Abstract
eNOS (endothelial nitric oxide synthase) activity is post-translationally regulated in a complex fashion by acylation, protein–protein interactions, intracellular trafficking and phosphorylation, among others. Signalling pathways that regulate eNOS activity include phosphoinositide 3-kinase/Akt, cyclic nucleotide-dependent kinases [PKA (protein kinase A) and PKG], PKC, as well as ERKs (extracellular-signal-regulated kinases). The role of ERKs in eNOS activation remains controversial. In the present study, we have examined the role of ERK1/2 in eNOS activation in HUVEC-CS [transformed HUVEC (human umbilical-vein endothelial cells)] as well as a widely used model for eNOS study, transiently transfected COS-7 cells. U0126 pretreatment of HUVEC-CS potentiated ATP-stimulated eNOS activity, independent of changes in intracellular Ca2+ concentration ([Ca2+]i). In COS-7 cells transiently expressing ovine eNOS, U0126 potentiated A23187-stimulated eNOS activity, but inhibited ATP-stimulated activity. Compensatory changes in phosphorylation of five key eNOS residues did not account for changes in A23187-stimulated activity. However, in the case of ATP, altered phosphorylation and changes in [Ca2+]i may partially contribute to U0126 inhibition of activity. Finally, seven eNOS alanine mutants of putative ERK1/2 targets were generated and the effects of U0126 pretreatment on eNOS activity were gauged with A23187 and ATP treatment. T97A-eNOS was the only construct significantly different from wild-type after U0126 pretreatment and ATP stimulation of eNOS activation. In the present study, eNOS activity was either potentiated or inhibited in COS-7 cells, suggesting agonist dependence for MEK/ERK1/2 signalling [where MEK is MAPK (mitogen-activated protein kinase)/ERK kinase] to eNOS and a complex mechanism including [Ca2+]i, phosphorylation and, possibly, intracellular trafficking.
Keywords: agonist, Ca2+, COS-7 cell, endothelial nitric oxide synthase, mitogen-activated protein kinase/extracellular-signal-regulated kinase (ERK) kinase (MEK), phosphorylation
Abbreviations: BAEC, bovine aorta endothelial cells; [Ca2+]i, intracellular Ca2+ concentration; CMV, cytomegalovirus; DMEM, Dulbecco's modified Eagle's medium; eNOS, endothelial nitric oxide synthase; ERK, extracellular-signal-regulated kinase; FBS, fetal bovine serum; HUVEC, human umbilical-vein endothelial cells; HUVEC-CS, transformed HUVEC; mAb, monoclonal antibody; MAPK, mitogen-activated protein kinase; MEK, MAPK/ERK kinase; pAb, polyclonal antibody; PKA, protein kinase A; UAEC, uterine artery endothelial cells; NP-UAEC, UAEC derived from non-pregnant ewes; pS617, phospho-Ser617; P-UAEC, UAEC derived from pregnant ewes; VEGF, vascular endothelial growth factor
INTRODUCTION
eNOS (endothelial nitric oxide synthase) is regulated by a complex combination of post-translational mechanisms, including acylation, intracellular trafficking, protein–protein interactions, as well as phosphorylation and S-nitrosylation [1–12]. Several signalling cascades and kinases have been implicated in eNOS regulation by phosphorylation, including phosphoinositide 3-kinase/Akt, PKA (protein kinase A), PKC and the ERKs (extracellular-signal-regulated kinases), among several others. Phosphorylation of eNOS on residues Tyr83, Ser116, Thr497, Ser617, Ser635 and Ser1179 (ovine and bovine numbering) has been demonstrated, and fluctuations in relative levels of phosphorylation on each amino acid residue have been observed after a variety of physiological stimuli [6,10,13–15].
The role of ERK1/2 in eNOS regulation remains controversial and not well understood at a mechanistic level. Several studies have been undertaken investigating how inhibition of this signalling pathway affects eNOS activity [18–30]. These studies have been performed using specific inhibitors of the kinases immediately upstream of ERK1/2, MEK1/2 [where MEK is MAPK (mitogen-activated protein kinase)/ERK kinase]. The compounds PD98059 and U0126 inhibit inactive MEK and both inactive and active MEKs respectively and have been used extensively to study the MEK/ERK1/2 signalling pathway. In addition, several studies of eNOS activation and phosphorylation both in vivo and in vitro have utilized these compounds to determine whether MEK/ERK1/2 signalling is necessary for eNOS activation.
Oestrogen stimulates acute eNOS activation in many cell types independent of any alteration in eNOS expression [16,17]. In pulmonary artery endothelial cells [18] and in UAEC (uterine artery endothelial cells) [19], inhibition of MEK1/2 with PD98059 attenuated oestrogen-stimulated eNOS activity. Oestrogen-stimulated phosphorylation of Ser1179 was also partly attenuated by PD98059 in UAEC. Recently, in vivo studies indicated that oestrogen-stimulated NO-dependent vasodilation in femoral and carotid arteries of mice was dependent on MEK/ERK1/2 signalling [20]. In addition to these studies with oestrogen, inhibition of MEK/ERK1/2 signalling has been observed to attenuate eNOS activity stimulated by a variety of factors in several cell lines [21–25]. In contrast, several studies performed in BAEC (bovine aorta endothelial cells) and another study in porcine pulmonary arteries have shown that inhibition of MEK/ERK1/2 signalling results in either no change [26–29] or an enhancement [30] of stimulated eNOS activity. Despite these many studies, a mechanism by which MEK/ERK1/2 signalling affects eNOS activity remains to be determined. It is unclear whether ERK1/2 directly phosphorylates eNOS and on which amino acid this may take place or whether these kinases signal indirectly to eNOS to alter activity.
ERKs phosphorylate many types of proteins including transcription factors, metabolic enzymes, cytoskeletal proteins and other protein kinases. They are proline-directed serine/threonine kinases, since their substrates have a proline residue in the S/T +1 position, where S/T denotes the phosphorylated serine or threonine residue. In the case of ERK1/2 in particular, many substrates contain proline in the S/T –2 position as well. The motif is then PX(S/T)P for ERK1/2 phosphorylation, and ovine eNOS contains seven such motifs.
In the present study, we utilized the COS-7 eNOS model to gain insight into potential molecular mechanisms for the effects of MEK/ERK1/2 signalling on eNOS. The literature contains studies where inhibition of MEK/ERK1/2 stimulates, inhibits or has no effect on eNOS activity, depending on the cell model and the agonists used [18–30]. We examined eNOS activity and changes in intracellular Ca2+ concentration ([Ca2+]i) in HUVEC-CS [transformed HUVEC (human umbilical-vein endothelial cells)] [31] to determine how our results would compare with previously published studies in untransformed HUVEC [22,23]. Little information is available on potential molecular mechanisms controlling the interaction of ERK1/2 with eNOS; therefore we used the COS-7 cells first to determine how inhibition of MEK/ERK1/2 affected A23187- and ATP-stimulated eNOS activity and phosphorylation on previously characterized amino acids. In addition, we determined how ATP stimulation of [Ca2+]i was altered under the same conditions. Finally, several eNOS alanine mutants were generated, based on the conservative ERK1/2 phosphorylation motif PX(S/T)P described above. Each mutant eNOS construct was transfected into COS-7 cells and stimulated activity in the presence and absence of U0126 was compared with wild-type eNOS. Our findings show that one residue in particular could indeed be a target for ERK1/2 phosphorylation that impacts on eNOS activity.
EXPERIMENTAL
Cell culture
COS-7 cells (A.T.C.C., Manassas, VA, U.S.A.) were cultured in DMEM (Dulbecco's modified Eagle's medium), high glucose (Invitrogen, Carlsbad, CA, U.S.A.) with 10% (v/v) FBS (fetal bovine serum; Invitrogen) and 50 units/ml penicillin and 50 μg/ml streptomycin (Invitrogen) and 4 μg/ml gentamicin (Sigma–Aldrich, St. Louis, MO, U.S.A.). Trypsin–EDTA (0.05% trypsin with EDTA; Invitrogen) was used to passage cells and COS-7 cells were used between passages 3 and 6. Cells were propagated and stored according to A.T.C.C. guidelines. Cells were serum-withdrawn for experiments as noted. Confluency at the time of experimentation varied, and was typically between 70 and 90%.
HUVEC (HUVEC-C, a spontaneously transformed cell line) were purchased from A.T.C.C. HUVEC-CS, derived from HUVEC-C [31], were plated on to gelatin-coated T75 flasks in MEM (minimal essential medium) D-Val (Gibco BRL) containing 20% FBS, 50 units/ml penicillin, 50 μg/ml streptomycin and 4 μg/ml gentamicin. Following culture to near confluence, cells were seeded to raw plastic for activity assays or glass coverslips for Ca2+ imaging.
eNOS activity assay
HUVEC-CS were grown in gelatin-coated T75 flasks and grown to approx. 70% confluence before seeding to 35 mm dishes and allowing to attach overnight before the activity assay. COS-7 cells were grown, transfected and plated as described above. Arginine-into-citrulline conversion assays were performed in intact cells as previously described [31,32]. The MEK inhibitor, U0126 (10 μM), was added 15 min before the 30 min treatment with A23187 or ATP.
Intracellular Ca2+ detection
Cells (COS-7 or HUVEC-CS) were plated on to 35 mm dishes with glass coverslips (MatTek, Ashland, MA, U.S.A.) with regular media changes before imaging was performed at the density stated in the individual experiments. At the time of imaging, cells were incubated in Krebs buffer [125 mM NaCl, 5 mM KCl, 1 mM MgSO4, 1 mM KH2PO4, 6 mM glucose, 25 mM Hepes and 2 mM CaCl2 (Calbiochem, San Diego, CA, U.S.A.), pH 7.4] containing 5 μM fura 2/AM (fura 2 acetoxymethyl ester; Invitrogen) and 0.05% Pluronic F127 (Invitrogen) for 45 min at 37 °C. The cells were washed twice and covered in 1 ml of Krebs buffer and then incubated for a further 30 min at 37 °C to allow complete ester hydrolysis. Cells were washed again and covered with 1–2 ml of Krebs buffer. fura 2 loading was verified by viewing at 380 nm UV excitation on a Nikon inverted microscope. The cells were recorded with a pixel-fly camera. By comparison with a standard curve established for the settings using buffers of known free [Ca2+] (Molecular Probes), the intracellular free [Ca2+] was calculated in real time. During optimization of [Ca2+]i imaging for COS-7 cells, it was determined that sham-transfected cells did not respond differently from untransfected cells.
Gene transfection
The pBK-CMV (Stratagene, La Jolla, CA, U.S.A.; CMV is cytomegalovirus) or plasmid containing wild-type [33] or mutant ovine eNOS cDNA was propagated in XL-10 Gold Ultracompetent (Stratagene) Escherichia coli cells. Transfection-quality plasmid DNA was purified with EndoFree Plasmid Maxi kit (Qiagen) following exactly the manufacturer's guidelines. Using GeneJammer reagent (Stratagene), plasmids were transiently transfected into COS-7 cells. Following the manufacturer's instructions, a 6:1 (v/w) ratio of GeneJammer to plasmid DNA was employed. Cells were transfected in either 60 mm dishes for studies of phosphoproteins (4×105 cells/dish; 12 μl of GeneJammer and 2.0 μg of plasmid DNA) or 100 mm dishes for study of eNOS activity (12×105 cells/dish; 36 μl of GeneJammer and 6.0 μg of plasmid DNA). After seeding COS-7 cells to 60 or 100 mm dishes and overnight incubation at 37 °C, cells were ideally 60% confluent at the time of transfection.
Approximately 48 h after transfection, the cells in 100 mm dishes were trypsinized and seeded to 12-well dishes to allow each treatment of the activity assay to be performed in triplicate. Some of the remaining cells were plated in a 60 mm dish to verify equal eNOS expression between wild-type and the various mutant eNOS constructs. Cells were allowed to attach for 8 h before they were serum-withdrawn in 0.01% BSA–DMEM overnight.
Investigation of phosphorylated eNOS, cell lysis and protein solubilization
COS-7 cells transiently expressing wild-type eNOS for 1.5 days were serum-withdrawn overnight in 0.01% BSA–DMEM. Cells were pretreated with vehicle or 10 μM U0126 for 15 min and then treated with 10 μM A23187 or 10 μM ATP for the indicated time. Following treatments as described above, cells were rinsed twice in ice-cold PBS, and frozen at −70 °C. Cells were then solubilized in lysis buffer (4 mM NaP2O7·H2O, 50 mM Hepes, 100 mM NaCl, 10 mM EDTA, 10 mM NaF and 2 mM Na3VO4, pH 7.5, with added 1 mM PMSF, 1% Triton X-100, 5 μg/ml leupeptin and 5 μg/ml aprotinin) before brief sonication (Sonifier Cell Disruptor, W185, Heat Systems; Ultrasonics). Cell lysates were centrifuged for 5 min at 16000 g to pellet cell debris. Solubilized whole cell lysate protein concentrations were determined by the BCA (bicinchoninic acid) assay (Sigma–Aldrich) with comparison with BSA standards.
SDS/PAGE/immunoblotting
Whole cell lysates from each protein sample (10 μg) were size-separated by SDS/PAGE on 7.5% (w/v) acrylamide/Tris/HCl Criterion gels (Bio-Rad) at 200 V for 1 h in Bio-Rad Criterion tank in 192 mM glycine, 25 mM Tris (pH 8.3) and 0.1% SDS (Bio-Rad, Hercules, CA, U.S.A.). Following transfer to an Immobilon P membrane at 100 V for 1 h in Bio-Rad Transblot with plate electrodes and cooling coil at 10 °C (150 mM glycine and 25 mM Tris), the blots were blocked in 5% (w/v) non-fat milk in TBS-T (500 mM NaCl, 25 mM Tris, pH 7.5, and 0.1% Tween 20). Primary antibodies against the following antigens were used in the experiments and diluted in 1% BSA–TBST: human p-eNOS Ser116 [Upstate Biotechnology; pAb (polyclonal antibody); 1:2500], human p-eNOS Thr495 (Upstate Biotechnology; pAb; 1:5000), bovine p-eNOS Ser617 (Upstate Biotechnology; pAb; 1:3000), bovine p-eNOS Ser635 (Upstate Biotechnology; pAb; 1:20000), human p-eNOS Ser1177 (Cell Signaling Technology; pAb; 1:5000), p-independent eNOS [BD Transduction Laboratories; mAb (monoclonal antibody); 1:5000], p-ERK1/2 Thr183/Tyr185 (Promega; pAb; 1:5000), and p-independent ERK1/2 (Cell Signaling Technology; pAb; 1:1000). Horseradish peroxidase-conjugated secondary antibodies (SαM Fab2 1:1000–1:10000; Amersham; and GαR 1:3000–1:5000; Cell Signaling Technology) were used for chemiluminescent detection with ECL® reagent (Amersham). Blots were exposed to Hyperfilm ECL® (Amersham), and autoradiography images were quantified using HP DeskScan and Molecular Analyst software V1.4 (Bio-Rad). All data collected with phosphorylation-state-dependent antibodies were normalized to the data collected with phosphorylation-state-independent antibodies for the same samples.
Site-directed mutagenesis
The QuikChange® Site-Directed Mutagenesis kit (Stratagene) was used to mutate pBK-CMV ovine eNOS following the manufacturer's instructions exactly as specified. The sense oligonucleotides used for mutagenesis are listed below according to the amino acid substitution and the mutated bases are indicated in bold-face: S33A, 5′-CAGGGCCCAGCCGCCCCGGCACCTGAGCCC-3′; T46A, 5′-CCCGCACCCGCCGCCCCGCACGCGCCAGAC-3′; S58A, 5′-CCAGCTCCCAACGCCCCCACGCTGACCCGG-3′; T97A, 5′-GACGGGCCCTGCGCTCCCAGGTGCTGCCTG-3′; S872A, 5′-CCTCCCCACCCGCCCCCCGGCTTCTCCG-3′; S1085A, 5′-CCCGGGAACCTGACGCCCCCAAGACCTACG-3′; T1202A, 5′-GCCCGGCCCAGACGCCCCCGGCCCCTG-3′.
Data analysis
Data are representative of at least n=3 separate experiments and are presented as the means±S.E.M. ANOVA was used for multiple comparisons, while paired Student's t test was used to determine differences between U0126 effects on individual mutants and paired wild-type controls (P values shown in Tables 1 and 2) as appropriate. Results were considered significant at P<0.05 level.
Table 1. U0126 influences A23187-stimulated citrulline production in intact COS-7 cells expressing wild-type and mutant eNOS.
Results for citrulline production are means±S.E.M. for four independent experiments performed in triplicate expressed as percentages of citrulline production after combined U0126/A23187 treatment compared with A23187 treatment alone. *P<0.05 compared with A23187 treatment alone (100% citrulline production). The far-right column represents the mean±S.E.M. of the ratio of wild-type/mutant citrulline production.
| Citrulline production (%): U0126+A23187/A23187 | ||||
|---|---|---|---|---|
| Construct | Mutant | Wild-type | P value (mutant versus wild-type) | Ratio of wild-type/mutant |
| S33A | 126±4* | 130±4* | 0.62 | 1.03±0.05 |
| T46A | 127±4* | 130±6* | 0.40 | 1.02±0.02 |
| S58A | 119±4* | 125±7* | 0.52 | 1.05±0.06 |
| T97A | 121±3* | 124±7* | 0.59 | 1.02±0.04 |
| S872A | 120±2* | 122±6* | 0.72 | 1.02±0.05 |
| S1085A | 119±6* | 119±1* | 0.97 | 1.01±0.04 |
| T1202A | 119±5* | 121±4* | 0.39 | 1.02±0.02 |
Table 2. U0126 influences ATP-stimulated citrulline production in intact COS-7 cells expressing wild-type and mutant eNOS.
Results for citrulline production are means±S.E.M. for four independent experiments performed in triplicate expressed as percentages of citrulline production after combined U0126/ATP treatment compared with ATP treatment alone. *P<0.05 compared with A23187 treatment alone (100% citrulline production). The far-right column represents the mean±S.E.M. of the ratio of wild-type/mutant citrulline production.
| Citrulline production (%): U0126+ATP/ATP | ||||
|---|---|---|---|---|
| Construct | Mutant | Wild-type | P value (mutant versus wild-type) | Ratio of wild-type/mutant |
| S33A | 94±6 | 84±4* | 0.31 | 0.91±0.07 |
| T46A | 94±3 | 86±4* | 0.33 | 0.92±0.07 |
| S58A | 92±2* | 86±4* | 0.30 | 0.94±0.05 |
| T97A | 101±3 | 88±1* | 0.03 | 0.87±0.02 |
| S872A | 93±3 | 92±2* | 0.74 | 0.99±0.04 |
| S1085A | 88±1* | 86±4* | 0.82 | 0.99±0.06 |
| T1202A | 88±1* | 86±4* | 0.67 | 0.98±0.05 |
RESULTS
U0126 potentiates ATP-stimulated eNOS activity in HUVEC-CS without significantly altering Ca2+ flux
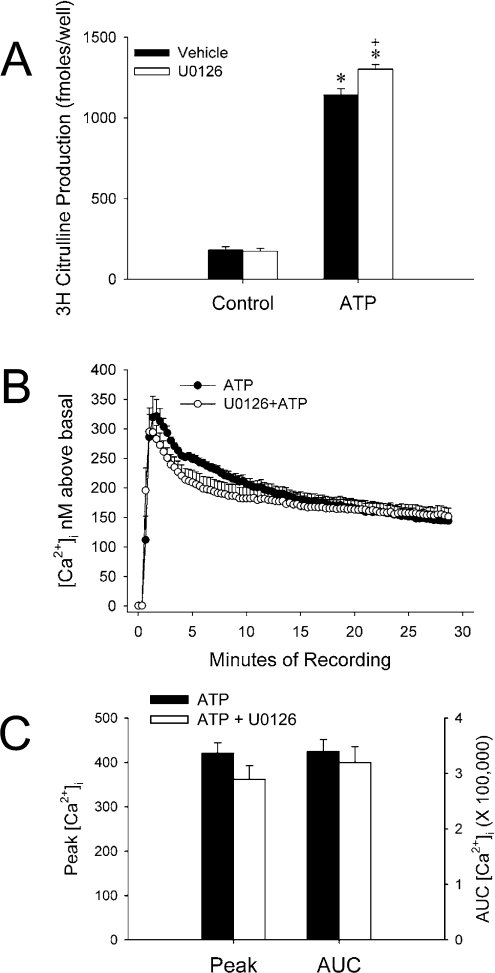
In previous studies of UAEC, we found that MEK/ERK1/2 inhibition significantly elevated ATP-stimulated eNOS activity in NP-UAEC (UAEC derived from non-pregnant ewes) and there was a similar trend in P-UAEC (UAEC derived from pregnant ewes) [34]. In addition, VEGF (vascular endothelial growth factor)-stimulated eNOS activity is enhanced in P-UAEC with U0126 pretreatment and tends towards this in NP-UAEC (M.A. Grummer, personal communication). However, not all endothelial cells share the same cell signalling properties; therefore we chose to investigate how MEK/ERK1/2 inhibition affected eNOS activity in another endothelial cell line commonly used in our laboratory. HUVEC-CS, a transformed cell line of HUVEC, were challenged with ATP with or without U0126 pretreatment to determine whether MEK/ERK1/2 inhibition affects eNOS activity in these cells. ATP-stimulated eNOS activity measured over 30 min was increased in cells pretreated with U0126 by approx. 15% over control pretreatment (Figure 1A). While this was consistent with results from UAEC, potential differences in cell signalling exist between endothelial cell types, and we were unsure whether increased eNOS activity was due to enhancement of HUVEC-CS [Ca2+]i response to ATP. Somewhat surprisingly, U0126 appeared to decrease the average [Ca2+]i trace over a 30 min recording period (Figure 1B); however, average peak [Ca2+]i was not significantly different for each treatment, nor was the total [Ca2+]i when calculated as area under the curve (Figure 1C). These results imply that MEK/ERK1/2 potentiation of eNOS activity involves a mechanism dependent on eNOS phosphorylation, protein–protein interactions or eNOS trafficking rather than indirect effects on [Ca2+]i. Further studies are necessary to determine the mechanism or mechanisms responsible in this cell line. In previously published studies performed in primary HUVEC, MEK/ERK1/2 inhibition with PD98059 decreased leptin-stimulated cell migration, an NO-dependent action [22]. In addition, Wyatt et al. [23] determined that cGMP production was inhibited in adenosine-stimulated HUVEC. Our results may contrast with previously published studies because we are working with an immortalized cell line instead of primary cells, but it seems more likely that this is due to a difference in the signalling mechanisms utilized by each of the different receptor classes, combined possibly with the more indirect assay methods used in those studies.
Figure 1. U0126 enhances ATP-stimulated eNOS activity HUVEC-CS despite a tendency towards decreased stimulation of [Ca2+]i.
(A) HUVEC-CS were seeded to 35 mm dishes and incubated overnight at 37 °C in 20% FBS/MEM. On the day of experiment, cells were rinsed with and incubated in Krebs buffer for 1 h at 37 °C. Before treatment, cells were incubated in 10 μM U0126 for 15 min at 37 °C. [3H]Arginine and either vehicle or ATP (final concentration 100 μM) in Krebs buffer were added to cells for 30 min. The results are expressed as means±S.E.M. for femtomoles of [3H]Citrulline and represent n=3 independent experiments (*P<0.05 when compared with ATP alone). (B) Cells were plated on to glass coverslips in 35 mm dishes and allowed to attach overnight. Cells incubated in Krebs buffer were inhibited with vehicle or 10 μM U0126 for 15 min at 37 °C before treatment with 100 μM ATP. The results are expressed as the means±S.E.M. for [Ca2+]i above basal (for each 20th data point) from n=5 (ATP) and n=6 (U0126+ATP) experiments. (C) Data from each recording were averaged for the net peak [Ca2+]i as well as area under the curve (AUC) for each cell.
U0126 pretreatment potentiates A23187-stimulated eNOS activity in transfected COS-7 cells without enhancing phosphorylation of key stimulatory residues
To date, no one has studied the role of ERK1/2 on eNOS in COS-7 cells, which is somewhat surprising considering the extensive work that has been done in COS-7 cells to describe and define the role of Akt on eNOS phosphorylation and activation [15,35–37]. After inhibiting endogenous MEK/ERK1/2 signalling in eNOS-transfected COS-7 cells with 10 μM U0126, we measured basal and A23187-stimulated eNOS activity. U0126 potentiated A23187-stimulated activity in COS-7 cells by approx. 30% (Figure 2A).
Figure 2. U0126 potentiates A23187-stimulated eNOS activity in COS-7 cells and decreases pS635.
(A) COS-7 cells were plated on to 100 mm dishes and incubated overnight at 37 °C in 10% FBS–DMEM. Cells were transfected with 6 μg of pBK-CMV-oeNOS. After 2 days of eNOS expression, cells were trypsinized and subcultured to 12-well plates. After plating for 8 h, cells were serum-withdrawn in 0.01% BSA–DMEM overnight. On the day of the experiment, the cells were rinsed with and incubated in Krebs buffer for 1 h at 37 °C and then [3H]Arginine was added for a 30 min incubation. Halfway through this incubation, 10 μM U0126 or vehicle was added. Cells were treated with vehicle or 10 μM A23187 for 30 min. The results are expressed as the means±S.E.M. for femtomoles of [3H]Citrulline and represent n=3 experiments where each treatment was performed in triplicate (+P<0.05 compared with A23187 alone; *P<0.05 compared with control). (B, C) Ovine eNOS-transfected COS-7 cells (60 mm dishes) were serum-starved overnight in 0.01% BSA–DMEM. Cells were treated with 10 μM U0126 or vehicle for 15 min and then stimulated with vehicle or 10 μM A23187 for 0–30 min. Whole cell lysates were collected and SDS/PAGE and Western blotting were performed with phospho-specific antibodies for ERK1/2 (B) and eNOS [pS116, pT497 (phospho-Thr497), pS617, pS635 and pS1179] (C) and also phosphorylation-state-independent antibodies to ERK1/2 and eNOS (+P<0.05 compared with A23187 alone; *P<0.05 compared with control).
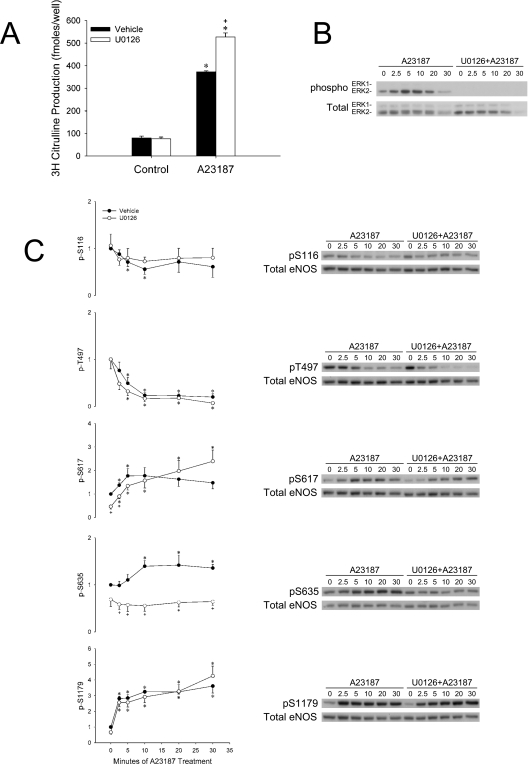
To investigate effects of MEK/ERK1/2 inhibition on eNOS phosphorylation, COS-7 cells were transfected with ovine eNOS and experiments similar to the activity assays were performed. Whole cell lysates were collected for SDS/PAGE and Western-blot analysis with phospho-specific antibodies. A23187 stimulated phosphorylation of ERK1/2 on activating sites (pTEpY Thr183/Tyr185) in COS-7 cells over time that was completely inhibited by U0126 (Figure 2B). Phosphorylation of Ser116 and Thr497 decreased with A23187 treatment, and U0126 pretreatment did not alter these levels (Figure 2C). In addition, increases in pS617 (phospho-Ser617) and pS1179 with A23187 treatment were not changed by inhibition of MEK/ERK1/2 (Figure 2C). In contrast, phosphorylation of Ser635 was inhibited both basally and after A23187 stimulation with MEK/ERK1/2 inhibition (Figure 2C).
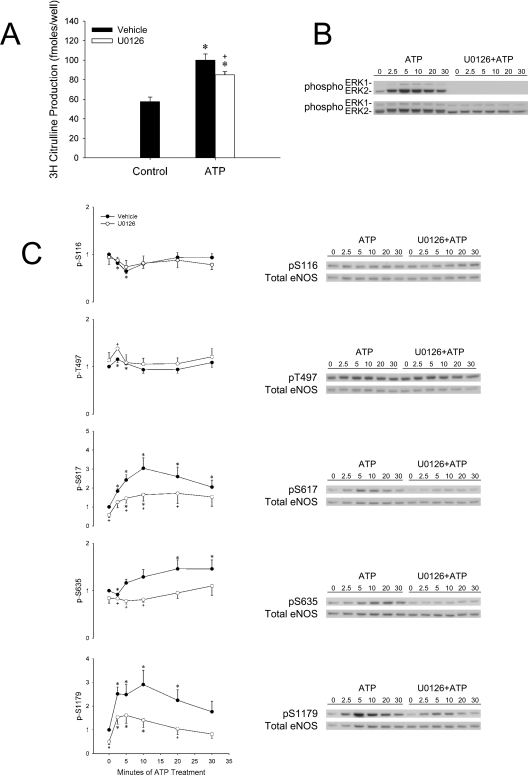
U0126 pretreatment inhibits ATP-stimulated eNOS activity in transfected COS-7 cells as well as phosphorylation of key stimulatory residues and ATP-stimulated [Ca2+]i
When eNOS-transfected COS-7 cells were treated with ATP after U0126 pretreatment, citrulline production was decreased compared with vehicle pretreatment (Figure 3A). This finding was especially interesting, considering the contrasting results observed with A23187 treatment. ATP stimulated ERK1/2 phosphorylation and was completely inhibited with U0126 pretreatment (Figure 3B). eNOS phosphorylation on Ser116 did not vary between U0126 and vehicle-pretreated cells. In contrast, significant differences were observed for at least one time point on the other phosphorylation sites (Thr497, Ser617, Ser635 and Ser1179). Importantly, phosphorylation of amino acids associated with increased activity (Ser617, Ser635 and Ser1179) was inhibited with U0126 pretreatment.
Figure 3. U0126 decreases ATP-stimulated eNOS activity in COS-7 cells and decreases pS617, pS635 and pS1179.
(A) COS-7 cells were plated on to 100 mm dishes and incubated overnight at 37 °C in 10% FBS–DMEM. Cells were transfected with 6 μg of pBK-CMV-oeNOS. After 2 days of eNOS expression, cells were trypsinized and subcultured to 12-well plates. After plating for 8 h, cells were serum-withdrawn in 0.01% BSA–DMEM overnight. On the day of the experiment, the cells were rinsed with and incubated in Krebs buffer for 1 h at 37 °C and then [3H]Arginine was added for a 30 min incubation. Halfway through this incubation, 10 μM U0126 or vehicle was added. Cells were treated with vehicle or 10 μM ATP for 30 min. The results are expressed as means±S.E.M. for femtomoles of [3H]Citrulline and represent n=3 experiments where each treatment was performed in triplicate (+P<0.05 compared with ATP alone; *P<0.05 compared with control). (B, C) Ovine eNOS-transfected COS-7 cells (60 mm dishes) were serum-starved overnight in 0.01% BSA–DMEM. Cells were treated with 10 μM U0126 or vehicle for 15 min and then stimulated with vehicle or 10 μM ATP for 0–30 min. Whole cell lysates were collected and SDS/PAGE and Western blotting were performed with phospho-specific antibodies for ERK1/2 (B) and eNOS (pS116, pT497, pS617, pS635 and pS1179) (C) and also phosphorylation-state-independent antibodies to ERK1/2 and eNOS (+P<0.05 compared with A23187 alone; *P<0.05 compared with control).
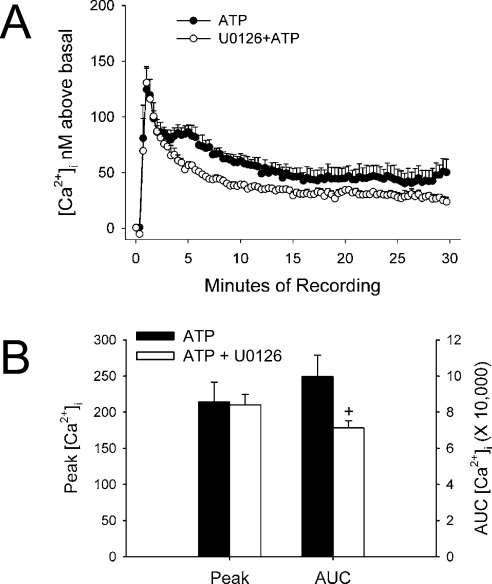
In addition to phosphorylation of key amino acid residues, we determined whether ATP-stimulated [Ca2+]i in COS-7 cells was altered with U0126 pretreatment. The sustained phase was somewhat decreased in response to ATP in cells pretreated with U0126 compared with cells stimulated with ATP alone (Figure 4A). Peak [Ca2+]i was not altered; however, the total area under the curve was indeed decreased with U0126 pretreatment (Figure 4B).
Figure 4. U0126 decreases ATP-stimulated extended phase of ATP stimulation of [Ca2+]i.
(A) Cells were plated on to glass coverslips in 35 mm dishes and allowed to attach overnight. Cells incubated in Krebs buffer were inhibited with vehicle or 10 μM U0126 for 15 min at 37 °C before treatment with 10 μM ATP. The results are expressed as the means±S.E.M. for [Ca2+]i above basal (for each 20th data point) from n=5 experiments. (B) Data from each recording were averaged for the net peak [Ca2+]i as well as area under the curve (AUC) for each cell (+P<0.05 when compared with ATP treatment alone).
Mutation of putative ERK1/2 phosphorylation sites on eNOS produced a candidate for further characterization with ATP
Seven eNOS alanine mutants were generated based on the PX(S/T)P ERK1/2 phosphorylation substrate motif: S33A, T46A, S58A, T97A, S872A, S1085A and T1202A. Each construct was transfected into COS-7 cells, and citrulline production in intact cells was compared with wild-type eNOS under the same conditions. Consistent expression levels were verified within each experiment by Western blotting (Figure 5).
Figure 5. Wild-type and mutant eNOS constructs were evenly expressed within each experiment.
Cells were plated, transfected, and experiments were performed as described for Figures 2 and 3. The strip blots indicate equal protein expression of eNOS between the different constructs transiently expressed by COS-7 cells and investigated in the same experiment (indicated by each solid bar over a group of two to three protein bands). The strip blots represent two of a total of eight blots performed to check for equal eNOS expression. wt, wild-type.
If ERK1/2 directly phosphorylated eNOS on one amino acid, we might expect to observe a loss of this effect with the target amino acid mutated to alanine. Since MEK/ERK1/2 inhibition potentiated A23187-stimulated eNOS activity, mutation to alanine would be expected to result in a loss of this potentiation with U0126 pretreatment. In contrast, MEK/ERK1/2 inhibition decreased ATP-stimulated eNOS activity; therefore mutation to alanine of a putative substrate amino acid would be expected to result in loss of this inhibition with U0126 pretreatment.
In the case of A23187 (Table 1), none of the mutants was observed to lose U0126 potentiation of A23187-stimulated eNOS activity. In the case of ATP, however, only three of the mutants (S58A, S1085A and T1202A) exhibited inhibition of stimulated activity with U0126 pretreatment comparable with wild-type eNOS (Table 2). Direct comparison of the activity ratios (U0126+ATP/ATP) demonstrated that only T97A-eNOS significantly differed from wild-type eNOS.
DISCUSSION
Our results have shown that U0126-mediated inhibition of MEK/ERK1/2 enhances ATP-stimulated eNOS activity without altering Ca2+ flux in a transformed endothelial cell line, HUVEC-CS. In addition, our studies characterized how inhibition of MEK/ERK1/2 alters eNOS activation and phosphorylation in a commonly used cell model of transiently transfected COS-7 cells treated with A23187 and ATP. The COS-7 eNOS model has been employed many times in past characterizations of molecular mechanisms of eNOS activation by acylation, protein–protein interactions and phosphorylation [1,13,38–42]. While even subtle differences in cell-specific signalling pathways mean that results from the COS-7 model cannot be directly extrapolated to endothelial cells (in vitro or in vivo) without further validation, these results provide a valuable basis for such future studies.
It is noteworthy that MEK/ERK inhibition attenuates eNOS activation after treatment with such diverse agonists as VEGF, oestrogen, adenosine, HDL (high-density lipoprotein) and leptin [19,21–23,25,43]. These studies were performed in a variety of cell types, including pulmonary artery endothelial cells, UAEC, HUVEC and even BeWo cells (a placental choriocarcinoma cell line available from A.T.C.C.). In contrast, intact BAEC do not exhibit decreased eNOS activation following MEK/ERK1/2 inhibition [26–28,30].
In the present study, eNOS activity was enhanced by MEK/ERK1/2 inhibition in ATP-stimulated HUVEC-CS and A23187-stimulated COS-7 cells transiently expressing eNOS. One other study observed enhancement of eNOS activity in cell lysates after MEK/ERK1/2 inhibition [30]. Bernier et al. [30] found evidence suggesting that ERK1/2 may directly phosphorylate eNOS in vitro on a site that is not Ser1179. They also found that Vmax, eNOS activity was decreased by incubating eNOS with recombinant ERK. eNOS immunoprecipitated from BAEC treated with bradykinin (with or without PD98059 pretreatment) had higher catalytic Vmax activity when MEK had been inhibited; however, these studies did not include an analysis of eNOS activity after MEK inhibition in intact cells. Our study is the first to show that MEK inhibition potentiates A23187-stimulated eNOS activity in intact cells, and further that this potentiation of eNOS activity occurs in the absence of compensatory changes in phosphorylation of five key eNOS residues. In fact, even in the face of increased activity, we observed a significant decrease in the level of stimulated Ser635 phosphorylation, an event previously correlated to increased eNOS activity [44–46].
Ser635 (KRKESSNTDS) is not a consensus site for ERK phosphorylation, implying a more complex role of MEK/ERK1/2 in the regulation of Ser635 phosphorylation. Others have determined that this amino acid is phosphorylated in a PKA-dependent manner [44,45]. Phosphorylation of this site may also depend on PKG activity [45]. The mechanistic role of Ser635 phosphorylation has not been completely elucidated, and studies of phosphonull and phosphomimetic mutants expressed in COS cells have not simplified the matter [47]. Under Vmax conditions, S635A exhibited enhanced activity compared with wild-type –eNOS, whereas S635D did not differ significantly from wild-type. In contrast, assays in intact cells showed that S635A was detrimental for basal NO production (as measured by nitrite accumulation), but did not affect ATP-stimulated NO production, despite increased ATP-stimulated Hsp90 (heat-shock protein 90) binding than wild-type eNOS, typically associated with increased eNOS activity. Finally, in intact cells, S635D-eNOS exhibited increased basal and ATP-stimulated NO production when compared with wild-type. Drawing on mutant experiments and considering that phosphorylation of Ser635 increases when the protein is activated, one might logically conclude that phosphorylation of this site augments eNOS activity. Before drawing that conclusion, it is important to consider that Ser635 phosphorylation has been shown to occur with a slower time course than Ser1179 in response to shear stress, VEGF and 8-bromo-cAMP [44] and ATP [32]. The time-course data are even more intriguing in light of recent experiments using Golgi- or plasma-membrane-targeted eNOS constructs expressed in COS-7 cells [48]. In these studies, the greatest phosphorylation of Ser635 occurred when eNOS was targeted to the trans-Golgi network and associated vesicles. However, basal NO production was lower than the wild-type with this construct and did not differ from wild-type after cells were stimulated with thapsigargin. Taking into account what is known about eNOS cycling and trafficking in the cell, pS635 may be more a measure of where eNOS is located in the cell rather than a stimulator of catalytic activity.
A key observation in this paper is the opposite effect observed between ATP and A23187 stimulation of eNOS activity in transiently transfected COS-7 cells pretreated with U0126. Whereas with A23187, potentiation of eNOS activity was observed, ATP-stimulated activity was actually decreased with MEK/ERK1/2 inhibition. Several potential explanations for this observation were determined by study of phosphorylation of key residues as well as changes in [Ca2+]i. U0126 decreased the stimulation of Ser617, Ser635 and Ser1179 phosphorylation by ATP. In addition, the overall change in [Ca2+]i over the sustained phase of intracellular Ca2+ release was decreased, yet this event clearly would have been bypassed when using a receptor-independent stimulus such as the ionophore A23187.
The present study is the first to undertake systematic mutation of conservative putative ERK1/2 phosphorylation sites on eNOS in hopes to find a candidate substrate residue. It has been supposed that Ser1179 could be a target for proline-directed kinases [49] due to its +1 P context (TRPSPSGPP). We have determined in the present study that inhibition of MEK/ERK1/2 does not subsequently lead to decreased basal or stimulated pS116 in transfected COS-7 cells, indicating that another proline-directed kinase could be responsible for this event in this cell type. We expected to find that if ERK1/2 directly phosphorylated one of the mutant amino acids, we should be able to see a loss of either potentiation (with A23187) or inhibition (with ATP) of eNOS activity observed with U0126 treatment. In the case of A23187, all of the constructs exhibited unaltered potentiation of activity compared with wild-type eNOS. This strongly suggests that none of these amino acid residues are phosphorylated in a manner that is a hindrance to A23187-stimulated activity. When studying these constructs' responses to ATP, however, the T97A mutant was the only one to exhibit significantly different behaviour compared with wild-type eNOS. Thr97 is adjacent to one of the cysteine residues (Cys96) responsible for co-ordination of a zinc ion in the dimer interface [50]. Phosphorylation of Thr97 could also affect S-nitrosylation of eNOS on Cys96 (evidenced in mutagenesis studies) and Cys101 (demonstrated by mass spectroscopy and mutagenesis) [11,12,51,52]. Nitrosylation in the zinc tetrathiolate cluster is thought to disrupt eNOS dimerization and moderately decrease eNOS activity [11,12], and any substantial charge alteration introduced by phosphorylation or dephosphorylation of Thr97 may affect eNOS S-nitrosylation [53].
The crystal structure of eNOS oxygenase dimer [54] shows that the T97 side chain is located on the surface of the eNOS protein, with the nearby (+1) proline accessible for targeting of kinases such as ERK1/2 (see Supplementary Figure at http://www.BiochemJ.org/bj/398/bj3980279add.htm). Thus the amino acid has both a highly appropriate location and accessibility to perform a regulatory role on eNOS activation. Clearly, further work is necessary to determine whether one or both such residues must be phosphorylated in an eNOS dimer for full effect, and whether such phosphorylation events are physiologically relevant in vivo. It would also be important to determine whether this mutation or the converse mutation to aspartate alters S-nitrosylation of eNOS.
The role of ERK1/2 in eNOS activation seems to be not only dependent on the cell and tissue in which eNOS activity is measured, but also depends on the agonist used to stimulate activity. While there is some evidence suggesting that eNOS could be directly phosphorylated by ERK1/2 in vitro [30], it is equally likely that ERK1/2 may affect eNOS activity by an indirect pathway, altering phosphorylation of eNOS residues or even Ca2+ flux. This is illustrated clearly when these studies are attempted in UAEC.
In UAEC, we previously reported that eNOS activation by a variety of agonists acting through growth-factor receptors or G-protein-coupled receptors was strongly correlated with ERK1/2 phosphorylation in P-UAEC, but not in NP-UAEC [55]. Therefore we went on to determine whether inhibition of MEK/ERK1/2 signalling would affect eNOS activity. Initial studies using detection of nitrate/nitrite production suggested that inhibition of MEK could block eNOS activation in response to ATP [56], but this method has been suggested to be prone to several errors [57]. Subsequent studies using arginine-into-citrulline conversion assays (also used herein) have instead shown that ATP-stimulated eNOS activity is somewhat potentiated by treatment with U0126 in P-UAEC and even more so in NP-UAEC [34]. It seems therefore that the same mechanisms operating in HUVEC-CS may be operating in UAEC. Nonetheless, the situation is complicated by effects on [Ca2+]i since when P-UAEC are used, U0126 has little effect on the Ca2+ response, but in NP-UAEC, potentiation of the Ca2+ response is clearly observed (F. X. Yi and I. M. Bird, unpublished work). Thus some degree of potentiation of eNOS activation in P-UAEC may be mediated at the level of eNOS itself, but in NP-UAEC, eNOS activation may also be mediated in part by a potentiated Ca2+ response. Further studies are now under way to determine at what level MEK inhibition impacts on Ca2+ signalling, particularly in NP-UAEC.
Online Data
Acknowledgments
This work was supported by NIH (National Institutes of Health; Bethesda, MD, U. S. A.) grants HD38843 and HL64601. J. M. C. was supported by NIH T32 Training Award (HD41921) and this work formed part of her Ph.D. studies in the Endocrinology Reproductive Physiology Training Program at the University of Wisconsin (Madison, WI, U. S. A.).
References
- 1.Shaul P. W., Smart E. J., Robinson L. J., German Z., Yuhanna I. S., Ying Y., Anderson R. G., Michel T. Acylation targets endothelial nitric-oxide synthase to plasmalemmal caveolae. J. Biol. Chem. 1996;271:6518–6522. doi: 10.1074/jbc.271.11.6518. [DOI] [PubMed] [Google Scholar]
- 2.Liu J., Hughes T. E., Sessa W. C. The first 35 amino acids and fatty acylation sites determine the molecular targeting of endothelial nitric oxide synthase into the Golgi region of cells: a green fluorescent protein study. J. Cell Biol. 1997;137:1525–1535. doi: 10.1083/jcb.137.7.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garcia-Cardena G., Fan R., Shah V., Sorrentino R., Cirino G., Papapetropoulos A., Sessa W. C. Dynamic activation of endothelial nitric oxide synthase by Hsp90. Nature (London) 1998;392:821–824. doi: 10.1038/33934. [DOI] [PubMed] [Google Scholar]
- 4.Michel J. B., Feron O., Sase K., Prabhakar P., Michel T. Caveolin versus calmodulin: counterbalancing allosteric modulators of endothelial nitric oxide synthase. J. Biol. Chem. 1997;272:25907–25912. doi: 10.1074/jbc.272.41.25907. [DOI] [PubMed] [Google Scholar]
- 5.Michel J. B., Michel T. The role of palmitoyl-protein thioesterase in the palmitoylation of endothelial nitric oxide synthase. FEBS Lett. 1997;405:356–362. doi: 10.1016/s0014-5793(97)00222-6. [DOI] [PubMed] [Google Scholar]
- 6.Fleming I., Fisslthaler B., Dimmeler S., Kemp B. E., Busse R. Phosphorylation of Thr495 regulates Ca2+/calmodulin-dependent endothelial nitric oxide synthase activity. Circ. Res. 2001;88:E68–E75. doi: 10.1161/hh1101.092677. [DOI] [PubMed] [Google Scholar]
- 7.Michell B. J., Griffiths J. E., Mitchelhill K. I., Rodriguez-Crespo I., Tiganis T., Bozinovski S., de Montellano P. R., Kemp B. E., Pearson R. B. The Akt kinase signals directly to endothelial nitric oxide synthase. Curr. Biol. 1999;9:845–848. doi: 10.1016/s0960-9822(99)80371-6. [DOI] [PubMed] [Google Scholar]
- 8.Chen Z. P., Mitchelhill K. I., Michell B. J., Stapleton D., Rodriguez-Crespo I., Witters L. A., Power D. A., Ortiz de Montellano P. R., Kemp B. E. AMP-activated protein kinase phosphorylation of endothelial NO synthase. FEBS Lett. 1999;443:285–289. doi: 10.1016/s0014-5793(98)01705-0. [DOI] [PubMed] [Google Scholar]
- 9.Harris M. B., Ju H., Venema V. J., Liang H., Zou R., Michell B. J., Chen Z. P., Kemp B. E., Venema R. C. Reciprocal phosphorylation and regulation of endothelial nitric-oxide synthase in response to bradykinin stimulation. J. Biol. Chem. 2001;276:16587–16591. doi: 10.1074/jbc.M100229200. [DOI] [PubMed] [Google Scholar]
- 10.Michell B. J., Harris M. B., Chen Z. P., Ju H., Venema V. J., Blackstone M. A., Huang W., Venema R. C., Kemp B. E. Identification of regulatory sites of phosphorylation of the bovine endothelial nitric-oxide synthase at serine 617 and serine 635. J. Biol. Chem. 2002;277:42344–42351. doi: 10.1074/jbc.M205144200. [DOI] [PubMed] [Google Scholar]
- 11.Ravi K., Brennan L. A., Levic S., Ross P. A., Black S. M. S-nitrosylation of endothelial nitric oxide synthase is associated with monomerization and decreased enzyme activity. Proc. Natl. Acad. Sci. U.S.A. 2004;101:2619–2624. doi: 10.1073/pnas.0300464101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Erwin P. A., Lin A. J., Golan D. E., Michel T. Receptor-regulated dynamic S-nitrosylation of endothelial nitric-oxide synthase in vascular endothelial cells. J. Biol. Chem. 2005;280:19888–19894. doi: 10.1074/jbc.M413058200. [DOI] [PubMed] [Google Scholar]
- 13.Fulton D., Church J. E., Ruan L., Li C., Sood S. G., Kemp B. E., Jennings I. G., Venema R. C. Src kinase activates endothelial nitric-oxide synthase by phosphorylating Tyr-83. J. Biol. Chem. 2005;280:35943–35952. doi: 10.1074/jbc.M504606200. [DOI] [PubMed] [Google Scholar]
- 14.Kou R., Greif D., Michel T. Dephosphorylation of endothelial nitric-oxide synthase by vascular endothelial growth factor: implications for the vascular responses to cyclosporin A. J. Biol. Chem. 2002;277:29669–29673. doi: 10.1074/jbc.M204519200. [DOI] [PubMed] [Google Scholar]
- 15.Fulton D., Gratton J. P., McCabe T. J., Fontana J., Fujio Y., Walsh K., Franke T. F., Papapetropoulos A., Sessa W. C. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature (London) 1999;399:597–601. doi: 10.1038/21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caulin-Glaser T., Garcia-Cardena G., Sarrel P., Sessa W. C., Bender J. R. 17β-estradiol regulation of human endothelial cell basal nitric oxide release, independent of cytosolic Ca2+ mobilization. Circ. Res. 1997;81:885–892. doi: 10.1161/01.res.81.5.885. [DOI] [PubMed] [Google Scholar]
- 17.Lantin-Hermoso R. L., Rosenfeld C. R., Yuhanna I. S., German Z., Chen Z., Shaul P. W. Estrogen acutely stimulates nitric oxide synthase activity in fetal pulmonary artery endothelium. Am. J. Physiol. 1997;273:L119–L126. doi: 10.1152/ajplung.1997.273.1.L119. [DOI] [PubMed] [Google Scholar]
- 18.Chen B. C., Lin L. L., Lin W. W. Protein kinase Cϵ-dependent pathway of extracellular signal-regulated protein kinase activation by P2Y1 and P2Y2 purinoceptors that activate cytosolic phospholipase A2 in endothelial cells. Eur. J. Pharmacol. 1999;373:101–110. doi: 10.1016/s0014-2999(99)00238-1. [DOI] [PubMed] [Google Scholar]
- 19.Chen D. B., Bird I. M., Zheng J., Magness R. R. Membrane estrogen receptor-dependent extracellular signal-regulated kinase pathway mediates acute activation of endothelial nitric oxide synthase by estrogen in uterine artery endothelial cells. Endocrinology. 2004;145:113–125. doi: 10.1210/en.2003-0547. [DOI] [PubMed] [Google Scholar]
- 20.Guo X., Razandi M., Pedram A., Kassab G., Levin E. R. Estrogen induces vascular wall dilation: mediation through kinase signaling to nitric oxide and estrogen receptors α and β. J. Biol. Chem. 2005;280:19704–19710. doi: 10.1074/jbc.M501244200. [DOI] [PubMed] [Google Scholar]
- 21.Mineo C., Yuhanna I. S., Quon M. J., Shaul P. W. High density lipoprotein-induced endothelial nitric-oxide synthase activation is mediated by Akt and MAP kinases. J. Biol. Chem. 2003;278:9142–9149. doi: 10.1074/jbc.M211394200. [DOI] [PubMed] [Google Scholar]
- 22.Goetze S., Bungenstock A., Czupalla C., Eilers F., Stawowy P., Kintscher U., Spencer-Hansch C., Graf K., Nurnberg B., Law R. E., et al. Leptin induces endothelial cell migration through Akt, which is inhibited by PPARγ-ligands. Hypertension. 2002;40:748–754. doi: 10.1161/01.hyp.0000035522.63647.d3. [DOI] [PubMed] [Google Scholar]
- 23.Wyatt A. W., Steinert J. R., Wheeler-Jones C. P., Morgan A. J., Sugden D., Pearson J. D., Sobrevia L., Mann G. E. Early activation of the p42/p44MAPK pathway mediates adenosine-induced nitric oxide production in human endothelial cells: a novel calcium-insensitive mechanism. FASEB J. 2002;16:1584–1594. doi: 10.1096/fj.01-0125com. [DOI] [PubMed] [Google Scholar]
- 24.Wei Z., Al-Mehdi A. B., Fisher A. B. Signaling pathway for nitric oxide generation with simulated ischemia in flow-adapted endothelial cells. Am. J. Physiol. Heart. Circ. Physiol. 2001;281:H2226–H2232. doi: 10.1152/ajpheart.2001.281.5.H2226. [DOI] [PubMed] [Google Scholar]
- 25.Cha M. S., Lee M. J., Je G. H., Kwak J. Y. Endogenous production of nitric oxide by vascular endothelial growth factor down-regulates proliferation of choriocarcinoma cells. Biochem. Biophys. Res. Commun. 2001;282:1061–1066. doi: 10.1006/bbrc.2001.4682. [DOI] [PubMed] [Google Scholar]
- 26.Kou R., Igarashi J., Michel T. Lysophosphatidic acid and receptor-mediated activation of endothelial nitric-oxide synthase. Biochemistry. 2002;41:4982–4988. doi: 10.1021/bi016017r. [DOI] [PubMed] [Google Scholar]
- 27.Igarashi J., Michel T. Sphingosine 1-phosphate and isoform-specific activation of phosphoinositide 3-kinase β: evidence for divergence and convergence of receptor-regulated endothelial nitric-oxide synthase signaling pathways. J. Biol. Chem. 2001;276:36281–36288. doi: 10.1074/jbc.M105628200. [DOI] [PubMed] [Google Scholar]
- 28.Lorenz M., Wessler S., Follmann E., Michaelis W., Dusterhoft T., Baumann G., Stangl K., Stangl V. A constituent of green tea, epigallocatechin-3-gallate, activates endothelial nitric oxide synthase by a phosphatidylinositol-3-OH-kinase-, cAMP-dependent protein kinase-, and Akt-dependent pathway and leads to endothelial-dependent vasorelaxation. J. Biol. Chem. 2004;279:6190–6195. doi: 10.1074/jbc.M309114200. [DOI] [PubMed] [Google Scholar]
- 29.Schmidt K., Gibraeil H. D., Mayer B. Lack of involvement of extracellular signal-regulated kinase (ERK) in the agonist-induced endothelial nitric oxide synthesis. Biochem. Pharmacol. 2002;63:1137–1142. doi: 10.1016/s0006-2952(01)00936-4. [DOI] [PubMed] [Google Scholar]
- 30.Bernier S. G., Haldar S., Michel T. Bradykinin-regulated interactions of the mitogen-activated protein kinase pathway with the endothelial nitric-oxide synthase. J. Biol. Chem. 2000;275:30707–30715. doi: 10.1074/jbc.M005116200. [DOI] [PubMed] [Google Scholar]
- 31.Gifford S. M., Grummer M. A., Pierre S. A., Austin J. L., Zheng J., Bird I. M. Functional characterization of HUVEC-CS: Ca2+ signaling, ERK 1/2 activation, mitogenesis and vasodilator production. J. Endocrinol. 2004;182:485–499. doi: 10.1677/joe.0.1820485. [DOI] [PubMed] [Google Scholar]
- 32.Cale J. M., Bird I. M. Dissociation of endothelial nitric oxide synthase phosphorylation and activity in uterine artery endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2006;290:H1433–H1445. doi: 10.1152/ajpheart.00942.2005. [DOI] [PubMed] [Google Scholar]
- 33.Cale J. M., Tsoi S. C., Toppe M., Grummer M. A., Ochiai M., Magness R. R., Bird I. M. Molecular cloning of ovine endothelial nitric oxide synthase and expression in COS-7 cells. J. Soc. Gynecol. Invest. 2005;12:156–168. doi: 10.1016/j.jsgi.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 34.Sullivan J. A., Grummer M. A., Yi F. X., Bird I. M. Pregnancy-enhanced endothelial nitric oxide synthase (eNOS) activation in uterine artery endothelial cells shows altered sensitivity to Ca2+, U0126, and wortmannin but not LY294002: evidence that pregnancy adaptation of eNOS activation occurs at multiple levels of cell signaling. Endocrinology. 2006;147:2442–2457. doi: 10.1210/en.2005-0399. [DOI] [PubMed] [Google Scholar]
- 35.Dimmeler S., Fleming I., Fisslthaler B., Hermann C., Busse R., Zeiher A. M. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature (London) 1999;399:601–605. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- 36.Brouet A., Sonveaux P., Dessy C., Balligand J. L., Feron O. Hsp90 ensures the transition from the early Ca2+-dependent to the late phosphorylation-dependent activation of the endothelial nitric-oxide synthase in vascular endothelial growth factor-exposed endothelial cells. J. Biol. Chem. 2001;276:32663–32669. doi: 10.1074/jbc.M101371200. [DOI] [PubMed] [Google Scholar]
- 37.Fontana J., Fulton D., Chen Y., Fairchild T. A., McCabe T. J., Fujita N., Tsuruo T., Sessa W. C. Domain mapping studies reveal that the M domain of hsp90 serves as a molecular scaffold to regulate Akt-dependent phosphorylation of endothelial nitric oxide synthase and NO release. Circ. Res. 2002;90:866–873. doi: 10.1161/01.res.0000016837.26733.be. [DOI] [PubMed] [Google Scholar]
- 38.Tesauro M., Thompson W. C., Moss J. Effect of staurosporine-induced apoptosis on endothelial nitric oxide synthase in transfected COS-7 cells and primary endothelial cells. Cell Death Differ. 2006;13:597–606. doi: 10.1038/sj.cdd.4401770. [DOI] [PubMed] [Google Scholar]
- 39.Fleming I., Mohamed A., Galle J., Turchanowa L., Brandes R. P., Fisslthaler B., Busse R. Oxidized low-density lipoprotein increases superoxide production by endothelial nitric oxide synthase by inhibiting PKCα. Cardiovasc. Res. 2005;65:897–906. doi: 10.1016/j.cardiores.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 40.Greif D. M., Kou R., Michel T. Site-specific dephosphorylation of endothelial nitric oxide synthase by protein phosphatase 2A: evidence for crosstalk between phosphorylation sites. Biochemistry. 2002;41:15845–15853. doi: 10.1021/bi026732g. [DOI] [PubMed] [Google Scholar]
- 41.Feron O., Michel J. B., Sase K., Michel T. Dynamic regulation of endothelial nitric oxide synthase: complementary roles of dual acylation and caveolin interactions. Biochemistry. 1998;37:193–200. doi: 10.1021/bi972307p. [DOI] [PubMed] [Google Scholar]
- 42.Lin M. I., Fulton D., Babbitt R., Fleming I., Busse R., Pritchard K. A., Jr, Sessa W. C. Phosphorylation of threonine 497 in endothelial nitric-oxide synthase coordinates the coupling of L-arginine metabolism to efficient nitric oxide production. J. Biol. Chem. 2003;278:44719–44726. doi: 10.1074/jbc.M302836200. [DOI] [PubMed] [Google Scholar]
- 43.Chen Z., Yuhanna I. S., Galcheva-Gargova Z., Karas R. H., Mendelsohn M. E., Shaul P. W. Estrogen receptor α mediates the nongenomic activation of endothelial nitric oxide synthase by estrogen. J. Clin. Invest. 1999;103:401–406. doi: 10.1172/JCI5347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boo Y. C., Hwang J., Sykes M., Michell B. J., Kemp B. E., Lum H., Jo H. Shear stress stimulates phosphorylation of eNOS at Ser635 by a protein kinase A-dependent mechanism. Am. J. Physiol. Heart Circ. Physiol. 2002;283:H1819–H1828. doi: 10.1152/ajpheart.00214.2002. [DOI] [PubMed] [Google Scholar]
- 45.Butt E., Bernhardt M., Smolenski A., Kotsonis P., Frohlich L. G., Sickmann A., Meyer H. E., Lohmann S. M., Schmidt H. H. Endothelial nitric-oxide synthase (type III) is activated and becomes calcium independent upon phosphorylation by cyclic nucleotide-dependent protein kinases. J. Biol. Chem. 2000;275:5179–5187. doi: 10.1074/jbc.275.7.5179. [DOI] [PubMed] [Google Scholar]
- 46.Boo Y. C., Sorescu G. P., Bauer P. M., Fulton D., Kemp B. E., Harrison D. G., Sessa W. C., Jo H. Endothelial NO synthase phosphorylated at Ser635 produces NO without requiring intracellular calcium increase. Free Radical Biol. Med. 2003;35:729–741. doi: 10.1016/s0891-5849(03)00397-6. [DOI] [PubMed] [Google Scholar]
- 47.Bauer P. M., Fulton D., Boo Y. C., Sorescu G. P., Kemp B. E., Jo H., Sessa W. C. Compensatory phosphorylation and protein–protein interactions revealed by loss of function and gain of function mutants of multiple serine phosphorylation sites in endothelial nitric-oxide synthase. J. Biol. Chem. 2003;278:14841–14849. doi: 10.1074/jbc.M211926200. [DOI] [PubMed] [Google Scholar]
- 48.Fulton D., Babbitt R., Zoellner S., Fontana J., Acevedo L., McCabe T. J., Iwakiri Y., Sessa W. C. Targeting of endothelial nitric-oxide synthase to the cytoplasmic face of the Golgi complex or plasma membrane regulates Akt- versus calcium-dependent mechanisms for nitric oxide release. J. Biol. Chem. 2004;279:30349–30357. doi: 10.1074/jbc.M402155200. [DOI] [PubMed] [Google Scholar]
- 49.Gallis B., Corthals G. L., Goodlett D. R., Ueba H., Kim F., Presnell S. R., Figeys D., Harrison D. G., Berk B. C., Aebersold R., Corson M. A. Identification of flow-dependent endothelial nitric-oxide synthase phosphorylation sites by mass spectrometry and regulation of phosphorylation and nitric oxide production by the phosphatidylinositol 3-kinase inhibitor LY294002. J. Biol. Chem. 1999;274:30101–30108. doi: 10.1074/jbc.274.42.30101. [DOI] [PubMed] [Google Scholar]
- 50.Raman C. S., Li H., Martasek P., Kral V., Masters B. S., Poulos T. L. Crystal structure of constitutive endothelial nitric oxide synthase: a paradigm for pterin function involving a novel metal center. Cell. 1998;95:939–950. doi: 10.1016/s0092-8674(00)81718-3. [DOI] [PubMed] [Google Scholar]
- 51.Erwin P. A., Mitchell D. A., Sartoretto J., Marletta M. A., Michel T. Subcellular targeting and differential S-nitrosylation of endothelial nitric-oxide synthase. J. Biol. Chem. 2006;281:151–157. doi: 10.1074/jbc.M510421200. [DOI] [PubMed] [Google Scholar]
- 52.Taldone F. S., Tummala M., Goldstein E. J., Ryzhov V., Ravi K., Black S. M. Studying the S-nitrosylation of model peptides and eNOS protein by mass spectrometry. Nitric Oxide. 2005;13:176–187. doi: 10.1016/j.niox.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 53.Stamler J. S., Toone E. J., Lipton S. A., Sucher N. J. (S)NO signals: translocation, regulation, and a consensus motif. Neuron. 1997;18:691–696. doi: 10.1016/s0896-6273(00)80310-4. [DOI] [PubMed] [Google Scholar]
- 54.Fischmann T. O., Hruza A., Niu X. D., Fossetta J. D., Lunn C. A., Dolphin E., Prongay A. J., Reichert P., Lundell D. J., Narula S. K., Weber P. C. Structural characterization of nitric oxide synthase isoforms reveals striking active-site conservation. Nat. Struct. Biol. 1999;6:233–242. doi: 10.1038/6675. [DOI] [PubMed] [Google Scholar]
- 55.Bird I. M., Sullivan J. A., Di T., Cale J. M., Zhang L., Zheng J., Magness R. R. Pregnancy-dependent changes in cell signaling underlie changes in differential control of vasodilator production in uterine artery endothelial cells. Endocrinology. 2000;141:1107–1117. doi: 10.1210/endo.141.3.7367. [DOI] [PubMed] [Google Scholar]
- 56.Di T., Sullivan J. A., Magness R. R., Zhang L., Bird I. M. Pregnancy-specific enhancement of agonist-stimulated ERK-1/2 signaling in uterine artery endothelial cells increases Ca2+ sensitivity of endothelial nitric oxide synthase as well as cytosolic phospholipase A2. Endocrinology. 2001;142:3014–3026. doi: 10.1210/endo.142.7.8278. [DOI] [PubMed] [Google Scholar]
- 57.Tsikas D. Methods of quantitative analysis of the nitric oxide metabolites nitrite and nitrate in human biological fluids. Free Radical Res. 2005;39:797–815. doi: 10.1080/10715760500053651. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.