Abstract
Ras is a major mediator of PE (phorbol ester) effects in mammalian cells. Various mechanisms for PE activation of Ras have been reported [Downward, Graves, Warne, Rayter and Cantrell (1990) Nature (London) 346, 719–723; Shu, Wu, Mosteller and Broek (2002) Mol. Cell. Biol. 22, 7758–7768; Roose, Mollenauer, Gupta, Stone and Weiss (2005) Mol. Cell. Biol. 25, 4426–4441; Grosse, Roelle, Herrlich, Höhn and Gudermann (2000) J. Biol. Chem. 275, 12251–12260], including pathways that target GAPs (GTPase-activating proteins) for inactivation and those that result in activation of GEFs (guanine nucleotide-exchange factors) Sos (son of sevenless homologue) or RasGRP (RAS guanyl releasing protein). However, a biochemical link between PE and GAP inactivation is missing and GEF stimulation is hard to reconcile with the observation that dominant-negative S17N-Ras does not compromise Ras-dependent ERK (extracellular-signal-regulated kinase) activation by PE. We have addressed this controversy and carried out an in-depth biochemical study of PE-induced Ras activation in COS-7 cells. Using a cell-permeabilization approach to monitor nucleotide exchange on Ras, we demonstrate that PE-induced Ras-GTP accumulation results from GEF stimulation. Nucleotide exchange stimulation by PE is prevented by PKC (protein kinase C) inhibition but not by EGFR [EGF (epidermal growth factor) receptor] blockade, despite the fact that EGFR inhibition aborts basal and PE-induced Shc (Src homology and collagen homology) phosphorylation and Shc–Grb2 (growth-factor-receptor-bound protein 2) association. In fact, EGFR inhibition ablates basal nucleotide exchange on Ras in growth-arrested COS-7 cells. These data disclose the existence of two separate GEF systems that operate independently from each other to accomplish PE-dependent formation of Ras-GTP and to maintain resting Ras-GTP levels respectively. We document that COS-7 cells do not express RasGRP and present evidence that the PE-responsive GEF system may involve PKC-dependent phosphorylation of Sos. More fundamentally, these observations shed new light on enigmatic issues such as the inefficacy of S17N-Ras in blocking PE action or the role of the EGFR in heterologous agonist activation of the Ras/ERK pathway.
Keywords: epidermal growth factor receptor (EGFR), nucleotide exchange, phorbol ester, Ras, RAS guanyl releasing protein (RasGRP), son of sevenless homologue (Sos)
Abbreviations: BIM, bisindolylmaleimide I; DAG, diacylglycerol; DMEM, Dulbecco's modified Eagle's medium; EGF, epidermal growth factor; EGFR, EGF receptor; ERK, extracellular-signal-regulated kinase; GAP, GTPase-activating protein; GEF, guanine nucleotide-exchange factor; Grb2, growth-factor-receptor-bound protein 2; GST, glutathione S-transferase; HB-EGF, heparin-binding EGF; H-Ras, v-Ha-ras Harvey rat sarcoma viral oncogene homologue; HRP, horseradish peroxidase; LPA, lysophosphatidic acid; K-Ras, Kirsten rat sarcoma viral oncogene homologue; MAPK, mitogen-activated protein kinase; MEK, MAPK/ERK kinase; NAC, N-acetylcysteine; NP40, Nonidet P40; PE, phorbol ester; PI3K, phosphoinositide 3-kinase; PKC, protein kinase C; PTP, protein tyrosine phosphatase; Raf-1, v-raf-1 murine leukaemia viral oncogene homologue 1; RasGRF, Ras protein-specific guanine nucleotide-releasing factor; RasGRP, RAS guanyl releasing protein; RBD, Ras binding domain; ROS, reactive oxygen species; Shc, Src homology and collagen homology; Sos, son of sevenless homologue
INTRODUCTION
Activation of K-Ras (Kirsten rat sarcoma viral oncogene homologue), H-Ras (v-Ha-ras Harvey rat sarcoma viral oncogene homologue) and N-Ras [neuroblastoma RAS viral (v-ras) oncogene homologue] GTPases (guanine nucleotide-binding proteins) (collectively referred to as Ras hereafter) results from an increase in the velocity ratio of two opposing reactions: the exchange of nucleotides on Ras resulting in GTP loading as catalysed by GEFs (guanine nucleotide-exchange factors) and the hydrolysis of Ras-bound GTP to GDP promoted by GAPs (GTPase-activating proteins). Hence, investigation of the biochemical mechanisms by which extracellular agonists elevate Ras-GTP levels boils down to elucidating how they affect GEF and/or GAP activities.
The tumour promoter and DAG (diacylglycerol) analogue PMA activates Ras in a limited number of cell types, including cardiac myocytes, COS fibroblast-like cells, T24 bladder carcinoma cells and leucocytes. Ras activation in response to PE (phorbol ester) in all these cell types is sensitive to PKC (protein kinase C) inhibition [1–6], but beyond this consensus reported mechanisms differ substantially. In T-cells, PE was originally proposed to down-regulate GAP activity via PKC, based on the finding that PE did not enhance the rate of nucleotide uptake by Ras in permeabilized cells [1]. PKC phosphorylates the p120GAP protein in vitro [7], providing a potential, albeit not yet proven, biochemical link for this scenario. Similar conclusions have been drawn for COS and T24 bladder carcinoma cells, based on the observation that PE activation of the Ras-effector ERK (extracellular-signal-regulated kinase) pathway was not affected by dominant-negative S17N-Ras [4,5,8,9]. Since S17N-Ras is believed to exert its blocking action by sequestering Ras-GEFs, these findings have been inferred to reinforce the notion that PE does not engage GEFs, but rather down-regulates GAP activity [4]. In T24 cells, intracellular sphingosine 1-phosphate as produced by PKC-activated sphingosine kinase has been put forward as a link between PE and Ras activation [4]. However, how this pathway ultimately feeds into Ras and whether or not this process is mediated by GAP inhibition is not known.
Opposed to this view, several lines of evidence argue for an involvement of Ras-GEFs in PE signalling to Ras. Recent biochemical and genetic evidence has made a strong case for members of the RasGRP (RAS guanyl releasing protein) family of Ras-GEFs as mediators of PE-induced Ras activation in lymphocytes [6,10–12]. RasGRP GEFs interact physically with DAG or PE via their C1 domain, and hence represent direct target proteins for PE. Mature T-cells from RasGRP1−/− knockout mice show dramatically reduced formation of Ras-GTP in response to PE [11], strongly arguing for RasGRP1 as a major mediator of PE- and DAG-elicited signalling to Ras. Analogous results from B-cells have shown that the B-cell receptor couples with the Ras/ERK pathway by means of the related GEF RasGRP3 [6,12]. Most recently, we have described the phosphorylation and concomitant activation of RasGRP1 and RasGRP3 by PKC, suggesting that PE feeds into RasGRP both through direct interaction and indirectly via PKC [6,13]. The appeal of this scenario resides in the fact that it provides a rationale for the well-established requirement for PKC in PE activation of Ras. However, RasGRP expression is largely limited to brain and lymphocytes [10,11,14], suggesting that this mechanism is unlikely to account for PE effects in other cell types.
Yet another line of evidence indicates that PE does access the Shc (Src homology and collagen homology)/Grb2 (growth-factor-receptor-bound protein 2)/Sos (son of sevenless homologue) pathway via PKC-mediated transactivation of the EGFR [EGF (epidermal growth factor) receptor]. PE induces tyrosine phosphorylation of the EGFR and Shc in a number of cell types [15–20]. In COS cells, this pathway proceeds via metalloprotease-mediated shedding of latent membrane-bound HB-EGF (heparin-binding EGF) [15], which consequently transactivates the EGFR in an autocrine/paracrine fashion. Available data on the role of the EGFR in PE signalling to the Ras/ERK pathway are controversial. EGFR has been reported to mediate PE activation of Ras or ERK in some cell types [16,18,21], yet fails to do so in other systems or in the hands of other investigators [22–24]. Moreover, while GEF (Sos or RasGRP) engagement by PE is documented in a number of settings, it is difficult to harmonize with the inefficacy of S17N-Ras in blocking PE activation of the Ras/ERK pathway, as observed in most (but not all [25,26]) of the cases where this has been investigated [4,5,8,9,27]. In summary, despite the documentation of a number of alternative mechanisms for PE-induced Ras activation, no singular pathway has been delineated as yet for a particular cell type.
In the present study, we investigated PE activation of Ras in more depth by looking at the actual rate of nucleotide turnover on Ras in permeabilized cells. We observed that PE elevates guanine nucleotide turnover on Ras in COS epithelial cells, demonstrating that PE engages Ras-GEFs to activate Ras. We present evidence for the existence of two Ras-GEF systems that operate independently from each other: one responds to PE-activated PKC and mediates agonist-induced acceleration of nucleotide exchange, and the other is fuelled by basal EGFR activity to maintain steady-state Ras-GTP levels in resting cells.
EXPERIMENTAL
Materials
Streptolysin O, the non-toxic diphtheria mutant CRM197, poly(Glu4-Tyr), iodoacetic acid, N-acetyl-L-cysteine, BIM (bisindolylmaleimide I), GDP, GTP, heparin–agarose, hydrogen peroxide and DMSO were purchased from Sigma–Aldrich (Munich, Germany). AG1478 (a tyrphostine-type inhibitor selective for the EGFR) was from Merck Biosciences (Bad Soden, Germany). PMA and PD98059 were from Alexis Biochemicals (Lausen, Switzerland). [α-32P]GTP (3000 Ci/mmol) was from NEN Life Science Products (Brussels, Belgium). Glutathione–Sepharose and GammaBind–Sepharose were from Amersham Biosciences (Freiburg, Germany).
Antibodies
Rabbit polyclonal serum 176 was raised against an N-terminal peptide of RasGRP1 which is conserved in mouse and human [10]. The L247-9 RasGRP3 polyclonal serum was obtained from rabbits immunized with a fragment of human RasGRP3 encompassing residues 544–689 [6]. These sera recognize both human and rodent RasGRP1 or RasGRP3 [6,10]. Anti-pan-Ras (Ab-4) was from Oncogene Science. Anti-H-Ras, K-Ras, N-Ras and anti-HB-EGF C-terminal domain were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, U.S.A.). Y13-259 rat monoclonal anti-Ras antibody was purified from the supernatant of hybridoma cells (A.T.C.C., Manassas, VA, U.S.A.). 4G10 anti-phosphotyrosine, polyclonal anti-Shc, anti-EGFR, anti-Grb2 and HRP (horseradish peroxidase)-conjugated rabbit anti-sheep IgG were from Upstate Biotechnology (Charlottesville, VA, U.S.A.). Anti-HB-EGF neutralizing antibody directed against the extracellular, secreted portion was from R&D Systems (Minneapolis, MN, U.S.A.). Anti-phospho-p44/42-ERK (Thr402/Tyr404) and anti-phospho-Akt (Thr308) were from Cell Signaling Technology (Beverly, MA, U.S.A.). Anti-pan-p44/42-ERK, monoclonal anti-Shc and anti-Sos antibody were from BD-Transduction Laboratories (San Jose, CA, U.S.A.). HRP-conjugated rabbit anti-mouse IgG was obtained from KPL (Gaithersburg, MD, U.S.A.).
Cell culture
COS-7 cells (a green-monkey kidney cell line; A.T.C.C.) were grown in DMEM (Dulbecco's modified Eagle's medium) supplemented with 10% (v/v) fetal calf serum at 37 °C in a 5%-CO2 incubator. For all experiments, cells were serum-starved for 24 h in DMEM.
Ras-GTP pull-down assay
Serum-starved COS-7 cells were treated/stimulated as appropriate and lysed in 1 ml (6-well plate) or 1.5 ml (10 cm dish, for up-scaling and visualization of basal Ras-GTP levels) of ice-cold lysis buffer [50 mM Tris, pH 7.5, 150 mM NaCl, 1 mM EGTA, 5 mM MgCl2, 1% NP40 (Nonidet P40), protease inhibitors and 100 μM GDP] supplemented with 20 μg/ml soluble GST (glutathione S-transferase)–Raf-1 (v-raf-1 murine leukaemia viral oncogene homologue 1)-RBD (Ras binding domain), which was produced in Escherichia coli by standard procedures. Cell material was scraped off and lysates were cleared by centrifugation. GST–Raf-1-RBD–Ras-GTP complexes were collected on glutathione–Sepharose, washed twice with 250 μl of lysis buffer lacking GDP and GST–Raf-1-RBD, followed by electrophoretic separation of the washed precipitates and Western-blot analysis with pan-Ras antibody alone (6-well plates) or in combination with K-Ras, H-Ras and N-Ras antibodies (10 cm dishes).
Permeabilization, nucleotide-exchange measurement and TLC analysis of Ras-bound nucleotides
COS-7 cell permeabilization was performed essentially as described previously [28]. Serum-starved subconfluent COS-7 cells in 6-well plates were incubated for at least 10 min in a buffer (50 mM Hepes, pH 7.5, 107 mM potassium glutamate, 15 mM NaCl, 3 mM MgCl2, 0.3 mM CaCl2 and 1 mM EGTA) at 37 °C in the presence or absence of relevant inhibitors. The medium was changed to 0.6 ml of the same solution at 37 °C containing 1 unit/ml streptolysin O and 500 μM Mg-ATP. For inhibitor-treated cells, this solution was supplemented with the relevant drugs. This solution was also supplemented with 100 nM PMA for analysis of nucleotide uptake 5 min post-stimulation. At 4 min after this addition, 100 μl of the same solution containing 400–500 μCi/ml [α-32P]GTP was added to each well. This solution was supplemented with 100 nM PMA for analysis of nucleotide uptake 1 min post-stimulation (if not indicated otherwise, PMA stimulation was always 5 min prior to starting the analysis of nucleotide uptake). Kinetics was started 1 min later (i.e. 5 min after the addition of streptolysin O): medium was removed and cells were lysed in 1 ml of ice-cold buffer (50 mM Hepes, pH 7.5, 100 mM NaCl, 10 mM MgCl2, 1% NP40, 100 μM GDP, 100 μM GTP and protease inhibitors) supplemented with 5 μg/ml Y13-259 antibody for Ras immunoprecipitation. Cells were scraped off and extracts were put on ice. Lysates were cleared by centrifugation and supernatants were made up to 500 mM NaCl, 0.5% sodium deoxycholate and 0.05% SDS. Immunocomplexes were collected on GammaBind–Sepharose by 45 min incubation at 4 °C. After six rounds of washing with 1 ml of an ice-cold buffer (50 mM Hepes, pH 7.5, 500 mM NaCl, 5 mM MgCl2, 0.1% Triton X-100 and 0.005% SDS), immunoprecipitates were sequentially subjected to Cerenkov counting and TLC analysis exactly as described by others [1]. In all panels, time point 0 as recorded is defined by quenching of the first assay point 5 min after streptolysin O addition.
Immunoprecipitation and Western blotting
For EGFR and Shc immunoprecipitation experiments, cells were treated as appropriate and lysed in a buffer (50 mM Tris, pH 7.5, 150 mM NaCl, 5 mM EDTA, 1% NP40, protease inhibitors and phosphatase inhibitors). Alternatively, in those cases where immunoprecipitation was combined with Ras-GTP pull-down assays, immunoprecipitations were performed on the supernatant of the Ras-GTP pull-down once GST–Raf-1-RBD had been collected on the glutathione–Sepharose. In either case, cell extracts were split and incubated on ice for 2 h with 1 μg of immunoprecipitating antibody for EGFR (sheep polyclonal) or Shc (rabbit polyclonal). Immunocomplexes were collected on GammaBind–Sepharose by an overnight incubation at 4 °C. Immunoprecipitates were washed three times with lysis buffer (50 mM Tris, pH 7.5, 150 mM NaCl, 5 mM EDTA, 1% NP40, protease inhibitors and phosphatase inhibitors) and resolved by SDS/PAGE. For immunoblotting, membranes were blocked and probed with 4G10 mouse anti-phosphotyrosine and mouse anti-Grb2. Blots were then incubated with the appropriate secondary antibodies, all conjugated with HRP and the immunoreactive bands were visualized by enhanced chemiluminescence. PVDF membranes probed with 4G10 anti-phosphotyrosine were stripped and reprobed with sheep anti-EGFR and mouse monoclonal anti-Shc.
Analysis of HB-EGF shedding
Serum-starved COS-7 cells [(2–3)×106 cells] were treated with 100 nM PMA for the indicated time periods. For detection of secreted HB-EGF, the culture medium was supplemented with 200 μM PMSF, 20 μg/ml aprotinin and 20 μg/ml leupeptin and incubated for 2 h with heparin–agarose beads at 4 °C. The heparin–agarose precipitates were washed once with ice-cold buffer (50 mM Tris, pH 7.5, 150 mM NaCl, 1 mM EGTA, 5 mM MgCl2, 1% NP40 and protease inhibitors), eluted with SDS/PAGE sample buffer [10% (v/v) glycerol, 2 % (w/v) SDS, 5% (v/v) 2-mercaptoethanol and 50 mM Tris/HCl, pH 6.8] and subjected to SDS/PAGE and Western blotting with goat anti-HB-EGF neutralizing antibody. For detection of proHB-EGF (HB-EGF precursor), cells were lysed in 500 μl of lysis buffer (50 mM Tris, pH 7.5, 150 mM NaCl, 1 mM EGTA, 5 mM MgCl2, 1% NP40 and protease inhibitors). Lysates were clarified by centrifugation and aliquots were subjected to SDS/PAGE and Western-blot analysis with goat anti-proHB-EGF directed against the C-terminal domain of proHB-EGF.
Reversible oxidation of PTPs (protein tyrosine phosphatases)
PTP oxidation was assayed by the method of Meng et al. [29]. Briefly, serum-starved COS-7 cells were challenged with 100 nM PMA or hydrogen peroxide for the indicated time periods. To prevent oxidation of the catalytic cysteine in PTPs, cells were carefully lysed in 150 μl of lysis buffer (25 mM sodium acetate, pH 5.5, 150 mM NaCl, 1% NP40, 10% glycerol and protease inhibitors) that had previously been vacuum-degassed for 4 h. Lysis was carried out in the presence or absence of 10 mM iodoacetic acid. Lysates were incubated for 30 min on ice to ensure complete alkylation and cleared by centrifugation at 20000 g for 15 min at 4 °C. Aliquots were then subjected to a PTP in-gel assay exactly as described in [29]. SDS/polyacrylamide gels were cast by standard procedures, except that 32P-labelled poly(Glu4-Tyr) was added to the polymerization mix at 104 Bq/ml gel solution. Gels were dried and exposed to a phosphoimager.
Northern blotting
Northern blotting for detection of RasGRP1 and RasGRP3 mRNAs was performed by routine methodologies. In brief, total RNA of COS-7, Jurkat T-cells, Ramos B-cells and cerebral cortex of the green monkey (Cercopithecus aethiops) (a gift from Dr Wolfgang Enard, Leipzig) was isolated using QIAamp RNA Mini kit (Qiagen, Hilden, Germany), separated by denaturing electrophoresis, vacuum-transferred to a nylon membrane and hybridized with [α-32P]dATP-labelled DNA probes derived from human RasGRP1 (encompassing bp 1–918; an alternative probe encompassing bp 918–2052 was used in a separate experiment, yielding the same results, as shown in Figure 8B) or human RasGRP3 (encompassing bp 1–1187). Labelling was done by random priming (Roche Molecular Biochemicals, Mannheim, Germany) and hybridization was performed under stringent conditions. After exposure, membranes were stripped and reprobed for β-actin.
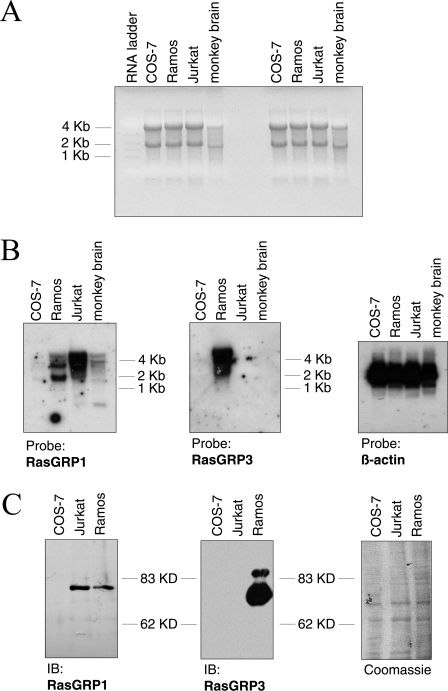
Figure 8. COS-7 cells do not express RasGRP1 and RasGRP3.
(A) Ethidium bromide stain of total RNA from COS-7, Ramos and Jurkat cells (all 6 μg) and green monkey brain cortex (2 μg) prior to processing for the Northern-blot analysis shown in (B). Molecular-mass marker is indicated in kilobases (kb). (B) Total RNA samples shown in (A) were transferred on to a nylon membrane and subjected to Northern hybridization with the probes indicated below the panels. Films were overexposed to avoid low transcript levels in COS-7 cells passing undetected. Molecular-mass marker is indicated in kilobases. (C) Cell lysates of COS-7, Ramos and Jurkat cells (30 μg of protein each) were analysed by Western blotting for the presence of RasGRP1 or RasGRP3. The membrane was subsequently stained with Coomassie Blue to ascertain equal loading (right panel). IB, immunoblot; kD, kDa.
RESULTS
PE stimulates nucleotide exchange on Ras
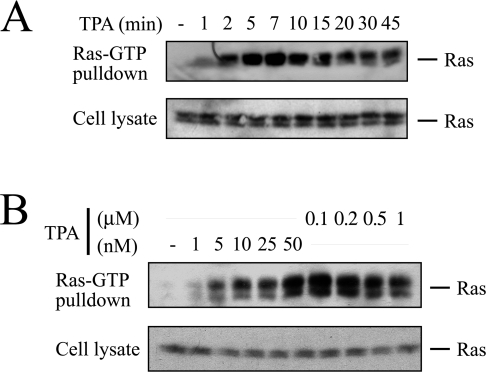
The PE PMA induces sustained and robust Ras activation in COS-7 cells (Figure 1A) in a dose-dependent manner (Figure 1B) [5]. We chose this cell type for the current study of PE-induced Ras activation because various mechanisms have been documented or inferred to operate in COS-7 cells: (i) PE-induced Ras activation is sensitive to PKC inhibitors [5], (ii) PE transactivates the EGFR, leading to tyrosine phosphorylation of Shc [15,18,21], (iii) PE has been inferred to activate Ras through direct activation of RasGRP1 [30], and finally, (iv) Ras and ERK activations in response to PE are not compromised by S17N-Ras in COS-7 cells [5,8,9], which has been interpreted by some investigators as an indication for PE-induced GAP down-regulation [4].
Figure 1. PMA activates Ras in COS-7 cells.
(A) Subconfluent serum-starved COS-7 cells were challenged with 100 nM PMA (TPA). After indicated time periods, cells were lysed in the presence of the Ras binding domain (RBD) of the Ras effector Raf-1 fused to GST and processed for detection of active GTP-loaded Ras as described in the Experimental section. Aliquots of the cell extracts were analysed in parallel to ascertain equal loading. (B) Same set-up as in (A), except that COS-7 cells were stimulated with the indicated concentrations of PMA for 10 min.
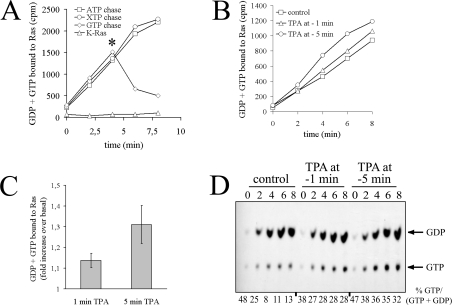
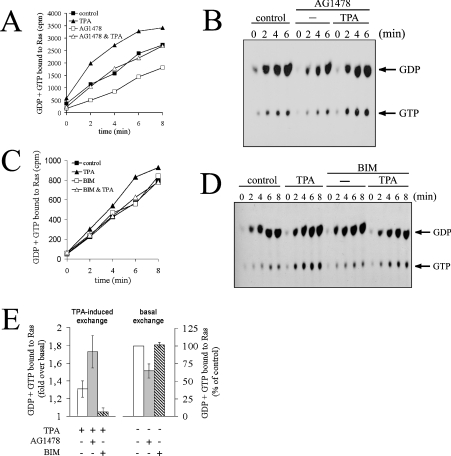
To systematically address the mechanism of PMA-induced Ras activation, we first designed experiments that would discriminate between GEF activation and GAP inhibition. To this end, we employed a cell permeabilization strategy that allows for the rapid delivery of radioactively labelled guanine nucleotides into cells. Quantification of radioactivity uptake by Ras over time provides information on the nucleotide-exchange rate of Ras proteins. Nucleotide uptake monitored by this assay is specific, since it is ablated by omission of the immunoprecipitating antibody Y13-259 or the permeabilizing agent streptolysin O, and reversible, since it is chased by recombinant Ras or unlabelled GTP, but not adenine or xanthine nucleotides (Figure 2A, and results not shown) [31]. Importantly, the Y13-259 antibody precipitated quantitatively K-, H- and N-Ras and, contrary to previous observations [32], it did not cross-react with endogenous TC21 or M-Ras proteins (results not shown). To evaluate whether or not PE stimulated nucleotide exchange on Ras, serum-starved COS-7 cells were challenged with PMA 1 or 5 min prior to permeabilization and quenching of the first assay point. As shown in Figure 2(B), PMA elevated the rate of nucleotide uptake by Ras. Stimulation of nucleotide uptake was more pronounced in cells challenged 5 min versus 1 min prior to pore formation, illustrating that stimulation of nucleotide exchange increased with time (quantified in Figure 2C). Increased uptake of guanine nucleotides in response to PE was accompanied by a percentage accumulation of Ras-GTP in the same immunoprecipitates (Figure 2D), strongly indicating that the PE-induced increase in nucleotide uptake was not an incidental event but was causally linked to the activation of Ras. Of note, initial values for % Ras-GTP/(GDP + GTP) started off high and levelled off only at later time points. This pattern most probably reflected the different time required for single Ras proteins to reach steady-state nucleotide turnover versus that needed for the whole Ras population to run through one round of nucleotide exchange. Taking all these results together, we conclude that PMA activates Ras-GEF(s) to stimulate nucleotide exchange on Ras.
Figure 2. PMA stimulates nucleotide exchange on Ras.
(A) Permeabilization assay for assessment of nucleotide exchange on Ras. Serum-starved COS-7 cells were permeabilized with streptolysin O in the presence of [α-32P]GTP. Ras proteins were immunoprecipitated at various time points with the Y13-259 antibody in the presence or absence of 5 μg/ml recombinant K-Ras protein, as indicated. [α-32P]GTP incorporation was chased by addition of 100 μM of indicated purine nucleotides at the time point marked by an asterisk. Washed immunoprecipitates were subjected to Cerenkov counting. (B) PMA (‘TPA’) elevates nucleotide exchange on Ras. Serum-starved COS-7 cells were challenged with 100 nM PMA 5 or 1 min prior to permeabilization in the presence of [α-32P]GTP and quenching of the first assay point (zero time as recorded). Ras proteins were immunoprecipitated with Y13-259 at the indicated time points and radioactivity associated with immunoprecipitates was evaluated by Cerenkov counting. (C) Quantification of results presented in (B). The amount of GDP + GTP bound to Ras 6 min after quenching the zero-time assay point (as recorded in B) was plotted as the fold increase of radioactivity bound to Ras in PMA-stimulated cells versus unstimulated cells. Results shown represent the means±S.E.M. for at least three independent experiments. (D) Separation of guanine nucleotides associated with Ras. Guanine nucleotides were eluted from the same immunoprecipitates as in (B) and separated by TLC. Values for % GTP/(GDP + GTP) are given below the Figure. Three independent experiments yielded similar results.
Transactivation of the EGFR/Shc pathway does not mediate Ras activation by PMA
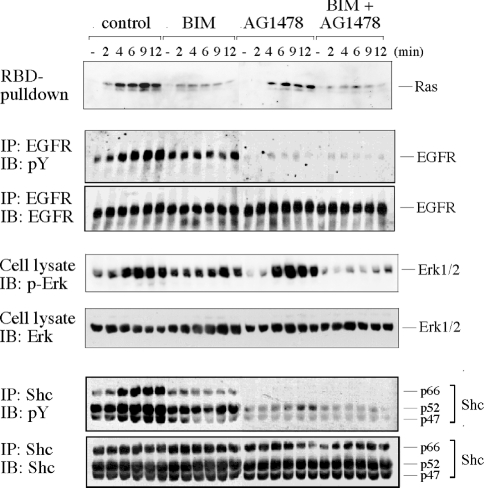
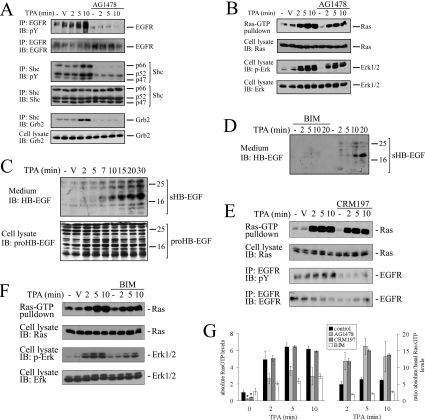
PMA elicits sequential tyrosine phosphorylation of the EGFR and the adapter protein Shc in COS cells [15,18]. Since it is well established that this pathway feeds into the Ras-GEF Sos, it was a likely mediator of PMA-induced nucleotide-exchange stimulation. To address this possibility, we used a pharmacological approach to inhibit EGFR activity. The well-characterized selective EGFR inhibitor AG1478 completely ablated PMA-induced EGFR and Shc phosphorylation (Figure 3), in agreement with a previous report [21]. AG1478 treatment also compromised basal levels of EGFR and Shc phosphorylation. In contrast, AG1478 caused only a partial attenuation of Ras-GTP accumulation in response to PMA (Figure 3; see quantification in Figure 4G), indicating that the bulk of Ras-GTP formation after PMA stimulation did not require the EGFR. Assessing the activation status of the Ras effector ERK pathway yielded an analogous picture, that is, application of AG1478 caused inhibition of basal ERK activity, but only minor mitigation of PMA-sparked ERK activation.
Figure 3. Role of EGFR and PKC in the activation of the Ras/ERK pathway by PMA.
Subconfluent, serum-starved COS-7 cells were treated with AG1478 (200 nM for 5 min) and/or BIM (500 nM for 10 min) or left untreated and challenged with 100 nM PMA. At the indicated time points, cells were lysed in the presence of GST–RBD and phosphatase inhibitors. The activation/phosphorylation status of Ras, EGFR, Shc and ERK was sequentially determined in the same lysates as described in the Experimental section. Antibodies used for immunoprecipitation (IP) or immunoblotting (IB) are indicated on the left-hand side of the panel. Four independent experiments yielded similar results. p-ERK, anti-phospho-ERK; pY, anti-phosphotyrosine.
Figure 4. Basal EGFR activity maintains basal Ras-GTP levels.
(A) EGFR inhibition disrupts basal Shc–Grb2 complex formation. Serum-starved COS-7 cells were treated with 200 nM AG1478 for 5 min or left untreated as indicated. Cells were challenged with 100 nM PMA (TPA) or vehicle DMSO and lysed at various time points. Cell extracts were split and subjected to EGFR or Shc immunoprecipitation. Tyrosine phosphorylation of both proteins or Shc–Grb2 association were assessed using phosphotyrosine (pY)- or Grb2-directed antibodies respectively. (B) Cells were challenged as in (A) and cell extracts were subjected to a Ras-GTP pull-down assay. Activation of ERK was assayed out of the same lysates as described in the Experimental section. (C) PMA induces shedding of endogenous proHB-EGF. Serum-starved COS-7 cells were challenged for the indicated time points with 100 nM PMA or the vehicle DMSO. The medium was collected and incubated for 2 h at 4 °C with heparin–agarose to collect secreted heparin-binding proteins. Proteins associated with the heparin beads were separated by SDS/PAGE and probed by immunoblotting with an antibody directed against the shed HB-EGF portion (sHB-EGF; top panel). In parallel, cells were lysed and cell lysates were analysed by Western blotting for the presence of membrane-bound proHB-EGF species by immunoblotting with a serum directed to the intracellular domain of HB-EGF (lower panel). (D) HB-EGF shedding in response to PMA is mediated by PKC. Serum-starved COS-7 cells were treated with BIM (500 nM for 10 min) or left untreated and challenged with PMA as indicated. Endogenous HB-EGF shedding was assessed as in (C). (E) Interruption of HB-EGF-mediated EGFR transactivation does not compromise Ras activation by PMA. Serum-starved COS-7 cells were treated with the diphtheria toxin mutant CRM197 (5 μg/ml for 30 min) to block HB-EGF function and challenged with 100 nM PMA or DMSO vehicle. At the indicated time points, cell extracts were prepared, split and subjected to a Ras-GTP pull-down assay or EGFR immunoprecipitation. Samples were processed as described in the Experimental section. (F) COS-7 cells were treated with 500 nM BIM for 10 min or left untreated prior to stimulation with 100 nM PMA. Samples were processed as described above for assessment of Ras-GTP formation and ERK activation. (G) Summary of effects of EGFR or PKC inhibition on Ras-GTP levels. Absolute Ras-GTP levels in experiments shown in (B, E, F) were quantified by densitometric analysis and are shown plotted on the left panel. The value for the unstimulated and untreated cells was set as 1. *P<0.05 compared with untreated. In the right panel, values are corrected for the basal Ras-GTP levels and plotted as the ratio absolute/basal Ras-GTP levels. Results shown represent the means±S.E.M. for three independent experiments. V, vehicle.
To investigate the role of PKC, a major target of PMA, we tested the effect of the PKC inhibitor BIM, which reportedly blocks PMA activation of Ras/ERK in COS cells [5] (Figure 3). In accordance with this and other reports [16,21], 500 nM BIM treatment strongly or fully impaired PMA-induced Ras-GTP accumulation, EGFR transactivation, tyrosine phosphorylation of Shc and ERK activation, suggesting that PMA signalled through PKC both to the EGFR/Shc and into the Ras/ERK pathway. Importantly, basal activation/phosphorylation levels of all parameters were not affected by BIM. In summary, while EGFR inhibition compromised basal levels of the Ras/ERK pathway but did not significantly impinge on PMA-induced Ras/ERK activation, PKC inhibition specifically interrupted PMA-dependent effects but not basal levels of activation. These results strongly indicated that PKCs acted as genuine mediators of PMA-elicited signalling, while EGFR activity sustained the steady-state levels of the Ras/ERK pathway. In addition, these observations illustrated that the EGFR/Shc and Ras/ERK pathways were engaged in parallel rather than in series downstream of PMA-activated PKC.
EGFR sustains basal Ras-GTP levels in serum-arrested cells
Figure 3 illustrates that EGFR inhibition with AG1478 compromised basal tyrosine phosphorylation of Shc, suggesting that the interruption of basal ERK-pathway activation occurred upstream of Ras. To address this hypothesis, we first investigated complex formation between Shc and Grb2 adapter proteins, a commonly used read-out for the engagement of the Grb2/Sos GEF system (Figure 4A). PMA-stimulated as well as basal Shc–Grb2 associations were ablated upon AG1478 treatment, indicating that inhibition of basal EGFR activity disrupted the Shc/Grb2/Sos GEF system in serum-arrested cells. To investigate whether disruption of the Shc–Grb2 complex formation impinged on basal Ras-GTP levels, we scaled up the Ras-GTP pull-down assay in order to detect basal levels of Ras-GTP (Figure 4B) (see the Experimental section). AG1478 treatment resulted in a marked reduction of basal Ras-GTP levels, confirming that basal EGFR activity drove steady-state Ras-GTP formation. The structurally related tyrphostine AG1296, a selective inhibitor of the platelet-derived growth factor receptor (which is not expressed in COS-7 cells), had no effect on basal Ras-GTP levels (results not shown). As shown above, PMA raised Ras-GTP levels in the background of AG1478 inhibition (Figure 4B). While Ras-GTP accumulation did not reach the level obtained from untreated cells, densitometric analysis of Ras-GTP signals and correction for the basal Ras-GTP values prior to PMA administration revealed that net accumulation of Ras-GTP in response to PMA was largely unaffected by AG1478 (Figure 4G). The same pattern was observed at the level of ERK (Figure 4B).
To underscore this finding with an alternative approach, we employed the diphtheria toxin mutant CRM197, which interrupts PMA-induced EGFR transactivation in COS-7 cells by scavenging HB-EGF [15]. In a preliminary experiment, we assessed whether or not PMA induced the processing of endogenous HB-EGF in COS-7 cells, since in this cell type, this process has as yet only been documented in cells engineered to overexpress HB-EGF [15,33]. Cells were stimulated with PMA for various time frames, and heparin-binding molecules released into the medium were collected by affinity purification on heparin–agarose. Immunoblotting with an antibody that targets the extracellular, secreted portion of HB-EGF (Figure 4; sHB-EGF) revealed time-dependent proHB-EGF processing which was detecTable 5–7 min after PMA administration (Figures 4C and 4D). Immunoblotting of the corresponding cell extracts with serum directed against the intracellular domain revealed a heterogeneous pattern of proHB-EGF polypeptides, as reported previously by others [15,34]. The observed shedding of HB-EGF suggested that sHB-EGF was indeed a potential mediator of PMA-elicited EGFR transactivation. In line with this notion and previous reports [33,34], PMA-induced processing of HB-EGF was highly sensitive to PKC inhibition (Figure 4D). A role for HB-EGF shedding in PMA-induced transactivation of the EGFR was further corroborated by the finding that sequestration of HB-EGF by CRM197 blocked EGFR transactivation in response to PMA (Figure 4E) [15]. However, CRM197 application resulted in only weak attenuation of PMA-induced Ras-GTP formation (Figures 4E and 4G) or ERK activation (results not shown), in line with previous observations made at the level of ERK [22]. These results confirmed that the EGFR did not transduce PMA signals to Ras, while it was critical for the maintenance of basal Ras-GTP levels.
In marked contrast, the effects of the PKC inhibitor BIM were the mirror image of those elicited by EGFR inhibition, that is, BIM did not affect basal Ras-GTP levels but strongly compromised PMA-induced Ras-GTP accumulation (Figures 4F and 4G). In Figure 4(G), Ras-GTP levels are plotted both in terms of absolute values and as fold of basal to highlight the fundamentally opposed consequences of EGFR versus PKC inhibition. Taken together, these results demonstrated that PKC and EGFR independently regulated PMA-induced and basal Ras-GTP formation respectively.
Two independent nucleotide-exchange factor systems regulate basal and PMA-driven nucleotide exchange on Ras
Since Ras activation by PMA proceeds via stimulation of GEFs (Figures 2B and 2C), we went on to determine the consequences of EGFR or PKC inhibition at the level of nucleotide exchange on Ras. The aim of these experiments was to determine whether the observed role of PKC and EGFR in PMA-induced versus basal Ras-GTP formation reflected the regulation of different Ras-GEF systems by PKC and EGFR. COS-7 cells were treated with AG1478 to inhibit the EGFR prior to PMA administration and permeabilization in the presence of radiolabelled GTP (Figure 5A). EGFR inhibition resulted in a decrease in the basal nucleotide exchange rate on Ras. Quantification revealed a reduction of overall basal nucleotide exchange by approx. 40% after EGFR inhibition (Figure 5E). However, since these values include the intrinsic nucleotide exchange rate of Ras proteins (the exact magnitude of which is arduous to estimate), the contribution of the EGFR to GEF-catalysed nucleotide exchange in resting COS-7 cells is bound to exceed 40%. This observation demonstrated that basal EGFR activity sustained steady-state Ras-GTP levels via stimulation of nucleotide exchange in serum-starved cells. Strikingly, the breakdown of basal nucleotide exchange caused by EGFR inhibition did not compromise PMA signalling to GEFs, since PMA was able to mount a response and raise nucleotide uptake in the presence of AG1478 (Figure 5A; quantified in Figure 5E). Remarkably, fold stimulation of overall nucleotide exchange by PMA was even increased in a background of EGFR inhibition (Figure 5E; see the Discussion section). Separation of guanine nucleotides associated with the same immunoprecipitates showed that PMA-induced accumulation of Ras-GTP was not compromised by the breakdown of basal nucleotide exchange elicited by AG1478 (Figure 5B), confirming the results of the Ras-GTP pull-down experiments shown above. Since EGFR and Shc are not phosphorylated/activated under these conditions (Figures 3 and 4A), the straightforward interpretation is that PMA addresses a Ras-GEF system different from the canonical Shc/Grb2/Sos module that acts downstream of the EGFR.
Figure 5. EGFR does not mediate GEF activation in response to PMA.
(A) Serum-starved COS-7 cells were pre-incubated with 200 nM AG1478 for 5 min at 37 °C or left untreated. Cells were challenged with 100 nM PMA (TPA) or left unstimulated, followed by permeabilization in the presence of [α-32P]GTP. Determination of guanine nucleotides associated with Ras was performed as described in the Experimental section. Note that PMA stimulation occurred 5 min prior to zero-time point as recorded in the plot. (B) Guanine nucleotides associated with Ras immunoprecipitates in (A) were separated by TLC. (C) The experiment was performed as described in (A), except that cells were pretreated with 500 nM BIM for 10 min. (D) Guanine nucleotides associated with Ras immunoprecipitates from the experiment shown in (C). (E) Quantification of nucleotide exchange for experiments shown in (A, C). Left panel: PMA-induced exchange. The amount of GDP + GTP bound to Ras 6 min after quenching the zero-time assay point (as recorded in A and C) was plotted as the fold increase in radioactivity associated with Ras in PMA-stimulated cells versus unstimulated cells for the various inhibitor treatments. Right panel: basal exchange rate. The amount of GDP + GTP bound to Ras in inhibitor-treated cells 6 min after quenching the zero-time assay point (as recorded in A and C) was plotted as percentage of radioactivity associated with Ras in untreated cells. Results shown represent the means and S.E. for at least three independent experiments.
In analogous experiments, the consequences of PKC inhibition with BIM were again the mirror image of AG1478 effects: BIM did not compromise basal nucleotide exchange on Ras, but it markedly impaired stimulation of nucleotide uptake and accumulation of GTP-loaded Ras in response to PMA (Figures 5C and 5D; quantified in Figure 5E), strongly supporting the idea that PKC is a genuine constituent of the PMA signalling pathway that feeds into Ras-GEF(s).
ROS (reactive oxygen species) mediate EGFR transactivation by PMA
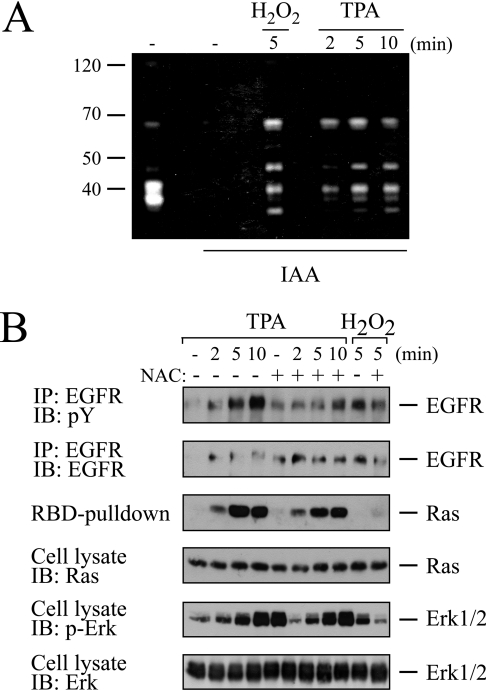
The experimental data presented so far indicated that PMA signals bifurcated downstream of PKC, leading to the parallel activation of the EGFR and the Ras/ERK pathway. This raised the question as to the molecular mechanism underlying this splitting of signals downstream of PKC. Several reports have documented that PMA potently induces ROS via PKC in a number of cell types [35,36]. An increasing body of evidence implicates reversible inhibition of PTPs as one major mode of action of ROS in growth-factor and PE signalling [35,36]. Reversible PTP inhibition results from the oxidation of the catalytic cysteine to sulphenic acid by ROS. To explore the involvement of ROS and ROS-dependent PTP down-regulation in PMA signalling to the EGFR or the Ras/ERK pathway, we investigated PTP inhibition using a recently developed technique that monitors the reversible oxidation/inhibition of PTPs [29]. Cell extracts of PMA-stimulated COS-7 cells were prepared in the presence of iodoacetic acid, which irreversibly alkylates the catalytic cysteine of virgin, non-oxidized PTPs, but not that of their oxidized counterparts. Extracts were separated by SDS/PAGE, followed by in-gel renaturation of PTPs and an in-gel phosphatase assay. This procedure therefore only scores the phosphatase activity of reversibly oxidized PTPs, since these species are protected against irreversible, post-lytic alkylation by iodoacetic acid. As shown in Figure 6(A), PMA induced the reversible oxidation/inhibition of several PTP species in a timedependency that matched both EGFR transactivation and Ras-GTP accumulation (Figure 3). To discern whether ROS-dependent processes were involved in activation of either of the two pathways, cells were treated with the thiol-group-containing reducing compound NAC (N-acetylcysteine) prior to PMA administration (Figure 6B). NAC almost completely abrogated PMA-induced EGFR transactivation. In contrast, Ras-GTP formation and ERK activation in response to PMA were not compromised by NAC treatment. Moreover, direct application of hydrogen peroxide resulted in tyrosine phosphorylation of the EGFR, but it did not promote Ras-GTP formation or ERK activation, indicating that COS-7 cells generally lacked the ability to couple ROS with Ras activation. Taken together, these observations identified ROS as a link between PKC and EGFR transactivation and underscored the conclusion that EGFR transactivation and activation of the Ras/ERK pathway proceeded via separate pathways downstream of PMA-activated PKC (Scheme 1).
Figure 6. PMA transactivates the EGFR via ROS.
(A) PMA (TPA) induces reversible PTP inhibition. Serum-starved COS-7 cells were treated with 0.5 mM H2O2 for 5 min or challenged for various time periods with 100 nM PMA. Cells were lysed in the absence or presence of iodoacetic acid (IAA), as indicated, to alkylate irreversibly the catalytic cysteine of non-oxidized PTPs. Extracts were separated by SDS/PAGE on a gel that had polymerized in the presence of a radiolabelled PTP substrate. Following in-gel renaturation of PTPs, renatured and non-alkylated (i.e. reversibly oxidized) PTPs hydrolyse the 32P-labelled substrate and give rise to negative (white) bands upon exposure to a film. (B) ROS production mediates EGFR transactivation by PMA. Serum-starved COS-7 cells were pretreated for 20 min with 20 mM NAC or left untreated. Cells were challenged with 100 nM PMA for various time points or with 0.5 mM H2O2 for 5 min. Cells were lysed and lysates were split for individual assessment of EGFR phosphorylation, Ras-GTP formation and ERK activation as described above.
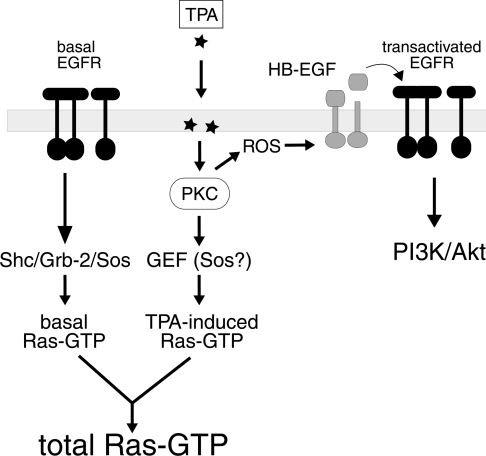
Scheme 1. Schematic representation of PMA signalling to Ras and the EGFR in COS-7 cells.
For reasons of clarity, two populations of EGFR are shown, one driving basal Ras-GTP formation, the second being targeted and transactivated in response to PMA (TPA). However, it is important to note that this study does not provide any evidence for the existence of different EGFR populations. The exact point of entry of ROS into the pathway is unknown.
EGFR mediates PI3K (phosphoinositide 3-kinase)/Akt activation by PE
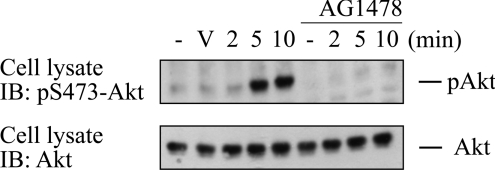
Since the EGFR became transactivated by PMA, but did not transduce the signal for Ras activation, we addressed the question whether PE-induced EGFR activation mediated PE effects other than Ras activation. As a second major signalling pathway, we investigated Akt activation by PE. PE elicited the time-dependent activation of Akt, as monitored by phosphorylation of Ser473 (Figure 7). PE-induced Akt activation was fully absent from AG1478-treated cells, showing that EGFR transactivation mediated Akt activation induced by PE. Hence, while EGFR transactivation by PE was unproductive in terms of Ras activation, it did mediate Akt activation in response to PE.
Figure 7. EGFR mediates AKT activation in response to PMA.
Serum-starved COS-7 cells were pretreated with AG1478 (200 nM for 10 min) or vehicle DMSO and stimulated with 100 nM PMA. Cell extracts were separated by SDS/PAGE, and Akt phosphorylation on Ser473 was assayed with a phosphorylation site-specific antibody (upper panel). Blot was stripped and reprobed for total load of Akt protein with pan-Akt serum (lower panel). V, vehicle.
COS-7 cells do not express RasGRP1 or RasGRP3
The current results illustrated that PE-induced nucleotide exchange on Ras and concomitant accumulation of Ras-GTP proceeded in the absence of tyrosine phosphorylation of Shc and Shc–Grb2 association. This observation excluded the canonical Shc/Grb2/Sos system as the GEF unit addressed by PE. Two other classes of Ras GEFs, RasGRF (Ras protein-specific guanine nucleotide-releasing factor) and RasGRP, could potentially mediate PE activation of Ras. RasGRP proteins are particularly attractive, since they interact physically with PMA and mediate PMA/DAG-induced Ras activation [6,10–14]. Recent work by Bivona et al. [30] has implicated RasGRP1 activity in DAG- and PMA-dependent signalling in COS cells by using two dominant-negative RasGRP versions. However, since endogenous expression of RasGRP1 was not demonstrated in that study, we investigated RasGRP1 expression in COS-7 cells by Northern blotting (Figure 8). In parallel we also assessed expression of the related GEF RasGRP3, because RasGRP3 shows the widest tissue distribution among RasGRP isoforms (see the Discussion section). To ascertain the functionality of the hybridization probes, we included two types of controls: firstly, we included total RNA from Jurkat T-cells and Ramos B-cells in this analysis, since these cell lines express RasGRP3 (Ramos) and RasGRP1 (Jurkat and, to a lesser extent, Ramos) [6,10,12]. Secondly, since COS-7 cells originate from green monkey, we prepared total RNA from the brain cortex of green monkey and included this sample in the experiment (see Figure 8A for total load of RNA). Various transcripts for RasGRP1 approx. 4 kb in size were detected in Jurkat and, with lower intensity, also in Ramos cells and green monkey brain (Figure 8B). The latter observation demonstrated that the hybridization probe was suited for the detection of monkey RasGRP1 mRNA, particularly if one considered that total RNA load was 3-fold less for the green monkey brain sample (see the legend to Figure 8A). A transcript for RasGRP3 of about the same size was detected only in Ramos cells (Figure 8B). As regards the two leucocyte cell lines, the pattern obtained here is virtually identical with previous results in terms both of the transcript size and the differential expression of RasGRP1 and RasGRP3 in the two cell types [6,10,12,14]. Importantly, neither RasGRP1 nor RasGRP3 mRNA was detected in COS-7 cells, indicating that neither GEF is expressed in this cell line. However, this conclusion was not straightforward in the case of RasGRP3, since a RasGRP3 transcript could not be detected in green monkey brain. Since this observation raised the possibility that the Northern-blot probe for human RasGRP3 did not hybridize to monkey mRNA, we went on to assess RasGRP1 and RasGRP3 expression in COS-7 cells at another level. COS-7, Jurkat and Ramos cell extracts were subjected to Western blotting using isoform-specific polyclonal antibodies (Figure 8C). Both antibodies have previously been shown to react with human and rodent RasGRP1/RasGRP3 proteins respectively [6,10], indicating that they should readily recognize the monkey orthologues. As shown in Figure 8(C), the result of the Western blot was essentially the same as that obtained in the Northern-blot experiment. Importantly, RasGRP1 and RasGRP3 were not detectable in COS-7 cell lysates. A commercially available antibody against RasGRP1 (Santa Cruz Biotechnology; H-120) which recognizes human and mouse RasGRP1 did also not detect RasGRP1 in COS-7 cells (results not shown). Taken together, these results strongly argued against the presence of RasGRP1 or RasGRP3 in COS-7 cells.
PE induces PKC-dependent phosphorylation of Sos
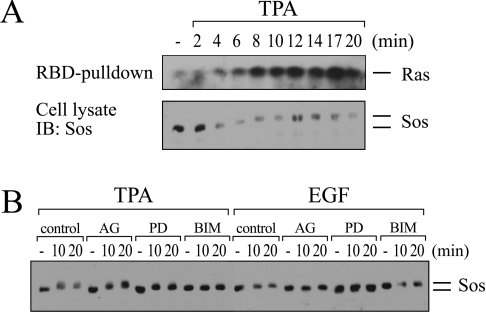
Since RasGRP1 and RasGRP3 are absent from COS-7 cells and RasGRF1/RasGRF2 proteins are similarly not expressed in this cell line [37], COS-7 cells are inferred to express only Sos among characterized Ras-GEF species. The most probable scenario involved an engagement of Sos by PMA-activated PKC, which did not include tyrosine phosphorylation of Shc or Shc–Grb2 complex formation. Sos might conceivably be recruited to another adapter- protein-containing complex in response to PMA. Alternatively, Sos activity might change as a result of phosphorylation events. To test this latter possibility, we stimulated COS-7 cells with PE and monitored changes in electrophoretic mobility of Sos, a reliable surrogate marker of Sos phosphorylation [38–41]. As shown in Figure 9(A), PE elicited a shift in Sos mobility, indicative of one or more phosphorylation events on Sos, which coincided temporally with the formation of Ras-GTP. To further characterize this phosphorylation event, we compared the effects of PE and EGF (Figure 9B). In agreement with previous findings obtained in COS-7 cells, EGF also induced a mobility shift of Sos [38], albeit of lower intensity than the one induced by PMA. The EGF-induced shift was blocked by AG1478 or the MEK [MAPK (mitogen-activated protein kinase)/ERK kinase] inhibitor PD98059, indicating that Sos phosphorylation occurred downstream of Ras via MEK-dependent feedback loops, as reported previously [38–41]. In contrast, the PMA-induced shift in Sos mobility was not affected by AG1478 treatment and was only partially sensitive to MEK inhibition, whereas PKC inhibition with BIM fully ablated the PE-induced shift. Taken together, these results indicated that Sos became phosphorylated in response to PE in a PKC-dependent fashion upstream of Ras.
Figure 9. PMA induces PKC-dependent phosphorylation of Sos.
(A) Serum-starved COS-7 cells were stimulated for the indicated time periods with 100 nM PMA (TPA). Cell extracts were split and assayed in parallel for Ras-GTP formation and Sos mobility shift. (B) Serum-starved COS-7 cells were treated with 200 nM AG1478, 50 μM PD98059, 500 nM BIM or vehicle DMSO (control) (all 10 min) followed by administration of 100 nM PMA or 10 ng/ml EGF. Cell extracts were prepared at the indicated time points, and Sos mobility shift was assayed by Western blotting.
DISCUSSION
Several mechanisms for Ras activation by PEs have been reported in past studies [1,4,13,18]. In peripheral T-cells, PKC-dependent inhibition of GAP proteins was originally put forward as the major pathway, as concluded from the observation that PMA did not increase nucleotide turnover on Ras [1]. In contrast, more recent reports have presented solid genetic and biochemical evidence arguing for a critical role of the GEF RasGRP1, implying that PE-induced Ras activation in T-cells proceeds via stimulation of nucleotide exchange [10–13]. Working on COS-7 cells, we demonstrate that PMA stimulates nucleotide exchange on Ras, strongly indicating that PMA targets Ras-GEF(s) in these cells. Previous studies have illustrated that PMA stimulates nucleotide exchange on Ras in permeabilized cardiac myocytes [2] and pancreatic acinar cells [42], indicating that GEF engagement may be the more widespread mechanism underlying PMA activation of Ras. With regard to the situation in T-cells, the reported absence of nucleotide-exchange stimulation and the increasing body of evidence arguing for RasGRP1 engagement by PMA are difficult to harmonize. One possible way of reconciling both lines of evidence relates to the particular features of Ras in those cells. Leucocytes exhibit a very high basal rate of GDP/GTP cycling on Ras [1,43], implying that a small fractional increase in nucleotide exchange could result in a large accumulation of Ras-GTP owing to signal amplification provided by the high Ras-GDP/GTP cycling rate. Based on our experience with the permeabilization methodology, we consider it possible that a low increase in nucleotide exchange, as conceivably mediated by RasGRP1, might have passed undetected in the relevant experiments. However, neither these considerations nor the experimental data presented here can rigorously rule out down-regulation of GAP activity by PMA, the more so since PMA stimulation reportedly reduces the amount of GAP activity that is recovered from T-cell extracts [1].
In COS-7 epithelial cells, PMA transactivates the EGFR via PKC and it has been proposed that this cross-talk culminates in Ras activation via the canonical Shc/Grb2/Sos GEF system [15,18,21]. However, while this pathway is blocked by dominant-negative S17N-Ras in COS-7 cells challenged with EGF, S17N-Ras does not affect Ras and ERK activation upon PMA stimulation of these cells [5,8,9]. These results indicated that a model in which PMA usurps the EGFR/Shc/Grb2/Sos pathway was unlikely to account for the Ras activation mechanism in COS-7 cells. The findings presented here are in line with these theoretical considerations, since they demonstrate that the EGFR is not an integral constituent of the signalling pathway linking PMA to Ras/ERK. Three experimental manipulations uncouple PMA-induced EGFR transactivation from Ras-GTP formation and hence illustrate that EGFR transactivation does not mediate Ras activation by PMA: (i) inhibition of the EGFR with the selective inhibitor AG1478, (ii) sequestration of HB-EGF with the diphtheria toxin mutant CRM197, and (iii) interference with ROS generation by the reducing agent NAC. In all three cases, Ras-GTP accumulation proceeds almost unaffected upon addition of PMA in the absence of EGFR transactivation or even in a background of complete EGFR inhibition. Moreover, EGFR inhibition with AG1478 did not compromise the up-regulation of nucleotide exchange induced by PMA, demonstrating unambiguously that engagement of RasGEFs by PMA is not mediated by the EGFR. Since PMA-induced tyrosine phosphorylation of Shc and Shc–Grb2 complex formation are totally dependent on EGFR activity (Figures 3 and 4A) [15], it follows that PMA targets a RasGEF system different from the canonical Shc/Grb2/Sos pathway.
It is noteworthy that previous investigations on the role of the EGFR in PMA signalling to the Ras downstream effector ERK in COS-7 cells had yielded seemingly discrepant results. Roudabush et al. [22] reported that activation of endogenous ERK by PMA was unaffected by AG1478 or CRM197, in agreement with the observations made here at the level of both ERK and Ras. In contrast, two laboratories have documented approx. 50% decrease in PMA-elicited ERK activation by AG1478 in COS-7 cells [18,21], leading to the conclusion that EGFR transactivation relays part of the PMA signal to the Ras/ERK pathway. It should be noted that one of the latter studies investigated activation of ectopically expressed ERK [18], while in the other a supra-optimal concentration of AG1478 was used (5 μM) [21], casting some doubts on the specificity of the reported effects. This notwithstanding, our finding that the EGFR maintains basal levels of ERK activation (which, as noticed previously by others [38], are rather high in COS cells compared with other commonly investigated fibroblast and epithelial cell lines) provides a plausible explanation for these divergent observations. As illustrated in Figure 3, ERK activation in response to PMA starts off from almost zero basal ERK activity levels in AG1478-treated cells, implying that ERK activity induced by PMA will not reach the value obtained in virgin cells. Indeed, in analogy with the results for Ras-GTP accumulation (Figure 4G), we find that maximum ERK phosphorylation upon PMA stimulation of AG1478-treated cells generally amounts to 80–90% of the value from AG1478-untreated cells. The present study highlights that this gap in ERK activity is attributable to the loss of basal ERK phosphorylation that occurs upon inhibition of basal EGFR activity, rather than to a defect in PMA signalling. This effect of AG1478 may be exacerbated by clonal differences among COS-7 batches affecting the relative magnitude of basal (AG1478-sensitive) versus PMA-responsive (AG1478-insensitive) Ras and ERK activities. In conclusion, we propose that a number of apparent discrepancies regarding the role of EGFR transactivation in Ras/ERK activation by PMA (and possibly also other agonists) can be rationalized by taking into consideration the contribution of basal levels of activation of the Ras/ERK pathway.
EGFR transactivation by heterologous extracellular agonists is a widespread phenomenon. A large body of evidence demonstrates that EGFR inhibition compromises ERK activation by numerous stimuli in a number of cell types, including COS-7 cells, arguing for the EGFR as a point of convergence for many agonists that signal to the Ras/ERK pathway. A previous study on the role of the EGFR in LPA (lysophosphatidic acid)-induced Ras activation has shown that EGFR inhibition impairs the net accumulation of Ras-GTP upon LPA stimulation of COS-7 cells [28], in apparent support of the mentioned scenario. However, EGFR inhibition did not prevent LPA-induced stimulation of nucleotide exchange [28]. This paradoxical effect of EGFR inhibition – blocking of LPA-induced Ras-GTP formation but no interference with nucleotide exchange stimulation – reflected a marked decrease in the rate of basal nucleotide exchange following EGFR inhibition, which could not be fully compensated by LPA despite unimpaired stimulation of nucleotide exchange. The same phenomenon, i.e. a decline in basal activity levels of the Ras/ERK pathway following EGFR inhibition, is also documented in the present paper at the level of Ras-GTP formation (Figures 4B and 4G), Ras nucleotide exchange (Figures 5A and 5E) and ERK activity (Figures 3 and 4B), and was previously illustrated by others at the level of ERK activation [22]. Taken together, all these observations indicated that it is the maintenance of basal nucleotide exchange on Ras by basal EGFR activity that is critical to the action of LPA. With regard to the present discussion, these considerations lend strong support to the notion that transactivation of the EGFR does not mediate Ras/ERK pathway activation by PMA in COS-7 cells. This conclusion is further reinforced by the converse observation, namely the fact that PMA induces pronounced tyrosine phosphorylation of the EGFR and/or Shc in cell types such as HEK-293 cells (human embryonic kidney cells) [15] or Rat-1 (a rat fibroblast cell line) fibroblasts [19], in which PMA fails to activate Ras [31,44].
The finding that the EGFR drives the formation of basal Ras-GTP levels, but not PMA or LPA-induced Ras-GTP accumulation, raises a further question: if EGFR inhibition does not compromise nucleotide-exchange stimulation in response to LPA [28] or PMA (the present study), why does AG1478 eradicate Ras-GTP formation only in the former case [28]? Two explanations can account for this observation: firstly, PMA but not LPA may down-regulate GAP activity in addition to stimulating GEFs. In consequence, PMA-induced GEF stimulation and GAP inhibition would amplify each other's effect and give rise to the full response of Ras-GTP formation that is observed throughout the present study. In contrast, if LPA action only relies on GEF stimulation, this effect may be strong enough to redress the balance of nucleotide exchange following its breakdown upon EGFR inhibition, as illustrated in [28], but it may not be potent enough to result in net Ras-GTP formation. Since several lines of evidence argue for GAP inactivation as a mechanism evoked by PMA [1,4], it is not unlikely that this scenario may apply to COS-7 cells.
Alternatively or additionally, PMA and LPA could address different pools of Ras within COS-7 cells. An increasing body of experimental evidence indicates that Ras proteins segregate into various subcellular organelles as well as into distinct plasma membrane microdomains, depending on the Ras isoform, activation status, palmitoylation status and possibly other as yet unknown cues [45]. It seems reasonable to assume that the function of the EGFR in maintaining steady-state nucleotide exchange on Ras applies to one or a subset, but not all, of the various compartments. In consequence, the breakdown of basal Ras nucleotide exchange upon EGFR inhibition would impinge on agonists that target the same Ras population (hypothetically: LPA), whereas Ras activation by cues that feed into a different Ras pool(s) (hypothetically: PMA) would not be affected. A particular appeal of this scenario resides in the fact that, additionally, it provides a rational explanation for the distinct sensitivity of PMA versus EGF and LPA signalling towards S17N-Ras: if S17N-Ras segregates exclusively into the EGF- and LPA-responsive Ras pool (following the line of reasoning started above), it should not affect nucleotide exchange in a separate Ras pool that is addressed by PMA. Circumstantial evidence in support of the existence of various Ras populations that are distinctly addressed by agonists is provided by the data shown in Figures 4(G) and 5(E), according to which the PMA-induced fold increase in nucleotide exchange and Ras-GTP formation is enhanced in a background of EGFR inhibition. The easiest interpretation of this surprising observation is that an EGFR-driven component of basal nucleotide exchange in a PMA-unresponsive Ras population had obscured the real magnitude of the PMA effect. In other words, the loss of basal nucleotide exchange upon AG1478 application reduces the overall basal rate without affecting PMA action, hence resulting in an apparent higher fold-stimulation of exchange and Ras-GTP formation.
The current data hence argue for the independent activation of the Ras/ERK and EGFR/Shc pathways downstream of PMA-activated PKC in COS-7 cells (Scheme 1). While PMA induces EGFR transactivation via PKC-dependent shedding of HB-EGF, this pathway does not mediate activation of Ras, but is responsible for activation of PI3K and Akt (Figure 7) and possibly other pathways. It is worth raising the point (previously noticed by others [46]) that the kinetics of PMA-induced HB-EGF processing, as illustrated here in Figure 4(C), at first sight appears to be in conflict with the time course of EGFR transactivation (e.g. Figure 3) and EGFR-dependent Akt phosphorylation (Figure 7), since both latter processes are switched on before HB-EGF release into the medium becomes detectable. However, HB-EGF processing is likely to proceed with faster kinetics than those illustrated in Figure 4(C), since the immediate release of shed HB-EGF to the medium is prevented by its association with heparan sulphate proteoglycans on the cell surface [15,47]. Indeed, initial studies on the mechanism of EGFR transactivation failed to detect soluble EGF ligands in the medium at all [17,48], and it was only upon more careful analysis that processing of HB-EGF and other latent membrane-bound EGF-like precursor proteins were detected and implicated in the process of EGFR transactivation [15,33,34]. In conclusion, as proposed previously by other investigators [15], these considerations suggest that the delayed kinetics of HB-EGF shedding as illustrated in the present study must not be at odds with a role for HB-EGF as a mediator of agonist-induced EGFR transactivation.
An unsolved issue concerns the identity of the RasGEF system(s) that is activated by PMA via PKC. The results presented here suggest that PMA does not address the canonical Shc/Grb2/Sos system. Moreover, RasGRF1 and RasGRF2 are both not expressed in COS cells [37]. On the other hand, members of the RasGRP family of RasGEFs are potential candidates, since they are direct target proteins of PMA/DAG. We focused our interest on the two family members RasGRP1 and RasGRP3 for a number of reasons: (i) RasGRP1 and RasGRP3 are the two species most solidly established to function as mediators of PMA-driven Ras activation, (ii) RasGRP1 and RasGRP3 are activated both by direct binding to PMA and indirectly via phosphorylation by PKC [6,13], (iii) RasGRP1 has been inferred (but not proven) to be present in COS cells in a recent study [30], (iv) RasGRP1 and RasGRP3 are the only members reported to be expressed in tissues other than brain and blood cells: RasGRP3 expression has been documented in mesangial cells of the kidney [49], endothelial cells [50] and endocrine cells [51]; RasGRP1 expression has been detected in skin keratinocytes [52], (v) RasGRP2 exhibits selectivity for Rap, does not respond to PMA and does not efficiently activate the Ras/ERK pathway [53], and (vi) RasGRP4 is a mast-cell-restricted GEF [54]. Although these considerations rendered RasGRP1 and RasGRP3 very attractive candidates for the elusive GEF activity addressed by PMA in COS-7 cells, we have been unable to detect expression of either RasGRP variant in COS-7 cells. The failure to detect RasGRP1 and RasGRP3 in cell extracts by Western blotting was unlikely to result from a failure of the antibodies to recognize the monkey orthologues, since both sera have been demonstrated to react with human and rodent RasGRP1 or RasGRP3 [6,10]. Similarly, since the hybridization probe used for detection of RasGRP1 mRNA by Northern blotting readily detected RasGRP1 transcripts from the brain cortex of green monkey (the animal species from which COS-7 cells originate), the negative result obtained for COS-7 cells unambiguously excluded RasGRP1 expression in this cell line. This degree of certainty could not be achieved in the RasGRP3 Northern blot, since no transcripts were detected in the monkey brain. One interpretation of this observation was that the Northern-blot probe did not hybridize to monkey RasGRP3 mRNA. Alternatively, RasGRP3 expression levels in cells of the cortex might be low and thus lie below the level of detection. We favoured the second possibility, since RasGRP3 expression within the brain has been mapped exclusively to oligodendroglia [49], and secondly, in agreement with the Northern-blot experiment, RasGRP3 protein was not detectable in COS-7 cells by Western blotting. In summary, these findings demonstrated that COS-7 cells do not express functionally significant levels of either RasGRP1 or RasGRP3.
These considerations indicated that COS-7 cells express only Sos among characterized Ras-GEF species, implying that Sos most likely represented the GEF activity stimulated by PMA via PKC. However, the presumptive Sos activation mechanism was unconventional in that it did not involve tyrosine phosphorylation of Shc or Shc–Grb2 complex formation. We present evidence that PE induces phosphorylation of Sos in a PKC-dependent fashion and that the features of this phosphorylation event(s) are consistent with it mediating the activation of Ras by PE. Thus, in three independent experiments of the kind shown in Figure 9(A), the initiation of the Sos mobility shift upon PMA administration correlated in time with the onset of Ras-GTP accumulation. PE and growth factors have previously been reported to induce phosphorylation at multiple sites within the proline-rich C-terminal region of Sos in various cell types [38–41]. As a major hallmark shared in all referred studies, Sos phosphorylation was fully sensitive to MEK inhibition, showing that it resulted in all cases from Ras-sparked negative feedback loops involving ERK, ERK-dependent kinases such as Rsk-2 (ribosomal protein S6 kinase, 90 kDa, polypeptide 1) or other as yet unidentified MEK-dependent kinases. In agreement with these findings, we observed full MEK-dependence of Sos phosphorylation in COS-7 cells stimulated with EGF. However, in marked contrast with all reported findings, the PE-induced shift in Sos mobility was only partially reverted after MEK inhibition, whereas it was fully absent from BIM-treated cells. It is important to note that whereas phosphorylation of some residues on Sos gives rise to reduced mobility in SDS/PAGE, other phosphorylation events do not induce changes in mobility [55]. Thus the findings presented in Figure 9 must be considered as preliminary and interpreted with care. This notwithstanding, the most plausible explanation was that the BIM-sensitive and PD98059-insensitive phosphorylation event(s) occurred downstream of PKC and upstream of Ras, whereas the PD98059-sensitive component of the mobility shift most likely reflected previously characterized MEK-dependent feedback phosphorylation of Sos. We are currently mapping the phosphorylation sites on Sos that become phosphorylated in response to PE in an attempt to clarify whether the observed PD98059-insensitive Sos phosphorylation is causally related to PE-driven Ras activation. Identification of the phosphorylation sites should also help to identify the PD98059-insensitive Sos kinase(s) engaged by PE. Since PKCα, PKCβ and PKCγ do not phosphorylate Sos in vitro [38], it seems more likely that PKC mediates activation of the elusive Sos kinase in response to PE.
In conclusion, the assessment of nucleotide exchange on Ras has enabled the detection of a Ras-GEF system that is addressed by PE via PKC and independently of the EGFR. In more general terms, the present study emphasizes the relevance of basal activation levels of signalling pathway constituents and the need to include this aspect in studies aimed at deciphering the topology of signal transduction pathways. Along this line of reasoning, the confirmation of a role for the EGFR in the maintenance of basal nucleotide exchange on Ras in growth-arrested cells ([28,46] and the present study) prompts the intriguing speculation that the EGFR may function to calibrate the Ras activation machinery in terms of a futile Ras-GDP/GTP cycle to regulate its responsiveness to LPA and other mitogens. Such a scenario could underlie the synergy between EGF and other mitogens that is observed in a large variety of cell types [56–59].
Acknowledgments
We thank Julian Downward (Cancer Research UK, London, U.K.) for helpful discussions and for sharing unpublished results. We acknowledge the gift of monkey brain tissue by Dr Wolfgang Enard (Max Planck Institute for Evolutionary Anthropology, Leipzig, Germany). We thank Frank Böhmer (Jena University, Jena, Germany) for critically reading this paper. This work was supported by grant number RU860/1-1 from Deutsche Forschungsgemeinschaft.
References
- 1.Downward J., Graves J. D., Warne P. H., Rayter S., Cantrell D. A. Stimulation of p21ras upon T-cell activation. Nature (London) 1990;346:719–723. doi: 10.1038/346719a0. [DOI] [PubMed] [Google Scholar]
- 2.Montessuit C., Thorburn A. Activation of Ras by phorbol esters in cardiac myocytes. Role of guanine nucleotide exchange factors. FEBS Lett. 1999;460:57–60. doi: 10.1016/s0014-5793(99)01223-5. [DOI] [PubMed] [Google Scholar]
- 3.Chiloeches A., Paterson H. F., Marais R., Clerk A., Marshall C. J., Sugden P. H. Regulation of Ras.GTP loading and Ras–Raf association in neonatal rat ventricular myocytes by G protein-coupled receptor agonists and phorbol ester. Activation of the extracellular signal-regulated kinase cascade by phorbol ester is mediated by Ras. J. Biol. Chem. 1999;274:19762–19770. doi: 10.1074/jbc.274.28.19762. [DOI] [PubMed] [Google Scholar]
- 4.Shu X., Wu W., Mosteller R. D., Broek D. Sphingosine kinase mediates vascular endothelial growth factor-induced activation of ras and mitogen-activated protein kinases. Mol. Cell. Biol. 2002;22:7758–7768. doi: 10.1128/MCB.22.22.7758-7768.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marais R., Light Y., Mason C., Paterson H., Olson M. F., Marshall C. J. Requirement of Ras-GTP–Raf complexes for activation of Raf-1 by protein kinase C. Science. 1998;280:109–112. doi: 10.1126/science.280.5360.109. [DOI] [PubMed] [Google Scholar]
- 6.Teixeira C., Stang S. L., Zheng Y., Beswick N. S., Stone J. C. Integration of DAG signaling systems mediated by PKC-dependent phosphorylation of RasGRP3. Blood. 2003;102:1414–1420. doi: 10.1182/blood-2002-11-3621. [DOI] [PubMed] [Google Scholar]
- 7.Gschwendt M., Kittstein W., Marks F. Protein kinase C forms a complex with and phosphorylates the GTPase activating protein GAP: phosphorylation by PKC is dependent on tyrosine phosphorylation of GAP and/or a GAP-associated protein. Biochem. Biophys. Res. Commun. 1993;194:571–576. doi: 10.1006/bbrc.1993.1858. [DOI] [PubMed] [Google Scholar]
- 8.Howe L. R., Leevers S. J., Gomez N., Nakielny S., Cohen P., Marshall C. J. Activation of the MAP kinase pathway by the protein kinase raf. Cell. 1992;71:335–342. doi: 10.1016/0092-8674(92)90361-f. [DOI] [PubMed] [Google Scholar]
- 9.Augsten M., Pusch R., Biskup C., Rennert K., Wittig U., Bayer K., Blume A., Wetzker R., Friedrich K., Rubio I. Live-cell imaging of endogenous Ras-GTP illustrates predominant Ras activation at the plasma membrane. EMBO Rep. 2006;7:46–51. doi: 10.1038/sj.embor.7400560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ebinu J. O., Bottorff D. A., Chan E. Y., Stang S. L., Dunn R. J., Stone J. C. RasGRP, a Ras guanyl nucleotide-releasing protein with calcium- and diacylglycerol-binding motifs. Science. 1998;280:1082–1086. doi: 10.1126/science.280.5366.1082. [DOI] [PubMed] [Google Scholar]
- 11.Dower N. A., Stang S. L., Bottorff D. A., Ebinu J. O., Dickie P., Ostergaard H. L., Stone J. C. RasGRP is essential for mouse thymocyte differentiation and TCR signaling. Nat. Immunol. 2000;1:317–321. doi: 10.1038/79766. [DOI] [PubMed] [Google Scholar]
- 12.Coughlin J. J., Stang S. L., Dower N. A., Stone J. C. RasGRP1 and RasGRP3 regulate B cell proliferation by facilitating B cell receptor–Ras signaling. J. Immunol. 2005;175:7179–7184. doi: 10.4049/jimmunol.175.11.7179. [DOI] [PubMed] [Google Scholar]
- 13.Roose J. P., Mollenauer M., Gupta V. A., Stone J., Weiss A. A diacylglycerol-protein kinase C–RasGRP1 pathway directs Ras activation upon antigen receptor stimulation of T cells. Mol. Cell. Biol. 2005;25:4426–4441. doi: 10.1128/MCB.25.11.4426-4441.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tognon C. E., Kirk H. E., Passmore L. A., Whitehead I. P., Der C. J., Kay R. J. Regulation of RasGRP via a phorbol ester-responsive C1 domain. Mol. Cell. Biol. 1998;18:6995–7008. doi: 10.1128/mcb.18.12.6995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prenzel N., Zwick E., Daub H., Leserer M., Abraham R., Wallasch C., Ullrich A. EGF receptor transactivation by G-protein-coupled receptors requires metalloproteinase cleavage of proHB-EGF. Nature (London) 1999;402:884–888. doi: 10.1038/47260. [DOI] [PubMed] [Google Scholar]
- 16.Chen N., Ma W.-Y., She Q.-B., Wu E., Liu G., Bode A. M., Dong Z. Transactivation of the epidermal growth factor receptor is involved in 12-O-tetradecanoylphorbol-13-acetate-induced signal transduction. J. Biol. Chem. 2001;276:46722–46728. doi: 10.1074/jbc.M107156200. [DOI] [PubMed] [Google Scholar]
- 17.Tsai W., Morielli A. D., Peralta E. G. The m1 muscarinic acetylcholine receptor transactivates the EGF receptor to modulate ion channel activity. EMBO J. 1997;16:4597–4605. doi: 10.1093/emboj/16.15.4597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grosse R., Roelle S., Herrlich A., Höhn J., Gudermann T. Epidermal growth factor receptor tyrosine kinase mediates Ras activation by gonadotropin-releasing hormone. J. Biol. Chem. 2000;275:12251–12260. doi: 10.1074/jbc.275.16.12251. [DOI] [PubMed] [Google Scholar]
- 19.Luttrell L. M., Daaka Y., Della Rocca G. J., Lefkowitz R. J. G protein-coupled receptors mediate two functionally distinct pathways of tyrosine phosphorylation in rat 1a fibroblasts. Shc phosphorylation and receptor endocytosis correlate with activation of Erk kinases. J. Biol. Chem. 1997;272:31648–31656. doi: 10.1074/jbc.272.50.31648. [DOI] [PubMed] [Google Scholar]
- 20.Dabrowski A., VanderKuur J. A., Carter-Su C., Williams J. A. Cholecystokinin stimulates formation of shc–grb2 complex in rat pancreatic acinar cells through a protein kinase C-dependent mechanism. J. Biol. Chem. 1996;271:27125–27129. doi: 10.1074/jbc.271.43.27125. [DOI] [PubMed] [Google Scholar]
- 21.Tebar F., Lladó A., Enrich C. Role of calmodulin in the modulation of the MAPK signalling pathway and the transactivation of epidermal growth factor receptor mediated by PKC. FEBS Lett. 2002;517:206–210. doi: 10.1016/s0014-5793(02)02624-8. [DOI] [PubMed] [Google Scholar]
- 22.Roudabush F. L., Pierce K. L., Maudsley S., Dad Khan K., Luttrell L. M. Transactivation of the EGF receptor mediates IGF-1-stimulated shc phosphorylation and ERK1/2 activation in COS-7 cells. J. Biol. Chem. 2000;275:22583–22589. doi: 10.1074/jbc.M002915200. [DOI] [PubMed] [Google Scholar]
- 23.Piiper A., Elez R., You S.-J., Kronenberger B., Loitsch S., Roche S., Zeuzem S. Cholecystokinin stimulates extracellular signal-regulated kinase through activation of the epidermal growth factor receptor, Yes, and protein kinase C. Signal amplification at the level of Raf by activation of protein kinase Cepsilon. J. Biol. Chem. 2003;278:7065–7072. doi: 10.1074/jbc.M211234200. [DOI] [PubMed] [Google Scholar]
- 24.Ma C., Bower K. A., Lin H., Chen G., Huang C., Shi X., Luo J. The role of epidermal growth factor receptor in ethanol-mediated inhibition of activator protein-1 transactivation. Biochem. Pharmacol. 2005;69:1785–1794. doi: 10.1016/j.bcp.2005.03.029. [DOI] [PubMed] [Google Scholar]
- 25.Thomas S. M., DeMarco M., D'Arcangelo G., Halegoua S., Brugge J. S. Ras is essential for nerve growth factor- and phorbol ester-induced tyrosine phosphorylation of MAP kinases. Cell. 1992;68:1031–1040. doi: 10.1016/0092-8674(92)90075-n. [DOI] [PubMed] [Google Scholar]
- 26.Wood K. W., Sarnecki C., Roberts T. M., Blenis J. ras mediates nerve growth factor receptor modulation of three signal-transducing protein kinases: MAP kinase, Raf-1, and RSK. Cell. 1992;68:1041–1050. doi: 10.1016/0092-8674(92)90076-o. [DOI] [PubMed] [Google Scholar]
- 27.de Vries-Smits A. M., Burgering B. M., Leevers S. J., Marshall C. J., Bos J. L. Involvement of p21ras in activation of extracellular signal-regulated kinase 2. Nature (London) 1992;357:602–604. doi: 10.1038/357602a0. [DOI] [PubMed] [Google Scholar]
- 28.Rubio I., Rennert K., Wittig U., Wetzker R. Ras activation in response to lysophosphatidic acid requires a permissive input from the epidermal growth factor receptor. Biochem. J. 2003;376:571–576. doi: 10.1042/BJ20031410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meng T. C., Fukada T., Tonks N. K. Reversible oxidation and inactivation of protein tyrosine phosphatases in vivo. Mol. Cell. 2002;9:387–399. doi: 10.1016/s1097-2765(02)00445-8. [DOI] [PubMed] [Google Scholar]
- 30.Bivona T. G., Perez De Castro I., Aheam I. M, Grana T. M., Chiu V., Lockyer P. J., Cullen P. J., Pellicer A., Cox A. D., Philips M. R. Phospholipase Cgamma activates Ras on the Golgi apparatus by means of RasGRP1. Nature (London) 2003;424:694–698. doi: 10.1038/nature01806. [DOI] [PubMed] [Google Scholar]
- 31.Buday L., Downward J. Epidermal growth factor regulates the exchange rate of guanine nucleotides on p21ras in fibroblasts. Mol. Cell. Biol. 1993;13:1903–1910. doi: 10.1128/mcb.13.3.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ehrhardt A., David M. D., Ehrhardt G. R. A., Schrader J. W. Distinct mechanisms determine the patterns of differential activation of H-Ras, N-Ras, K-Ras 4B, and M-Ras by receptors for growth factors or antigen. Mol. Cell. Biol. 2004;24:6311–6323. doi: 10.1128/MCB.24.14.6311-6323.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mifune M., Ohtsu H., Suzuki H., Nakashima H., Brailoiu E., Dun N. J., Frank G. D., Inagami T., Higashiyama S., Thomas W. G., et al. G protein coupling and second messenger generation are indispensable for metalloprotease-dependent, heparin-binding epidermal growth factor shedding through angiotensin II type-1 receptor. J. Biol. Chem. 2005;280:26592–26599. doi: 10.1074/jbc.M502906200. [DOI] [PubMed] [Google Scholar]
- 34.Goishi K., Higashiyama S., Klagsbrun M., Nakano N., Umata T., Ishikawa M., Mekada E., Taniguchi N. Phorbol ester induces the rapid processing of cell surface heparin-binding EGF-like growth factor: conversion from juxtacrine to paracrine growth factor activity. Mol. Biol. Cell. 1995;6:967–980. doi: 10.1091/mbc.6.8.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Datta R., Yoshinaga K., Kaneki M., Pandey P., Kufe D. Phorbol ester-induced generation of reactive oxygen species is protein kinase Cbeta-dependent and required for SAPK activation. J. Biol. Chem. 2000;275:41000–41003. doi: 10.1074/jbc.M009322200. [DOI] [PubMed] [Google Scholar]
- 36.Zor U., Ferber E., Gergely P., Szucs K., Dombradi V., Goldman R. Reactive oxygen species mediate phorbol ester-regulated tyrosine phosphorylation and phospholipase A2 activation: potentiation by vanadate. Biochem. J. 1993;295:879–888. doi: 10.1042/bj2950879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arozarena I., Matallanas D., Berciano M. T., Sanz-Moreno V., Calvo F., Munoz M. T., Egea G., Lafarga M., Crespo P. Activation of H-Ras in the endoplasmic reticulum by the RasGRF family guanine nucleotide exchange factors. Mol. Cell. Biol. 2004;24:1516–1530. doi: 10.1128/MCB.24.4.1516-1530.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Porfiri E., McCormick F. Regulation of epidermal growth factor receptor signaling by phosphorylation of the ras exchange factor hSOS1. J. Biol. Chem. 1996;271:5871–5877. doi: 10.1074/jbc.271.10.5871. [DOI] [PubMed] [Google Scholar]
- 39.Langlois W. J., Sasaoka T., Saltiel A. R., Olefsky J. M. Negative feedback regulation and desensitization of insulin- and epidermal growth factor-stimulated p21ras activation. J. Biol. Chem. 1995;270:25320–25323. doi: 10.1074/jbc.270.43.25320. [DOI] [PubMed] [Google Scholar]
- 40.Holt K. H., Kasson B. G., Pessin J. E. Insulin stimulation of a MEK-dependent but ERK-independent SOS protein kinase. Mol. Cell. Biol. 1996;16:577–583. doi: 10.1128/mcb.16.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Douville E., Downward J. EGF induced SOS phosphorylation in PC12 cells involves P90 RSK-2. Oncogene. 1997;15:373–383. doi: 10.1038/sj.onc.1201214. [DOI] [PubMed] [Google Scholar]
- 42.Duan R.-D., Zheng C.-F., Guan K.-L., Williams J. A. Activation of MAP kinase kinase (MEK) and Ras by cholecystokinin in rat pancreatic acini. Am. J. Physiol. 1995;268:G1060–G1065. doi: 10.1152/ajpgi.1995.268.6.G1060. [DOI] [PubMed] [Google Scholar]
- 43.Rubio I., Wetzker R. A permissive function of phosphoinositide 3-kinase in ras activation mediated by inhibition of GTPase-activating proteins. Curr. Biol. 2000;10:1225–1228. doi: 10.1016/s0960-9822(00)00731-4. [DOI] [PubMed] [Google Scholar]
- 44.Lorenzo P. S., Kung J. W., Bottorf D. A., Garfield S. A., Stone J. C., Blumberg P. M. Phorbol esters modulate the Ras exchange factor RasGRP3. Cancer Res. 2001;61:943–949. [PubMed] [Google Scholar]
- 45.Quatela S. E., Philips M. R. Ras signaling on the Golgi. Curr. Opin. Cell Biol. 2006;18:162–167. doi: 10.1016/j.ceb.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 46.Downward J. Role of receptor tyrosine kinases in G-protein-coupled receptor regulation of Ras: transactivation or parallel pathways? Biochem. J. 2003;376:e9–e10. doi: 10.1042/BJ20031745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Raab G., Klagsbrun M. Heparin-binding EGF-like growth factor. Biochim. Biophys. Acta. 1997;1333:F179–F199. doi: 10.1016/s0304-419x(97)00024-3. [DOI] [PubMed] [Google Scholar]
- 48.Eguchi S., Numaguchi K., Iwasaki H., Matsumoto T., Yamakawa T., Utsunomiya H., Motley E. D., Kawakatsu H., Owada K. M., Hirata Y., et al. Calcium-dependent epidermal growth factor receptor transactivation mediates the angiotensin II-induced mitogen-activated protein kinase activation in vascular smooth muscle cells. J. Biol. Chem. 1998;273:8890–8896. doi: 10.1074/jbc.273.15.8890. [DOI] [PubMed] [Google Scholar]
- 49.Yamashita S., Mochizuki N., Ohba Y., Tobiume M., Okada Y., Sawa H., Nagashima K., Matsuda M. CalDAG-GEFIII activation of Ras, R-ras, and Rap1. J. Biol. Chem. 2000;275:25488–25493. doi: 10.1074/jbc.M003414200. [DOI] [PubMed] [Google Scholar]
- 50.Roberts D. M., Anderson A. L., Hidaka M., Swetenburg R. L., Patterson C., Stanford W. L., Bautch V. L. A vascular gene trap screen defines RasGRP3 as an angiogenesis-regulated gene required for the endothelial response to phorbol esters. Mol. Cell. Biol. 2004;24:10515–10528. doi: 10.1128/MCB.24.24.10515-10528.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ozaki N., Miura Y., Yamada T., Kato Y., Oiso Y. RasGRP3 mediates phorbol ester-induced, protein kinase C-independent exocytosis. Biochem. Biophys. Res. Commun. 2005;329:765–771. doi: 10.1016/j.bbrc.2005.02.031. [DOI] [PubMed] [Google Scholar]
- 52.Rambaratsingh R. A., Stone J. C., Blumberg P. M., Lorenzo P. S. RasGRP1 represents a novel non-protein kinase C phorbol ester signaling pathway in mouse epidermal keratinocytes. J. Biol. Chem. 2003;278:52792–52801. doi: 10.1074/jbc.M308240200. [DOI] [PubMed] [Google Scholar]
- 53.Caloca M. J., Zugaza J. L., Bustelo X. R. Exchange factors of the RasGRP family mediate Ras activation in the Golgi. J. Biol. Chem. 2003;278:33465–33473. doi: 10.1074/jbc.M302807200. [DOI] [PubMed] [Google Scholar]
- 54.Yang Y., Li L., Wong G. W., Krilis S. A., Madhusudhan M. S., Sali A., Stevens R. L. RasGRP4, a new mast cell-restricted Ras guanine nucleotide-releasing protein with calcium- and diacylglycerol-binding motifs. Identification of defective variants of this signaling protein in asthma, mastocytosis, and mast cell leukemia patients and demonstration of the importance of RasGRP4 in mast cell development and function. J. Biol. Chem. 2002;277:25756–25774. doi: 10.1074/jbc.M202575200. [DOI] [PubMed] [Google Scholar]
- 55.Corbalan-Garcia S., Yang S. S., Degenhardt K. R., Bar-Sagi D. Identification of the mitogen-activated protein kinase phosphorylation sites on human Sos1 that regulate interaction with Grb2. Mol. Cell. Biol. 1996;16:5674–5682. doi: 10.1128/mcb.16.10.5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhao D., Letterman J., Schreiber B. M. beta-Migrating very low density lipoprotein (beta VLDL) activates smooth muscle cell mitogen-activated protein (MAP) kinase via G protein-coupled receptor-mediated transactivation of the epidermal growth factor (EGF) receptor: effect of MAP kinase activation on beta VLDL plus EGF-induced cell proliferation. J. Biol. Chem. 2001;276:30579–30588. doi: 10.1074/jbc.M103761200. [DOI] [PubMed] [Google Scholar]
- 57.Sakai T., de la Pena J. M., Mosher D. F. Synergism among lysophosphatidic acid, beta1A integrins, and epidermal growth factor or platelet-derived growth factor in mediation of cell migration. J. Biol. Chem. 1999;274:15480–15486. doi: 10.1074/jbc.274.22.15480. [DOI] [PubMed] [Google Scholar]
- 58.Li J., Kim Y. N., Bertics P. J. Platelet-derived growth factor-stimulated migration of murine fibroblasts is associated with epidermal growth factor receptor expression and tyrosine phosphorylation. J. Biol. Chem. 2000;275:2951–2958. doi: 10.1074/jbc.275.4.2951. [DOI] [PubMed] [Google Scholar]
- 59.Cunnick J. M., Dorsey J. F., Standley T., Turkson J., Kraker A. J., Fry D. W., Jove R., Wu J. Role of tyrosine kinase activity of epidermal growth factor receptor in the lysophosphatidic acid-stimulated mitogen-activated protein kinase pathway. J. Biol. Chem. 1998;273:14468–14475. doi: 10.1074/jbc.273.23.14468. [DOI] [PubMed] [Google Scholar]