Abstract
Matrix metalloproteinase (MMP)-mediated degradation of the extracellular matrix is a major factor for tumor development and expansion. This study analysed MMP-10 protein expression and activity in human lung tumors of various grade, stage, and type to address the relationship between MMP-10 and tumor characteristics and to evaluate MMP-10 as a therapeutic target in non small cell lung carcinoma (NSCLC). Unlike the majority of MMPs, MMP-10 was located in the tumor mass as opposed to tumor stroma. MMP-10 protein was observed at low levels in normal human lung tissues and at significantly higher levels in all types of NSCLC. No correlation was observed between MMP-10 protein expression and tumor type, stage, or lymph node invasion. To discriminate between active and inactive forms of MMP-10 in samples of human NSCLC, we have developed an ex vivo fluorescent assay. Measurable MMP-10 activity was detected in 42 of 50 specimens of lung cancer and only 2 of 10 specimens of histologically normal lung tissue. No relationship was observed between MMP-10 activity levels and clinicopathologic characteristics. Our results suggest that MMP-10 is expressed and active at high levels in human NSCLC compared to normal lung tissues, and, as such, is a potential target for the development of novel therapeutics for lung cancer treatment.
Keywords: Matrix metalloproteinase, MMP-10, non small cell lung carcinoma, tumor development, molecular target
Introduction
The term non small cell lung cancer (NSCLC) covers a group of different histopathologic characteristics. A number of features of NSCLC at presentation, namely, primary tumor size, regional lymph node involvement, presence of metastases, and tumor grade, have been shown to be related to survival. Early-stage NSCLC, without distant metastases or heavy regional nodal involvement, may be amenable to surgical resection. The role of adjuvant chemotherapy in this setting is becoming more established, but response to chemotherapy, whether adjuvant or palliative, is often poor and may relate to the pathologic heterogeneity of NSCLC among other factors. As a consequence, a better understanding of the molecular pathology of NSCLC, determination of novel tumor markers, and identification of targets for therapeutic exploitation are central to improving diagnosis and treatment.
In order that a tumor may grow, spread, and metastasise, it must possess the ability to breakdown and degrade both the extracellular matrix (ECM) and basement membranes, promote an angiogenic response, evade immune surveillance, and avoid elimination [1]. Numerous proteolytic enzymes have been implicated in these processes, most prominent among which are the matrix metalloproteinases (MMPs) [2–4]. The MMPs are a family of at least 24 structurally related zincdependent endopeptidases central to tumor invasion, metastasis, angiogenesis, and malignant cell proliferation [5–7]. Historically, the MMPs were classified into subgroups based on their specificity for ECM proteins (collagenases, gelatinases, matrilysins, stromelysins, and so on), which is reflected in their names [1,5–7]. However, as the number of MMPs identified and their respective substrates has grown, a sequential numbering system for their identity is now being adopted, and the MMPs are now grouped according to their structure [1]. Currently, the MMPs are classified into eight distinct groups, with MMP-10 now being classified into the simple hemopexin domain structural class [1].
All MMPs are produced as inactive zymogens (proMMPs), which must be subsequently activated by removal of the propeptide domain to generate catalytically active MMPs [1,4,5,8,9]. With the exception of membrane-type MMPs (MT-MMPs), this conversion of inactive proMMP to active MMP occurs extracellularly. Activation of MMPs is regulated at two levels within tissues: transcriptionally in response to cytokines and growth factors [10] and proteolytically at the level of proMMP activation [5]. Once secreted and activated, MMP activity is regulated in situ by endogenous inhibitors termed tissue inhibitors of metalloproteinase (TIMPs) [5]. Therefore, within tissues, the balance between proMMP, active MMP, and TIMPs determines the overall MMP activity [5].
Several studies have addressed the expression of the stromelysin subgroup of MMPs in NSCLC to ascertain their role in tumor development and progression, and as prognostic markers [11–13]. A strong expression of MMP-3 (stromelysin-1) and MMP-11 (stromelysin-3) is observed in the stromal compartment of NSCLC [11–13]. The observation that MMP-3 is expressed throughout the majority of these tumors suggested that it may be a central regulator of local growth of the neoplastic cell mass [11]. Conversely, MMP-11 expression is related to local lymph node tumor invasion [12,13]. In contrast to MMP-3 and MMP-11, the role of MMP-10 (stromelysin-2) in human NSCLC has not been extensively evaluated.
Although MMP-10 is closely related to MMP-3 with respect to structure and substrate specificity [14,15], differences in inducibility, activity, and cellular distribution exist, suggesting distinct roles for both MMP-3 and MMP-10 [8,16–19]. MMP-10 has a relatively broad substrate specificity, including proMMPs, ECM proteins, proteoglycans, glycoproteins, and collagens [1,14,15]. Unlike several other MMPs that are localised predominantly in tumor stroma [7,20], tumor cells themselves appear to express MMP-10 [8,19–21]. Overexpression of MMP-10 has been demonstrated in several human tumors of epithelial origin including head and neck, esophageal and oral squamous cell carcinoma (SCC), and squamous and basal cell carcinomas of the skin [19,20,22,23]. Recently, a small study (six cases) also demonstrated increased levels of MMP-10 mRNA in recurrences of human NSCLC following surgical resection (five of six cases) [24], indicating a potential role for MMP-10 in the development of human NSCLC. The expression profile of MMP-10 in several human tumors, coupled with broad substrate specificity [14,15] and expression in several stages of the tumorigenic process [20,22,23], suggests MMP-10 as a potential tumor marker and a predictor of clinical behavior, and supports MMP-10 inhibition as a valid therapeutic strategy.
The aim of this study was to determine the protein expression and activity of MMP-10 in NSCLC, the relationship between MMP-10 and clinicopathologic factors, and the potential for utilising MMP-10 activity as a therapeutic strategy for the treatment of human NSCLC.
Materials and Methods
Human Tissue Samples
A total of 66 formalin-fixed, paraffin-embedded NSCLC specimens and 50 freshly resected specimens of NSCLC was used for this study. In addition, the study also includes 11 histologically normal human lung tissue specimens, excised from patients with early-stage lung cancer but distant to the tumor mass. Table 1 shows the histologic and physical characteristics of the tumors analysed, all of which were obtained following surgical resection. A number of neuroendocrine carcinoma (six samples) and sarcomas (three samples) were diagnosed among the specimens, and, although not part of the NSCLC classification, are included for comparison. All experiments were performed after first obtaining consent from the local research and ethics committee according to Medical Research Council regulations. Prior informed consent was also obtained from patients for studies involving fresh tissues. Patient details were anonymised to ensure confidentiality.
Table 1.
Relationship between Immunohistochemical MMP-10 Expression and Clinicopathologic Features of Human NSCLC.
| Characteristic | All Samples (n = 84) | Number of Paraffin-Embedded Samples (n = 71) | Median MMP-10 Protein Expression (± Interquartile Range) | P | |
| Histologic type | |||||
| Normal tissue | 11 | 5 | 1.00 (0.67–1.00) | ||
| Adenocarcinoma | 35 | 31 | 2.00 (2.00–3.85) | ||
| SCC | 24 | 22 | 3.00 (2.00–4.00) | ||
| Large cell carcinoma | 2 | 2 | 2.38 (2.00–2.75) | ||
| Neuroendocrine | 6 | 6 | 2.00 (2.00–4.00) | ||
| Sarcoma | 3 | 2 | 3.50 (3.00–4.00) | ||
| Unclassified carcinoma | 3 | 3 | 2.00 (1.50–2.50) | ||
| Differentiation status | |||||
| Normal tissue | 11 | 5 | 1.00 (0.67–1.00) | ||
| Poor | 24 | 21 | 3.00 (2.00–4.00) | ||
| Moderate | 20 | 17 | 2.00 (2.00–3.35) | ||
| Well | 2 | 2 | 3.25 (3.00–3.50) | ||
| Not stated | 27 | 26 | 2.00 (2.00–3.75) | ||
| Tumor stage* | |||||
| Normal tissue | 11 | 5 | 1.00 (0.67–1.00) | ||
| pT1 | 6 | 5 | 3.00 (3.00–3.50) |  |
.18 |
| pT2 | 44 | 33 | 2.00 (2.00–3.25) | ||
| pT3 | 5 | 5 | 1.00 (0.75–2.50) | ||
| Not stated | 18 | 12 | 4.00 (3.00–4.00) | ND | |
| Lymph node status* | |||||
| Normal tissue | 11 | 5 | 1.00 (0.67–1.00) | ||
| pN0 | 26 | 21 | 3.00 (2.00–3.50) |  |
.93 |
| pN1 | 17 | 14 | 2.63 (2.00–3.70) | ||
| pN2 | 13 | 11 | 2.88 (2.00–4.00) | ||
| pNx | 17 | 9 | 3.0 (2.00–3.88) | ND | |
Values represent median expression level of MMP-10 (± interquartile range). Statistical analyses were undertaken to assess differences between pathologic factors in relation to MMP-10 expression. Values of P ≤ .05 were considered significant.
ND = Not included in statistical analyses.
Neuroendocrine tumors, unclassified carcinomas, and sarcomas not included in these analyses.
Human Tumor Xenografts
Human tumor cell lines were xenografted in mice under a project license issued by the UK Home Office, following UKCCCR guidelines. Female mice (nu/nu from an inbred colony; B&K Universal, Hull, UK) 6 to 8 weeks old were implanted subcutaneously with 2 to 3 mm3 fragments of H460 (non small cell lung carcinoma, or NSCLC), COLO 205 (colon adenocarcinoma), or PC3 (prostate adenocarcinoma) tumors. Resultant tumors were removed, and either snap-frozen in liquid nitrogen or formalin-fixed and embedded in paraffin wax.
Immunohistochemistry
Localisation of MMP-10 was assessed by immunohistochemistry in 66 formalin-fixed, paraffin-embedded tissue blocks of primary lung tumors and five blocks of histologically normal lung tissue and the H460 human NSCLC tumor xenograft. Briefly, sections were dewaxed in xylene and rehydrated to water through graded alcohols. Heat-mediated antigen retrieval was performed by microwaving the slides for 20 minutes in citric acid buffer (0.01 M, pH 6.0). Endogenous peroxidase activity was quenched with freshly prepared 1% hydrogen peroxide for 30 minutes at room temperature. Nonspecific antibody binding was inhibited using 5% normal horse serum for 20 minutes at room temperature. Sections were then incubated for 90 minutes at room temperature in a humidified atmosphere with the primary monoclonal antibody raised against amino acids 342 to 476 of human MMP-10 (Clone 5E4; Novocastra Laboratories, Newcastle, UK) diluted 1:50 in PBS. This antibody was previously shown in our laboratory to be specific for MMP-10, detecting both active and pro forms of the enzyme by Western blot analysis. Negative staining controls were performed using normal mouse IgG (Dako, Ely, UK) in place of the primary antibody. After washing in PBS, sections were incubated for 30 minutes at room temperature with an anti-mouse biotinylated secondary antibody (Vector Laboratories, Peterborough, UK), diluted 1:200 in PBS, washed in PBS, and followed by amplification and detection using a Vectastain ABC kit according to the manufacturer's instructions (Vector Laboratories). Immunocomplex visualisation was performed using 3,3′ -diaminobenzidine (DAB) according to the manufacturer's instructions (Vector Laboratories). Sections were then counterstained with Harris' hematoxylin and mounted in DPX mountant (Sigma, UK).
Semiquantitative Analysis of Immunohistochemistry
Positive immunostaining was scored semiquantitatively by two independent observers. The epithelial compartment of each tumor section was assigned a score for intensity and distribution of staining on a scale of 0 (no staining) to 4 (intense staining throughout section). A note of any specific staining pattern was recorded for each section. Histologically normal human lung sections were analysed in the same manner and assigned a score. The results were compared for any relationships to clinicopatholigic parameters.
Isolation and Immunocapture of MMP-10
For analysis of the levels of active enzyme, MMP-10 protein was isolated from freshly resected clinical samples of human NSCLCs (50 samples), histologically normal lung tissues (10 samples), and human tumor xenografts (H460, COLO 205, and PC3). All samples were immediately snapfrozen in liquid nitrogen following excision and stored at -80°C until required. For MMP-10 isolation, each specimen was weighed and homogenised in 4 vol (wt/vol) of homogenisation buffer (20 mM Tris-HCl, pH 7.5, 125 mM NaCl, and 0.01% Triton X-100) using a dounce homogeniser on ice. Cell debris was then removed by centrifugation at 9000g for 15 minutes at 4°C. To 200 µl of supernatant (representing 40 mg of tissue), 3 µg of primary anti-MMP-10 antibody (rabbit polyclonal; NeoMarkers, Fremont, CA) was added and incubated overnight at 4°C with constant agitation. Fifty microliters of anti-rabbit secondary antibody conjugated to magnetic beads (sheep anti-rabbit IgG magnetic beads; Dynal, UK) was added to the suspension and the complexincubated at 4°C for 30 minutes. Immunocomplexed MMP-10 was isolated by applying a magnetic field to the tube to attract the magnetic beads. The isolated beads were washed five times in PBS to remove any unbound material. Once cleaned, the MMP-10 complex was resuspended in 100 µl of assay buffer (50 mM Tris-HCl, pH 7.5, 10 mM CaCl2, 150 mM NaCl, and 0.05% Brij 35) and either analysed immediately or stored at -20°C for later use.
Validation of the immunocaptured protein as being MMP-10 was performed using sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot analysis of xenograft immunoprecipitates, a panel of clinical samples, and recombinant MMP-10 protein (R&D Systems, Abingdon, UK). Briefly, 20 µl of immunocaptured protein solution was separated on SDS-PAGE gels. The gel was either stained using Coomassie Blue solution (Sigma) to identify isolated protein bands, or transferred to polyvinylidenedifluoride (PVDF) for Western blot analysis. For Western blot analysis, nonspecific protein binding to the membranes was blocked using 2% enhanced chemiluminescence (ECL) advanced blocking reagent (Amersham, Bucks, UK) in PBS. The blots were then incubated with anti-MMP-10 monoclonal antibody (5E4) diluted 1:1000 in PBS overnight followed by HRP-conjugated rabbit anti-mouse secondary antibody (Dako) diluted 1:2000 in PBS for 1 hour. Immunoreactive bands were visualised using an ECL detection kit according to the manufacturer's instructions. Stained gels and chemiluminescence were detected using the Bio-Rad FX imaging system.
Assay of MMP-10 Activity
MMP-10 activity was defined as the ability of the isolated fraction to hydrolyse the fluorescent MMP-10 substrate, Pro-Lys-Pro-Val-Glu-nVal-Trp-Arg-Lys(Dnp)-NH2 (R&D Systems). The assay was performed in a 96-well plate by incubating 100 µl of tumor immunoprecipitate with 20 µl of substrate (final concentration, 100 µM), for a total volume of 120 µl. Fluorescence was measured (excitation = 320 nm; emission = 405 nm) following a 240-minute incubation using a Cary Eclipse fluorescence spectrophotometer (Varian, Walton-on-Thames, UK). Continuous monitoring showed this to be the linear part of the enzymatic curve. A negative reaction blank by omission of tumor homogenate was included in all plates. For each sample, the relative activity was calculated as the fluorescence obtained from the sample minus that obtained in the reaction blank following the 240-minute incubation. Activity was expressed as change in fluorescence per hour per gram of tissue. The reproducibility and reliability of the assay were determined using samples obtained from three independent H460 preparations.
Because MMP-10 can exist in both pro and active forms, the total potential MMP-10 activity was calculated from samples demonstrating a range of MMP-10 protein expression as measured by immunohistochemistry. Analysis was performed on five NSCLC tumor samples (three adenocarcinomas, two SCCs; all pT2 N0) and four histologically normal tissues. Tumor immunoprecipitates were preincubated with 1 mM APMA (amino-phenylmercuric acetate) for 2 hours at 37°C to activate all pro-MMP-10 to active MMP-10. Activity of MMP-10 was then measured as previously described.
Statistical Analyses
Statistical analysis was undertaken using the SPSS software package, version 11.0 (SPSS Inc., Chicago, IL). In the immunohistochemical study, because MMP-10 expression is not normally distributed, the average expression values for each category were reported as medians with interquartile ranges. Differences between two independent groups were determined by the Mann-Whitney U test, and the significance of differences between more than two groups was determined by Kruskal-Wallis one-way analysis. The threshold for statistical significance was assigned at P = .05.
Results
Expression of MMP-10 in NSCLC
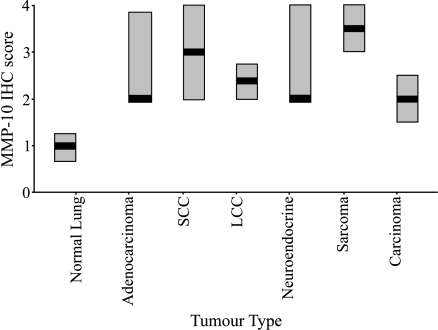
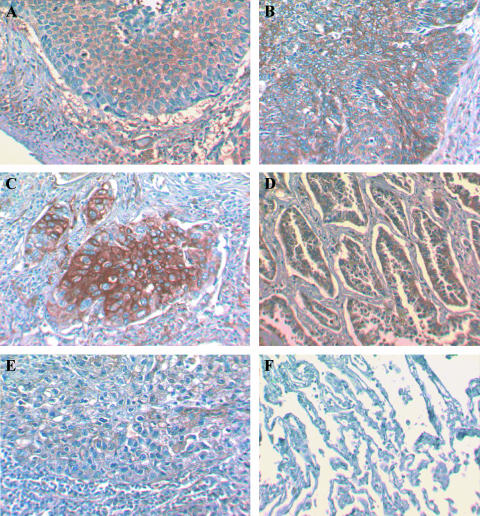
The immunostaining analysis of MMP-10 protein in human NSCLC (66 cases; summarised in Table 1) of various histologies and histologically normal lung tissues (five cases) is summarised in Figure 1. The majority of histologically normal lung tissue sections analysed showed very low MMP-10 expression (median = 1.00; Figure 1). In contrast, MMP-10 was expressed at much higher levels in all histologic type of NSCLC (Figure 1). The levels of MMP-10 were found to be statistically higher in NSCLC than in histologically normal lung tissue ( P = .01). Analysis in terms of histologic tumor type showed that there was no significant difference of MMP-10 expression according to histologic type of tumor. Similarly, no statistical differences were observed in MMP-10 expression between the types of carcinoma analysed, adenocarcinoma, SCC, and large cell carcinoma (LCC). Figure 2 shows representative images of MMP-10 expression in the various types of NSCLC and normal lung tissues.
Figure 1.
Immunohistochemical expression of MMP-10 protein in human lung cancers and histologically normal lung tissues. In addition to samples of NSCLC (adenocarcinoma, SCC, LCC, carcinoma), MMP-10 expression was also analysed in both tumors of neuroendocrine origin and sarcomas as a comparison. On the boxchart, thick horizontal lines through the boxes represent median expression and the boxes represent the interquartile range. SCC = squamous cell carcinoma; LCC = large cell carcinoma; carcinoma = unclassified carcinoma.
Figure 2.
Immunohistochemical results of MMP-10 protein expression in NSCLC (A–E) and histologically normal lung tissues (F). MMP-10 was expressed predominantly in the tumor mass in all types of NSCLC; nonkeratinising (A) and keratinising (B) squamous lung carcinoma, poorly differentiated (C) and welldifferentiated (D) adenocarcinoma, and large cell carcinoma (E). No immunoreactivity for MMP-10 is observed in a histologically normal lung tissue specimen (F).
Expression of MMP-10 in all the tumors analysed herein was primarily in the tumor mass rather than the surrounding tumor stroma (Figure 2). Occasionally, expression could be observed within the stromal region of the tumor, but in these cases, staining appeared to be both weak and extracellular, possibly relating to secreted active MMP-10.
Although a high expression of MMP-10 was observed in NSCLC, no correlation was observed with evidence of lymph node metastasis (P = 0.93; Table 1). Expression of MMP-10 appeared to show a negative correlation with increasing pathologic tumor stage (pT1-pT2-pT3), but this was not found to be statistically significant (P = 0.18; Table 1).
Presence of Active MMP-10 in NSCLC
Because MMP-10 can exist as both an inactive proenzyme and an active enzyme, we assessed the presence of active MMP-10 in NSCLC (50 samples) of various histologies (Table 1) and histologically normal lung tissues (10 cases). Such analyses could not be achieved immunohistochemically due to the inability of this technique to discriminate between the active and inactive forms of the enzyme; therefore, an assay of function was required. MMP-10 activity was defined as the ability of MMP-10 protein immunoprecipitated from tumor lysates to cleave a fluorescently labeled substrate. Activity was quantified as the fluorescence detected after the incubation of the substrate with MMP-10 protein for 240 minutes and was expressed as change in fluorescence per hour per gram of tissue.
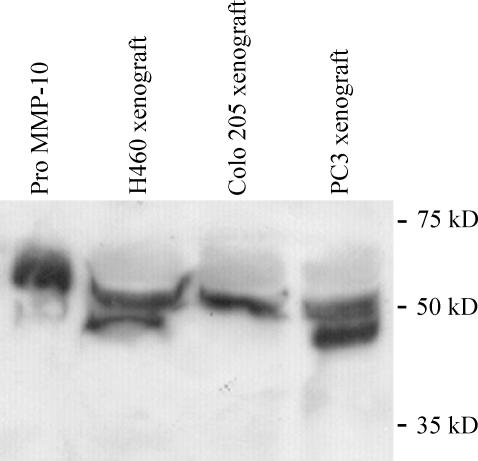
Validation of the assay was performed using human tumor xenograft tissues and recombinant MMP-10 proteins. Immunoprecipitated protein separated by SDS-PAGE electrophoresis demonstrated protein bands corresponding with the size of pro- and active MMP-10 (recombinant) and IgG (antibody) (data not shown). Western blot analysis confirmed the identity of these protein bands as MMP-10 (Figure 3). Although bands corresponding to pro-MMP-10 were detected in all lanes, active MMP-10 protein was detected only in the H460 (lung) and PC3 (prostate) xenograft lysates (Figure 3), in agreement with the high levels of enzyme activity detected in these xenografts. Reproducibility of the activity assay was confirmed using independent H460 lysate preparations (n = 3), with a mean activity of 221.3 ± 10.3 fluorescence units/hr per gram of tissue.
Figure 3.
Specificity of immunocapture technique for MMP-10. Western blot analyses were undertaken of proteins isolated from both human tumor xenograft lysates and recombinant MMP-10 protein. Pro-MMP-10 is present in all lanes and active MMP-10 is present in only H460 and PC3 lysates, in agreement with activity assays. Colo205 = human colon adenocarcinoma xenograft; H460 = human non small cell lung carcinoma xenograft; PC3 = prostate adenocarcinoma xenografts.
Measurable MMP-10 activity was detected in 42 of 50 specimens of lung cancer (median = 2.90 fluorescence units). No relationship was observed between histologic type of tumor and levels of active MMP-10 (Table 2). In contrast, measurable MMP-10 activity was detected in 2 of 10 specimens of histologically normal lung tissues (median activity = 0.0; Table 2). Although the majority of normal lung tissues examined demonstrated low or negative protein expression (Figure 1) and activity (Table 2) of MMP-10, one sample showed an activity higher than the rest (31.2 fluorescence units/hr per gram of tissue). This sample was obtained from the same patient as the SCC, demonstrating the highest activity (265 fluorescence units/hr per gram of tissue). Although histologically normal, the enzyme activity in this sample is likely to be as a consequence of the adjacent highly active tumor.
Table 2.
Relationship between Activity Levels of MMP-10 and Clinicopathologic Features of Human NSCLC.
| Pathologic Parameter | Number of Cases (n = 59) | Median MMP-10 Activity [Δfluorescence/hr Per Gram of Tissue] (Range of Activity) | P |
| Histologic type | |||
| Normal lung | 10 | 0 (0–31.2) | |
| Adenocarcinoma | 29 | 10.0 (0.0–216.3) | |
| SCC | 13 | 24.4 (0.0–265.0) | |
| Neuroendocrine | 5 | 16.9 (1.9–28.1) | |
| Sarcoma | 2 | 78.4 (50.6–106.3) | |
| Tumor stage* | |||
| pT1 | 4 | 40.0 (6.25–121.88) | |
| pT2 | 36 | 15.3 (0.0–265.0) | .55 |
| pT3 | 2 | 12.2 (10.0–14.4) | |
| Lymph node status* | |||
| pN0 | 17 | 20.6 (0.0–200.6) | |
| pN1 | 10 | 10.3 (0.0–265.0) | .46 |
| pN2 | 8 | 27.2 (0.0–123.1) | |
| pNX | 8 | 59.7 (0.0–216.3) | ND |
Values are expressed as the median proteolytic activity (and range) in the clinical samples. Activity is expressed as change in fluorescence units per hour per gram of tissue. Statistical analyses were undertaken to assess differences in MMP-10 activity between clinicopathologic features. Values of P ≤ .05 were considered significant.
ND = Not done.
Neuroendocrine tumors and sarcomas were not included in these analyses.
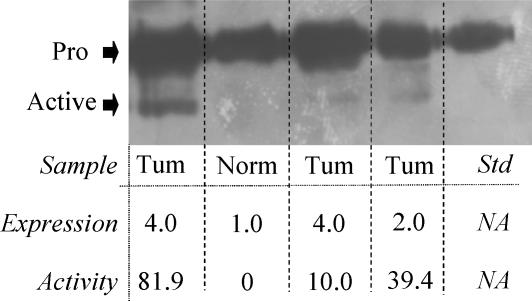
Verification of a relationship between measured fluorescent cleavage activity and the appearance of active MMP-10 in the NSCLC tumor samples was undertaken using a representative panel of human clinical samples. Protein bands corresponding to both pro and active MMP-10 were detected by Western blot analysis in the clinical samples (Figure 4). Protein expression levels, as measured by immunohistochemistry, correlated with the amount of pro- MMP-10 detected by Western blot analysis (Figure 4). The presence and levels of active MMP-10 detected by Western blot analysis also corresponded with the proteolytic activity detected in the various samples (Figure 4). This further supports the measured proteolytic activity as being representative of MMP-10 activity.
Figure 4.
Relationship between the presence of pro- and active MMP-10, immunohistochemical analyses, and proteolytic activity in human clinical samples. Pro-MMP-10 is present in all lanes and corresponds to the expression levels of pro-MMP-10 detected by immunohistochemistry. Proteolytic activity of the immunocaptured protein, as measured by change in fluorescence per hour per gram of tissue, relates well to the presence of active MMP-10. Tum = NSCLC specimen; Norm = histologically normal lung tissue; pro = pro -MMP-10; active = active MMP-10.
In agreement with the immunohistochemical analysis, no relationship was observed between MMP-10 activity and either the tumor stage (pT status) or presence of local invasion to the lymph node (pN status) (Table 2). To measure total potential MMP-10 activity, selected samples were incubated with APMA to activate pro-MMP-10 to active MMP-10. No significant increase in MMP-10 activity was observed in those samples exhibiting low MMP-10 expression by immunohistochemistry (Table 3). Conversely, samples expressing high levels of MMP-10 by immunohistochemistry demonstrated a higher level of potential MMP-10 activity (Table 3). In the histologically normal tissues examined, only one sample demonstrated a significant increase in activity following APMA incubation. In contrast, total levels of MMP-10 activity were significantly higher in the tumor samples analysed.
Table 3.
Measurement of Total Potential MMP-10 Activity and Relationship to Immunohistochemical MMP-10 Expression.
| Tumor Type | MMP-10 Protein Expression | MMP-10 Activity Prior to APMA Incubation [Δfluorescence/hr Per Gram of Tissue] | MMP-10 activity After APMA Incubation (Potential Activity) [Δfluorescence/hr Per Gram of Tissue] |
| Normal lung | 0.7 | 0 | 0 |
| Normal lung | 1.0 | 0 | 0 |
| Normal lung | 1.3 | 0 | 0.6 |
| Normal lung | 3.0 | 0 | 7.6 |
| Adenocarcinoma | 1.2 | 0 | 0 |
| Adenocarcinoma | 2.0 | 20.6 | 42.4 |
| SCC | 2.0 | 24.4 | 36.3 |
| Adenocarcinoma | 3.25 | 60.6 | 76.6 |
| SCC | 3.0 | 121.3 | 121.3 |
APMA activation was used to convert inactive pro-MMP-10 into its active form, allowing the measurement of the total potential MMP-10 activity of the sample. The ability of the sample to cleave a specific fluorogenic substrate was used to assess the levels of active MMP-10 and total potential MMP-10 activity in each sample. Values are expressed as the change in fluorescence units per hour per gram of tissue.
Discussion
Degradation of the ECM is essential for growth, vascularisation, spread, and invasion of tumors. Although many enzymes are known to be involved, the MMPs are believed to be the central mediators of this process. Within tumors, MMPs can exist in two forms: as an inactive proenzyme or as a proteolytically activated form. To address whether MMP-10 was a suitable target for therapeutic intervention, expression and activity of MMP-10 were assessed in NSCLCs and compared to that of histologically normal lung tissues.
Overexpression of MMP-10 has been shown in several malignancies including head and neck carcinoma, oral carcinoma, esophageal carcinoma, skin cancer [19–21,23,24] and, now, in several histologic types of human lung cancer. The expression of MMP-10 protein in human NSCLC, its relationship to tumor characteristics, and the presence of active MMP-10 have not been previously reported. In this study, MMP-10 expression and activity were significantly higher in all histologic types of NSCLC compared to histologically normal lung tissues (Figure 1). In contrast to several other MMPs [15], MMP-10 protein was predominantly expressed in the tumor mass rather than the surrounding stromal fibroblasts (Figure 2), an observation previously reported in other tumor types [19,20]. Although the antibodies used in this study had previously been shown to detect both pro and active MMP-10, the cytoplasmic distribution of MMP-10 observed in the archival wax embedded samples is likely to reflect only inactive pro-MMP-10 and not secreted active MMP-10, as suggested by Western blot analysis (Figure 4). This theory is supported by the low levels of extracellular MMP-10 protein detected by immunohistochemistry in this study. Furthermore, even though both MMP-10 expression and activity were increased in NSCLC over normal lungs, in the several cases where both fresh and wax-embedded tissue were available, only a weak correlation was observed between MMP-10 expression and activity (data not shown). In the samples treated with APMA, in which pro-MMP-10 is converted to active MMP-10, samples demonstrating a higher expression of MMP-10 showed a greater ability to cleave the fluorogenic substrate. In contrast, samples demonstrating absent or low expression of MMP-10 showed no significant increase in fluorescence. This result further confirmed the clear differential in MMP-10 activity between histologically normal lungs and NSCLC, and supported the presence of both pro and active MMP-10 in NSCLC samples. These observations highlight the caution that should be employed when attempting to relate mRNA or immunohistochemical expression studies of MMP to clinical features or function without prior consideration of proteolytic MMP activity.
Conversely to the majority of MMPs that show a relationship to tumor invasion or tumor stage, we observed no such relationship between either MMP-10 expression or activity, and either tumor histology, pT status, or pN status in NSCLC. This observation is in agreement with previous studies of cutaneous and oral SCC in which MMP-10 was not associated with malignant cell invasion [19,20]. Recently, upregulated MMP-10 expression was suggested to relate to the recurrence of stage IB (pT2, pN0, and pM0) lung cancer [24]. Although this conclusion was based on only a small sample population, no such analysis could be undertaken in our study due to the management of recurrent tumors by chemotherapy as opposed to surgical resection. Taken together, our data, demonstrating a lack of a correlation between MMP-10 and either pT or pN status and that of others, suggests that MMP-10 is involved in remodelling of the ECM associated with the growth and expansion of the neoplastic cell mass, rather than with either invasion to other sites or progression to metastatic disease in several tumor types [19,20], including NSCLC [24].
During the course of reepithelialisation of cutaneous wounds, MMP-10 protein is expressed by migrating keratinocytes and is central to successful wound healing [25]. In this situation, MMP-10 expression is induced by cytokines rather than cell contact with the dermal matrix. Interestingly, those cells shown to express MMP-10 in the wound were also shown to synthesize laminin-5 [25], an ECM protein central to cell migration and wound closure following tissue injury [26]. Importantly for this study, laminin-5 overexpression has been observed at the invasive tumor-stromal interface of several different tumor types, including NSCLC, implicating a role for laminin-5 in tumor growth and stromal invasion [19,26–28]. In epithelial skin cancers, MMP-10 expression is only observed in laminin-5-positive tumor cells [20], an observation in agreement with wound healing studies [25] and indicative of a close relationship between these two proteins. Although coexpression of these proteins is often observed, cleavage of laminin-5 by MMP-10 and the interplay between the proteins have not yet been shown in vivo [20,25]. With reference to this study, laminin-5 is frequently overexpressed in lung adenocarcinomas and is associated with vascular invasion but not with nodal involvement, lymphatic invasion, pleural invasion, or pathologic tumor stage [26]—a profile similar to that observed with MMP-10 in our study. In lung adenocarcinomas, laminin-5 overexpression is associated with disease recurrence and a poor prognosis [26]. Based on these studies, laminin-5 overexpression was suggested to occur at a relatively early stage of lung tumor formation and shows no relationship to lymphatic invasion or metastases [26]—a theory supportive of our observations with MMP-10. Therefore, if MMP-10 is an early event in lung tumor formation, is involved in tumor growth rather than invasion, and associates with a poor prognosis, targeting of MMP-10 using either specific inhibitors or targeted therapeutics may be a valuable approach for the treatment of NSCLC. Additional studies are necessary to address these questions.
The observation that MMPs themselves can activate one another suggests a hierarchy among the MMP family and implies the existence of an MMP activation cascade [8,29]. A central role for MMP-10 in this process is demonstrated by its ability to cleave several proMMPs including MMP-1, MMP-7, MMP-8, MMP-9, and MMP-13 [8,29]. Taken together, the localisation of MMP-10 to the tumor mass and the lack of correlation to tumor clinicopathologic factors, this broad activity suggests an upstream and potential “initiator” role for MMP-10 in the MMP cascade. This hypothesis is further supported by proteolytic activation of MMP-10 by urokinase plasminogen activator (uPA) coupled with an apparent lack of evidence supporting MMP-10 activation by other MMPs [30,31]. Taken together with the observation that MMP-10 is expressed and active in the majority of NSCLC samples analysed, this further supports MMP-10 as a potential target for therapeutic intervention.
Over the last few years, the identification of MMPs as being tumor-specific and central to tumor development and spread resulted in the development of MMP inhibitors as anticancer therapeutics. Until recently, clinical trials using these inhibitors have been disappointing, with very little success [32]. One of the main problems attributed to the failure of these compounds was their broad-spectrum inhibition of MMPs and subsequent activity against normal cellular processes [32]. In addition, these drugs were tested in late-stage cancer trials and so would bypass MMPs involved in the early stages of cancer, such as MMP-10 [32]. Therefore, it is reasonable to postulate that inhibitors with specificity against specific MMPs, such as MMP-10, may be a better approach for the future. Recently, new classes of MMP mediated therapeutics have been suggested, including gene-targeted strategies and MMP-activated cytotoxics, which offer alternative approaches for anticancer drug development. A greater understanding of MMP expression, their activity profiles, and interrelationships between one another is required to design more specific, viable, and potent MMP-mediated therapies for the treatment of human tumors, including NSCLC [32,33].
In conclusion, this is the first study showing the expression and activity of MMP-10 in human NSCLC. Unlike the majority of MMPs, MMP-10 does not associate with tumor invasion but instead may play a role in the growth and development of the tumor mass in NSCLC. Further evaluation of MMP-10 activity is required to address the proteolytic levels of MMP-10 in tumor tissue, quantify MMP-10 levels, establish any relationship between MMP-10 and prognosis, and establish the viability of MMP-10 as a target for therapeutic intervention in human NSCLC.
Acknowledgements
The authors thank B. Cronin and S. Shnyder for technical help with this study and members of the Tom Connors Cancer Research Centre for critical reading of the manuscript.
Abbreviations
- NSCLC
non small cell lung carcinoma
- MMP
matrix metalloproteinase
Footnotes
This work was supported by Cancer Research UK grant C459/A2579.
References
- 1.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 2.Duffy MJ, Duggan C, Maguire T, Mulcahy K, Elvin P, McDermott E, Fennelly JJ, O'Higgins N. Urokinase plasminogen activator as a predictor of aggressive disease in breast cancer. Enzyme Protein. 1996;49:85–93. doi: 10.1159/000468618. [DOI] [PubMed] [Google Scholar]
- 3.Duffy MJ, Duggan C, Mulcahy HE, McDermott EW, O'Higgins NJ. Urokinase plasminogen activator: a prognostic marker in breast cancer including patients with axillary node-negative disease. Clin Chem. 1998;44:1177–1183. [PubMed] [Google Scholar]
- 4.Puente XS, Sanchez LM, Overall CM, Lopez-Otin C. Human and mouse proteases: a comparative genomic approach. Nat Rev Genet. 2003;4:544–558. doi: 10.1038/nrg1111. [DOI] [PubMed] [Google Scholar]
- 5.Stamenkovic I. Extracellular matrix remodelling: the role of matrix metalloproteinases. J Pathol. 2003;200:448–464. doi: 10.1002/path.1400. [DOI] [PubMed] [Google Scholar]
- 6.Overall CM. Molecular determinants of metalloproteinase substrate specificity: matrix metalloproteinase substrate binding domains, modules, and exosites. Mol Biotechnol. 2002;22:51–86. doi: 10.1385/MB:22:1:051. [DOI] [PubMed] [Google Scholar]
- 7.Foda HD, Zucker S. Matrix metalloproteinases in cancer invasion, metastasis and angiogenesis. Drug Discov Today. 2001;6:478–482. doi: 10.1016/s1359-6446(01)01752-4. [DOI] [PubMed] [Google Scholar]
- 8.Nakamura H, Fujii Y, Ohuchi E, Yamamoto E, Okada Y. Activation of the precursor of human stromelysin 2 and its interactions with other matrix metalloproteinases. Eur J Biochem. 1998;253:67–75. doi: 10.1046/j.1432-1327.1998.2530067.x. [DOI] [PubMed] [Google Scholar]
- 9.Murphy G, Stanton H, Cowell S, Butler G, Knauper V, Atkinson S, Gavrilovic J. Mechanisms for pro matrix metalloproteinase activation. APMIS. 1999;107:38–44. doi: 10.1111/j.1699-0463.1999.tb01524.x. [DOI] [PubMed] [Google Scholar]
- 10.Stamenkovic I. Matrix metalloproteinases in tumor invasion and metastasis. Semin Cancer Biol. 2000;10:415–433. doi: 10.1006/scbi.2000.0379. [DOI] [PubMed] [Google Scholar]
- 11.Bodey B, Bodey B, Jr, Groger AM, Siegel SE, Kaiser HE. Invasion and metastasis: the expression and significance of matrix metalloproteinases in carcinomas of the lung. In Vivo. 2001;15:175–180. [PubMed] [Google Scholar]
- 12.Delebecq TJ, Porte H, Zerimech F, Copin MC, Gouyer V, Dacquembronne E, Balduyck M, Wurtz A, Huet G. Overexpression level of stromelysin 3 is related to the lymph node involvement in non-small cell lung cancer. Clin Cancer Res. 2000;6:1086–1092. [PubMed] [Google Scholar]
- 13.Karameris A, Panagou P, Tsilalis T, Bouros D. Association of expression of metalloproteinases and their inhibitors with the metastatic potential of squamous-cell lung carcinomas. A molecular and immunohistochemical study. Am J Respir Crit Care Med. 1997;156:1930–1936. doi: 10.1164/ajrccm.156.6.9612046. [DOI] [PubMed] [Google Scholar]
- 14.Duffy MJ, Maguire TM, Hill A, McDermott E, O'Higgins N. Metalloproteinases: role in breast carcinogenesis, invasion and metastasis. Breast Cancer Res. 2000;2:252–257. doi: 10.1186/bcr65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vihinen P, Kahari VM. Matrix metalloproteinases in cancer: prognostic markers and therapeutic targets. Int J Cancer. 2002;99:157–166. doi: 10.1002/ijc.10329. [DOI] [PubMed] [Google Scholar]
- 16.Saghizadeh M, Brown DJ, Castellon R, Chwa M, Huang GH, Ljubimova JY, Rosenberg S, Spirin KS, Stolitenko RB, Adachi W, Kinoshita S, Murphy G, Windsor LJ, Kenney MC, Ljubimov AV. Overexpression of matrix metalloproteinase-10 and matrix metalloproteinase-3 in human diabetic corneas: a possible mechanism of basement membrane and integrin alterations. Am J Pathol. 2001;158:723–734. doi: 10.1016/S0002-9440(10)64015-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bord S, Horner A, Hembry RM, Compston JE. Stromelysin-1 (MMP-3) and stromelysin-2 (MMP-10) expression in developing human bone: potential roles in skeletal development. Bone. 1998;23:7–12. doi: 10.1016/s8756-3282(98)00064-7. [DOI] [PubMed] [Google Scholar]
- 18.Saarialho-Kere UK, Pentland AP, Birkedal-Hansen H, Parks WC, Welgus HG. Distinct populations of basal keratinocytes express stromelysin-1 and stromelysin-2 in chronic wounds. J Clin Invest. 1994;94:79–88. doi: 10.1172/JCI117351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Impola U, Uitto VJ, Hietanen J, Hakkinen L, Zhang L, Larjava H, Isaka K, Saarialho-Kere U. Differential expression of matrilysin-1 (MMP-7), 92 kD gelatinase (MMP-9), and metalloelastase (MMP-12) in oral verrucous and squamous cell cancer. J Pathol. 2004;202:14–22. doi: 10.1002/path.1479. [DOI] [PubMed] [Google Scholar]
- 20.Kerkela E, Ala-aho R, Lohi J, Grenman R, V MK, Saarialho-Kere U. Differential patterns of stromelysin-2 (MMP-10) and MT1-MMP (MMP-14) expression in epithelial skin cancers. Br J Cancer. 2001;84:659–669. doi: 10.1054/bjoc.2000.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muller D, Breathnach R, Engelmann A, Millon R, Bronner G, Flesch H, Dumont P, Eber M, Abecassis J. Expression of collagenase-related metalloproteinase genes in human lung or head and neck tumours. Int J Cancer. 1991;48:550–556. doi: 10.1002/ijc.2910480412. [DOI] [PubMed] [Google Scholar]
- 22.O-Charoenrat P, Rhys-Evans PH, Eccles SA. Expression of matrix metalloproteinases and their inhibitors correlates with invasion and metastasis in squamous cell carcinoma of the head and neck. Arch Otolaryngol Head Neck Surg. 2001;127:813–820. [PubMed] [Google Scholar]
- 23.Mathew R, Khanna R, Kumar R, Mathur M, Shukla NK, Ralhan R. Stromelysin-2 overexpression in human esophageal squamous cell carcinoma: potential clinical implications. Cancer Detect Prev. 2002;26:222–228. doi: 10.1016/s0361-090x(02)00035-1. [DOI] [PubMed] [Google Scholar]
- 24.Cho NH, Hong KP, Hong SH, Kang S, Chung KY, Cho SH. MMP expression profiling in recurred stage IB lung cancer. Oncogene. 2003;23:845–851. doi: 10.1038/sj.onc.1207140. [DOI] [PubMed] [Google Scholar]
- 25.Rechardt O, Elomaa O, Vaalamo M, Paakkonen K, Jahkola T, Hook-Nikanne J, Hembry RM, Hakkinen L, Kere J, Saarialho-Kere U. Stromelysin-2 is upregulated during normal wound repair and is induced by cytokines. J Invest Dermatol. 2000;115:778–787. doi: 10.1046/j.1523-1747.2000.00135.x. [DOI] [PubMed] [Google Scholar]
- 26.Moriya Y, Niki T, Yamada T, Matsuno Y, Kondo H, Hirohashi S. Increased expression of laminin-5 and its prognostic significance in lung adenocarcinomas of small size. An immunohistochemical analysis of 102 cases. Cancer. 2001;91:1129–1141. doi: 10.1002/1097-0142(20010315)91:6<1129::aid-cncr1109>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 27.Maatta M, Soini Y, Paakko P, Salo S, Tryggvason K, Autio-Harmainen H. Expression of the laminin gamma2 chain in different histological types of lung carcinoma. A study by immunohistochemistry and in situ hybridization. J Pathol. 1999;188:361–368. doi: 10.1002/(SICI)1096-9896(199908)188:4<361::AID-PATH363>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 28.Giannelli G, Antonaci S. Biological and clinical relevance of Laminin-5 in cancer. Clin Exp Metastasis. 2000;18:439–443. doi: 10.1023/a:1011879900554. [DOI] [PubMed] [Google Scholar]
- 29.Ramos-DeSimone N, Hahn-Dantona E, Sipley J, Nagase H, French DL, Quigley JP. Activation of matrix metalloproteinase-9 (MMP-9) via a converging plasmin/stromelysin-1 cascade enhances tumor cell invasion. J Biol Chem. 1999;274:13066–13076. doi: 10.1074/jbc.274.19.13066. [DOI] [PubMed] [Google Scholar]
- 30.Vaalamo M, Weckroth M, Puolakkainen P, Kere J, Saarinen P, Lauharanta J, Saarialho-Kere UK. Patterns of matrix metalloproteinase and TIMP-1 expression in chronic and normally healing human cutaneous wounds. Br J Dermatol. 1996;135:52–59. [PubMed] [Google Scholar]
- 31.Nagase H. Human stromelysins 1 and 2. Methods Enzymol. 1995;248:449–470. doi: 10.1016/0076-6879(95)48029-3. [DOI] [PubMed] [Google Scholar]
- 32.Overall CM, Lopez-Otin C. Strategies for MMP inhibition in cancer: innovations for the post-trial era. Nat Rev Cancer. 2002;2:657–672. doi: 10.1038/nrc884. [DOI] [PubMed] [Google Scholar]
- 33.Zucker S, Cao J, Chen WT. Critical appraisal of the use of matrix metalloproteinase inhibitors in cancer treatment. Oncogene. 2000;19:6642–6650. doi: 10.1038/sj.onc.1204097. [DOI] [PubMed] [Google Scholar]