Abstract
The origins of the “endocrine disrupter hypothesis” may be traced to reports on adolescent daughters born to women who had taken the highly potent synthetic estrogen, diethylstilbestrol, while pregnant, and who developed a rare form of vaginal cancer and adenocarcinoma. Bisphenol A (BPA) is an estrogenic chemical that is highly employed in the manufacture of a wide range of consumer products. Some observational studies have suggested that the amounts of BPA to which we are exposed could alter the reproductive organs of developing rodents. We examined the influence of BPA at low doses to address the questions of (a) whether in utero exposure affects the vagina of the offspring and (b) which mechanisms cause the toxic effects. Gravid Sprague-Dawley dams were administered either 0.1 (low dose) or 50 mg/kg per day BPA, the no observed effect level, or 0.2 mg/kg per day 17α-ethinyl estradiol by gavage. Striking morphological changes were observed in the vagina of postpubertal offspring leading us to examine vaginal estrogen receptor (ER) expression because BPA binds to the ERα, which is important for growth of the vaginal epithelium. We show that the full -length ERα is not expressed during estrus in the vagina of female offspring exposed to either dose of BPA when compared to the control group, whereas ERα expression does not differ from the control group during the diestrus stage. ERα downregulation seems to be responsible for the observed altered vaginal morphology.
Keywords: bisphenol A, low dose, estrogen receptor, rat vagina, endocrine disrupter
Introduction
Endocrine-disrupting industrial chemicals (EDCs) are released into the environment and interfere with normal hormonal processes. Some of these chemicals can produce toxic effects at surprisingly low doses. Many researchers hypothesize that exposure to these EDCs during critical periods of development, i.e., early postnatal or in utero, could result in adverse effects to wildlife and humans [1,2].They may influence growth, reproduction, and development.
The origins of the “endocrine disrupter hypothesis” may be traced to reports on adolescent daughters born to women who had taken the highly potent synthetic estrogen, diethylstilbestrol (DES), while pregnant. These daughters developed a wide range of reproductive tract abnormalities, including a rare form of vaginal cancer, vaginal adenocarcinoma [3].
Bisphenol A (BPA) is a chemical that has been widely discussed as a prime candidate for endocrine disruption. BPA is composed of two unsaturated phenolic rings that resemble DES. It is among those estrogenic industrial compounds that are highly and widely used in the manufacture of epoxy, polyester- styrene, and polycarbonate resins and which are used for the manufacture of dental fillings, baby bottles, and food packaging. The ability of BPA to migrate from polymer to food has been described [4,5]. In vitro studies demonstrated that BPA binds to the estrogen receptors (ERs), induces estrogen-dependent gene expression/responses and is weakly estrogenic when compared to 17β-estradiol (E2) or DES [6–8].
Minuscule amounts of EDCs were shown to alter the reproductive organs of developing mice [9,10], sparking alarm within the scientific community and regulatory agencies. Fred vom Saal's group at the University of Missouri, Columbia showed that early developmental exposure to low doses of DES increased prostate weight in offspring, but levels above this had the opposite effect [11]. A second study by vom Saal showed even more disturbing effects. It demonstrated that exposure of female mouse fetuses to a very low dose of BPA alters their postnatal growth rate and results in precocious puberty [12]. They found significantly reduced number of days between vaginal opening and first vaginal oestrus. Alarmed about the implications of these results, some laboratories, mainly industrial ones, tried to reproduce these data but failed [13–15]. Faced with conflicting studies and aware of other new research that might resolve the question, the Environmental Protection Agency (EPA) enlisted the help of an expert panel, which met and conducted an extensive review of raw data from submitted studies [16]. The expert panel finally concluded that some estrogenic chemicals can cause biologic effects at levels below those normally found safe. Discrepancies between the studies may be attributed to variable sensitivity to estrogenic chemicals by laboratory animals. Indeed, a study has demonstrated that rodent strains can vary dramatically in their response to estrogenic compounds [17]. Furthermore, the issues of dose and binding affinities to the ERs seem to be the heart of the controversy regarding xenoestrogens. Pointing to these uncertainties, the expert panel recommended new screening and testing procedures because traditional toxicology tests, particularly standard reproductive and developmental assays, appear to be inadequate for screening programs. The scope of the new testing program should include assays for (1) in vitro and in vivo screening of ER expression and functions and (2) genetic analysis. Robert Kavlock, director of the reproductive division of the National Health and Environmental Effects Research Laboratory at the U.S. EPA, currently stated that the inconclusive results concerning effects of BPA on reprotoxicology, can only be solved by understanding the mechanisms [2].
We examined the influence of BPA on several reproductive endpoints during early development and adulthood at low doses to address the questions of (1) whether in utero exposure interferes with the reproductive system of the offspring and (2) what are the mechanisms leading to longterm deleterious effects after exposure of the developing fetus to exogenous estrogens.
Materials and Methods
Female Sprague-Dawley rats with sperm-positive vaginal smears were treated with either 2% cornstarch (Mondamin) at 10 ml/kg per day, 0.1 mg/kg per day BPA, 50 mg/kg per day BPA or 0.2 mg/kg per day E2. Cornstarch served as the vehicle for BPA and pharmacological grade peanut oil was used as the vehicle for E2. The gravid dams were treated by gavage on gestation days 6 through 21.
Intact female offspring were maintained on a 12/12 hour light:dark cycle, and beginning at approximately 3 months of age, estrous cycle stage was determined by vaginal swabbing for 3 weeks. Each estrus group contained 22 offspring in the cornstarch group, 13 offspring in the 0.1 mg/kg per day, and 12 offspring in the 50 mg/kg per day BPA group, as well as 19 offspring in the 0.2 mg/kg per day E2 group. The diestrus group contained 20 offspring in the cornstarch group, 8 offspring in the 0.1 mg/kg per day and 6 offspring in the 50 mg/kg per day BPA group, as well as 5 offspring in the 0.2 mg/kg per day group. At approximately 4 months of age, female offspring were killed by decapitation in either estrus or diestrus between 0930 and 1600 hour of each day. Body weight and reproductive organ and liver weights were determined. Animals were maintained in accordance with the Guide for the Care and Use of Laboratory Animals from the physiological society of Germany. BPA was purchased from Sigma-Aldrich Chemicals (Steinheim, Germany) and E2 from Aldrich Chemical (Milwaukee, WI).
Histology
Vaginal tissue was fixed in Bouin solution, embedded in paraffin, and sectioned at 3 µm. After processing tissue sections were stained with hematoxylin and eosin for light microscopy. The thickness of vaginal epithelium and the number of vaginal epithelial cell layers were measured using a microscope (Axiophot, Zeiss, Göttingen, Germany) and the public domain Scion image analysis software (Scion Image 1.62c MacOs, Scion, Frederick, MD).
Western Blot Analyses
Western blot analyses were performed on vaginal tissue of each in utero treated offspring. Homogenization of rat vaginal tissue samples was performed for 15 minutes in an ultrasonic bath on ice in homogenization buffer including a cocktail of protease inhibitors (complete; Boehringer Mannheim, Germany). Homogenates were centrifuged at 20,000xg for 30 minutes. The resulting supernatants were retained for further experiments. Protein concentrations were determined in triplicate according to Bradford using BSA as a reference standard. Fifteen micrograms protein was separated by SDS-PAGE using 10% gels and then electrotransferred to nitrocellulose membranes using a semidry blot transfer method. The quality as well as equal loading of protein blots was determined by Ponceau S staining of nitrocellulose and using a monoclonal antibody against β-actin (Sigma, Germany) at 1:15.000. Blots were incubated overnight with polyclonal antibodies against ERα (MC-20, sc-542; Santa Cruz, Heidelberg, Germany) at a 1:100 dilution. The Mr of the immunoreactive bands were determined using molecular weight marker protein stocks SDS-PAGE 7b (Sigma, Germany). After washing, blots were incubated for 90 minutes with anti-rabbit or anti-mouse IgG-conjugated peroxidase antibody at a 1:5000 dilution (Biogenes, Berlin, Germany). Blots were finally rinsed, and immune complexes were visualized using ECL reagent (Amersham, Freidburg, Germany). Specificity of the obtained immunoreactive bands was assessed by using peptide absorbed antiserum against ERα-peptide (MC-20, sc-542P; Santa Cruz).
Results
Histology of Vaginal Epithelium
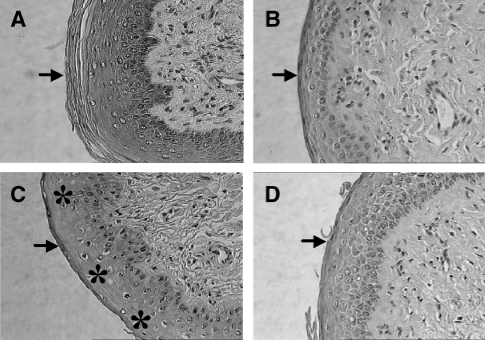
Striking morphologic changes in the differentiation, stratification, and cornification of the vaginas could be observed during estrus from the same in utero treated animals when compared to the negative control group (Figure 1A). No keratinization at the surface of the epithelium was observed following exposure to 0.1 mg/kg BPA (Figure 1B) when compared to the control group (Figure 1A). The thickness of the total epithelium was reduced following exposure to 0.1 mg/kg BPA (Figure 1B) when compared to the control group (Figure 1A). The 50-mg dose caused a similar effect, but was less pronounced (Figure 1D). Exposure to 0.2 mg/kg E2 (positive control; Figure 1B) resulted in a morphologic picture similar to the 0.1 mg/kg BPA group (Figure 1B) with slight desquamation of the superficial layers. In diestrus, no major differences of the vaginal epithelium could be observed between the control and treated animals (data not shown).
Figure 1.
Representative histology of the rat offspring vagina at estrus. (A) Control (cornstarch-treated animals) group. The vaginal epithelium is formed by 6–10 layers, of which superficial layers are cornified (↓). x400. (B) Rat vagina at estrus. 0.1 mg/kg BPA. No keratinization of the superficial layers (↓). Vaginal epithelium is formed by less then four layers. x400 (C) Rat vagina at estrus. 0.2 mg/kg E2 (positive control). No keratinization at the surface of the epithelium (↓). Slight desquamation of the superficial layers (*). Vaginal epithelium is formed by ≤6 layers. x400. (D) Rat vagina at estrus. 50 mg/kg BPA. Process of slight keratinization at the surface of the superficial layers (↓). Mostly no keratinization can be observed. Vaginal epithelium is formed by ≤6 layers. x400.
We could not observe any further morphological and structural changes of the vagina in offspring.
Western Blot Analyses
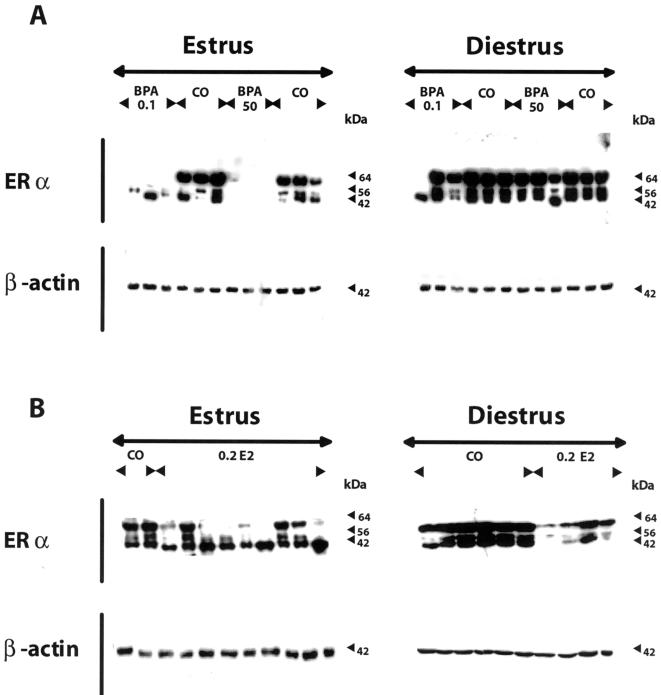
We clearly demonstrate that the full-length ERα variant at 64 kDa is not expressed during estrus at the protein level in the vagina of all female offspring exposed to either dose of BPA compared to the negative control group, whereas ERα expression does not differ from the control group during the diestrus stage (Figure 2A). The anti-ERα antibody specifically reacted with three bands at 64, 56, and 42 kDa from homogenates of rat vagina from control animals (Figure 2A). Offspring exposed to 0.2 mg E2/kg body weight during gestation showed either a decreased or no expression of ERα in their vagina during estrus (Figure 2B). Binding to all immunopositive bands was eliminated when antibody was preincubated with antigen peptide (not shown).
Figure 2.
Representative Western blot analyses of ERα expression of vaginal protein during the estrous cycle of female Sprague-Dawley offspring exposed to BPA in utero. Gravid dams were fed by gavage on gestation days 6 through 21 with either 2% cornstarch (negative control; CO) at 10 ml/kg per day, 0.1 mg/kg per day BPA (BPA 0.1), a low dose, or 50 mg/kg per day BPA (BPA 50), the NOEL, or 0.2 mg/kg per day E2 (0.2 E2), used as a positive control. The female offspring were then sacrificed either in estrus or diestrus at 4 months of age. (A) The full-length ERα variant at 64 kDa is not expressed during estrus at the protein level in the vagina of all female offspring exposed to either dose of BPA compared to the negative control group, whereas ERα expression does not differ to the negative control group during the diestrus stage. The anti-ERα antibody reacted specifically with three bands at 64, 56, and 42 kDa from homogenates of rat vagina from control animals (CO). (B) Offspring exposed to 0.2 mg E2/kg body weight during gestation showed either a decreased or no expression of the full-length ERα variant at 64 kDa in their vagina during the estrus phase compared to the negative control group (CO). In contrast to the BPA group the 42-kDa variant of the ERα is expressed during estrus in the vagina of all offspring exposed to 0.2 mg E2/kg body weight. The expression of the ERα variants was decreased compared to the negative control group during the diestrus stage. Protein loading was normalized to β-actin using a monoclonal primary antibody at 1:15,000 (Sigma, Deisenhofen, Germany), which was specific for a band at 42 kDa (A and B).
In addition, we could not observe ERβ expression at the protein level in the rat vaginal tissue from any group (data not shown).
Discussion
Vaginal epithelium cornifies or mucifies in response to the changing levels of circulating steroids during the estrous cycle [18]. Estrogens stimulate vaginal epithelium proliferation in vivo [19] and play a critical role in vaginal growth, epithelial morphogenesis, cytodifferentiation, and secretory activity. EDCs have been suspected to have adverse environmental effects because prenatal exposure to DES, another synthetic estrogen, was reported to be associated with clear cell adenocarcinoma of the vagina in female offspring. We performed Western blot analyses to study the expression of ERs and their variants in the vagina of 4-month-old (postpubertal) offspring exposed to BPA in utero, because ERα expression is important for growth, differentiation, and cornification of the vaginal epithelial cells [20]. The expression of ERα was investigated because we recently demonstrated [21] that those in utero treated animals exhibited altered times to vaginal opening and striking morphologic changes in the differentiation, stratification, and cornification of the vaginas during estrus. Western blot analyses were used to elucidate the possible mechanisms for the effects we observed in our observational studies. For the first time, we clearly demonstrate that the full-length ERα variant at 64 kDa is not expressed during estrus at the protein level in the vagina of all female offspring exposed to either dose of BPA compared to the negative control group, whereas ERα expression does not differ from the control group during the diestrus stage (Figure 2A). The anti-ERα antibody specifically reacted with three bands at 64, 56, and 42 kDa from homogenates of rat vagina from control animals (Figure 2A). These shorter bands (56 and 42 kDa) are derived from the alternative usage of initiation ATG or splicing [22,23]. Specificity of immunoreactivity was analyzed by using peptide-preabsorbed antibodies. Furthermore, offspring exposed to 0.2 mg E2/kg body weight during gestation showed either a decreased or no expression of ERα in their vagina during estrus (Figure 2B). Our findings clearly indicate that in utero exposure, not neonatal exposure [24], of rats to low doses of BPA, similar to amounts typically found in the environment, elicits long-term changes in ERα expression. BPA significantly downregulates the expression of ERα leading to our findings showing striking morphologic changes in the differentiation and cornification of vaginas. Our data are consistent with previous reports showing a downregulation of ERα expression in the uterus after neonatal exposure to DES [24,25]. The mechanisms underlying the downregulation of ERα expression may be complex, as is the case with the tissue-specific agonist/antagonist action of tamoxifen [26]. BPA may antagonize the action of endogenous estrogens because there are differences in the existence of tissue-specific ER coactivators [27]. Adult organisms have compensatory mechanisms that may minimize disturbances; these mechanisms may be imperfect in the very young or unborn who are still in the process of developing homeostasis and undergoing genetic imprinting.
In summary, this is the first study that shows that the full-length ERα is not expressed during estrus in the vagina of female offspring exposed in utero to either dose of BPA when compared to the control group, whereas ERα expression does not differ to the control group during the diestrus stage. ERα downregulation seems to be responsible for the observed altered vaginal morphology.
Acknowledgement
The authors thank Helga Stürje for technical assistance.
Abbreviations
- BPA
bisphenol A
- DES
diethylstilbestrol
- EDCs
endocrine-disrupting industrial chemicals
- EPA
Environmental Protection Agency
- ER
estrogen receptor
- E2
17β-estradiol
Footnotes
This work was supported by grants from the Bundesministerium für Bildung und Forschung (BMBF) awarded to I. Chahoud and M. Paul.
References
- 1.Loder N. Royal society warns on hormone disrupters. Nature. 2000;406:4. doi: 10.1038/35017709. [DOI] [PubMed] [Google Scholar]
- 2.Triendl R. Genes may solve hormone-disrupter debate. Nature. 2001;409:274. doi: 10.1038/35053303. [DOI] [PubMed] [Google Scholar]
- 3.Herbst AL, Ulfelder H, Poskanzer DC. Adenocarcinoma of the vagina. Association of maternal stilbestrol therapy with tumor appearance in young women. N Engl J Med. 1971;284:878–881. doi: 10.1056/NEJM197104222841604. [DOI] [PubMed] [Google Scholar]
- 4.Yamamoto T, Yasuhara A. Quantities of bisphenol A leached from plastic waste samples. Chemosphere. 1999;38:2569–2576. doi: 10.1016/s0045-6535(98)00464-0. [DOI] [PubMed] [Google Scholar]
- 5.Yoshida T, Horie M, Hoshino Y, Nakazawa H. Determination of bisphenol A in canned vegetables and fruit by high performance liquid chromatography. Food Addit Contam. 2001;18:69–75. doi: 10.1080/026520301446412. [DOI] [PubMed] [Google Scholar]
- 6.Gaido KW, Leonard LS, Lovell S, Gould JC, Babai D, Portier CJ, McDonnell DP. Evaluation of chemicals with endocrine modulating activity in a yeast-based steroid hormone receptor gene transcription assay. Toxicol Appl Pharmacol. 1997;143:205–212. doi: 10.1006/taap.1996.8069. [DOI] [PubMed] [Google Scholar]
- 7.Tong W, Perkins R, Xing L, Welsh WJ, Sheehan DM. QSAR models for binding of estrogenic compounds to estrogen receptor alpha and beta subtypes. Endocrinology. 1997;138:4022–4025. doi: 10.1210/endo.138.9.5487. [DOI] [PubMed] [Google Scholar]
- 8.Kuiper GG, Carlsson B, Grandien K, Enmark E, Haggblad J, Nilsson S, Gustafsson JA. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology. 1997;138:863–870. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- 9.vom Saal FS, Cooke PS, Buchanan DL, Palanza P, Thayer KA, Nagel SC, Parmigiani S, Welshons WV. A physiologically based approach to the study of bisphenol A and other estrogenic chemicals on the size of reproductive organs, daily sperm production, and behavior. Toxicol Ind Health. 1998;14:239–260. doi: 10.1177/074823379801400115. [DOI] [PubMed] [Google Scholar]
- 10.Welshons WV, Nagel SC, Thayer KA, Judy BM, vom Saal FS. Low-dose bioactivity of xenoestrogens in animals: fetal exposure to low doses of methoxychlor and other xenoestrogens increases adult prostate size in mice. Toxicol Ind Health. 1999;15:12–25. doi: 10.1177/074823379901500103. [DOI] [PubMed] [Google Scholar]
- 11.vom Saal FS, Timms BG, Montano MM, Palanza P, Thayer KA, Nagel SC, Dhar MD, Ganjam VK, Parmigiani S, Welshons WV. Prostate enlargement in mice due to fetal exposure to low doses of estradiol or diethylstilbestrol and opposite effects at high doses. Proc Natl Acad Sci USA. 1997;94:2056–2061. doi: 10.1073/pnas.94.5.2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Howdeshell KL, Hotchkiss AK, Thayer KA, Vandenbergh JG, vom Saal FS. Exposure to bisphenol A advances puberty. Nature. 1999;401:763–764. doi: 10.1038/44517. [DOI] [PubMed] [Google Scholar]
- 13.Cagen SZ, Waechter JM, Dimond SS, Breslin WJ, Butala JH, Jekat FW, Joiner RL, Shiotsuka RN, Veenstra GE, Harris LR. Normal reproductive organ development in CF-1 mice following prenatal exposure to bisphenol A. Toxicol Sci. 1999;50:36–44. doi: 10.1093/toxsci/50.1.36. [DOI] [PubMed] [Google Scholar]
- 14.Cagen SZ, Waechter JM, Dimond SS, Breslin WJ, Butala JH, Jekat FW, Joiner RL, Shiotsuka RN, Veenstra GE, Harris LR. Normal reproductive organ development in Wistar rats exposed to bisphenol A in the drinking water. Regul Toxicol Pharmacol. 1999;30:130–139. doi: 10.1006/rtph.1999.1340. [DOI] [PubMed] [Google Scholar]
- 15.Ashby J, Tinwell H, Haseman J. Lack of effects for low dose levels of bisphenol A and diethylstilbestrol on the prostate gland of CF1 mice exposed in utero. Regul Toxicol Pharmacol. 1999;30:156–166. doi: 10.1006/rtph.1999.1317. [DOI] [PubMed] [Google Scholar]
- 16.Kaiser J. Endocrine disrupters. Panel cautiously confirms low-dose effects. Science. 2000;290:695–697. doi: 10.1126/science.290.5492.695. [DOI] [PubMed] [Google Scholar]
- 17.Spearow JL, Doemeny P, Sera R, Leffler R, Barkley M. Genetic variation in susceptibility to endocrine disruption by estrogen in mice. Science. 1999;285:1259–1261. doi: 10.1126/science.285.5431.1259. [DOI] [PubMed] [Google Scholar]
- 18.Boutin EL, Battle E, Cunha GR. The germ layer origin of mouse vaginal epithelium restricts its responsiveness to mesenchymal inductors: uterine induction. Differentiation. 1992;49:101–107. doi: 10.1111/j.1432-0436.1992.tb00774.x. [DOI] [PubMed] [Google Scholar]
- 19.Galand P, Leroy F, Chretien J. Effect of oestradiol on cell proliferation and histological changes in the uterus and vagina of mice. J Endocrinol. 1971;49:243–252. doi: 10.1677/joe.0.0490243. [DOI] [PubMed] [Google Scholar]
- 20.Couse JF, Curtis SW, Washburn TF, Lindzey J, Golding TS, Lubahn DB, Smithies O, Korach KS. Analysis of transcription and estrogen insensitivity in the female mouse after targeted disruption of the estrogen receptor gene. Mol Endocrinol. 1995;9:1441–1454. doi: 10.1210/mend.9.11.8584021. [DOI] [PubMed] [Google Scholar]
- 21.Talsness C, Fialkowski O, Gericke C, Merker HJ, Chahoud I. The effects of low and high doses of Bisphenol A on the reproductive system of female and male rat offspring. Congenital Anomalies. 2000;40:S94–S107. [Google Scholar]
- 22.Friend KE, Ang LW, Shupnik MA. Estrogen regulates the expression of several different estrogen receptor mRNA isoforms in rat pituitary. Proc Natl Acad Sci USA. 1995;92:4367–4371. doi: 10.1073/pnas.92.10.4367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Friend KE, Resnick EM, Ang LW, Shupnik MA. Specific modulation of estrogen receptor mRNA isoforms in rat pituitary throughout the estrous cycle and in response to steroid hormones. Mol Cell Endocrinol. 1997;131:147–155. doi: 10.1016/s0303-7207(97)00098-1. [DOI] [PubMed] [Google Scholar]
- 24.Khurana S, Ranmal S, Ben-Jonathan N. Exposure of newborn male and female rats to environmental estrogens: delayed and sustained hyperprolactinemia and alterations in estrogen receptor expression. Endocrinology. 2000;141:4512–4517. doi: 10.1210/endo.141.12.7823. [DOI] [PubMed] [Google Scholar]
- 25.Medlock KL, Branham WS, Sheehan DM. Long-term effects of postnatal exposure to diethylstilbestrol on uterine estrogen receptor and growth. J Steroid Biochem Mol Biol. 1992;42:23–28. doi: 10.1016/0960-0760(92)90007-6. [DOI] [PubMed] [Google Scholar]
- 26.Gallo MA, Kaufman D. Antagonistic and agonistic effects of tamoxifen: significance in human cancer. Semin Oncol. 1997;24:S1. [PubMed] [Google Scholar]
- 27.Horwitz KB, Jackson TA, Bain DL, Richer JK, Takimoto GS, Tung L. Nuclear receptor coactivators and corepressors. Mol Endocrinol. 1996;10:116–177. doi: 10.1210/mend.10.10.9121485. [DOI] [PubMed] [Google Scholar]