Abstract
Bid is the only known Bcl-2 family member that can function as an agonist of proapoptotic Bcl-2-related proteins such as Bax and Bak. Expression of the proapoptotic Bcl-2 family protein Bid was assessed by immunoblotting and immunohistochemical methods in normal murine and human-tissues and in several types of human cancers and tumor cell lines. Bid expression in normal tissues varied widely, with prominent Bid immunostaining occurring in several types of short-lived cells (e.g., germinal center B cells, peripheral blood granulocytes, differentiated keratinocytes) and in apoptosissensitive cells (e.g., adult neurons). Analysis of Bid expression by immunostaining of 100 colon, 95 ovarian, and 254 prostate cancers, as well as 59 brain tumors and 50 lymphomas, revealed evidence of altered Bid regulation in sometypes of cancers. Correlations with clinical outcome data revealed association of higher levels of Bid with longer recurrence-free survival in men with locally advanced (T3 stage) prostate cancer (P=0.04). Immunoblot analysis of Bid protein levels in the NCI's panel of 60 human tumor cell lines revealed a correlation between higher levels of Bid and sensitivity to ribonucleotide reductase (RR)-inhibiting drugs (P<0.0005). Overexpression of Bid in a model tumor cell line by gene transfection resulted in increased sensitivity to apoptosis induction by a RR inhibitor. Taken together, these observations suggest a potential role for Bid in tumor responses to specific chemotherapeutic drugs, and lay a foundation for future investigations of this member of the Bcl-2 family in healthy and diseased tissues.
Keywords: Bid, Bcl-2, apoptosis, cancer, tissue microarrays
Introduction
Apoptosis is an evolutionary conserved process by which multicellular organisms cleanly remove noninstructed, misinstructed, misinstructed, and damaged cells to ensure proper embryonic development, maintenance of tissue homeostasis, and immunologic defense [1]. The Bcl-2 family of proteins plays a key role in processes underlying programmed cell death. This gene family is comprised of antiapoptotic members such as Bcl-2, Bcl-XL, Bcl-w, Mcl-1, Bfl-1, and Bcl-B and proapoptotic proteins, which include Bax, Bak, Bad, Bok, Bik, Bid, Bim, Hrk, Blk, Bnip3, Noxa, Puma, and Bcl-G. One of the features of Bcl-2 family proteins is the formation of homo-and heterodimers, whose relative abundance coincides with either cell death or survival [2,3]. Four conserved domains, called Bcl-2 Homology (BH) domains have been identified in the members of this family [4,5]. The BH3 domain, in addition to being a protein-protein interaction domain, is also required for the death-promoting function of many proapoptotic molecules [6-8]. Several proapoptotic members of the Bcl-2 family possess sequence homology only to the BH3 amphipathic α helical domain. These proteins, termed “BH3 only,” include Bid, Bik, Bad, Bim, Blk, Hrk, Noxa, Puma, and Bcl-GS of mammals and EGL-1 of Caenorhabditis elegans [9–19].
Bid is a BH3-only protein that promotes cell death. Of all of the 26 human Bcl-2 family members, Bid is the only one that has been determined to function as an agonist of proapoptotic Bcl-2 family proteins Bax and Bak [11]. Thus, Bid has special status among the Bcl-2 family members. The Bid protein shares structural similarity with multidomain Bcl-2 family proteins, which are known to be capable of integrating into membranes and forming ion channels or pores [20,21]. Pore formation by Bid, however, requires removal of an N-terminal autorepression domain [22].
One in vivo mechanism for activating Bid involves apoptosis-inducing members of the tumor necrosis factor (TNF) family of cytokine receptors. Unlike many Bcl-2 family proteins, Bid lacks a carboxyl-terminal membrane-anchoring segment and resides mostly in the cytosol of healthy cells [11]. However, the Bid protein can be activated by proteolysis, resulting in its targeting to membranes. Inactive cytosolic p22 Bid is activated through cleavage by caspase-8 following triggering of Fas or TNF receptor 1 (TNFR1) [23–25]. The caspase-8 mediated activation of Bid represents an amplification step in the presence of low caspase-8 levels, recruiting mitochondria into the cell death mechanism [26]. Specifically, caspase-mediated cleavage results in a rapid translocation of the p15 active COOH fragment of Bid (containing the BH3 domain) to mitochondria. Truncated Bid binds latent proapoptotic Bcl-2 family members Bax and Bak, inducing their oligomerization in mitochondrial membranes, and leading to formation of pores that release cytochrome c [23–25,27–31]. The release of cytochrome c from mitochondria promotes the oligomerization in the cytosol of a cytochrome c/Apaf-1/caspase-9 complex, which activates caspase-9 and results in the cleavage of downstream effector proteases, caspase-3, -6 and -7 [32]. An intact BH3 domain within tBid is required for cytochrome c release, but not for targeting mitochondria [31]. Truncated Bid has at least a 10-fold higher affinity toward Bcl-XL and is 100 times more efficient in inducing cytochrome c release from mitochondria compared to its full-length precursor [23]. Thus, Bid connects the TNF family death receptor (extrinsic) pathway for apoptosis and the mitochondria (intrinsic) pathway. Cardiolipin, present in the mitochondrial membranes, has been reported to mediate targeting of tBid to mitochondria by providing a membrane environment conducive to Bid insertion [33]. The posttranslational N-myristoylation of Bid also enhances insertion of this protein into the outer mitochondrial membrane, release of cytochrome c, and cell death [34].
Another mechanism for Bid activation involves granzyme B, a serine aspartyl protease injected into target cells by cytosolic T cells and natural killer (NK) cells [35,36]. Granzyme B directly cleaves Bid, generating a 14-kDa truncated Bid fragment (“gtBid” for granzyme B truncated), and causing caspase-independent disruption of mitochondrial function and cytochrome c release [23,37–39].
A third mechanism for Bid activation involves a rather indirect route. Several reports have demonstrated that caspase-8, and its substrate Bid, are activated in response to certain apoptotic stimuli in a death receptor-independent manner, representing a relatively late event in the mitochondrial pathways for apoptosis [40,41]. Thus, in some cell- and/or signal-type specific scenarios, cytochrome c-mediated activation of caspase-9 and-3 can lead to downstream activation of caspase-8 and subsequent cleavage of Bid [42].
Finally, in addition to caspase-dependent cleavage of Bid, lysosomal proteases have also been shown recently to generate similar length cleaved fragments of this protein, which are competent to induce cytochrome c release from mitochondria [43]. Thus, Bid proteolytic cleavage can be executed in four distinct apoptotic pathways: 1) as an early event in the extrinsic death receptor pathway, 2) as a late event in the intrinsic mitochondrial pathway, 3) granzyme B, and 4) lysosomal proteases [43–45].
The aim of the present study was to provide an overview of the in vivo expression of Bid in normal tissues and cancers, thus providing a better understanding of its contribution to normal tissue homeostasis and human malignancies.
Materials and Methods
Antibodies
Polyclonal antisera for Bid were generated in rabbits using synthetic peptides or recombinant protein immunogens. A peptide (NH2-CSDNSFRRELDALGHELPVLAPQ-amide) corresponding to residues 28 to 50 of huBid, was synthesized with an N-terminal cysteine appended to permit conjugation to maleimide-activated carrier proteins KLH and OVA (Pierce, Rockford, IL), as described previously [46,47]. This peptide conjugate was used to generate a polyclonal antiserum (AR-54) in rabbits. Two additional anti-Bid sera were generated in rabbits using recombinant protein immunogens. Wild-type mouse BID (AR-52) and BIDΔ1–55 (AR-53) were produced as GST fusion proteins from pGEX vectors using Escherichia coli BL21 (DE3) as the host strain. The protein purification method has been described previously [22].
New Zealand white female rabbits were injected subcutaneously with a mixture of 0.25 ml KLH-peptide (1 mg/ml), 0.25 ml OVA-peptide (1 mg/ml), or recombinant protein (0.1 to 0.25 µg protein per immunization) and 0.5 ml Freund's complete adjuvant (dose divided over 10 injection sites) and then boosted three times at weekly intervals, followed by another 3 to 20 boostings at monthly intervals with 0.25 mg each of KLH-peptide, OVA-peptide, or recombinant protein immunogens in Freund's incomplete adjuvant, collecting blood at 1 to 3 weeks after each boosting to obtain immune serum.
Tissue Preparation
Normal tissues for immunohistochemical analysis were derived either from human biopsy and autopsy material (n≥5; Department of Pathology, UCSD, San Diego) or from adult mice of various strains. The tissues were fixed in either neutral-buffered formalin, zinc-buffered formalin (Z-fix; Anatech, Battle Creek, MI), B5, or Bouin's solution (Sigma, St. Louis, MO), and embedded in paraffin.
Archival Tumor Specimens
Ovarian cancer specimens derived from 95 patients presenting to the Department of Gynecology and Obstetrics of the University of Freiburg/Germany between 1993 and 1998 were included in this study. Clinical data including survival and chemoresponse data were available until July 2000, representing a median follow-up of 35 months.
The prostate cancer specimens were obtained from the archives of the Institutes for Pathology, University of Basel (Basel, Switzerland), the Cantonal Institute for Pathology (Liestal, Switzerland) and the Tampere University Hospital (Tampere, Finland). Tissue samples included 137 primary tumors with stage T1 according to International Union Against Cancer criteria [48], incidentally discovered after transurethral resection for presumed BPH, and 117 primary tumors treated by radical prostatectomy, including 38 specimens with clinical stage T1 to T2 and 79 locally advanced tumors (clinical stage T3).
The colon carcinoma specimens derived from 100 patients were received from the Department of Pathology, Yonsei University, College of Medicine, Seoul, Korea. All specimens presented stage II tumors (AJCC/UICC). The patients were treated by surgical resection of the involved segment of colon. No postoperative chemotherapy was performed.
Archival paraffin blocks from 59 glioma cases, including 15 astrocytomas, 11 glioblastomas, 20 oligodendrogliomas, and 13 ependymomas were obtained from the Department of Neuropathology at the University of Düsseldorf, Germany.
Non-Hodgkin's lymphoma (NHL) specimens employed for these studies represented archival paraffin blocks obtained from ECOG trial (#6491). The material included 29 diffuse large lymphomas, 12 follicular lymphomas and 9 cases containing both components.
Tissue Array Construction
For the characterization of Bid expression in normal tissues, we constructed two tissue microarrays each containing 130 specimens, representing 0.6- or 1-mm (diameter) cylindrical cores acquired from paraffin blocks of normal human or mouse tissues which were sectioned at 4- to 5-µm thickness. To construct ovarian cancer, colon cancer, and glioma tissue microarrays, two to five cylinders of 1-mm-diameter tissue were taken from representative areas of each archival paraffin block and arrayed into a new recipient paraffin block with a custom-built precision instrument (Beecher Instruments, Silver Spring, MD). Serial sections (4 µm) were applied to 3-aminopropyltriethoxysilane (APES)-coated slides (Sigma), as described [49]. These materials had been fixed in 8% formalin and paraffin-embedded according to routine procedures. The prostate tissue microarray was constructed as described previously [50].
Immunohistochemistry
Dewaxed tissue sections were exposed to polyclonal antibodies (PABs) generated against synthetic peptides and confirmed to be specific for Bid. The sections were immunostained using a diaminobenzidine (DAB)-based detection method as described in detail, employing either an avidin-biotin complex reagent (Vector Laboratories, Burlingame, CA) [47] or the Envision-plus-horse radish peroxidase (HRP) system (DAKO, Carpinteria, CA) using an automated immunostainer (Dako universal staining system) [51]. The dilutions of antisera typically employed were 1:3000 (v/v) for AR-54, and 1:8000 for AR-52 and AR-53.
For all tissues examined, the immunostaining procedure was performed in parallel using preimmune serum to verify specificity of the results. Initial confirmations of antibody specificity also included experiments in which antiserum was preabsorbed with 5 to 10 µg/ml of either synthetic peptide immunogen or recombinant protein immunogen.
The immunostaining results were arbitrarily scored according to intensity as 0, negative; 1+, weak; 2+, moderate; and 3+, strong. For lymphomas, samples were additionally scored for percentage of immunopositive malignant cells, estimating the percentage in increments of 10% (0%, 10%, 20%, 30%, and so on) from a minimum of five representative medium-power fields. Comparisons were made with the germinal centers from non-neoplastic lymph nodes (n=6). The scoring of immunostaining for all other tumors was based on the percentage of immunopositive cells (0 to 100) multiplied by staining intensity score (0/1/2/3), yielding scores of 0 to 300.
Statistical Analysis
Data were analyzed using the JMP Statistics software package (SAS Institute, Cary, NC), and STATISTICA Software (StatSoft, Tulsa, OK). Comparisons of Bid immunostaining data with patient survival were made using the Kaplan-Meier method. An unpaired t test method was used for correlation of Bid immunoscores with the available patient data. For the ovarian cancer analysis, statistical significance of differences in clinical responses to chemotherapy was assessed using the unpaired t test and χ2 test, where nonresponders (NRs) were compared with patients who achieved either partial response or complete response (R for responders).
Cell Isolation and Culture
Established tumor cell lines were cultured in either RPMI or DMEM with 10% FBS [52]. These cell lines included the NCI panel of 60 human tumor cell lines [53], plus 13 additional human cancer cell lines maintained in our laboratory: 7 breast cancer cell lines (231, BT474, HS574, A1N4, 10A, 468 and ZR751), 5 prostate cancer lines (PPC1, ALVA31, °CA1, LNCap and TSU-PRL) and the lymphoma line RS11846.
A total of 4x104 PC-3 cells were seeded in six-well plates (30 mm diameter) and transfected with 3 µg of either pcDNA3 or pcDNA3-Bid using lipofectamine-plus reagent (Gibco-BRL, Grand Island, NY). After 24 h, cells were left untreated or were treated with 10 nM pyrimidine 5-glycodialdehyde, and incubated for another 24 hours before assessing the induction of apoptosis by staining of cells with 5 µg/ml of Hoechst reagent (Aldrich, Milwaukee, WI).
Immunoblotting
Mouse and human tissue lysates as well as lysates from cultured cells were normalized for total protein content (50 µg and 100 µg per lane, respectively) and subjected to SDSPAGE/immunoblot analysis, using 1:1000 to 1:5000 (v/v) dilutions of anti-Bid antisera, and secondary HRP conjugated goat anti-rabbit antibody (1:3000 v/v dilution) (Bio-rad, Hercules, CA), with detection accomplished using an enhanced chemiluminescence (ECL) (Amersham-based) multiple antigen detection (MAD) immunoblotting method that allows for multiple reprobings of blots without antibody stripping, as described previously [54,55].
Plasmids encoding Bcl-2, Bcl-XL, Bax, Bak, Bid, and Bad were translated in TNT-reticulocyte lysates using either T3 or T7 RNA polymerase (Promega) according to the manufacturer's protocol, in the presence of [35S]-labeled l-methionine (∼1 mCi/mmol) (Amersham, Piscataway, NJ). Translation reactions (5 µl) were subjected to SDS-PAGE/immunoblot analysis (Figure 1A), using polyclonal Bid antiserum (AR-54) and ECL (Amersham) detection method.
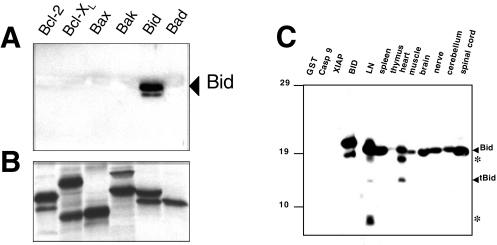
Figure 1.
Bid antibody specificity and expression of Bid protein in normal mouse tissues characterized by immunoblotting. The in vitro translated Bcl-2, Bcl-XL, Bax, Bak, Bid, and Bad proteins were subjected to SDS-PAGE/immunoblot analysis (panel A), using polyclonal Bid antiserum (AR-54). Incubation with Bid antiserum detected only Bid in vitro translated protein. Panel B shows the analysis by SDS-PAGE and fluorography of each translation reaction, confirming synthesis of the Bcl-2 family proteins. In (C), lysates from various mouse tissues were normalized for total protein content (50 µg per lane) and subjected to SDS-PAGE/immunoblot analysis using mouse-specific AR-52. As a control, recombinant GST, caspase-9, XIAP, and Bid proteins were also included in the gel (10 ng). Arrowheads indicate the positions of the full-length (uncleaved) ∼22-kDa Bid and the ∼15-kDa form of Bid typical of the caspase-cleavage. Additional bands representing partial degradation products are indicated by asterisks (*).
Statistical Analysis of Cell Line Data
Comparisons of Bid protein levels and in vitro chemosensitivity data for the NCI panel of 60 human tumor cell lines resulted in a rank-order correlation. P values were calculated from two-tailed t distributions and Pearson correlation coefficients and adjusted using a Bonferroni correction for multiple correlations [53].
Results
Characterization of Anti-Bid Antibodies
The antisera were raised against huBid amino acids 28 to 50 (AR-54), and against recombinant proteins fusing GST to either a full-length mouse Bid (AR-52) or a fragment of mouse Bid containing amino acids 1 to 55 (AR-53). When tested against several in vitro translated Bcl-2 family proteins by immunoblotting, these antisera reacted only with Bid (Figure 1A). Figure 1B shows the analysis by SDS-PAGE and fluorography of each translation reaction, confirming synthesis of the [35S]-labeled Bcl-2 family proteins. These anti-Bid antisera also displayed specific reactivity against recombinant Bid protein produced in bacteria (Figure 1C).
Immunoblot Analysis of Bid in Normal Tissues
Detergent lysates were prepared from normal mouse tissues, normalized for total protein content, and analyzed by SDS-PAGE/immunoblotting using antisera specific for Bid. The most abundant amounts of the Bid protein were detected in lymph node, spleen, spinal cord, cerebrum, cerebellum, peripheral nerve, and heart. Bid was far less prevalent in mouse thymus and skeletal muscle (Figure 1C).
The endogenous ∼22-kDa mouse Bid protein was the most abundant band detected in SDS-PAGE/immunoblot assays (Figure 1C). However, additional minor bands of lower molecular weight were seen in some cases, presumably representing cleaved or partial degradation Bid products, which may arise during tissue harvesting or processing. Some of these smaller forms of Bid do not appear to represent caspase-cleaved Bid, based on comparisons of their mobility in SDS-PAGE with recombinant tBid (∼15 kDa).
Immunohistochemical Analysis of Bid in Normal Mouse and Human Tissues
Using antisera specific for Bid, the in vivo patterns of expression of this protein were examined in normal human and murine tissues by immunohistochemistry. The expression pattern was very similar in human and mouse tissues. The Bid immunostaining was confined to the cytosol, frequently revealing a coarse granular pattern. Representative examples of immunostaining results are provided in Figure 2. It should be noted that because of the nonquantitative nature of immunohistochemistry, these data should be interpreted as only approximations of the relative amounts of Bid in various types of cells in vivo.
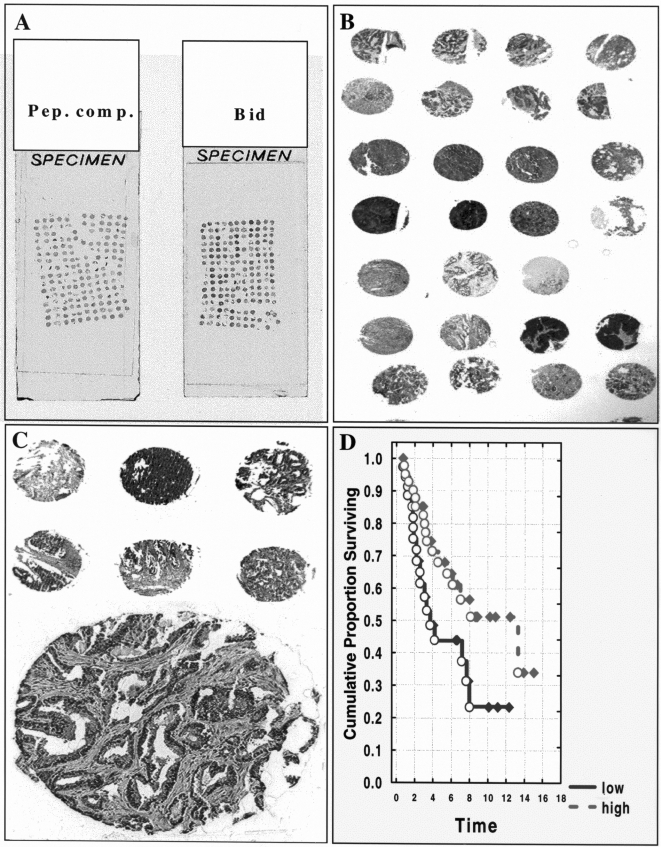
Figure 2.
Immunohistochemical analysis of Bid expression in normal mouse and human tissues. (A) In mouse spleen, Bid is expressed in the marginal zone where T and B lymphocytes are loosely arranged encircling the parietal lymphatic sheath (original magnification, x100). (B, C) Bid immunostaining is shown for a human reactive lymph node. In (B), Bid staining predominates in the large lymphocytes within the germinal center of a secondary follicle. In contrast to the germinal center, lymphocytes in the mantle zone are mostly negative for Bid (original magnification, x400). In (C), interfollicular dendritic cells contain high levels of Bid (x1000). (D) Mouse brain (cortex) is shown. The cell bodies and dendrites of cortical pyramidal neurons display intense staining for Bid (x1000). (E, F) Bid immunostaining in stratified squamous epithelium of the epidermis and cervix are shown, respectively (x1000). Note strong Bid immunoreactivity in cells of the upper layers of these epithelia, and absence of Bid staining in undifferentiated cells along the basement membrane.
Bid immunoreactivity was detected in neurons in the peripheral and central nervous system. The peripheral ganglia of the submucosal (Meissner's) and myenteric (Auerbach's) plexus of the gastrointestinal tract, dorsal root ganglia of spinal nerves, and axons of peripheral nerves contained moderate levels of this protein. In the spinal cord, motoneurons in the ventral horns, sensory neurons in the dorsal horns, and intermediate neurons showed intense Bid staining. In addition to neuronal cytosolic staining, Bid was also present in dendrites (Figure 2D). This proapoptotic protein was also seen in glial cells. The most pronounced expression was found in fibrillary astrocytes of the white matter. The oligodendroglia contained lower levels of Bid and microglial cells were negative.
The lymphoid system is another site of prominent Bid expression. The highly apoptosis-prone germinal center B cells (both small and large cells) of secondary lymphoid follicles in lymph nodes, tonsils, and spleen contained moderate to strong Bid immunostaining, whereas the surrounding long-lived mantle zone lymphocytes were either only weakly positive or immunonegative (Figure 2B). The weak to moderate staining was present in some of the large transformed lymphocytes but small resting lymphocytes in this region were typically immunonegative for Bid. Thus, expression of Bid in lymphocytes may be dynamically regulated in vivo with changes in lymphocyte activation. In the interfollicular region of lymph nodes, sinus histiocytes and interdigitating dendritic cells contained high levels of Bid protein (Figure 2C). Macrophages in the splenic red pulp showed very intense Bid immunoreactivity. In the white pulp, lymphocytes constituting the marginal zone contained moderate to high Bid staining (Figure 2A). Strong staining of macrophages was also found in other organs, such as lung (alveolar macrophages). In the thymus, the cortical thymocytes were typically negative or weakly positive, whereas roughly half of the medullary thymocytes contained low to moderate levels of Bid. Thus, Bid expression may be modulated during thymocyte differentiation.
Among the hematopoietic cells of the bone marrow, Bidpositive monocytes and mature neutrophils dominated the immunostaining results, followed by weakly positive megakaryocytes. The majority of myeloid precursors contained only weak or no immunostaining. In the peripheral blood, monocytes, granulocytes, and eosinophils showed moderate Bid immunoreactivity.
Bid was expressed at immunodetectable levels in several epithelial tissues. The expression of this protein was strongest in the upper layers of the epidermis and was only weakly present in the basal cells lining the basement membrane (Figure 2E). Similar observations were made for the stratified squamous epithelium of esophagus (not shown) and cervix (Figure 2F). The pseudostratified tracheal and bronchial epithelium, and simple ductal epithelia of the salivary glands, pancreas, liver, and endometrium in uterus, as well as absorptive epithelium in the gastrointestinal tract also stained positive for Bid. The connective tissue fibroblasts contained weak to moderate Bid immunoreactivity.
Hepatocytes and occasional Kupfer cells in the liver demonstrated immunodetectable levels of Bid. In the kidney, the epithelial cells in the thin loops of Henle, distal tubule, and collecting ducts contained moderate levels of this protein.
In the testis, cells in various stages of spermato- and spermiogenesis in the seminiferous tubules showed variable Bid content, with the highest observed in spermatids. In the prostate, the androgen-dependent secretory epithelial cells lining the lumen contained low levels of this protein, whereas the androgen-independent basal cells were immunonegative. Bid expression in the ovary was found in the granulosa cells of the growing and mature follicles, whereas germinal epithelium, oocytes, and stromal cells were mostly immunonegative (not shown). In the breast, weak to moderate immunoreactivity was detected in the cuboidal epithelium lining the alveoli, and in the myoepithelial cells.
Cardiomyocytes and arterial smooth muscle contained weak to moderate immunostaining, whereas resting endothelial cells of established vessels expressed very low or no Bid. Activated endothelial cells at sites of inflammation or angiogenesis were not examined.
Bid Expression in Human Malignancies
The expression of Bid was examined in several types of human cancers by immunohistochemical methods using archival paraffin-embedded tumor specimens. Representative examples of immunostaining results are presented in Figure 3.
Figure 3.
Immunohistochemical analysis of Bid expression in tumors. Representative Bid immunostaining results are presented for microarrays of ovarian (A–B) and prostate (C) cancer specimens. Tissue microarray blocks were prepared using 1-mm-diameter cylindrical punches of fixed tissue from 95 ovarian tumor specimens or 0.4-mm-diameter punches from 175 prostate cancers. (D) Kaplan-Meier analysis is presented for prostate (T3 stage disease) cancer patients, showing the proportion of patients surviving alive without disease (relapse-free survival [RFS]), for data dichotomized into high and low Bid immunoscore groups. In (A–C), examples of Bid immunostaining are presented at original magnifications ranging from 2x to 200x. In (A), replicates of the ovarian cancer array were stained using Bid peptide-preadsorbed antiserum (left), as a control, or with AR-54 anti-Bid antiserum (right).
We determined the immunohistochemical expression of Bid in 59 gliomas, including 15 astrocytotic, 11 glioblastomas, 20 oligodendroglial, and 13 ependymal tumors obtained from surgical resection. The level of Bid expression did not correlate with the grade of malignancy in astrocytic tumors. In ependymomas, high levels of Bid were found in anaplastic ependymomas (mean score=156), whereas low-grade ependymal tumors expressed less protein (mean score=96), although the results are not statistically significant. Comparisons of the immunoscore data for oligodendrogliomas and astrocytomas revealed a statistically significant higher expression of Bid in oligodendroglial tumors (t test P=0.04). No correlation was found between progression of tumors (primary tumors versus recurrences) and Bid immunoscore results when examining either all tumors together or separately by histologic subtype.
The immunoreactivity of Bid was scored in 100 primary colorectal adenocarcinomas, 60 of which contained residual non-neoplastic epithelium for direct comparisons. Compared to normal epithelium, Bid immunoscores were elevated in 34 of 60 carcinomas (57%) (P=0.0002). Patients were divided into three groups based on clinical outcome: 1) those who survived without disease (n=59); 2) survived with disease recurrence (n=10); and 3) those who died from disease (n=31). Of the 34 cases with higher Bid expression 17 (50%) died from disease, 1 (3%) survived with disease recurrence, and 16 (47%) survived without disease. Of the 26 cases with lower Bid expression, 4 (15%) died from disease, 3 (12%) survived with disease recurrence, and 19 (73%) survived without disease. At ∼6.5 years of follow-up, half of the patients with high levels of Bid were alive, whereas 70% of patients with low Bid levels remained alive. However, these differences in survival for high and low Bid expressors did not reach statistical significance. The comparison of mean Bid scores among the three groups revealed no significant differences.
Of the 95 ovarian cancers analyzed for Bid expression (Figure 3A and B), nearly all (94 of 95 cases) contained at least 20% immunopositive cells. Examination of the immunoscoring data as a continuous variable failed to reveal a significant correlation of Bid immunostaining with patient age (≤60 vs. >60 years), clinical stage (FigO I/II vs. III/IV), or response to chemotherapy (responder [CR, PR, NED] versus nonresponder [PD, NC]). The follow-up time was too short for this cohort of patients to assess correlations of Bid with survival.
Tissue samples from 254 primary prostate cancers with stage T1 to T3 disease were scored for Bid protein content (Figure 3C). Bid immunoscores were significantly higher in advanced prostate cancers, as defined by clinical stage (T1 vs. T3; P=0.0002). In the T1 and T2 groups, Bid immunoreactivity did not correlate with survival. However, higher Bid immunoscores were associated with longer recurrence-free survival (RFS) in the T3 group of prostate cancer patients (P=0.04) (Figure 3D). No significant correlation of Bid immunostaining with patient age (≤70 vs. >70 years), or Gleason grading was found.
The expression of Bid was explored in B-cell NHLs. Patient specimens employed for these studies represented archival paraffin blocks obtained from a variety of ECOG trials (EST 6494). A total of 50 lymphoma specimens were immunostained. Based on comparisons with non-neoplastic nodes, the presence of ≥20% immunopositive malignant lymphocytes was chosen as a cut-off for dichotomizing immunostaining data into positive versus negative groups. Using this method, 33 of 50 (66%) lymphomas were determined to be Bid-positive. Larger proportions of lymphomas with diffuse (22 of 29; 76%) versus nodular (follicular) (3 of 12; 25%) cytoarchitecture were Bid immunopositive (P=0.002). Also, when immunoscore data (intensityxpercentage) were analyzed, diffuse lymphomas were found to have an average of five-fold higher expression of Bid compared to follicular lymphomas (scores 138±14.5 vs. 27±22.5) (P=0.0007). The expression of Bid in lymphomas with mixed follicular and diffuse histology (n=9; score 113) was similar to diffuse lymphomas (score 113±26.0 [n=9] vs. 138±14.5 [n=29]).
Immunoblot Analysis of Bid Protein in the NCI Panel of Tumor Cell Lines
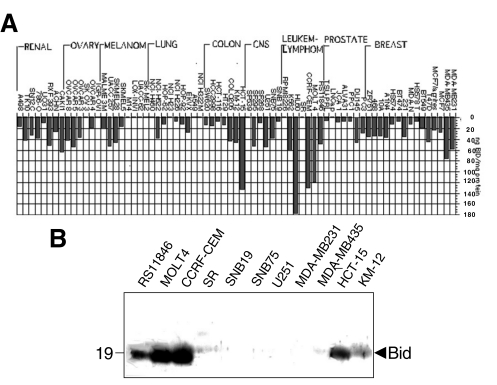
The relative levels of Bid protein in the NCI 60 tumor cell line panel and in additional prostate and breast cancer cell lines were characterized by immunoblot assay. ECL data on X-ray films were quantified by scanning densitometry and the relative amounts of Bid were estimated for each tumor cell line, using recombinant GST-Bid protein to generate standard curves for extrapolation, essentially as described [52]. Figure 4A summarizes the results, expressing the data as nanograms of Bid protein per milligram of total protein. Among the various tumor cell lines tested, leukemia and lymphoma cell lines contained the highest amounts of Bid (Figure 4A and B). The promyelotic leukemia line HL60, acute lymphoblastic leukemia line CCRF-CEM, and T-cell acute lymphoblastic leukemia MOLT 4 expressed the highest levels of this protein among all tumor lines examined.
Figure 4.
Immunoblot analysis of Bid protein in the NCI panel of tumor cell lines. The relative levels of Bid protein in the NCI 60 tumor cell line panel and in additional prostate and breast cancer lines were characterized by immunoblot assay. ECL data on X-ray films were quantified by scanning densitometry and the relative amounts of Bid were estimated for each tumor cell line, using recombinant GST-Bid protein to generate standard curves for extrapolation, essentially as described [52]. (A) summarizes the results, expressing the data as nanograms of Bid protein per milligram of total protein. (B) provides examples of Bid immunoblot data for 11 tumor lines.
Bid protein was expressed at low to moderate levels in most tumor cell lines, including breast, ovarian, colon, prostate cancer, lung cancer, and CNS tumors. However, some tumor cell lines lacked a detectable expression of Bid protein, including 4 of 7 melanomas, 4 of 9 lung cancers, and 2 of 6 ovarian cancer cell lines (Figure 4A).
Bid Expression Correlates with Cell Sensitivity to Ribonucleotide Reductase Inhibitors
Bid protein levels were correlated with the chemosensitivity data for compounds tested on the NCI 60 cell line panel (Table 1). Bid expression positively correlated with sensitivity to pyrimidine 5-glycodialdehyde and diglycoaldehyde, two ribonucleotide reductase inhibitors, as well as to pyrazine diazohydroxide (PZDH), an antitumor agent that forms DNA adducts through the reactive pyrazine diazonium ion.
Table 1.
Bid Expression Correlates with Tumor Cell Line Sensitivity to Ribonucleotide Reductase Inhibitors
| Rank | Name of drug | Pearson | P (two tail) |
| 1 | Pyrimidine 5-glycodialdehyde | 0.5 | 0.00004 |
| 2 | Diglycoaldehyde | 0.49 | 0.00008 |
| 3 | PZDH | 0.47 | 0.00016 |
| 4 | Fluorodopan | 0.44 | 0.00037 |
| 5 | Hydroxyurea | 0.43 | 0.00062 |
| 6 | Asaley | 0.4 | 0.00173 |
| 7 | Batracyclin | 0.39 | 0.00211 |
| 8 | Carmethizole | 0.39 | 0.00221 |
| 9 | Melphalan | 0.38 | 0.00257 |
| 10 | BCNU | 0.38 | 0.00291 |
| 13 | Chlorambucil | 0.35 | 0.00632 |
| 27 | Bryostatin 1 | 0.29 | 0.02534 |
| 34 | Fludarabine-PO4 | 0.27 | 0.03943 |
| 73 | VP-16 | 0.16 | 0.22201 |
| 92 | Methotrexate | 0.12 | 0.36811 |
| 109 | Cisplatinum | 0.09 | 0.45489 |
| 113 | Flavopiridol | 0.09 | 0.47077 |
| 120 | Tamoxifen | 0.09 | 0.50519 |
| 127 | Vincristine sulfate | 0.07 | 0.56246 |
| 150 | Paclitaxel | 0.03 | 0.81474 |
Bid protein levels were correlated with chemosensitivity data for compounds tested on the NCI 60 cell line panel, and the results were analyzed using a Bonferroni correction for multiple correlations and calculating P values from two-tailed t distributions and Pearson correlation coefficients, essentially as described [53]. Because of the large number of comparisons performed, P≤0.0003 was considered statistically significant, as explained previously [53]. Results were ranked according to Pearson coefficients.
Note that Bid expression positively correlated with the sensitivity to pyrimidine 5-glycodialdehyde and diglycoaldehyde, two ribonucleotide reductase inhibitors, as well as to PZDH, an antitumor agent that forms DNA adducts through the reactive pyrazine diazonium ion.
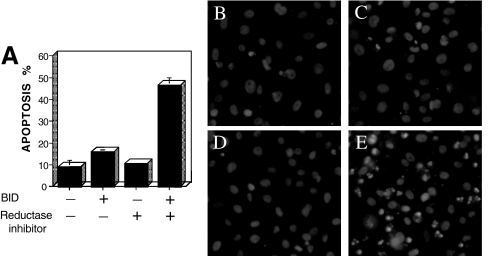
To explore the role of Bid in cell death induction by ribonuclease inhibitors, we tested whether Bid overexpression sensitized cells to pyrimidine 5-glycodialdehyde. Prostate cancer PC-3 cell were transfected with pcDNA3 or with pcDNA3-Bid and treated with this compound. As shown in Figure 5A – D, neither the transfection with pcDNA3-Bid alone (panel B) nor the incubation of pcDNA3 PC-3 cells with 10 nM pyrimidine 5-glycodialdehyde (panel C) led to cell death. However, incubation of the Bid-transfected cells with 10 nM pyrimidine 5-glycodialdehyde induced apoptosis (panel D), thus confirming a potential role of the Bid protein in sensitizing tumors to this agent.
Figure 5.
Bid overexpression sensitizes tumor cells to pyrimidine 5-glycodialdehyde. Prostate cancer PC-3 cells (4x104 cells in six-well plates) were transfected with 3 µg pcDNA3 or pcDNA3-Bid, and cultured for 24 hours, before adding 10 nM pyrimidine 5-glycodialdehyde (PGA) or control diluent and 5 µg/ml of Hoechst reagent (Aldrich). (A) After 24 hours, the percentage of cells with apoptotic morphology (condensed chromatin and fragmented nuclei) was determined (mean+SE; n=3). (B–D) Representative photomicrographs of Hoechst-stained cells are presented for PC3 cells, as follows (B) PC3/pcDNA3; (C) PC3/pcDNA3 treated with PGA; (D) PC3/pcDNA3-Bid; and (E) PC3/pcDNA3-Bid treated with PGA.
Discussion
By generating specific anti-Bid sera, we have explored the in vivo expression of this Bcl-2 family protein in normal tissues and several types of cancer using an immunohistochemical approach. As implied by previous Northern blotting experiments [11], the expression of Bid is widespread, suggesting a role for this protein in the regulation of cell life and death in various types of cells. The survey of Bid expression in normal tissues suggests a prominent role for this protein in the nervous and lymphoid systems.
Interestingly, Bid appears to be present in cells that are prone to apoptosis, such as the cells in upper layers of stratified epithelia, enterocytes toward the apex of the intestinal villi, terminally differentiated neurons, maturing spermatids, and germinal center B cells. In spite of a relatively ubiquitous presence of this proapoptotic protein in normal tissues, mice deficient for Bid have no apparent developmental abnormalities [56], thus illustrating the redundancy of mechanisms regulating cell death. However, it is possible that defects in apoptosis could occur in Bid-deficient mice during times of stress. For example, when Bid-deficient mice were challenged with anti-Fas antibody, hepatocyte apoptosis was reduced compared to normal animals [56]. Thus, although not required for developmental cell death or for normal cell turnover in the adult, the Bid protein may assume important roles in disease situations, particularly where inflammatory stimuli invoke elaboration of ligands of TNF family death receptors that are known to activate Bid through caspase-8-mediated cleavage.
Immunohistochemical comparisons of Bid immunointensity suggest that the expression of Bid may become elevated in some tumors, such as gliomas, colon carcinomas, and prostate cancers. Higher levels of Bid were also found in more advanced (T3) prostate cancers compared to earlier stage (T1, T2) tumors and in lymphomas with more advanced histology (diffuse large cell versus follicular small cell). It is possible that elevated levels of this proapoptotic protein are an indication of other compensatory defects in apoptosis mechanisms that allow tumor cells to tolerate high levels of Bid. For example, Bid cannot kill cells if either Bax or Bak is not intact [57]. Also, phosphorylation of Bid by casein kinases has recently been shown to render it resistant to cleavage by caspase-8 [58]. Given that roughly half of aggressive lymphomas are generally curable with combination chemotherapy, whereas low-grade lymphomas are not [59], it is interesting that Bid was higher in the lymphomas with more advanced histology.
In the NCI panel of 60 human tumor cell lines, Bid expression correlated positively with the sensitivity to pyrimidine 5-glycodialdehyde and diglycoaldehyde, two ribonucleotide reductase inhibitors, as well as to PZDH, an antitumor agent that forms DNA adducts through the reactive pyrazine diazonium ion. Statistical comparisons of Bid expression versus the chemosensitivity to another ribonucleotide reductase inhibitor, hydroxyurea, were of borderline significance, but this agent also inhibits DNA polymerases [60]. These findings are unlikely to be fortuitous, given that such correlations were not seen for several other Bcl-2 family proteins, including BAD, Bcl-2, Bcl-XL, Bax, and Bak [52] (and unpublished observations). Further evidence of a role for Bid in dictating sensitivity of tumor cells to ribonucleotide reductase inhibitors was obtained through gene transfer experiments. Whereas control PC3 prostate cancer cells were insensitive to cell death induction by 10 nM pyrimidine 5-glycodialdehyde, incubation of the Bid-transfected PC-3 cells with 10 nM pyrimidine 5-glycodialdehyde induced apoptosis, thus confirming a role of this protein in apoptosis mediated by ribonucleotide reductase inhibitors.
Upregulation of enzymes of nucleotide metabolism and DNA biosynthesis, such as ribonucleotide reductase, together with the concomitant down-modulation of the purine and pyrimidine degradation enzymes, helps to support the aberrant proliferation of transformed cells [61]. Ribonucleotide reductases, therefore, are considered excellent targets for cancer chemotherapy. However, like all anticancer drugs, clinical responses to these agents are varied. It will therefore be interesting in future studies to inquire whether differences in tumor levels of Bid represent one of the determinants of variations in clinical responses to ribonucleotide reductase inhibitors. Also of interest is the mechanistic basis for why Bid correlates with sensitivity to ribonucleotide reductase inhibitors and not other classes of anticancer drugs. In this regard, it has been suggested that some anticancer drugs in some types of tumors invoke a pathway for apoptosis involving TNF family death receptors, resulting in activation of caspase-8, and thus creating a scenario where Bid might assume an important role [41,62–68]. Further studies are thus required to gain greater insights into the biologic repercussions and the clinical significance of the variability of Bid expression in human neoplasms.
Acknowledgements
We thank E. Godi for acquisition of clinical data for ovarian cancers, X. Xiao for technical assistance, A. Grotting for initial work on tissue arraying, and R. Cornell for manuscript preparation.
Footnotes
J.M.Z. is currently supported by the Lady Tata Memorial Foundation, and I.M.H. by Deutsche Krebshilfe-Mildred Scheel Stiftung (#D/98/02201). This work was generously supported by National Institutes of Health (NIH) grant NS36821 (S.K.), and GM60554 (J.C.R.), and an award from CaP-CURE.
References
- 1.Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456–1462. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- 2.Chao DT, Korsmeyer SJ. BCL-2 family: regulators of cell death. Annu Rev Immunol. 1998;16:395–419. doi: 10.1146/annurev.immunol.16.1.395. [DOI] [PubMed] [Google Scholar]
- 3.Reed JC. Bcl-2 family proteins. Oncogene. 1998;17:3225–3236. doi: 10.1038/sj.onc.1202591. [DOI] [PubMed] [Google Scholar]
- 4.Reed JC. Double identity for proteins of the Bcl-2 family. Nature. 1997;387:773–776. doi: 10.1038/42867. [DOI] [PubMed] [Google Scholar]
- 5.Kelekar A, Thompson CB. Bcl-2-family proteins: the role of the BH3 domain in apoptosis. Trends Cell Biol. 1998;8:324–330. doi: 10.1016/s0962-8924(98)01321-x. [DOI] [PubMed] [Google Scholar]
- 6.Chittenden T, Flemington C, Houghton AB, Ebb RG, Gallo GJ, Elangovan B, Chinnadurai G, Lutz RJ. A conserved domain in Bak, distinct from BH1 and BH2, mediates cell death and protein binding functions. EMBO J. 1995;14:5589–5596. doi: 10.1002/j.1460-2075.1995.tb00246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hunter JJ, Parslow TG. A peptide sequence from Bax that converts Bcl-2 into an activator of apoptosis. J Biol Chem. 1996;271:8521–8524. doi: 10.1074/jbc.271.15.8521. [DOI] [PubMed] [Google Scholar]
- 8.Zha H, Aime-Sempe C, Sato T, Reed JC. Proapoptotic protein Bax heterodimerizes with Bcl-2 and homodimerizes with Bax via a novel domain (BH3) distinct from BH1 and BH2. J Biol Chem. 1996;271:7440–7444. doi: 10.1074/jbc.271.13.7440. [DOI] [PubMed] [Google Scholar]
- 9.Boyd JM, Gallo GJ, Elangovan B, Houghton AB, Malstrom S, Avery BJ, Ebb RG, Subramanian T, Chittenden T, Lutz RJ, et al. Bik, a novel death-inducing protein shares a distinct sequence motif with Bcl-2 family proteins and interacts with viral and cellular survival-promoting proteins. Oncogene. 1995;11:1921–1928. [PubMed] [Google Scholar]
- 10.Yang E, Zha J, Jockel J, Boise LH, Thompson CB, Korsmeyer SJ. Bad, a heterodimeric partner for Bcl-XL and Bcl-2, displaces Bax and promotes cell death. Cell. 1995;80:285–291. doi: 10.1016/0092-8674(95)90411-5. [DOI] [PubMed] [Google Scholar]
- 11.Wang K, Yin XM, Chao DT, Milliman CL, Korsmeyer SJ. BID: a novel BH3 domain-only death agonist. Genes Dev. 1996;10:2859–2869. doi: 10.1101/gad.10.22.2859. [DOI] [PubMed] [Google Scholar]
- 12.Inohara N, Ding L, Chen S, Nunez G. Harakiri, a novel regulator of cell death, encodes a protein that activates apoptosis and interacts selectively with survival-promoting proteins Bcl-2 and Bcl-X(L) EMBO J. 1997;16:1686–1694. doi: 10.1093/emboj/16.7.1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Conradt B, Horvitz H. The C. elegans protein EGL-1 is required for programmed cell death and interacts with the Bcl-2-like protein CED-9. Cell. 1998;93:519–529. doi: 10.1016/s0092-8674(00)81182-4. [DOI] [PubMed] [Google Scholar]
- 14.Hegde R, Srinivasula SM, Ahmad M, Fernandes-Alnemri T, Alnemri ES. Blk, a BH3-containing mouse protein that interacts with Bcl-2 and Bcl-xL, is a potent death agonist. J Biol Chem. 1998;273:7783–7786. doi: 10.1074/jbc.273.14.7783. [DOI] [PubMed] [Google Scholar]
- 15.OConnor L, Strasser A, O'Reilly LA, Hausmann G, Adams JM, Cory S, Huang DC. Bim: a novel member of the Bcl-2 family that promotes apoptosis. EMBO J. 1998;17:384–395. doi: 10.1093/emboj/17.2.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo B, Godzik A, Reed JC. Bcl-G: a novel proapoptotic member of the Bcl-2 family. J Biol Chem. 2000;27:27. doi: 10.1074/jbc.M005889200. [DOI] [PubMed] [Google Scholar]
- 17.Oda E, Ohki R, Murasawa H, Nemoto J, Shibue T, Yamashita T, Tokino T, Taniguchi T, Tanaka N. Noxa, a BH3-only member of the Bcl-2 family and candidate mediator of p53-induced apoptosis. Science. 2000;288:1053–1058. doi: 10.1126/science.288.5468.1053. [DOI] [PubMed] [Google Scholar]
- 18.Nakano K, Vousden KH. PUMA, a novel proapoptotic gene, is induced by p53. Mol Cell. 2001;7:683–694. doi: 10.1016/s1097-2765(01)00214-3. [DOI] [PubMed] [Google Scholar]
- 19.Yu J, Zhang L, Hwang PM, Kinzler KW, Vogelstein B. PUMA induces the rapid apoptosis of colorectal cancer cells. Mol Cell. 2001;7:673–682. doi: 10.1016/s1097-2765(01)00213-1. [DOI] [PubMed] [Google Scholar]
- 20.Chou JJ, Li H, Salvesen GS, Yuan J, Wagner G. Solution structure of BID, an intracellular amplifier of apoptotic signaling. Cell. 1999;96:615–624. doi: 10.1016/s0092-8674(00)80572-3. [DOI] [PubMed] [Google Scholar]
- 21.McDonnell JM, Fushman D, Milliman CL, Korsmeyer SJ, Cowburn D. Solution structure of the proapoptotic molecule BID: a structural basis for apoptotic agonists and antagonists. Cell. 1999;96:625–634. doi: 10.1016/s0092-8674(00)80573-5. [DOI] [PubMed] [Google Scholar]
- 22.Schendel SL, Azimov R, Pawlowski K, Godzik A, Kagan BL, Reed JC. Ion channel activity of the BH3 only Bcl-2 family member, BID. J Biol Chem. 1999;274:21932–21936. doi: 10.1074/jbc.274.31.21932. [DOI] [PubMed] [Google Scholar]
- 23.Li H, Zhu H, Xu CJ, Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94:491–501. doi: 10.1016/s0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- 24.Luo X, Budihardjo I, Zou H, Slaughter C, Wang X. Bid, a Bcl-2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell. 1998;94:481–490. doi: 10.1016/s0092-8674(00)81589-5. [DOI] [PubMed] [Google Scholar]
- 25.Gross A, Yin XM, Wang K, Wei MC, Jockel J, Milliman C, Erdjument-Bromage H, Tempst P, Korsmeyer SJ. Caspase cleaved BID targets mitochondria and is required for cytochrome c release, while BCL-XL prevents this release but not tumor necrosis factor-R1/Fas death. J Biol Chem. 1999;274:1156–1163. doi: 10.1074/jbc.274.2.1156. [DOI] [PubMed] [Google Scholar]
- 26.Budihardjo I, Oliver H, Lutter M, Luo X, Wang X. Biochemical pathways of caspase activation during apoptosis. Annu Rev Cell Dev Biol. 1999;15:269–290. doi: 10.1146/annurev.cellbio.15.1.269. [DOI] [PubMed] [Google Scholar]
- 27.Desagher S, Osen-Sand A, Nichols A, Eskes R, Montessuit S, Lauper S, Maundrell K, Antonsson B, Martinou JC. Bid-induced conformational change of Bax is responsible for mitochondrial cytochrome c release during apoptosis. J Cell Biol. 1999;144:891–901. doi: 10.1083/jcb.144.5.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eskes R, Desagher S, Antonsson B, Martinou JC. Bid induces the oligomerization and insertion of Bax into the outer mitochondrial membrane. Mol Cell Biol. 2000;20:929–935. doi: 10.1128/mcb.20.3.929-935.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Korsmeyer SJ, Wei MC, Saito M, Weiler S, Oh KJ, Schlesinger PH. Proapoptotic cascade activates BID, which oligomerizes BAK or BAX into pores that result in the release of cytochrome c. Cell Death Differ. 2000;7:1166–1173. doi: 10.1038/sj.cdd.4400783. [DOI] [PubMed] [Google Scholar]
- 30.Ruffolo SC, Breckenridge DG, Nguyen M, Goping IS, Gross A, Korsmeyer SJ, Li H, Yuan J, Shore GC. BID-dependent and BID-independent pathways for BAX insertion into mitochondria. Cell Death Differ. 2000;7:1101–1108. doi: 10.1038/sj.cdd.4400739. [DOI] [PubMed] [Google Scholar]
- 31.Wei MC, Lindsten T, Mootha VK, Weiler S, Gross A, Ashiya M, Thompson CB, Korsmeyer SJ. tBID, a membrane-targeted death ligand, oligomerizes BAK to release cytochrome c. Genes Dev. 2000;14:2060–2071. [PMC free article] [PubMed] [Google Scholar]
- 32.Zou H, Henzel WJ, Liu X, Lutschg A, Wang X. Apaf-1, a human protein homologous to C. elegans CED-4, participates in cytochrome c-dependent activation of caspase-3. Cell. 1997;90:405–413. doi: 10.1016/s0092-8674(00)80501-2. [DOI] [PubMed] [Google Scholar]
- 33.Lutter M, Fang M, Luo X, Nishijima M, Xie X, Wang X. Cardiolipin provides specificity for targeting of tBid to mitochondria. Nat Cell Biol. 2000;2:754–761. doi: 10.1038/35036395. [DOI] [PubMed] [Google Scholar]
- 34.Zha J, Weiler S, Oh KJ, Wei MC, Korsmeyer SJ. Posttranslational N-myristoylation of BID as a molecular switch for targeting mitochondria and apoptosis. Science. 2000;290:1761–1765. doi: 10.1126/science.290.5497.1761. [DOI] [PubMed] [Google Scholar]
- 35.Berke G. The CTL's kiss of death. Cell. 1995;81:9–12. doi: 10.1016/0092-8674(95)90365-8. [DOI] [PubMed] [Google Scholar]
- 36.Shresta S, Pham CT, Thomas DA, Graubert TA, Ley TJ. How do cytotoxic lymphocytes kill their targets? Curr Opin Immunol. 1998;10:581–587. doi: 10.1016/s0952-7915(98)80227-6. [DOI] [PubMed] [Google Scholar]
- 37.Barry M, Heibein JA, Pinkoski MJ, Lee SF, Moyer RW, Green DR, Bleackley RC. Granzyme B short-circuits the need for caspase 8 activity during granule-mediated cytotoxic T-lymphocyte killing by directly cleaving Bid. Mol Cell Biol. 2000;20:3781–3794. doi: 10.1128/mcb.20.11.3781-3794.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heibein JA, Goping IS, Barry M, Pinkoski MJ, Shore GC, Green DR, Bleackley RC. Granzyme B-mediated cytochrome c release is regulated by the Bcl-2 family members Bid and Bax. J Exp Med. 2000;192:1391–1402. doi: 10.1084/jem.192.10.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sutton VR, Davis JE, Cancilla M, Johnstone RW, Ruefli AA, Sedelies K, Browne KA, Trapani JA. Initiation of apoptosis by granzyme B requires direct cleavage of Bid, but not direct granzyme B- mediated caspase activation. J Exp Med. 2000;192:1403–1414. doi: 10.1084/jem.192.10.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Siegel RM, Martin DA, Zheng L, Ng SY, Bertin J, Cohen J, Lenardo MJ. Death-effector filaments: novel cytoplasmic structures that recruit caspases and trigger apoptosis. J Cell Biol. 1998;141:1243–1253. doi: 10.1083/jcb.141.5.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun XM, MacFarlane M, Zhuang J, Wolf BB, Green DR, Cohen GM. Distinct caspase cascades are initiated in receptor-mediated and chemical-induced apoptosis. J Biol Chem. 1999;274:5053–5060. doi: 10.1074/jbc.274.8.5053. [DOI] [PubMed] [Google Scholar]
- 42.Tang D, Lahti JM, Kidd VJ. Caspase-8 activation and bid cleavage contribute to MCF7 cellular execution in a caspase-3-dependent manner during staurosporine-mediated apoptosis. J Biol Chem. 2000;275:9303–9307. doi: 10.1074/jbc.275.13.9303. [DOI] [PubMed] [Google Scholar]
- 43.Stoka V, Turk B, Schendel SL, Kim TH, Chirman T, Snipas SJ, Ellerby LM, Bredesen D, Freeze H, Abrahamson M, Bromme D, Krajewski S, Reed JC, Yin XM, Turk V, Salvesen GS. Lysosomal protease pathways to apoptosis: cleavage of bid, not Pro-caspases, is the most likely route. J Biol Chem. 2000;9:9. doi: 10.1074/jbc.M008944200. [DOI] [PubMed] [Google Scholar]
- 44.Salvesen GS, Dixit VM. Caspases: intracellular signaling by proteolysis. Cell. 1997;91:443–446. doi: 10.1016/s0092-8674(00)80430-4. [DOI] [PubMed] [Google Scholar]
- 45.Thornberry NA, Lazebnik Y. Caspases: enemies within. Science. 1998;281:1312–1316. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- 46.Reed JC, Meister L, Tanaka S, Cuddy M, Yum S, Geyer C, Pleasure D. Differential expression of Bcl2 protooncogene in neuroblastoma and other human tumor cell lines of neural origin. Cancer Res. 1991;51:6529–6538. [PubMed] [Google Scholar]
- 47.Krajewski S, Krajewska M, Shabaik A, Miyashita T, Wang HG, Reed JC. Immunohistochemical determination of in vivo distribution of Bax, a dominant inhibitor of Bcl-2. Am J Pathol. 1994;145:1323–1336. [PMC free article] [PubMed] [Google Scholar]
- 48.Sobin LH, Wittekind C. TNM Classification of Malignant Tumours. 5th Edition. New York: Wiley; 1997. [Google Scholar]
- 49.Rentrop M, Knapp B, Winter H, Schweizer J. Aminoalkylsilane-treated glass slides as support for in situ hybridization of keratin cDNAs to frozen tissue sections under varying fixation and pretreatment conditions. Histochem J. 1986;18:271–276. doi: 10.1007/BF01676237. [DOI] [PubMed] [Google Scholar]
- 50.Kononen J, Bubendorf L, Kallioniemi A, Barlund M, Schraml P, Leighton S, Torhorst J, Mihatsch MJ, Sauter G, Kallioniemi OP. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med. 1998;4:844–847. doi: 10.1038/nm0798-844. [DOI] [PubMed] [Google Scholar]
- 51.Krajewski S, Krajewska M, Ellerby LM, Welsh K, Xie Z, Deveraux QL, Salvesen GS, Bredesen DE, Rosenthal RE, Fiskum G, Reed JC. Release of caspase-9 from mitochondria during neuronal apoptosis and cerebral ischemia. Proc Natl Acad Sci USA. 1999;96:5752–5757. doi: 10.1073/pnas.96.10.5752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kitada S, Krajewska M, Zhang X, Scudiero D, Zapata JM, Wang HG, Shabaik A, Tudor G, Krajewski S, Myers TG, Johnson GS, Sausville EA, Reed JC. Expression and location of proapoptotic Bcl-2 family protein BAD in normal human tissues and tumor cell lines. Am J Pathol. 1998;152:51–61. [PMC free article] [PubMed] [Google Scholar]
- 53.Weinstein JN, Myers TG, O'Connor PM, Friend SH, Fornace AJ, Kohn KW, Fojo T, Bates SE, Rubinstein LV, Anderson NL, Buolamwini JK, van Osdol WW, Monks AP, Scudiero DA, Sausville EA, Zaharevitz DW, Bunow B, Viswanadhan VN, Johnson GS, Wittes RE, Paull KD. An information-intensive approach to the molecular pharmacology of cancer. Science. 1997;275:343–349. doi: 10.1126/science.275.5298.343. [DOI] [PubMed] [Google Scholar]
- 54.Krajewski S, Zapata JM, Reed JC. Detection of multiple antigens on western blots. Anal Biochem. 1996;236:221–228. doi: 10.1006/abio.1996.0160. [DOI] [PubMed] [Google Scholar]
- 55.Krajewski S, Zapata JM, Krajewska M, VanArsdale T, Shabaik A, Gascoyne RD, Reed JC. Immunohistochemical analysis of in vivo patterns of TRAF-3 expression, a member of the TNF receptor-associated factor family. J Immunol. 1997;159:5841–5852. [PubMed] [Google Scholar]
- 56.Yin XM, Wang K, Gross A, Zhao Y, Zinkel S, Klocke B, Roth KA, Korsmeyer SJ. Bid-deficient mice are resistant to Fas-induced hepatocellular apoptosis. Nature. 1999;400:886–891. doi: 10.1038/23730. [DOI] [PubMed] [Google Scholar]
- 57.Cheng EH, Wei MC, Weiler S, Flavell RA, Mak TW, Lindsten T, Korsmeyer SJ. Bcl-2, Bcl-XL sequester BH3 domain-only molecules preventing Bax- and Bak-mediated mitochondrial apoptosis. Mol Cell. 2001;8:705–711. doi: 10.1016/s1097-2765(01)00320-3. [DOI] [PubMed] [Google Scholar]
- 58.Desagher S, Osen-Sand A, Montessuit S, Magnenat E, Vilbois F, Hochmann A, Journot L, Antonsson B, Martinou J. Phosphorylation of Bid by casein kinases I and II regulates its cleavage by caspase 8. Mol Cell. 2001;8:601–611. doi: 10.1016/s1097-2765(01)00335-5. [DOI] [PubMed] [Google Scholar]
- 59.Longo DL. Non-Hodgkin's lymphomas. Curr Opin Hematol. 1994;1:295–302. [PubMed] [Google Scholar]
- 60.Yamanaka K, Hayashi H, Tachikawa M, Kato K, Hasegawa A, Oku N, Okada S. Metabolic methylation is a possible genotoxicity-enhancing process of inorganic arsenics. Mutat Res. 1997;394:95–101. doi: 10.1016/s1383-5718(97)00130-7. [DOI] [PubMed] [Google Scholar]
- 61.Hatse S, De Clercq E, Balzarini J. Role of antimetabolites of purine and pyrimidine nucleotide metabolism in tumor cell differentiation. Biochem Pharmacol. 1999;58:539–555. doi: 10.1016/s0006-2952(99)00035-0. [DOI] [PubMed] [Google Scholar]
- 62.Friesen C, Herr I, Krammer PH, Debatin K-M. Involvement of the CD95 (APO-1/Fas) receptor/ligand system in drug induced apoptosis in leukemia cells. Nat Med. 1996;2:574–577. doi: 10.1038/nm0596-574. [DOI] [PubMed] [Google Scholar]
- 63.Fulda S, Sieverts H, Friesen C, Herr I, Debatin KM. The CD95 (APO-1/Fas) system mediates drug-induced apoptosis in neuroblastoma cells. Cancer Res. 1997;57:3823–3829. [PubMed] [Google Scholar]
- 64.Herr I, Wilhelm D, Bohler T, Angel P, Debatin KM. Activation of CD975 (APO-1/Fas) signaling by ceramide mediates cancer therapy-induced apoptosis. EMBO J. 1997;16:6200–6208. doi: 10.1093/emboj/16.20.6200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fulda S, Los M, Friesen C, Debatin KM. Chemosensitivity of solid tumor cells in vitro is related to activation of the CD95 system. Int J Cancer. 1998;76:105–114. doi: 10.1002/(sici)1097-0215(19980330)76:1<105::aid-ijc17>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 66.Arita K, Utsumi T, Kato A, Kanno T, Kobuchi H, Inoue B, Akiyama J, Utsumi K. Mechanism of dibucaine-induced apoptosis in promyelocytic leukemia cells (HL-60) Biochem Pharmacol. 2000;60:905–915. doi: 10.1016/s0006-2952(00)00406-8. [DOI] [PubMed] [Google Scholar]
- 67.Eichhorst ST, Muerkoster S, Weigand MA, Krammer PH. The chemotherapeutic drug 5-fluorouracil induces apoptosis in mouse thymocytes in vivo via activation of the CD95(APO-1/Fas) system. Cancer Res. 2000;61:243–248. [PubMed] [Google Scholar]
- 68.Engels IH, Stepczynska A, Stroh C, Lauber K, Berg C, Schwenzer R, Wajant H, Janicke RU, Porter AG, Belka C, Gregor M, Schulze-Osthoff K, Wesselborg S. Caspase-8/FLICE functions as an executioner caspase in anticancer drug-induced apoptosis. Oncogene. 2000;19:4563–4573. doi: 10.1038/sj.onc.1203824. [DOI] [PubMed] [Google Scholar]