Abstract
SER virus, a paramyxovirus closely related to simian virus 5, induces no syncytium formation. The SER virus F protein has a long cytoplasmic tail (CT), and truncation or mutations of the CT result in enhanced syncytium formation (S. Seth, A. Vincent, and R. W. Compans, J. Virol. 77:167-178, 2003; S. Tong, M. Li, A. Vincent, R. W. Compans, E. Fritsch, R. Beier, C. Klenk, M. Ohuchi, and H.-D. Klenk, Virology 301:322-333, 2002). We hypothesized that the presence of the long CT serves to stabilize the metastable conformation of the F protein. We observed that the hemifusion, cytoplasmic content mixing, and syncytium formation ability of the wild-type SER virus F coexpressed with the SER virus hemagglutinin-neuraminidase (HN) protein was enhanced, both qualitatively and quantitatively, at elevated temperatures. We also observed enhanced hemifusion, content mixing, and syncytium formation in SER virus F- and HN-expressing cells at reduced pH conditions ranging between 4.8 and 6.2. We have obtained evidence that in contrast to other paramyxoviruses, entry of SER virus into cells occurs by a low-pH-dependent process, indicating that the conversion to the fusion-active state for SER virus F is triggered by exposure to reduced pH.
Enveloped viruses enter host cells by fusion of the viral membrane and a cellular membrane. For many enveloped viruses, such as orthomyxoviruses, rhabdoviruses, and togaviruses, membrane fusion occurs in the endosome after the viral fusion protein has been activated at low pH. However, other viruses such as paramyxoviruses and some retroviruses fuse with the cellular plasma membrane at neutral pH (3, 4, 36). Fusion mediated by most paramyxoviruses requires the interaction of two virion-associated glycoproteins, the hemagglutinin-neuraminidase (HN) that mediates viral attachment and the F protein that mediates subsequent fusion (11, 21). The events that trigger membrane fusion activity by viruses differ significantly for various fusion glycoproteins. In influenza virus, the hemagglutinin (HA) protein is activated to a fusion-competent state by exposure to an acidic environment to refold into its minimal energy state (5, 30). Human immunodeficiency virus (HIV) fusion activity is triggered at neutral pH by association of gp120 with its cellular receptor-coreceptor complex (16, 24). The paramyxovirus F protein is thought to be triggered upon receptor binding by the HN protein (23, 33). The cytoplasmic tail (CT) domain of viral fusion proteins has also been shown to play a regulatory role in membrane fusion. For simian immunodeficiency virus, HIV type 1 (HIV-1), and HIV-2, truncation of the CT causes increased syncytium formation (10, 13, 25, 29, 49). Elongation of the CT of influenza virus HA has been shown to interfere with the formation and enlargement of fusion pores (27), and deacylation of the CT of HA suppresses syncytium formation but has no effect on lipid mixing and calcein transfer (15). For paramyxoviruses, F protein CT truncations in Newcastle disease virus (NDV) and human parainfluenza virus type 3 resulted in greatly reduced syncytium formation (34, 47), whereas truncations of the CT of measles virus caused increased syncytium formation (6). Deletion of the CT of the simian virus 5 (SV5) F protein inhibited fusion pore enlargement (12). The R peptide, consisting of the C-terminal 16 amino acids of the envelope protein of murine leukemia virus, is known to be inhibitory to membrane fusion (18, 22, 32, 45, 46). Truncation and mutations in the murine leukemia virus Env protein showed that the CT and the membrane-spanning region of Env can influence the overall structure of the ectodomain of the protein and that the R-peptide-truncated form of Env exhibited a conformation markedly different from that of the full-length protein (1). Truncation of the CT region of the gB protein of herpesvirus also results in an increase in its cell surface expression and syncytium formation (14).
SER virus is a recently identified virus (20) that was isolated from aborted pigs with porcine respiratory and reproductive syndrome. This virus belongs to the family Paramyxoviridae, genus Rubulavirus, and is very closely related to SV5 but replicates without causing syncytium formation (39). Comparison of the CT sequences between SV5 F (WR strain) and SER virus F revealed the presence of a stop codon at amino acid position 530 in the SV5 F CT that is followed by a 21-amino-acid sequence identical to that of the extended CT of SER virus F. This suggests that SV5 evolved as a more fusogenic virus from a progenitor virus resembling SER virus. In previous studies, it was found that truncation or specific mutations in the long CT domain of SER virus F enhanced the ability to cause syncytium formation (35, 39). Since SER virus is infectious and grows in MDBK cells to titers similar to those seen for SV5, its glycoprotein must be able to support virus entry even though it is unable to induce syncytium formation. We hypothesized that the presence of the long CT in the SER virus F protein serves to stabilize the metastable conformation of the F protein and hence restrict syncytium formation. If this is the case, the conversion to the fusion-active conformation may not occur as efficiently at neutral pH or physiological temperature but may be triggered by exposure to reduced pH or increased temperature. For the present report, we studied the effects of elevated temperature and various pH conditions on SER virus F-induced membrane fusion and we also determined whether the entry of SER virus into cells occurs by a low-pH-dependent process.
BHK21 and MDBK cells were maintained in Dulbecco's modified minimal essential medium supplemented with 10% fetal calf serum (HyClone Laboratories, Logan, Utah). The recombinant vaccinia virus vTF7-3 was kindly provided by Bernard Moss (National Institutes of Health, Bethesda, Md.). The pGEM-3-SER F, pGEM-3-SER HN, and pGEM-3-L539,548A plasmids characterized previously (37, 39) were expressed in BHK21 cells. Rabbit anti-SV5 antibody was a kind gift from R.A. Lamb (Northwestern University). SER virus was propagated in MDBK cells, and the virus titers were determined (using guinea pig erythrocytes [RBCs]) by hemagglutination assay (39). Bafilomycin A and ammonium chloride were obtained from Sigma (St. Louis, Mo.).
Guinea pig RBCs were labeled with the hydrophobic fluorescent dye R18 (Molecular Probes, Eugene, Oreg.) or calcein AM (Molecular Probes, Leiden, The Netherlands) as described by Bagai and Lamb (2). Hemifusion, calcein transfer, and syncytium formation assays were performed in various temperature and pH conditions with BHK21 cells coexpressing the wild-type (wt) or mutant F and HN proteins.
Effect of temperature on hemifusion, content mixing, and syncytium formation.
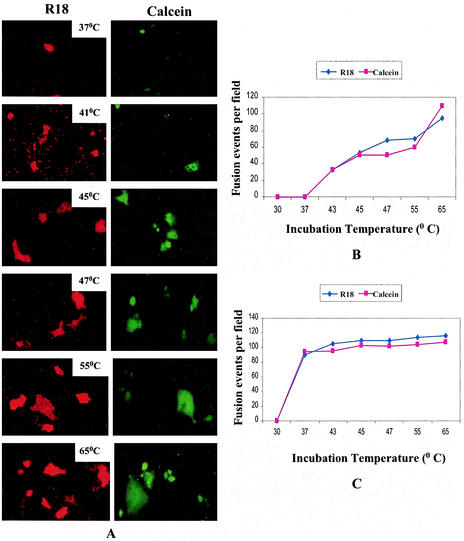
To study whether the SER virus F protein requires additional energy for fusion activation of the metastable state, the effect of elevated temperature on the fusion activity of the SER virus F protein and coexpressed SER virus F and HN proteins was determined at various temperatures from 22 to 65°C. Extensive dye transfer of both R18 and calcein to large syncytia was seen in cells expressing recombinant F/HN that were briefly incubated at temperatures of 45°C or higher (Fig. 1A), whereas no transfer was observed when cells with labeled adsorbed RBCs were incubated at 22 or 30°C. At incubation temperatures of 37 and 39°C, some dye transfer (restricted to single cells) was observed. Dye transfer to smaller syncytia was observed at 41 and 43°C. The labeled syncytia appeared larger at temperatures of 55 or 65°C than at 45 to 47°C. At temperatures higher than 65°C, data were not obtained due to increased cytopathology. Vaccinia virus-infected and mock-transfected cells served as negative controls and showed no dye transfer. In the absence of HN, the SER virus F-expressing cells did not show any lipid mixing or calcein binding at higher temperatures (data not shown).
FIG. 1.
Effect of elevated temperature on hemifusion and content mixing induced by SER virus F/HN. BHK21 cells expressing SER virus F and HN were incubated with guinea pig RBCs labeled with either R-18 or calcein AM at 4°C for 1 h and were then washed to remove unbound RBCs. Cells were further incubated at a temperature of 22, 30, 37, 41, 45, 47, 55, or 65°C for 2 min in a water bath, shifted back to 37°C for 30 min, and observed for dye transfer under an inverted fluorescence microscope (magnification, ×200). Vaccinia virus-infected, mock-transfected cells served as negative controls. (A) Left panels, R-18 transfer (hemifusion); right panels, calcein transfer (content mixing). The incubation temperature for each panel is shown on the panel. (B and C) Extent of fusion expressed as number of lipid-mixing or content-mixing events per microscopic field (averaged over 10 fields) in SER virus F/HN-expressing cells (B) and in SER virus F-L539,548A/HN-expressing cells (C). Differently sized syncytia were scored the same, as we were unable to determine the number of nuclei in the labeled syncytia.
The extent of hemifusion and calcein-binding activities was assayed quantitatively by counting the number of labeled syncytia averaged over 10 fields, and fusion was expressed as the number of lipid-mixing and content-mixing events per microscopic field (Fig. 1B and C). Both hemifusion and content mixing showed an increase at elevated temperatures. It was observed that the extents of lipid-mixing and content-mixing events were fairly coincident and were found to increase with temperature, reaching maximal level at 65°C. In contrast, a mutant SER virus F, L539,548A, which had been shown earlier to induce extensive dye transfer at 37°C (35), showed no significant increase at higher temperatures (Fig. 1C). These results provide evidence that increased temperature enhances the quality and the quantity of the hemifusion and content-mixing events.
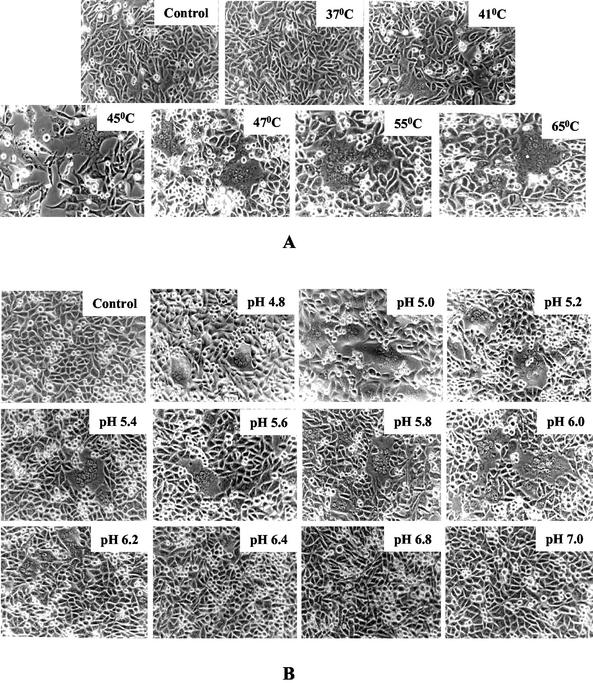
To study the effect of elevated temperature on cell-cell fusion, BHK21 cells which transiently expressed SER virus F/HN glycoproteins were exposed to various temperatures for a brief interval. Extensive cell-cell fusion was observed when cells expressing SER virus F/HN glycoproteins were exposed to higher temperatures of 45°C and above (Fig. 2A). As observed in hemifusion and calcein-binding experiments, the syncytia were found to be larger with incubation at 55 or 65°C. These results indicate that polykaryon formation in SER virus F/HN-expressing cells is also triggered by an increase in temperature.
FIG. 2.
(A) Effect of temperature on syncytium formation. At 16 h posttransfection, BHK21 cells expressing SER virus F and HN were incubated at a temperature of 37, 41, 45, 47, 55, or 65°C for 2 min, shifted back to 37°C, and observed under an inverted light microscope. Vaccinia virus-infected, mock-transfected cells served as negative controls, as shown on the top left panel. Magnification, ×200. (B) Effect of pH on SER virus F/HN-induced syncytium formation. At 16 h posttransfection, BHK21 cells expressing SER virus F and HN were incubated for 2 min at 37°C with various pH buffers as indicated, neutralized with neutral pH buffer, and observed under an inverted light microscope for syncytium formation. Vaccinia virus-infected, mock-transfected cells served as negative controls (top left panel). Magnification, ×200.
Effect of pH on hemifusion, content mixing, and syncytium formation.
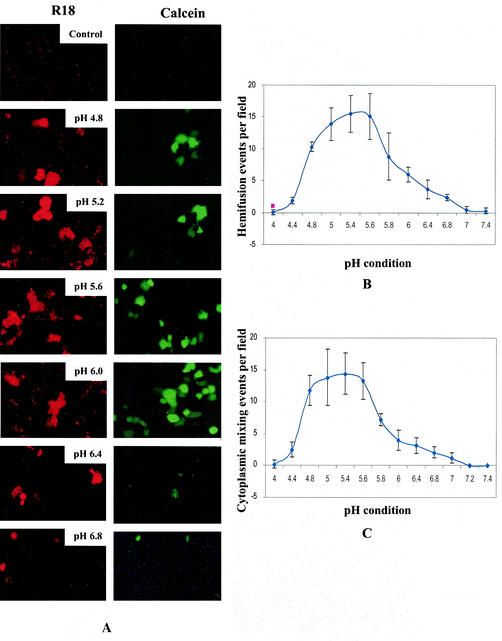
To study the effect of reduced pH on different stages of cell fusion, we assayed syncytium formation, lipid mixing, and content mixing under various pH conditions ranging from pH 4.0 to 7.4 on BHK21 cells coexpressing wt SER virus F/HN proteins (Fig. 2B and 3). Vaccinia recombinant virus-infected BHK21 cells expressing wt or mutant influenza virus HA proteins have been used to study the effect of pH change on membrane fusion, and it was shown that vaccinia virus does not induce low-pH-dependent fusion in this cell type (7, 38). Extensive syncytia were seen in SER virus F/HN-expressing BHK21 cells exposed to buffers with pH ranging from 4.8 to 6.0, but the size and the number of syncytia decreased at pH above 6.0 and no syncytia were observed at pH 6.4 or above (Fig. 2B). No syncytium formation was observed at pH of 4.6 or below (data not shown). These results indicate that a decrease in pH to the range of 4.8 to 6.0 is a trigger for SER virus F-induced syncytium formation activity. We observed significant dye transfer from labeled RBCs to SER virus F/HN-expressing cells incubated at pH 4.8 to 6.2 (Fig. 3A). We observed minimal dye transfer (of both R18 and calcein) restricted to single cells in pH conditions between pH 6.4 and 7.2. Cells expressing SER virus F in the absence of SER virus HN did not show any dye transfer (data not shown). The SER virus L539,548A mutant, which has been found to trigger extensive dye transfer under normal physiological pH conditions, showed extensive lipid mixing, calcein transfer, and cell fusion under a broad range of pH conditions (data not shown). The extent of hemifusion and content mixing was further assessed by determining the number of labeled syncytia averaged over 10 fields; the fusion results are expressed as the number of lipid-mixing and content-mixing events per microscopic field. The maximal dye transfer (representing the fusion events) was observed between pH 5.0 and 5.6, whereas, with an increase in pH to 7.2, dye transfer was significantly reduced (Fig. 3B and C). These results suggest that there is an acid-induced change of the metastable conformation of the SER virus F protein to a fusion-active state.
FIG.3.
Effect of pH on hemifusion and content mixing. BHK21 cells expressing SER virus F and HN were incubated with guinea pig RBCs labeled with either R-18 or calcein AM at 4°C for 1 h and then washed with fusion buffer (pH 7.4) (HEPES-saline buffered with sodium citrate and citric acid) to remove unbound RBCs as described by Steinhauer et al. (38), washed, and incubated for 2 min at 37°C with the buffer adjusted to various pH conditions as shown, followed by a wash with neutral pH buffer and further incubation for 30 min. Cells were observed for dye transfer under a fluorescence microscope (magnification, ×200). (A) Top two panels, vaccinia virus-infected, mock-transfected cells with R18- and calcein-labeled RBCs; other panels, SER virus F/HN-expressing cells with R18- and calcein-labeled RBCs treated under the indicated pH conditions. (B and C) Extent of fusion expressed as number of lipid-mixing (B) and content-mixing (C) events per microscopic field, as averaged over 10 fields. Each error bar represents the standard deviation from the mean.
SER virus entry process.
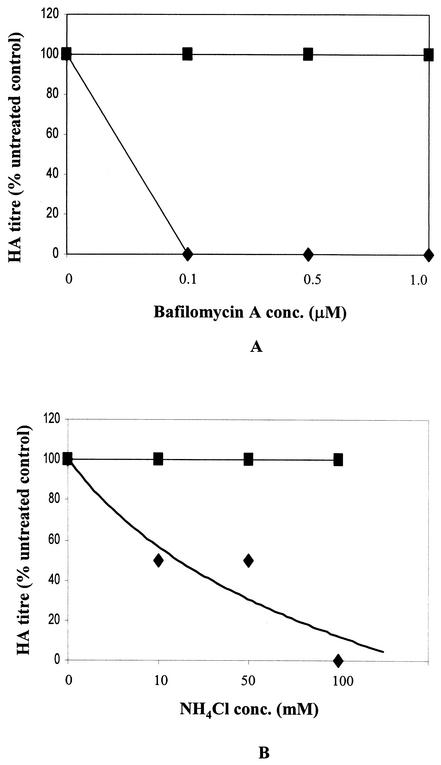
We further investigated the process of virus entry into host cells to determine whether SER virus entry is low-pH dependent. A low-pH-dependent step in virus entry can be demonstrated by the use of compounds that raise the pH in endosomes. The high specificity of bafilomycin A1 (BFLA1) in the inhibition of vacuolar ATPase (9) and the fact that very low concentrations of this compound were observed to inhibit entry of influenza virus, Semliki Forest virus, and Sindbis virus (17, 19) indicate that this compound is useful for investigating enveloped virus entry. The effects of the lysosomotropic agent ammonium chloride on SER virus/SV5-infected cells were also compared. Virus replication was found to be completely inhibited in cells infected with SER virus in the presence of BFLA1 at concentrations as low as 0.1 μM, whereas BFLA1-treated SV5-infected cells showed no change in viral titers (Fig. 4A). SER virus replication was also inhibited in the presence of ammonium chloride at a concentration of 100 mM, whereas SV5 replication was not inhibited (Fig. 4B). SER virus replication was unaffected when the inhibitors were added at 1 h postinfection, indicating that the drugs affect an early step during SER virus entry (data not shown). These results provide evidence that SER virus entry is low-pH dependent.
FIG. 4.
Effect of BFLA1 and ammonium chloride on SER virus infection. MDBK cells were mock infected or infected with SER virus or SV5 at a multiplicity of infection of 10 PFU/cell for 2 h at 37°C in the presence of different concentrations of BFLA1 (A) or ammonium chloride (B). Cells were washed to remove excess virus and inhibitor, followed by incubation with fresh medium. Results for HA titers at 48 h postinfection on culture medium from cells infected or treated with BFLA1 or ammonium chloride at different concentrations or from untreated infected cells are shown as percentages of control (HA titer of untreated SER virus-infected cells, 256 hemagglutinating units; HA titer of SV5-infected cells, 4,096 hemagglutinating units).
The present study analyzed the effect of temperature on different aspects of SER virus F-induced membrane fusion, including hemifusion, content mixing, and syncytium formation. We previously found that cells coexpressing wt SER virus F and HN protein showed minimal lipid mixing or content mixing with labeled RBCs which was restricted to single cells at 37°C (35). At elevated temperatures, we found enhanced dye transfer to syncytia, and at 55°C or 65°C, the dyes were transferred to larger syncytia. A similar temperature dependence was observed when cell-cell fusion was assayed, which supports the conclusion that the extended CT results in an increased requirement for thermal energy for conversion of SER virus F to a fusion-active state. It was previously reported that the SV5 WR strain F protein caused fusion with coexpressed influenza virus HA only at elevated temperatures, indicating that a metastable native fusion-inactive conformation is triggered by elevated temperatures to change to a fusion-active state (28).
We also observed that cells in which SER virus F was coexpressed with SER virus HN showed low-pH-dependent hemifusion activity and content mixing with labeled RBCs as well as syncytium formation under pH conditions ranging between pH 4.8 and 6.2, but no fusion was observed under conditions of higher pH (pH 6.4 to 7.4). Neither the wt SER virus F nor an SER virus F mutant that showed extensive fusion at neutral pH was able to mediate fusion under pH conditions below pH 4.8. The SER virus F protein expressed alone did not exhibit fusion activity under reduced pH conditions, indicating that the trigger for fusion activity of the F protein also requires the presence of HN-receptor interaction under conditions of reduced pH.
Conformational changes within viral fusion proteins have been postulated to play an important role in the promotion of membrane fusion. The influenza virus HA protein is the best-characterized example of a fusion protein triggered by low pH (4, 37). The observation of changes in antigenicity of HA with a change in pH indicates that structural rearrangements occur throughout the molecule (8, 26, 40, 41, 48). It has also been shown that activation of HA membrane fusion in vitro is temperature dependent; influenza viruses that fuse membranes at 37°C and pH 5.6 can also fuse at 62°C and pH 7.0 (5, 30, 42). Changes in the susceptibility of the Sendai virus F glycoprotein to proteolysis following incubation at an elevated temperature which promoted fusion suggested that a change in conformation also occurs (43).
The finding of a low-pH-dependent fusion activity in the SER virus F protein is unusual for a paramyxovirus. The fusion activity of other paramyxoviruses is pH independent, and virus entry is thought to occur by fusion of the viral envelope with the plasma membrane (44). Many other enveloped viruses (such as orthomyxoviruses, alphaviruses, flaviviruses, and rhabdoviruses) with low-pH-dependent fusion activity enter host cells by endocytosis. The acidification which occurs in the endosome triggers conformational changes in the fusion proteins, exposing the hydrophobic portions of the protein and leading to fusion of the viral envelope with the endosomal membrane. A previous study of fusion of NDV with COS-7 cells reported an enhancement in fusion at acidic pH (31). However, unlike SER virus, NDV also induces fusion at neutral pH, and it was suggested that NDV has a dual mode of entry (via the plasma membrane or an endocytic pathway) (31). The present results, showing that proton ATPases block SER virus infection, support the conclusion that a low-pH entry mechanism operates for SER virus.
Acknowledgments
This study was supported by grant CA 18611 from the National Institutes of Health.
We thank Tanya Cassingham for assistance in preparing the manuscript and David Steinhauer (Emory University) for helpful comments.
REFERENCES
- 1.Aguilar, H. C., W. F. Anderson, and P. M. Cannon. 2003. Cytoplasmic tail of Moloney murine leukemia virus envelope protein influences the conformation of the extracellular domain: implications for mechanism of action of the R peptide. J. Virol. 77:1281-1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bagai, S., and R. A. Lamb. 1995. Quantitative measurement of paramyxovirus fusion: differences in requirements of glycoproteins between simian virus 5 and human parainfluenza virus 3 or Newcastle disease virus. J. Virol. 69:6712-6719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bizebard, T., B. Gigant, P. Rigolet, B. Rasmussen, O. Diat, P. Bosecke, S. A. Wharton, J. J. Skehel, and M. Knossow. 1995. Structure of influenza virus haemagglutinin complexed with a neutralizing antibody. Nature 376:92-94. [DOI] [PubMed] [Google Scholar]
- 4.Bullough, P. A., F. M. Hughson, A. C. Treharne, R. W. Ruigrok, J. J. Skehel, and D. C. Wiley. 1994. Crystals of a fragment of influenza haemagglutinin in the low pH induced conformation. J. Mol. Biol. 236:1262-1265. [DOI] [PubMed] [Google Scholar]
- 5.Carr, C. M., C. Chaudhry, and P. S. Kim. 1997. Influenza hemagglutinin is spring-loaded by a metastable native conformation. Proc. Natl. Acad. Sci. USA 94:14306-14313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cathomen, T., H. Y. Naim, and R. Cattaneo. 1998. Measles viruses with altered envelope protein cytoplasmic tails gain cell fusion competence. J. Virol. 72:1224-1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cross, K. J., S. A. Wharton, J. J. Skehel, D. C. Wiley, and D. A. Steinhauer. 2001. Studies on influenza haemagglutinin fusion peptide mutants generated by reverse genetics. EMBO J. 20:4432-4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daniels, R. S., A. R. Douglas, J. J. Skehel, and D. C. Wiley. 1983. Analyses of the antigenicity of influenza haemagglutinin at the pH optimum for virus-mediated membrane fusion. J. Gen. Virol. 64:1657-1662. [DOI] [PubMed] [Google Scholar]
- 9.Drose, S., K. U. Bindseil, E. J. Bowman, A. Siebers, A. Zeeck, and K. Altendorf. 1993. Inhibitory effect of modified bafilomycins and concanamycins on P- and V-type adenosinetriphosphatases. Biochemistry 32:3902-3906. [DOI] [PubMed] [Google Scholar]
- 10.Dubay, J. W., S. J. Roberts, B. H. Hahn, and E. Hunter. 1992. Truncation of the human immunodeficiency virus type 1 transmembrane glycoprotein cytoplasmic domain blocks virus infectivity. J. Virol. 66:6616-6625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dutch, R. E., S. B. Joshi, and R. A. Lamb. 1998. Membrane fusion promoted by increasing surface densities of the paramyxovirus F and HN proteins: comparison of fusion reactions mediated by simian virus 5 F, human parainfluenza virus type 3 F, and influenza virus HA. J. Virol. 72:7745-7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dutch, R. E., and R. A. Lamb. 2001. Deletion of the cytoplasmic tail of the fusion protein of the paramyxovirus simian virus 5 affects fusion pore enlargement. J. Virol. 75:5363-5369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Earl, P. L., S. Koenig, and B. Moss. 1991. Biological and immunological properties of human immunodeficiency virus type 1 envelope glycoprotein: analysis of proteins with truncations and deletions expressed by recombinant vaccinia viruses. J. Virol. 65:31-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fan, Z., M. L. Grantham, M. S. Smith, E. S. Anderson, J. A. Cardelli, and M. I. Muggeridge. 2002. Truncation of herpes simplex virus type 2 glycoprotein B increases its cell surface expression and activity in cell-cell fusion, but these properties are unrelated. J. Virol. 76:9271-9283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fischer, C., B. Schroth-Diez, A. Herrmann, W. Garten, and H. D. Klenk. 1998. Acylation of the influenza hemagglutinin modulates fusion activity. Virology 248:284-294. [DOI] [PubMed] [Google Scholar]
- 16.Furuta, R. A., C. T. Wild, Y. Weng, and C. D. Weiss. 1998. Capture of an early fusion-active conformation of HIV-1 gp41. Nat. Struct. Biol. 5:276-279. [DOI] [PubMed] [Google Scholar]
- 17.Glomb-Reinmund, S., and M. Kielian. 1998. The role of low pH and disulfide shuffling in the entry and fusion of Semliki Forest virus and Sindbis virus. Virology 248:372-381. [DOI] [PubMed] [Google Scholar]
- 18.Gray, K. D., and M. J. Roth. 1993. Mutational analysis of the envelope gene of Moloney murine leukemia virus. J. Virol. 67:3489-3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guinea, R., and L. Carrasco. 1995. Requirement for vacuolar proton-ATPase activity during entry of influenza virus into cells. J. Virol. 69:2306-2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heinen, E., W. Herbst, and N. Schmeer. 1998. Isolation of a cytopathogenic virus from a case of porcine reproductive and respiratory syndrome (PRRS) and its characterization as parainfluenza virus type 2. Arch. Virol. 143:2233-2239. [DOI] [PubMed] [Google Scholar]
- 21.Hu, X. L., R. Ray, and R. W. Compans. 1992. Functional interactions between the fusion protein and hemagglutinin-neuraminidase of human parainfluenza viruses. J. Virol. 66:1528-1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Januszeski, M. M., P. M. Cannon, D. Chen, Y. Rozenberg, and W. F. Anderson. 1997. Functional analysis of the cytoplasmic tail of Moloney murine leukemia virus envelope protein. J. Virol. 71:3613-3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lamb, R. A. 1993. Paramyxovirus fusion: a hypothesis for changes. Virology 197:1-11. [DOI] [PubMed] [Google Scholar]
- 24.Moore, J. P., J. A. McKeating, R. A. Weiss, and Q. J. Sattentau. 1990. Dissociation of gp120 from HIV-1 virions induced by soluble CD4. Science 250:1139-1142. [DOI] [PubMed] [Google Scholar]
- 25.Mulligan, M. J., G. V. Yamshchikov, G. D. Ritter, Jr., F. Gao, M. J. Jin, C. D. Nail, C. P. Spies, B. H. Hahn, and R. W. Compans. 1992. Cytoplasmic domain truncation enhances fusion activity by the exterior glycoprotein complex of human immunodeficiency virus type 2 in selected cell types. J. Virol. 66:3971-3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nestorowicz, A. N., D. O. White, and D. C. Jackson. 1985. Conformational changes in influenza virus haemagglutinin and its monomer detected by monoclonal antibodies. Vaccine 3:175-181. [DOI] [PubMed] [Google Scholar]
- 27.Ohuchi, M., C. Fischer, R. Ohuchi, A. Herwig, and H. -D. Klenk. 1998. Elongation of the cytoplasmic tail interferes with the fusion activity of influenza virus hemagglutinin. J. Virol. 72:3554-3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paterson, R. G., C. J. Russell, and R. A. Lamb. 2000. Fusion protein of the paramyxovirus SV5: destabilizing and stabilizing mutants of fusion activation. Virology 270:17-30. [DOI] [PubMed] [Google Scholar]
- 29.Ritter, G. D., Jr., M. J. Mulligan, S. L. Lydy, and R. W. Compans. 1993. Cell fusion activity of the simian immunodeficiency virus envelope protein is modulated by the intracytoplasmic domain. Virology 197:255-264. [DOI] [PubMed] [Google Scholar]
- 30.Ruigrok, R. W., N. G. Wrigley, L. J. Calder, S. Cusack, S. A. Wharton, E. B. Brown, and J. J. Skehel. 1986. Electron microscopy of the low pH structure of influenza virus haemagglutinin. EMBO J. 5:41-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.San Roman, K., E. Villar, and I. Munoz-Barroso. 1999. Acidic pH enhancement of the fusion of Newcastle disease virus with cultured cells. Virology 260:329-341. [DOI] [PubMed] [Google Scholar]
- 32.Schultz, A., and A. Rein. 1985. Maturation of murine leukemia virus env proteins in the absence of other viral proteins. Virology 145:335-339. [DOI] [PubMed] [Google Scholar]
- 33.Sergel, T., L. W. McGinnes, and T. G. Morrison. 1993. The fusion promotion activity of the NDV HN protein does not correlate with neuraminidase activity. Virology 196:831-834. [DOI] [PubMed] [Google Scholar]
- 34.Sergel, T., and T. G. Morrison. 1995. Mutations in the cytoplasmic domain of the fusion glycoprotein of Newcastle disease virus depress syncytia formation. Virology 210:264-272. [DOI] [PubMed] [Google Scholar]
- 35.Seth, S., A. Vincent, and R. W. Compans. 2003. Mutations in the cytoplasmic domain of a paramyxovirus fusion glycoprotein rescue syncytium formation and eliminate the hemagglutinin-neuraminidase protein requirement for membrane fusion. J. Virol. 77:167-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Skehel, J. J., P. M. Bayley, E. B. Brown, S. R. Martin, M. D. Waterfield, J. M. White, I. A. Wilson, and D. C. Wiley. 1982. Changes in the conformation of influenza virus hemagglutinin at the pH optimum of virus-mediated membrane fusion. Proc. Natl. Acad. Sci. USA 79:968-972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Skehel, J. J., T. Bizebard, P. A. Bullough, F. M. Hughson, M. Knossow, D. A. Steinhauer, S. A. Wharton, and D. C. Wiley. 1995. Membrane fusion by influenza hemagglutinin. Cold Spring Harbor Symp. Quant. Biol. 60:573-580. [DOI] [PubMed] [Google Scholar]
- 38.Steinhauer, D. A., S. A. Wharton, J. J. Skehel, D. C. Wiley, and A. J. Hay. 1991. Amantadine selection of a mutant influenza virus containing an acid-stable hemagglutinin glycoprotein: evidence for virus-specific regulation of the pH of glycoprotein transport vesicles. Proc. Natl. Acad. Sci. USA 88:11525-11529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tong, S., M. Li, A. Vincent, R. W. Compans, E. Fritsch, R. Beier, C. Klenk, M. Ohuchi and H.-D. Klenk. 2002. Regulation of fusion activity by the cytoplasmic domain of a paramyxovirus F protein. Virology 301:322-333. [DOI] [PubMed] [Google Scholar]
- 40.Webster, R. G., L. E. Brown, and D. C. Jackson. 1983. Changes in the antigenicity of the hemagglutinin molecule of H3 influenza virus at acidic pH. Virology 126:587-599. [DOI] [PubMed] [Google Scholar]
- 41.Wharton, S. A., L. J. Calder, R. W. Ruigrok, J. J. Skehel, D. A. Steinhauer, and D. C. Wiley. 1995. Electron microscopy of antibody complexes of influenza virus haemagglutinin in the fusion pH conformation. EMBO J. 14:240-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wharton, S. A., J. J. Skehel, and D. C. Wiley. 1986. Studies of influenza haemagglutinin-mediated membrane fusion. Virology 149:27-35. [DOI] [PubMed] [Google Scholar]
- 43.Wharton, S. A., J. J. Skehel, and D. C. Wiley. 2000. Temperature dependence of fusion by sendai virus. Virology 271:71-78. [DOI] [PubMed] [Google Scholar]
- 44.White, J., M. Kielian, and A. Helenius. 1983. Membrane fusion proteins of enveloped animal viruses. Q. Rev. Biophys. 16:151-195. [DOI] [PubMed] [Google Scholar]
- 45.Yang, C., and R. W. Compans. 1996. Analysis of the cell fusion activities of chimeric simian immunodeficiency virus-murine leukemia virus envelope proteins: inhibitory effects of the R peptide. J. Virol. 70:248-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang, C., and R. W. Compans. 1997. Analysis of the murine leukemia virus R peptide: delineation of the molecular determinants which are important for its fusion inhibition activity. J. Virol. 71:8490-8496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yao, Q., and R. W. Compans. 1995. Differences in the role of the cytoplasmic domain of human parainfluenza virus fusion proteins. J. Virol. 69:7045-7053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yewdell, J. W., W. Gerhard, and T. Bachi. 1983. Monoclonal anti-hemagglutinin antibodies detect irreversible antigenic alterations that coincide with the acid activation of influenza virus A/PR/834-mediated hemolysis. J. Virol. 48:239-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zingler, K., and D. R. Littman. 1993. Truncation of the cytoplasmic domain of the simian immunodeficiency virus envelope glycoprotein increases Env incorporation into particles and fusogenicity and infectivity. J. Virol. 67:2824-2831. [DOI] [PMC free article] [PubMed] [Google Scholar]