Abstract
Tetrathiomolybdate (TM) is a potent nontoxic orally delivered copper complexing agent under development for the last several years for the treatment of Wilson's disease. It has been shown to block angiogenesis in primary and metastatic tumors. Therefore, the combination of cytotoxic radiotherapy (RT) and antiangiogenic TM could target both the existing tumor and the tumor microvasculature in a comprehensive strategy. Using a Lewis lung high metastatic (LLHM) carcinoma mouse tumor model, we demonstrate that the combination of TM and RT is more effective than either used as monotherapy. We also show that their therapeutic effects are additive, with no additional toxicity. We show that TM has no significant cytotoxicity in vitro against LLHM tumor cells, further supporting the antiangiogenic mechanism for its action.
Keywords: angiogenesis, radiation, cancer, lung, copper
Introduction
The tumor microvasculature can serve as an excellent target for the treatment of all types of tumors [1,2]. Compounds that interfere with critical steps in the angiogenic cascade are making their way into the clinical arena as potential treatment options for patients with cancer [3,4]. The steps required for successful tumor angiogenesis are diverse and depend on an imbalance [5,6] between angiogenesis activators such as vascular endothelial growth factor (VEGF) [7], basic fibroblast growth factor (bFGF) [8–11], and transforming growth factor-beta (TGF-beta) [12], and inhibitors such as thrombospondin 1 [13], angiostatin [14], and endostatin [15]. In tumors, this imbalance serves to tilt the angiogenic switch in favor of angiogenesis and is thought to be important in regulating both primary and metastatic tumor growth [6].
A strategy that affects multiple activators of angiogenesis may be highly applicable for the treatment of human tumors and could potentially suppress tumor angiogenesis more powerfully than single target strategies. Copper is a required cofactor for the function of many key mediators of angiogenesis including bFGF [16–19], VEGF and angiogenin [20,21]. Several animal studies have demonstrated that copper is required for angiogenesis [22,23]. Studies in animal models using the copper chelator penicillamine and a low copper diet reported a decrease in tumor size relative to controls [23,24]. In two animal models, a VX2 carcinoma implanted in the brains of rabbits and a 9L gliosarcoma implanted in the brains of rats, copper chelation was shown to inhibit both angiogenesis and tumor growth [25,26].
Tetrathiomolybdate (TM) is a copper reducing agent used in the treatment of Wilson's disease [27], an autosomal recessive disorder of copper transport that results in abnormal copper accumulation primarily in the liver and brain. There is extensive clinical experience with TM as a copper-lowering drug in the treatment of Wilson's disease [27]. TM is the most potent copper-binding agent known, forming a stable tripartite complex with copper and protein. When given with food, TM complexes dietary copper with food protein and prevents absorption of copper from the gastrointestinal tract. When given between meals, TM is absorbed into the blood stream where it complexes with free (or loosely bound) copper and serum albumin. This TMbound copper fraction is no longer available for cellular uptake, has no biologic activity, and is slowly cleared from the body in bile and urine. Thus, patients taking TM are placed in a negative copper balance immediately. The best measure of the level of biologically active copper in the body is obtained by the measurement of the protein ceruloplasmin (Cp) [28], a protein made by the liver in amounts that directly reflect the level of biologically active copper present in the body.
It has recently been shown that TM can impede the development of mammary tumors in Her2-neu transgenic mice [29]. The safety and antitumor effects of TM therapy alone in patients with solid tumors have been evaluated in a Phase I clinical trial [30]. This phase I trial demonstrated that TM could modulate copper stores to impair angiogenesis, but leave other copper-dependent cellular processes largely intact, so that no significant clinical toxicity occurred. There was only a small percentage of grade 1–2 hematologic toxicity ( easily reversible changes in hematocrit or white count) noted.
Radiotherapy against primary tumors has been shown to be improved by the addition of antiangiogenic agents using animal models [31–35]. This has been accomplished without significant side effects or increased toxicity noted. In this paper we present preclinical studies that test whether antiangiogenic therapy with the copper lowering agent TM can be combined with radiation therapy to improve the efficiency of the radiotherapy (RT) of primary tumors in mice. We demonstrate that angiogenic inhibition through copper reduction can be combined with cytotoxic RT to improve tumor control.
Materials and Methods
In Vivo Therapy with Radiation or TM
Lewis lung high metastatic (LLHM) carcinoma cells were derived from Lewis lung low metastatic (LLLM) tumors by serial passage in C57BL6 mice and selection for lung metastases that grow with large primary tumors present (i.e., primary tumors that no longer produce angiostatin, which inhibits metastatic growth). The LLHM cell line was maintained by serial passage in C57BL6/J mice (Jackson Laboratories, Bar Harbor, ME). The phenotype of lung metastasis was assessed at each passage. Male 6-to 9-week-old C57BL6/J mice were used for experiments. The mice were acclimated and caged in groups of five or less and then were shaved. All mice were fed a diet of animal chow ad libitum. They were anesthetized using isoflurane delivered through a ventilation chamber before all procedures and were observed until fully recovered.
Tumors were isolated from carrier mice and implanted into experimental animals as previously described [14]. In summary, animals with 1000-mm3 tumors were sacrificed, and the skin overlying the tumor cleaned with betadine and ethanol. In a laminar flow hood, tumor tissue was excised under aseptic conditions. A suspension of tumor cells in phosphate-buffered saline (PBS) was made by passage of viable tumor tissue through a sieve and a series of sequentially smaller hypodermic needles. The final concentration was adjusted to 1x107 cells/ml and the suspension placed on ice. The subcutaneous dorsa (for passage) of carrier mice, or upper leg dorsa (for radiation experiments), were injected with 1x106 cells in 0.1 ml of PBS after cleaning the injection site with ethanol.
Initial experiments using 60 mice were carried out to determine the average volume of water consumed per mouse per day. The volume of water imbibed in each 24-hour period was measured for each cage, and the volume per mouse determined. The concentration of TM in the water bottles was then calculated to deliver the appropriate dose of TM. Freshly mixed TM was placed into the water bottles each day.
The mice receiving RT were irradiated with a gamma-cell 40 cesium irradiator (100 cGy/min). The unit has two Cs sources creating a more homogeneous field between the sources where the specimen is placed. A specially designed metal cylindrical jig apparatus was used to immobilize the mouse with the right (tumor-bearing) leg/flank region extended. The jig was placed into a cylindrical lead shield encasing the bodies of the mice while exposing the leg/flank tumor to be irradiated. No anesthesia was necessary. Measurements demonstrate that the exposed leg receives the full dose of RT, whereas the shielded body receives <2% of the total dose given.
Copper Status of Mice
Blood was collected from mice treated with TM by anesthetizing the mice with isoflurane and performing cardiac puncture. The blood was centrifuged at 3000g, 4°C, and the serum collected and frozen at -20°C until the assays were run.
The Cp levels were measured as per a standard assay [36]. Twenty-five microliters of serum from an individual mouse were placed into each of two tubes and 375 µl of 0.1 M sodium acetate buffer (pH 5.0, stored at 4°C) was immediately added. Tubes were placed in a 30°C water bath. After 5 minutes, 100 µl of o-dianisidine dihydrochloride (7.88 mM, Sigma, St. Louis, MO) reagent (preincubated at 30°C) was pipetted into each tube. After 30 minutes one of the two tubes for each group was removed from the water bath, and 1 ml of 9 M sulfuric acid was added to quench the reaction. After 45 minutes, the second tube was quenched and the absorbency of both tubes at 540 nm was analyzed using a 1-cm path length cuvette in a spectrophotometer. The Cp concentration in international units was calculated by the formula: Cp oxidase activity=(A45-A30)x0.625 U/ml.
Microvessel Density Counting
Tumors were preserved in 10% neutral buffered formalin, and embedded in paraffin. Five-micrometer sections were immunohistochemically stained for CD31. In summary: The sections were deparaffinized (xylene and then ethanol), and then hydrated with water. They were placed in 1% hydrogen peroxide in methanol for 30 minutes at room temperature. The sections were washed with double-distilled water, and then Tris buffer (pH 7.2) for 5 minutes. Next, they were treated with proteinase K 36 µg/ml) in 0.2 M Tris buffer (pH 7.2) at 37°C for 30 minutes, and then were washed in Tris buffer (pH 7.2), 5 minutes. The sections were then blocked in TNB buffer (0.1M Tris-HCl pH 7.5, 0.15 M NaCl, 0.5% blocking reagent), 30 minutes at room temperature (TSA-Indirect kit, NEN Life Science Products, Boston, MA). The primary antibody (rat anti-mouse CD31, Pharmingen, San Diego, CA) was added at 1:250 dilution in TNB buffer overnight at 4°C. They were then rinsed with TNT wash buffer (NEN TSA-Indirect kit 0.1M Tris-HCl pH 7.5, 0.15 M NaCl, 0.05% Tween-20). Secondary antibody was then added (biotinylated rabbit anti-rat, Vector #BA-4001) at a 1:200 dilution in TNB buffer for 30 minutes at room temperature. They were then rinsed with TNT buffer, and incubated in streptavidin horseradish peroxidase (SAHRP), at a 1:100 dilution in TNB buffer for 30 minutes at room temperature, and then washed again. Biotinylated tyramide in the amplification diluent was added for 7 minutes at room temperature, and washes done. Incubation in SAHRP in 1:100 TNB for 30 minutes at room temperature was done and washes carried out. DAB was then added, and the slides were counterstained. Hot spots were counted as previously described [37]. The slides were viewed at 100x magnification to determine “hot spots” of angiogenesis, and the vessels present were counted. Generally, five of these were counted per mouse sample. Two different investigators did the counting (M.K.K. and N.K.G.), and the values were averaged.
In Vitro Tumor Cell Proliferation Assay (MTT Assay)
In vitro experiments were carried out on the LLHM tumor cell line, using an MTT (3-(4,5-dimethylthiazol-2yl)-2,5 diphenyltetrazolium bromide, Sigma) proliferation assay. MTT stains live cells, and the A595 absorbency correlates with cell number. TM was added to the LLHM cells at concentrations of 0, 0.001, 0.01, 0.1, 1, or 10 nM. The proliferation was carried out for 5 days with daily measurements of absorbency. A flat-bottomed 96-well Falcon microtest tissue culture plate was used for each time point with six replicate wells used for each TM concentration. One thousand cells were added to each well. TM was added to bring the concentration to 0, 0.001, 0.02, 0.01, 0.0.1, 1.0, and 10 nM per well. On days 0 to 4, 50 µl of MTT was added to each well using a repeat pipetter and the plate was incubated for 2 hours at 37°C. The plates were centrifuged for 5 minutes at 2200 rpm in a 4°C Sorvall RT 6000B centrifuge. One hundred microliters of dimethyl sulfoxide was added to each well using a repeat pipetter and the plate agitated for 5–10 minutes. The absorbency at 595 nm was read by a Dynatek MR 5000 plate reader. An absorbency titration curve was generated using the MTT assay carried out with 10-fold increases in cells from 0 to 1 million cells and the absorbency at 595 nm measured.
Statistical Methods
The tumor volume measurements were analyzed using growth curve random effects models. The model was fit to the logarithm of the tumor size measurements between 10 and 16 days after subtracting the logarithm of the baseline volume. The models allowed for an exponential growth of the tumors and the evaluation of the difference between treatments. The method gave estimates of the treatment effects and interactions between the treatments. Each of the four experiments was analyzed separately and in a combined analysis that allowed for differences in growth rate and intercept between the experiments. A repeated measures mixed model was applied to tumor values to analyze tumor growth.
Kaplan-Meier plots and Cox proportional hazards models were used to compare the survival times in the first 16 days of the four treatment groups. Deaths after 16 days were reclassified as censored at 16 days due to the rapid onset of death due to lung metastases after 16 days particularly in the untreated control group. For the Cox model, the experiment number was included as a baseline hazard stratification variable, and log of initial tumor volume and treatment group were the covariates. Likelihood ratio tests were used to test the null hypothesis that the four treatment groups gave equal survival after adjusting for initial tumor volume and experiment number.
Results
In Vivo TM and Radiotherapy Experiments
One million cells were injected into the flanks of C57BL6 mice, and the tumors grown to an average of 291 mm3 (212±53, 288±82, 305±68, and 359±113 mm3 in experiments 1 to 4, respectively). In each experiment the tumor volumes were kept to within 20% of the mean volume on day zero. The mice were then separated into four groups and treated as follows: (a) no therapy, (b) TM alone, (c) RT alone, (d) TM+RT. TM was continued for the duration of the experiment in groups b and d. Radiotherapy was delivered to all of the tumors in groups c and d on the same day when the tumors reached an average of 522 mm3 (493±76, 523±117, 520±160, and 552±236 mm3 in experiments 1 to 4, respectively). Mice were observed clinically for any evidence of copper reduction toxicity, and the dose of TM was adjusted as needed throughout the experiment. A dose of 15 Gy was selected for RT, as this dose was shown in previous experiments (data not shown) to inhibit tumor growth but not produce cures. If the tumors are cured, there is no way to assess interaction with another agent, and if too little dose is given, the growth curves would look too much like the untreated arm. The dose of 15 Gy, therefore, permitted analysis of the interaction of RT and TM.
The total number of mice in experiments 1 to 4 at the beginning of each experiment was (a) 38 mice in the untreated group, (b) 38 mice in the TM alone group, (c) 35 mice in the RT alone group, and 35 mice in the RT + TM group. The exact number of mice in each arm of each experiment over time is shown in Figure 1. In experiment 1, the mice received 0.75 mg/mouse per day of TM orally for 15 days, then 0.5 mg/mouse per day for the duration of the experiment. In experiments 2–4, they received 1 mg/mouse per day of TM orally for 2 days, then 0.5 mg/mouse per day for the duration of the experiment. The effectiveness of the TM doses was assessed by measuring the copper chelation status (reflected in the Cp levels) shown below.
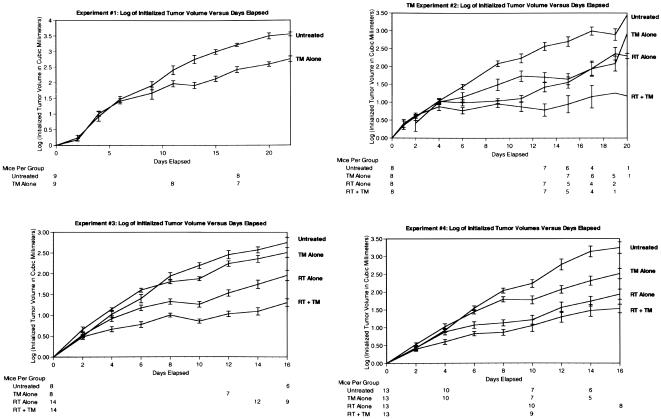
Figure 1.
The logarithm of the initialized tumor volumes versus the days of treatment are shown for four experiments. One million LLHM tumor cells were injected into the flanks of mice and grown to an average of 291 mm3 (tumor volumes were calculated by 0.52xlxw2. At this point the mice were sorted into their respective groups, and started on TM if appropriate. When the mice in the RT or RT + TM groups reached an average of 522 mm3 (2 days later) they all received RT on the same day to the tumor-injected flanks using a gamma-cell Cs irradiator and specially designed shielding. The number of mice still living are shown for all time points. Statistical analysis was done from days 10–16. The y axis represents the logarithm of the tumor volumes initialized for the starting tumor volume on day 0. All mice getting TM began on day 0. In experiment 1, they received 0.75 mg/mouse per day of TM orally for 15 days, then 0.5 mg/mouse per day for the duration of the experiment. In experiments 2–4, they received 1 mg/mouse per day of TM orally for 2 days, then 0.5 mg/mouse per day for the duration of the experiment.
The statistical analysis was performed using a growth curve random effects model between 10 and 16 days. Figure 1 shows the tumor growth data, plots of log volume change from baseline versus time, for four separate experiments. The relative difference between the tumor volumes in the period 10–16 days was similar across the four experiments. In all the experiments, there was a significant treatment effect (P<0.001 for all four experiments). The analysis of the combined data shows the decrease in tumor volume compared to control in the period 10–16 days (Table 1).
Table 1.
Analysis of the Combined Tumor Growth Data.
| Treatment | Ratio of Tumor Volume Compared to Untreated Control on Days 10–16 (95% Confidence Interval) |
| Untreated control | 1 |
| TM | 0.55 (0.47, 0.64) |
| RT | 0.34 (0.20, 0.41) |
| TM+RT | 0.22 (0.19, 0.26) |
A comparison of the combined tumor growth data from all four experiments and for the day 10–16 time period demonstrated the relative differences shown. All tumor volumes are shown relative to the untreated controls.
The effect of RT and TM appeared to be close to additive on the scale of the logarithm of tumor volume. In experiments 2 and 4 there was a weak suggestion of subadditivity, whereas in experiment 3 there was a suggestion of superadditivity. Overall there was no statistically significant interaction between TM and RT for any of the experiments (P=0.27 for experiment 2, P=0.06 for experiment 3, and P=0.16 for experiment 4). This indicates that the effects of TM and RT are at least additive with no definite evidence of synergy.
There was no increased toxicity (retraction, ulceration, or death) in the mice receiving TM+RT compared to mice receiving either therapy alone. The most common clinical signs of TM toxicity occurred when the mice did not drink, and they then appeared dehydrated (decreased skin turgor, hair with ruffled appearance, and eventually less movement and a hunched-over posture). The mice were assessed throughout the experiment to note any differences in fibrosis or swelling or infection in the radiation field with none apparent.
An analysis of survival revealed that 42 of the 146 animals died by day 16. The initial tumor volume was highly statistically significantly associated with survival time (P=0.006). There were significant differences between the four treatment groups (P=0.03). The estimated relative hazard and 95% confidence intervals relative to the control group were 0.59 (0.22, 1.58) for TM, 1.62 (0.72, 3.70) for RT and 0.50 (0.19, 1.32) for TM+RT. Thus, there is marginal evidence that the addition of TM may improve the survival of these animals.
Copper Status
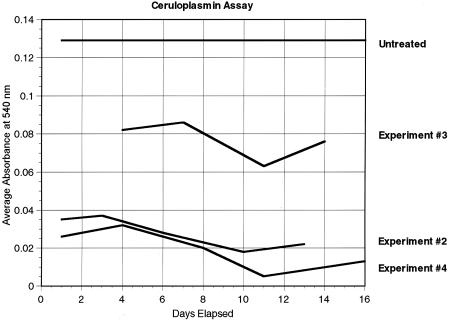
Figure 2 shows the Cp levels measured in experiments 2, 3, and 4. Each Cp level is the average of measurements from the sera of at least two mice. The average Cp levels of mice measured in experiments 2, 3, and 4 were 0.028 U/ml (range 0.018–0.037), 0.077 U/ml (range 0.063–0.086), and 0.019 U/ml (range 0.005–0.032), respectively. The average Cp level of untreated tumor-bearing mice was found to be 0.129 U/ml (range 0.102–0.147). Reduction of copper levels was obtained in these experiments and to the level previously demonstrated to inhibit tumor growth in mice and humans [29,30]. In the mice, copper reduction could be obtained within 2 days, and the Cp was reduced to target levels by the time the radiation was delivered.
Figure 2.
Cp assay. Serum Cp levels (as represented by the average absorbency at 540-nm wavelength) were measured at various time points in TM-treated tumor-bearing mice. Each point represents an average measurement of at least two mice. As a comparison, the top curve (labeled untreated) represents the baseline measurement of Cp from five tumor-bearing untreated mice.
Microvessel Density Counting of Tumors
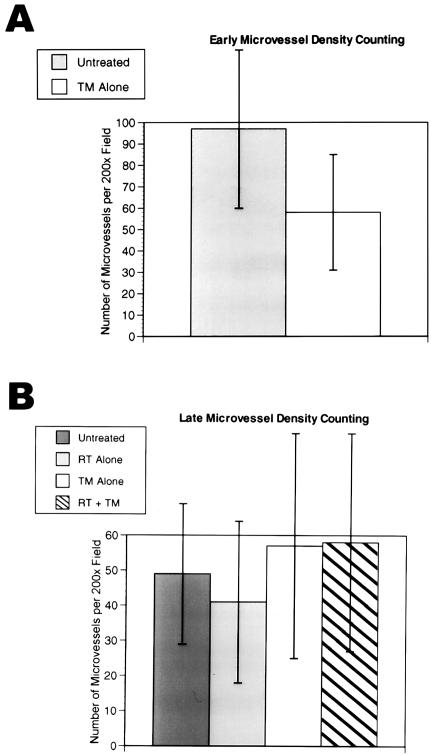
Microvessel density at day 2 of TM therapy indicated a trend for decreased microvessel density in the TM-treated tumors, but it did not reach statistical significance (Figure 3A). There was no difference in microvessel density between the untreated, RT alone, TM alone, and RT+TM samples at the end of the experiment on day 16 (Figure 3B). Microvessel density counting has been shown to have prognostic significance in numerous studies. However, recent studies are seriously calling into question the ability of microvessel density to correlate with response to therapy (Lynn Hlatky, personal communication). The density is dependent on the number of microvessels and on the number of tumor cells in a given field. Although initially there may be a difference in microvessel density between the treated and control groups, as the antiangiogenic factors take effect, and the tumor cell growth slows down or apoptosis ensues, the density can change to a higher value as the relative number of microvessels to tumor cells increases.
Figure 3.
Microvessel density counting of mouse tumors. The chart in (A) shows a trend for TM-treated tumors to have decreased microvessel density counts. The difference is not statistically significant as demonstrated by the overlapping standard deviation error bars. In (B) the average microvessel density counts for untreated, RT-treated, TM-treated, and RT+TM-treated tumors show not statistically significant differences. Each bar represents the average microvessel counts at 200x magnification from three mice (with two to five hot spots counted per mouse tumor), and with two independent investigators counting. The same results occur for when the data for each investigator are analyzed separately (data not shown).
Effects of TM Concentration on LLHM Proliferation In Vitro
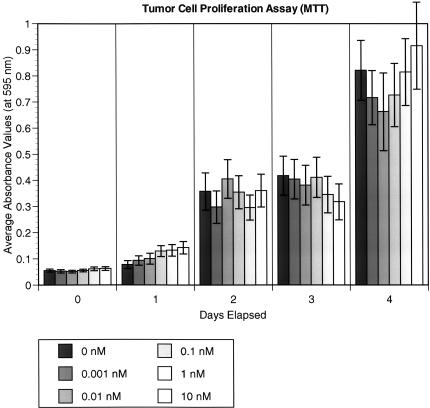
We carried out MTT assays on the LLHM tumor cell line to determine whether escalating concentrations of TM would effect tumor growth in vitro. There was no statistically significant difference at any TM concentration. Figure 4 shows the growth curve of the tumor cells with varying concentrations of TM (0.001–10 nM). The physiologic concentration of TM in human patients is estimated to be 0.01 nM, indicating that there is no change in cancer cell survival even at 1000 times (10 nM) this concentration of TM in vitro.
Figure 4.
MTT assay in vitro tumor cell proliferation analyses were carried out on LLHM tumor cells from day 0 to day 4. The y axis absorbency values are proportional to the number of tumor cells. Each color represents a different concentration of TM (ranging from 0 to 10 nM) being added to the LLHM cells. Each bar represents the average of five microtiter well values. The error bars represent standard error. There is no statistically significant difference in tumor cell number noted at any time point and at any concentration of TM.
Discussion
The combination of the antiangiogenic copper complexing agent TM with RT caused a greater decrease in the size of primary LLHM carcinoma tumors in mice than either used as monotherapy. In addition, TM had no affect on the proliferation of LLHM cells in vitro, even at concentrations of TM 1000-fold over those expected in the tumors. There was no increased toxicity with the combination therapy (RT+TM) in these preclinical studies. This is the first report that the combination of antiangiogenic therapy by copper-lowering therapy has been demonstrated to have efficacy in combination with RT to increase the control of tumor growth.
There is a small amount of published data indicating that copper chelation may help induce apoptosis in tumor cells treated with DNA-damaging agents like bleomycin. This would yield a model where copper reduction helps RT (a DNA-damaging agent) to induce apoptosis in tumor cells [38,39]. There is also, however, published data indicating that copper appears to increase tumor kill through oxidative radical formation (a key mechanism of RT-induced cell killing), and this would not support the role of copper reduction in increasing tumor cell kill by RT [40]. We also demonstrate that copper reduction with TM does not effect tumor cell proliferation in our in vitro system.
Radiotherapy against primary tumors has been shown to be improved by the addition of antiangiogenic agents [31–35]. The work of Teicher et al. is particularly intriguing, as it demonstrated an increase in the concentration of oxygen occurs with antiangiogenic therapy [32]. The mechanism for this is unknown, but there are many postulated possibilities.
We postulate that a significant component of the effect of RT on tumor growth could be due to the antiangiogenic effects of RT on the tumor endothelium. Radiotherapy has been shown to damage endothelial cells (particularly capillary endothelial cells) in vivo, and many effects of RT are postulated to be due to this [41,42]. Radiotherapy could be acting as a spatially specific antiangiogenic agent. Thus, when RT is then combined with another antiangiogenic agent like TM, it is in principle possible to greatly increase the effects on tumor growth. In theory, one could use a different dosing (or fractionation) of RT to treat tumor endothelium, and possibly have a large effect on tumor growth. This would be analogous to the use of different dosing of chemotherapy (monotonic dosing) to best treat the tumor endothelium, as was first done by Browder et al. [43]. The combination of this new antiangiogenic dosing of chemotherapy with a weak antiangiogenic agent has also been shown to have at least an additive effect in long-term mouse models by Kerbel [44]. It is possible that different RT schedules, or different methods for specifically targeting the microvasculature with RT, could disrupt angiogenesis more effectively.
Copper reduction with TM can affect multiple angiogenesis activators. A strategy that affects multiple activators of angiogenesis could potentially suppress tumor angiogenesis more powerfully than single target strategies. Numerous animal studies demonstrate that copper lowering is antiangiogenic, and can therefore suppress tumor growth. TM is an orally delivered copper complexing drug and has been used successfully for several years in experimental studies to treat Wilson's disease in humans. The exact copper status of patients can be easily and accurately determined using a simple blood test (Cp). A phase I study demonstrated that the copper reduction to levels necessary to inhibit angiogenesis have little toxicity in humans [30].We now present preclinical data demonstrating that antiangiogenic copper lowering with TM can be combined with cytotoxic (and probably also antiangiogenic) RT, and that their contributions are additive with respect to tumor control with no noticeable increase in toxicity. We are currently developing phase I clinical trials examining the combination of TM with RT in human cancers at the University of Michigan.
Acknowledgements
Thank you to Michelle LeBlanc (University of Michigan Comprehensive Cancer and Geriatrics Center research histology and immunohistochemical laboratory) for doing the immunohistochemical staining and to Evelyn Flynn (Children's Hospital, Boston, J. Folkman laboratory) for her incredible procedures. Thank you also to Xaio Ming (SDM laboratory technician) for her teaching us how to properly do the MTT assay. Thank you to James MacDonald and Jennifer Besse (University of Michigan Medical Center) for statistical assistance. Thank you also to Theodore Lawrence (University of Michigan Medical Center) for helpful discussions and a review of this manuscript. A special thank you to Judah Folkman for taking me (M.K.K.), at the time a young radiation oncologist and molecular biologist, into his laboratory (as a postdoctoral student) for the most inspirational and educational time of my life. J.F. is a truly a scientist of the highest caliber, and a caring physician, helping the smallest and weakest of patients at Children's Hospital. I (M.K.K.) also thank Michael O'Reilly for teaching me how to be a “mouse angiogenesis” guy, and for years of friendship. I (M.K.K.) also give a special thank you to another incredible scientist, Peter Polverini (TOFC), who has been a mentor, a collaborator on new angiogenic projects, and a friend since I have moved to Michigan. S.D.M. is supported in part by National Institutes of Health-RO1 CA77612, NIH 5T32 CA09537-16, and DOD-DAMD17-OD-1-0345.
Abbreviations
- Cp
ceruloplasmin
- MTT
(3-4,5-dimethlythiazol-2yl)-2,5 diphenyltetrazolium bromide
- bFGF
basic fibroblastic growth factor
- LLHM
Lewis lung high metastatic carcinoma
- RT
radiotherapy
- TGF-beta
transforming growth factor-beta
- TM
tetrathiomolybdate
- VEGF
vascular endothelial growth factor
References
- 1.Folkman J. Anti-angiogenesis: new concept for therapy of solid tumors. Ann Surg. 1972;175:409–416. doi: 10.1097/00000658-197203000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perez-Atayde AR, Sallan SE, Tedrow U, Connors S, Allred E, Folkman J. Spectrum of tumor angiogenesis in the bone marrow of children with acute lymphoblastic leukemia. Am J Pathol. 1997;150:815–821. [PMC free article] [PubMed] [Google Scholar]
- 3.Nelson NJ. Inhibitors of angiogenesis enter phase III testing. J Natl Cancer Inst. 1998;90:960–963. doi: 10.1093/jnci/90.13.960a. [DOI] [PubMed] [Google Scholar]
- 4.Kerbel RS. Tumor angiogenesis: past, present and the near future. Carcinogenesis. 2000;21:505–515. doi: 10.1093/carcin/21.3.505. [DOI] [PubMed] [Google Scholar]
- 5.Iruela-Arispe ML, Dvorak HF. Angiogenesis: a dynamic balance of stimulators and inhibitors. Thromb Haemostasis. 1997;78:672–677. [PubMed] [Google Scholar]
- 6.Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 7.Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1997;18:4–25. doi: 10.1210/edrv.18.1.0287. [DOI] [PubMed] [Google Scholar]
- 8.Shing Y, Folkman J, Sullivan R, Butterfield C, Hurray J, Klagsbrun M. Heparin affinity: purification of a tumor-derived capillary endothelial cell growth factor. Science. 1984;223:1296–1299. doi: 10.1126/science.6199844. [DOI] [PubMed] [Google Scholar]
- 9.Shing Y, Folkman J, Haudenschild C, Lund D, Crum R, Klagsbrun M. Angiogenesis is stimulated by a tumor-derived endothelial cell growth factor. J Cell Biochem. 1985;29:275–287. doi: 10.1002/jcb.240290402. [DOI] [PubMed] [Google Scholar]
- 10.Gospodarowicz D, Cheng J, Lui GM, Baird A, Bohlent P. Isolation of brain fibroblast growth factor by heparin-Sepharose affinity chromatography: identity with pituitary fibroblast growth factor. Proc Natl Acad Sci USA. 1984;81:6963–6967. doi: 10.1073/pnas.81.22.6963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Esch F, Ueno N, Baird A, Hill F, Denoroy L, Ling N, Gospodarowicz D, Guillemin R. Primary structure of bovine brain acidic fibroblast growth factor (FGF) Biochem Biophys Res Commun. 1985;133:554–562. doi: 10.1016/0006-291x(85)90942-8. [DOI] [PubMed] [Google Scholar]
- 12.Pertovaara L, Kaipainen A, Mustonen T, Orpana A, Ferrara N, Saksela O, Alitalo K. Vascular endothelial growth factor is induced in response to transforming growth factor-beta in fibroblastic and epithelial cells. J Biol Chem. 1994;269:6271–6274. [PubMed] [Google Scholar]
- 13.Rastinejad F, Polverini PJ, Bouck NP. Regulation of the activity of a new inhibitor of angiogenesis by a cancer suppressor gene. Cell. 1989;56:345–355. doi: 10.1016/0092-8674(89)90238-9. [DOI] [PubMed] [Google Scholar]
- 14.O'Reilly MS, Holmgren L, Shing Y, Chen C, Rosenthal RA, Moses M, Lane WS, Cao Y, Sage EH, Folkman J. Angiostatin: a novel angiogenesis inhibitor that mediates the suppression of metastases by a Lewis lung carcinoma. [see comments] Cell. 1994;79:315–328. doi: 10.1016/0092-8674(94)90200-3. [DOI] [PubMed] [Google Scholar]
- 15.O'Reilly MS, Boehm T, Shing Y, Fukai N, Vasios G, Lane WS, Flynn E, Birkhead JR, Olsen BR, Folkman J. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88:277–285. doi: 10.1016/s0092-8674(00)81848-6. [DOI] [PubMed] [Google Scholar]
- 16.Watanabe T, Seno M, Sasada R, Igarashi K. Molecular characterization of recombinant human acidic fibroblast growth factor produced in E. coli: comparative studies with human basic fibroblast growth factor. Mol Endocrinol. 1990;4:869–879. doi: 10.1210/mend-4-6-869. [DOI] [PubMed] [Google Scholar]
- 17.Engleka KA, Maciag T. Inactivation of human fibroblast growth factor-1 (FGF-1) activity by interaction with copper ions involves FGF-1 dimer formation induced by copper-catalyzed oxidation. 1994 [PubMed] [Google Scholar]
- 18.Shing Y. Heparin-copper biaffinity chromatography of fibroblast growth factors. J Biol Chem. 1988;263:9059–9062. [PubMed] [Google Scholar]
- 19.Patstone G, Maher P. Copper and calcium binding motifs in the extracellular domains of fibroblast growth factor receptors. J Biol Chem. 1996;271:3343–3346. doi: 10.1074/jbc.271.7.3343. [DOI] [PubMed] [Google Scholar]
- 20.Soncin F, Guitton JD, Cartwright T, Badet J. Interaction of human angiogenin with copper modulates angiogenin binding to endothelial cells. Biochem Biophys Res Commun. 1997;236:604–610. doi: 10.1006/bbrc.1997.7018. [DOI] [PubMed] [Google Scholar]
- 21.Badet J, Soncin F, Guitton JD, Lamare O, Cartwright T, Barritault D. Specific binding of angiogenin to calf pulmonary artery endothelial cells. Proc Natl Acad Sci USA. 1989;86:8427–8431. doi: 10.1073/pnas.86.21.8427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parke A, Bhattacherjee P, Palmer RM, Lazarus NR. Characterization and quantification of copper sulfate-induced vascularization of the rabbit cornea. Am J Pathol. 1988;130:173–178. [PMC free article] [PubMed] [Google Scholar]
- 23.Raju KS, Alessandri G, Ziche M, Gullino PM. Ceruloplasmin, copper ions, and angiogenesis. J Natl Cancer Inst. 1982;69:1183–1188. [PubMed] [Google Scholar]
- 24.Brem SS, Zagzag D, Tsanaclis AM, Gately S, Elkouby MP, Brien SE. Inhibition of angiogenesis and tumor growth in the brain. Suppression of endothelial cell turnover by penicillamine and the depletion of copper, an angiogenic cofactor. Am J Pathol. 1990;137:1121–1142. [PMC free article] [PubMed] [Google Scholar]
- 25.Brem S, Tsanaclis AM, Zagzag D. Anticopper treatment inhibits pseudopodial protrusion and the invasive spread of 9L gliosarcoma cells in the rat brain. Neurosurgery. 1990;26:391–396. doi: 10.1097/00006123-199003000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Yoshida D, Ikeda Y, Nakazawa S. Suppression of tumor growth in experimental 9L gliosarcoma model by copper depletion. Neurol Med-Chir. 1995;35:133–135. doi: 10.2176/nmc.35.133. [DOI] [PubMed] [Google Scholar]
- 27.Brewer GJ, Johnson V, Dick RD, Kluin KJ, Fink JK, Brunberg JA. Treatment of Wilson disease with ammonium tetrathiomolybdate: II. Initial therapy in 33 neurologically affected patients and follow-up with zinc therapy. Arch Neurol. 1996;53:1017–1025. doi: 10.1001/archneur.1996.00550100103019. [DOI] [PubMed] [Google Scholar]
- 28.Linder MC, Houle PA, Isaacs E, Moor JR, Scott LE. Copper regulation of ceruloplasmin in copper-deficient rats. Enzyme. 1979;24:23–35. doi: 10.1159/000458625. [DOI] [PubMed] [Google Scholar]
- 29.Merajver SD, Irani J, van Golen K, Brewer G. In Proc AACR Special Conference on Angiogenesis and Cancer. 1998 [Google Scholar]
- 30.Brewer GJ, Dick RD, Grover DK, LeClaire V, Tseng M, Wicha M, Pienta K, Redman BG, Jahan T, Sondak VK, Strawderman M, LeCarpentier G, Merajver SD. Treatment of metastatic cancer with tetrathiomolybdate, an anticopper, antiangiogenic agent: phase I study. Clin Cancer Res. 2000;6:1–10. [PubMed] [Google Scholar]
- 31.Teicher BA, Holden SA, Dupuis NP, Kakeji Y, Ikebe M, Emi Y, Goff D. Potentiation of cytotoxic therapies by TNP-470 and minocycline in mice bearing EMT-6 mammary carcinoma. Breast Cancer Res Treat. 1995;36:227–236. doi: 10.1007/BF00666043. [DOI] [PubMed] [Google Scholar]
- 32.Teicher BA, Depuis NP, Kusumoto T, Robinson MF, Liu F, Menon K, Coleman N. Antiangiogenic agents can increase tumor oxygenation and response to radiation therapy. Radiat Oncol Invest. 1995;2:269–276. [Google Scholar]
- 33.Mauceri HJ, Hanna NN, Beckett MA, Gorski DH, Staba MJ, Stellato KA, Bigelow K, Heimann R, Gately S, Dhanabal M, Soff GA, Sukhatme VP, Kufe DW, Weichselbaum RR. Combined effects of angiostatin and ionizing radiation in antitumour therapy. Nature. 1998;394:287–291. doi: 10.1038/28412. [DOI] [PubMed] [Google Scholar]
- 34.Gorski DH, Beckett MA, Jaskowiak NT, Calvin DP, Mauceri HJ, Salloum RM, Seetharam S, Koons A, Hari DM, Kufe DW, Weichselbaum RR. Blockage of the vascular endothelial growth factor stress response increases the antitumor effects of ionizing radiation. Cancer Res. 1999;59:3374–3378. [PubMed] [Google Scholar]
- 35.Kishi K, Petersen S, Petersen C, Hunter N, Mason K, Masferrer JL, Tofilon PJ, Milas L. Preferential enhancement of tumor radioresponse by a cyclooxygenase-2 inhibitor. Cancer Res. 2000;60:1326–1331. [PubMed] [Google Scholar]
- 36.Schosinsky KH, Lehmann HP, Beeler MF. Measurement of ceruloplasmin from its oxidase activity in serum by use of o-dianisidine dihydrochloride. Clin Chem. 1974;20:1556–1563. [PubMed] [Google Scholar]
- 37.Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis — correlation in invasive breast carcinoma. N Engl J Med. 1991;324:1–8. doi: 10.1056/NEJM199101033240101. [DOI] [PubMed] [Google Scholar]
- 38.Umezawa K, Nakazawa K, Uchihata Y, Otsuka M. Screening for inducers of apoptosis in apoptosis-resistant human carcinoma cells. Adv Enzyme Regul. 1999;39:145–156. doi: 10.1016/s0065-2571(98)00022-3. [DOI] [PubMed] [Google Scholar]
- 39.Suginaka R, Izui R, Inoue J, Muraoka Y, Yamaguchi K, Otsuka M, Umezawa K. Induction of apoptosis in human pancreatic carcinoma cells by a synthetic bleomycin-like ligand. Jpn J Cancer Res. 1998;89:947–953. doi: 10.1111/j.1349-7006.1998.tb00653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ho YS, Liu HL, Duh JS, Chen RJ, Ho WL, Jeng JH, Wang YJ, Lin JK. Induction of apoptosis by S-nitrosoglutathione and Cu2+ or Ni2+ ion through modulation of bax, bad, and bcl-2 proteins in human colon adenocarcinoma cells. [PubMed] [Google Scholar]
- 41.Fajardo LF, Berthrong M. Vascular lesions following radiation. Pathol Annu. 1988;23:297–330. [PubMed] [Google Scholar]
- 42.Fajardo LF. The unique physiology of endothelial cells and its implications in radiobiology. Front Radiat Ther Oncol. 1989;23:96–112. doi: 10.1159/000416574. [DOI] [PubMed] [Google Scholar]
- 43.Browder T, Butterfield CE, Kraling BM, Shi B, Marshall B, O'Reilly MS, Folkman J. Antiangiogenic scheduling of chemotherapy improves efficacy against experimental drug-resistant cancer. Cancer Res. 2000;60:1878–1886. [PubMed] [Google Scholar]
- 44.Klement G, Baruchel S, Rak J, Man S, Clark K, Hicklin DJ, Bohlen P, Kerbel RS. Continuous low-dose therapy with vinblastine and VEGF receptor-2 antibody induces sustained tumor regression without overt toxicity. J Clin Invest. 2000;105:R15–R24. doi: 10.1172/JCI8829. [DOI] [PMC free article] [PubMed] [Google Scholar]