode of activation independent of ADP-ribose. J. Biol. Chem. 2002;277:23150–23156. doi: 10.1074/jbc.M112096200. [DOI] [PubMed] [Google Scholar]
- 10.McHugh D., Flemming R., Xu S. Z., Perraud A. L., Beech D. J. Critical intracellular Ca2+ dependence of transient receptor potential melastatin 2 (TRPM2) cation channel activation. J. Biol. Chem. 2003;278:11002–11006. doi: 10.1074/jbc.M210810200. [DOI] [PubMed] [Google Scholar]
- 11.Kühn F. J., Lückhoff A. Sites of the NUDT9-H domain critical for ADP-ribose activation of the cation channel TRPM2. J. Biol. Chem. 2004;279:46431–46437. doi: 10.1074/jbc.M407263200. [DOI] [PubMed] [Google Scholar]
- 12.Ame J. C., Spenlehauer C., de Murcia G. The PARP superfamily. BioEssays. 2004;26:882–893. doi: 10.1002/bies.20085. [DOI] [PubMed] [Google Scholar]
- 13.Sanghavi D. M., Thelen M., Thornberry N. A., Casciola-Rosen L., Rosen A. Caspase-mediated proteolysis during apoptosis: insights from apoptotic neutrophils. FEBS Lett. 1998;422:179–184. doi: 10.1016/s0014-5793(98)00004-0. [DOI] [PubMed] [Google Scholar]
- 14.Schuber F., Lund F. E. Structure and enzymology of ADP-ribosyl cyclases: conserved enzymes that produce multiple calcium mobilizing metabolites. Curr. Mol. Med. 2004;4:249–261. doi: 10.2174/1566524043360708. [DOI] [PubMed] [Google Scholar]
- 15.Partida-Sanchez S., Cockayne D. A., Monard S., Jacobson E. L., Oppenheimer N., Garvy B., Kusser K., Goodrich S., Howard M., Harmsen A., et al. Cyclic ADP-ribose production by CD38 regulates intracellular calcium release, extracellular calcium influx and chemotaxis in neutrophils and is required for bacterial clearance in vivo. Nat. Med. 2001;7:1209–1216. doi: 10.1038/nm1101-1209. [DOI] [PubMed] [Google Scholar]
- 16.Guse A. H., Cakir-Kiefer C., Fukuoka M., Shuto S., Weber K., Bailey V. C., Matsuda A., Mayr G. W., Oppenheimer N., Schuber F., Potter B. V. Novel hydrolysis-resistant analogues of cyclic ADP-ribose: modification of the ‘northern’ ribose and calcium release activity. Biochemistry. 2002;41:6744–6751. doi: 10.1021/bi020171b. [DOI] [PubMed] [Google Scholar]
- 17.Howard M., Grimaldi J. C., Bazan J. F., Lund F. E., Santos-Argumedo L., Parkhouse R. M., Walseth T. F., Lee H. C. Formation and hydrolysis of cyclic ADP-ribose catalyzed by lymphocyte antigen CD38. Science. 1993;262:1056–1059. doi: 10.1126/science.8235624. [DOI] [PubMed] [Google Scholar]
- 18.De Flora A., Franco L., Guida L., Bruzzone S., Usai C., Zocchi E. Topology of CD38. Chem. Immunol. 2000;75:79–98. doi: 10.1159/000058764. [DOI] [PubMed] [Google Scholar]
- 19.Krause K. H., Campbell K. P., Welsh M. J., Lew D. P. The calcium signal and neutrophil activation. Clin. Biochem. 1990;23:159–166. doi: 10.1016/0009-9120(90)80030-m. [DOI] [PubMed] [Google Scholar]
- 20.Jüngling E., Kammermeier H. Rapid assay of adenine nucleotides or creatine compounds in extracts of cardiac tissue by paired-ion reverse-phase high-performance liquid chromatography. Anal. Biochem. 1980;102:358–361. doi: 10.1016/0003-2697(80)90167-0. [DOI] [PubMed] [Google Scholar]
- 21.Graeff R., Lee H. C. A novel cycling assay for cellular cADP-ribose with nanomolar sensitivity. Biochem. J. 2002;361:379–384. doi: 10.1042/bj3610379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Folley L. S., Power S. D., Poyton R. O. Separation of nucleotides by ion-pair, reversed-phase high-performance liquid chromatography: use of Mg(II) and triethylamine as competing hetaerons in the separation of adenine and guanine nucleotides. J. Chromatogr. A. 1983;281:199–207. [Google Scholar]
- 23.Jankowski A., Grinstein S. A noninvasive fluorimetric procedure for measurement of membrane potential. Quantification of the NADPH oxidase-induced depolarization in activated neutrophils. J. Biol. Chem. 1999;274:26098–26104. doi: 10.1074/jbc.274.37.26098. [DOI] [PubMed] [Google Scholar]
- 24.Guse A. H., da Silva C. P., Berg I., Skapenko A. L., Weber K., Heyer P., Hohenegger M., Ashamu G. A., Schulze-Koops H., Potter B. V., Mayr G. W. Regulation of calcium signalling in T lymphocytes by the second messenger cyclic ADP-ribose. Nature (London) 1999;398:70–73. doi: 10.1038/18024. [DOI] [PubMed] [Google Scholar]
- 25.Gasser A., Guse A. H. Determination of intracellular concentrations of the TRPM2 agonist ADP-ribose by reversed-phase HPLC. J. Chromatogr. B. 2005;821:181–187. doi: 10.1016/j.jchromb.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 26.Gasser A., Glassmeier G., Fliegert R., Langhorst M. F., Meinke S., Hein D., Kruger S., Weber K., Heiner I., Oppenheimer N., et al. Activation of T cell calcium influx by the second messenger ADP-ribose. J. Biol. Chem. 2006;281:2489–2496. doi: 10.1074/jbc.M506525200. [DOI] [PubMed] [Google Scholar]
- 27.Pusch M., Neher E. Rates of diffusional exchange between small cells and a measuring patch pipette. Pflügers Arch. 1988;411:204–211. doi: 10.1007/BF00582316. [DOI] [PubMed] [Google Scholar]
- 28.Partida-Sanchez S., Iribarren P., Moreno-Garcia M. E., Gao J. L., Murphy P. M., Oppenheimer N., Wang J. M., Lund F. E. Chemotaxis and calcium responses of phagocytes to formyl peptide receptor ligands is differentially regulated by cyclic ADP ribose. J. Immunol. 2004;172:1896–1906. doi: 10.4049/jimmunol.172.3.1896. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.