Abstract
Existing prostate cancer cell lines have been derived from late stages of human prostate cancer. In this paper, we present two cell lines generated from prostatic intraepithelial neoplasia (PIN), the precursor lesion for prostate adenocarcinoma. Pr-111 and Pr-117 were established from PIN lesions that developed in the C3(1)/Tag transgenic model of prostate cancer. Pr-111 and Pr-117 cells express simian virus 40 large T antigen (SV40 Tag) and are immortalized in culture, distinguishing them from normal prostate cells. The growth rates of these two cell lines are quite different; with Pr-111 cells growing much more slowly (doubling time approximately 40 hours) compared to Pr-117 cells (doubling time approximately 22 hours), and also show significantly different growth rates in different media. Both prostate cell lines express cytokeratin and androgen receptor (AR) with Pr-111 cells demonstrating androgen-dependent growth and Pr-117 cells exhibiting androgen-responsive growth characteristics. Athymic nude mice injected with Pr-111 cells either do not develop tumors or develop tumors after a long latency period of 14 weeks. Pr-117 cells, however, develop tumors by 3 to 6 weeks, suggesting that Pr-117 cells represent a later stage of tumor progression. These two novel cell lines will be useful for studying early stages of prostate tumor development and androgen responsiveness.
Keywords: prostate, PIN, adenocarcinoma, cell lines, mouse
Abbreviations: AR, androgen receptor; BSA, bovine serum albumin; CS-FBS, charcoal-stripped fetal bovine serum; DHT, dihydrotestosterone; DMEM, Dulbecco's modified Eagle's medium; ECM, extracellular matrix; FBS, fetal bovine serum; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; GM, growth medium; H&E, hematoxylin and eosin; HPV, human papilloma virus; MTT, 3-(45-dimethylthiazol-2-yl)-25-diphenyltetrazolium bromide; NDS, normal donkey serum; PIN, prostatic intraepithelial neoplasia; RT-PCR, reverse transcription-polymerase chain reaction; s.c., subcutaneous; SV40 Tag, simian virus 40 large T antigen
Introduction
It is estimated that about 192,000 new cases of prostate cancer will be diagnosed in the United States during the year 2001, and almost 32,000 deaths will result from the disease [1]. These statistics place prostate cancer as the second leading cause of cancer death in men in the United States. As a result, there is an increasing need to develop early detection techniques and assays to distinguish normal from cancerous tissues. To improve current techniques in prostate cancer diagnosis, additional tumor model systems need to be developed, especially those focusing on early stages of prostate cancer formation.
Prostatic intraepithelial neoplasia (PIN) is considered by most to be the precursor lesion for prostate adenocarcinoma [2]. In prostate tissue with developed carcinoma, evidence of PIN lesions may be seen, indicating a progression from normal glandular epithelium through PIN, to fully dedifferentiated prostate tissue [3]. Additionally, it has been shown that PIN has a high predictive value as a marker for adenocarcinoma [4]. Although mounting evidence suggests that PIN is the precursor to adenocarcinoma, little is known about the molecular biology of PIN and what determines whether PIN lesions will transition into invasive carcinomas [5]. Characterizations of PIN have generally been limited to histologic analysis because there are few in vitro and in vivo model systems representing this stage of prostate cancer progression. To our knowledge, only one PIN cell line has been established through immortalization with human papilloma virus (HPV) [6].
To develop a better understanding of the progression of PIN to adenocarcinoma, animal models must be established to facilitate analyses both in vitro and in vivo. Of the established animal models, the transgenic mouse systems are the most promising. Cell lines established from these murine models can be studied in vivo without the added complexity of using immunosuppressants in immunocompetent animals receiving xenografts. An intact immune system is essential to accurately study the growth of cell lines in the natural host. Several transgenic prostate cancer mouse models have been developed that express simian virus 40 large T antigen (SV40 Tag) under the control of different promoter regulatory sequences. These include the mouse cryptidin-2 regulatory gene [7], the rat probasin promoter [8], the human insulin growth factor-1 regulatory sequences [9], the fetal globin promoter [10], and the model developed by Maroulakou et al. [11], where SV40 Tag is expressed under the regulatory control of the rat steroid binding promoter gene, C3(1) [12]. The first four of these models develop fully dedifferentiated prostate tumors within 2 to 3 months, making it difficult to reliably pinpoint early tumor stages. However, C3(1)/Tag transgenic mice develop PIN lesions from 2 to 7 months of age and invasive carcinomas after 6 months of age [13], making the study of PIN lesions and the establishment of cell lines from early stages of prostate cancer feasible.
The C3(1)/Tag mouse model is the source of several cell lines representing different levels of prostate tumor progression. These cell lines have undergone extensive characterization and may represent an in vitro animal model system for the study of prostate tumor progression. Cell lines from C3(1)/Tag prostate lesions have been developed from different stages of prostate cancer development including prostate adenocarcinomas (Pr-142) [14] and metastases (Pr-14C1and Pr-14C2) (unpublished data). In this study, we describe the establishment of cell lines, Pr-111 and Pr-117, derived from PIN lesions. In vitro analyses of these cell lines demonstrate an epithelial origin and response to androgen. These cell lines are valuable new reagents for studying the PIN stage of prostate cancer development. This system may provide extensive data on protein expression correlating to cancer grade, as well as, a therapeutic model for an effective treatment.
Materials and Methods
Establishment and Culture of Cell Lines
Prostates were collected immediately following sacrifice of C3(1)/Tag transgenic male mice and carefully resected to avoid other glandular tissues such as the urethral and bulbourethral glands. Each prostate was divided in half; one half was fixed in 4% paraformaldehyde for histologic analysis and the other half was prepared for tissue culture. For tissue culture, prostates were rinsed in phosphate-buffered saline (PBS) (Gibco BRL, Gaithersburg, MD), minced into ∼1-mm3 pieces, and centrifuged (1100 rpm at 15°C for 3 minutes). The pieces of tissue were resuspended in growth medium (GM), PMFR-4A [15] supplemented with 3x10−11 M retinoic acid (Sigma, St. Louis, MO), 2.3x10−6 M α-tocopherol (Sigma), 10 µg/l epidermal growth factor (Collaborative, Bedford, MA), 10 µg/l cholera toxin (List Biological, Campbell, CA), 10 mg/l bovine pituitary extract (Hammond Cell Tech, Alameda, CA), 1 µg/l hydrocortisone (Sigma), 4 mg/l insulin (Gibco), 100 mg/l gentamycin (Sigma), 0.1 mM phosphoethanolamine (Sigma), and 3x10−8 M selenous acid (Sigma). One hundred units per milliliter of collagenase (Sigma) was added to the medium/tissue and gently rocked overnight at 37°C. After centrifugation, remaining cells were plated on 30-mm2 dishes coated with collagen (Vitrogen 100, Collagen; Santa Clara, CA) in GM. Cells were trypsinized from organoids and grown in GM+2% fetal bovine serum (FBS; Gibco) + 10 nM dihydrotestosterone (DHT) on collagen.
Immunofluorescence
Cells were plated on eight-well chamber slides, grown to 80% confluency, fixed in -20°C methanol, and washed with cold PBS. The slides were incubated 1 hour at room temperature with blocking buffer, 5% bovine serum albumin (BSA), 0.02% sodium azide in 1xPBS, and 5% normal donkey serum (NDS). Blocking buffer was removed from the wells and primary antibody, diluted in primary antibody diluting buffer (Biomed, Foster City, CA), was added and incubated overnight at 4°C. Both SV40 Tag mouse monoclonal antibody (Ab-2; Calbiochem, San Diego, CA) and pan-cytokeratin rabbit polyclonal antibody (detects a range of cytokeratins; Zymed, San Francisco, CA) were used at 1:20 dilutions. After incubation, slides were washed three times with 1xPBS and secondary antibody with either trimethylrhodamine isothiocyanate (TRITC)- or fluorescein isothiocyanate (FITC)-conjugated IgG (Jackson Immuno Research, West Grove, PA) was incubated in a slide humidifying chamber for 1 hour at room temperature in the dark. Following incubation, the slides were washed three times with 1xPBS and allowed to dry. Two drops of Gel-Mount (Biomed) were added to each of the slides followed by a coverslip. The stained slides were visualized under fluorescent microscopy (Olympus BX60) and pictures were taken with an Olympus C-35AD-4 automatic camera with 200 ASA Kodak color print film with a 40x objective lens.
Western Blot Analysis
Total cell extracts were isolated using the RIPA protocol (Santa Cruz, Santa Cruz, CA). Cell extract concentrations were determined by a BioRad protein assay method (BioRad, Hercules, CA) and compared to a BSA standardization curve. Ten micrograms total protein extracts in 3x sample buffer (150 mM Tris, pH 6.8; 6% SDS; 30% glycerol; 6 mM EDTA; 10% β-mercaptoethanol; 0.01% bromophenol blue) was loaded on an 8% tris-glycine denaturing gel (iGels, Salt Lake City, UT). Gels were stained with coomassie blue (Sigma) or transferred to a nitrocellulose membrane filter (Novex, San Diego, CA) for immunoblotting. After transfer, the filter was blocked in 1xPBS plus 0.5% Tween-20 (PBS-T) and 5% nonfat dry milk at room temperature for 1 hour. Filters were washed with 1xPBS-T and incubated overnight at 4°C with either a 1:100 dilution of anti-SV40 Tag mouse mAb or 1:200 dilution of anti-vimentin mouse mAb (Zymed) in 1xPBS-T with 0.02% sodium azide. The filter was washed and incubated with goat anti-mouse IgG peroxidase-conjugated secondary antibody (Boehringer Mannheim, Indianapolis, IN) diluted 1:6667 in 1xPBS-T for 1 hour at room temperature. The filter was exposed to autoradiography following treatment with enhanced chemiluminescence according to the manufacturer's protocol (NEN, Boston, MA).
Proliferation Assay
Cells were plated at 1x104 cells/well in 24-well dishes in either GM+2% FBS + DHT (GM+2%) or Dulbecco's modified Eagle's medium (DMEM) + 10% FBS (DMEM + 10%). Every 24 hours, cells were trypsinized and counted using a hemacytometer. Experiments were based on an average of six wells in two separate experiments. The doubling times were determined for each cell line in each medium.
Growth on Matrigel
Cells (1x104) were plated on Matrigel (Becton Dickinson, Bedford, MA) in 30-mm2 dishes (400 µl Matrigel/30 mm2) in DMEM + 10% or GM+2% medium. Cells were fed every 3 days before photographing on a Zeiss Axiovert 25 microscope with a Nikon FE2 35-mm camera using TMAX 100 ASA Kodak black and white professional film.
Nude Mice Studies
Cells (1x106 cells/0.2 ml PBS) were injected subcutaneously (s.c.) into each of five, 8-week-old male athymic mice. Tumors were measured weekly and size was determined by the formula: largest diameterx(smallest diameter)2x0.4 [16].
Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
The presence of androgen receptor (AR) was determined by RT-PCR using mouse-specific PCR primers. One microgram of total RNA was purified with amplification-grade DNAse I (Gibco) according to manufacturer's directions and reverse transcribed into cDNA using Superscript II Reverse Transcriptase (Gibco). One microliter of cDNA (1:5 dilution) was amplified with mouse-specific AR primers (5′ primer: CGACTACTACAACTTTCCGC; 3′ primer: TCATCTCCACAGATCAGGCA) with the following conditions: 35 cycles at 94°C for 1 minute, 58°C for 2 minutes, and 72°C for 3 minutes. The internal control, glyceraldehyde 3-phosphate dehydrogenase (GAPDH), was amplified with mouse-specific PCR primers (Clontech, Palo Alto, CA). One microliter of cDNA (1:5 dilution) was amplified for 28 cycles at 94°C for 30 seconds, 60°C for 1 minute, and 72°C for 2 minutes. The PCR products were run on 1% agarose gels stained with ethidium bromide.
Androgen Dependence Assay
Cells (1x103) were plated in each of eight wells on 96-well plates. The cells were incubated in medium (GM or DMEM) supplemented with charcoal-stripped FBS (CS-FBS; Cocalico Biologicals, Reamstown, PA) and in control medium: GM+2% or DMEM+10%. Cells were fed with appropriate medium (with and without androgens) before the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay [17], which was performed on a 96-well plate every 4 days. Media were aspirated and replaced with medium containing 0.5 mg/ml MTT (Sigma) and incubated for 3 hours. After incubation, the medium was removed and replaced with isopropanol to dissolve the formazan crystals. The wells were read and analyzed at 570 nm on a Model 550 Microplate Reader (BioRad) using the Microplate Manager 4.0 software (BioRad).
Results
Establishment of Cell Lines from C3(1)/Tag Transgenic Mice PIN Lesions
C3(1)/Tag transgenic mouse prostate tissue was carefully resected from 3-and 5 1/2-month-old mice. One half of each section was stained with hematoxylin and eosin (H&E) and histologic analysis showed that the 3-month-old prostate tissue contained low-grade PIN lesions exhibiting irregular spacing and cell crowding (arrow, Figure 1A), whereas the 5 1/2-month-old tissue contained high-grade PIN lesions with some basal cell layer disruption and cell stratification (arrows, Figure 1B). The remaining prostate tissue from each mouse was used to establish two cell lines, Pr-111 (from low-grade PIN) and Pr-117 (from high-grade PIN). The cell lines were isolated and maintained in culture on collagen in GM+2% FBS. GM is a growth medium designed to encourage growth of normal prostate epithelial cells and inhibit growth of stromal cells [15]. In addition, our standard GM+2% medium contains 10 nM DHT. The Pr-111 and Pr-117 cells acquire a cobblestone-like morphology, typical of epithelial cells, when grown to confluence on collagen (Figure 1, C and D).
Figure 1.
Histology of C3(1)/Tag transgenic mouse prostates and growth of cell lines in culture on collagen. H&E sections of prostates from 3- and 5 1/2-month-old male transgenic mice show consecutively, (A) low-grade PIN demonstrating irregular spacing and cell crowding (arrow) and (B) high-grade PIN with some basal cell layer disruption and cell stratification (arrows) (light, x 100). Subsequent culture of isolated cells on collagen in GM+2% FBS resulted in two cell lines: (C) Pr-111 (passage 9) cell line from the low-grade PIN lesion and (D) Pr-117 (passage 9) cell line from the high-grade PIN lesion (phase contrast, x 200).
Immunofluorescent Staining of Pr-111 and Pr-117 Cells
After establishing the cell lines in culture, Pr-111 and Pr-117 cells were analyzed for cell marker expression by immunofluorescence. As expected for cells of epithelial origin, both Pr-111 and Pr-117 cells express cytokeratins in the cytoplasm as detected by a pan-cytokeratin primary antibody (Figure 2). In contrast, mouse NIH 3T3 fibroblasts show no cytokeratin expression, whereas, positive control cell lines, A549 (a human lung epithelial cell line) and Pr-142 (a mouse prostate adenocarcinoma cell line derived from a C3(1)/Tag transgenic mouse [14]), exhibit cytokeratin immunofluorescent staining.
Figure 2.
Cytokeratin and SV40 Tag expression in Pr-111 and Pr-117 cells using immunofluorescence. Cells (1x105 cells/well) were grown on eight-well chamber slides and exposed to antibodies for pan-cytokeratin (antibody that detects a range of cytokeratins) and SV40 Tag. Pr-111 and Pr-117 cells show expression of cytokeratin and the SV40 Tag transgene. The epithelial A549 cell line (human lung carcinoma) represents a positive control for cytokeratin; NIH3T3 (mouse fibroblast cell line) is a negative control for cytokeratin; Pr-142 (mouse prostate adenocarcinoma cell line) is the positive control for SV40 Tag (fluorescent, x 400).
To show transgene expression, immunofluorescence on Pr-111 and Pr-117 cells was performed using a monoclonal antibody to SV40 Tag. Both Pr-111 and Pr-117 cells exhibit 100% nuclear SV40 Tag expression (Figure 2). As a positive control, Pr-142 cells show SV40 Tag expression, whereas NIH3T3 and A549 are negative, indicating that these cell lines did not originate from a SV40 Tag model system (Figure 2).
Western Blot Analysis of Pr-111 and Pr-117 Cells
Total cell protein was examined by Western blot analysis using a monoclonal antibody for the nuclear protein, SV40 Tag. Figure 3A demonstrates expression of the 92-kDa SV40 Tag protein in Pr-111 and Pr-117 cell lines as well as in Pr-142 (positive control). SV40 Tag expression levels appeared lower in Pr-111 cells (potential PIN cell line) compared with Pr-117 and Pr-142 cells. As expected, no SV40 Tag expression was detected in NIH3T3 and A549 cells.
Figure 3.
Protein expression analysis of transgenic mouse prostate cell lines. Western blot analysis shows that the Pr-111 and Pr-117 cell lines express SV40 Tag (A), and do not express the stromal cell marker, vimentin (B). (C) A matched gel was stained with coomassie blue for loading efficiency. Pr-142 is used as the negative control for vimentin and positive control for SV40 Tag, whereas NIH3T3 is used as the positive control for vimentin and negative control for SV40 Tag.
Western blot analysis was also used to detect vimentin, a protein expressed by cells of mesenchymal origin, such as fibroblasts and smooth muscle cells. Figure 3B shows that Pr-111 and Pr-117 cell lines do not express vimentin. In contrast, the fibroblastic NIH3T3 cells show the expected 57-kDa vimentin band (Figure 3B). The amount of protein loaded in each lane was compared with a coomassie blue staining of a matched SDS-PAGE gel (Figure 3C).
Growth Rates for Pr-111 and Pr-117 Cell Lines
Once isolated from PIN lesions, Pr-111 and Pr-117 cells were cultured on collagen in GM+2%. Growth rates were determined for the cell lines grown in two different media, GM+2% or DMEM+10%, and counted every 24 hours.
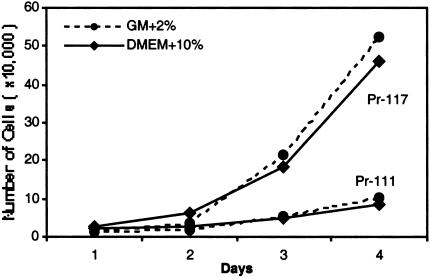
The doubling time for the Pr-111 cell line in GM+2% is 37.2 hours, whereas it is only 20.8 hours for the Pr-117 cell line. When grown in DMEM+10%, the doubling times were 46.4 hours for Pr-111 cells and 25.6 hours for Pr-117 cells. In both media, Pr-111 cells show a significantly slower growth rate compared with Pr-117 cells (Figure 4). In addition to having a higher growth rate than Pr-111 cells, Pr-117 cells grow at a rate considerably greater than Pr-142 cells (data not shown).
Figure 4.
Growth curve for Pr-111 and Pr-117 cell lines. Twenty-four-well dishes coated with collagen were seeded with 5x103 cells/well in GM+2% or DMEM+ 10%. Cells were trypsinized and counted with a hemocytometer every 24 hours. Pr-111 cells have a significantly slower growth rate compared to Pr-117 cells in both media. Each point represents an average of six wells in three independent experiments.
Morphological Characteristics of Pr-111 and Pr-117 on Matrigel
Pr-111 and Pr-117 cells were grown on two different substrates [plastic or the extracellular matrix (ECM), Matrigel] to determine if the cells would undergo morphologic alterations depending on the media.
When grown on plastic in either GM+2% and DMEM+10%, both Pr-111 and Pr-117 cell lines appear as cuboidal monolayers (Figure 5, A and B). However, when grown on Matrigel, the morphology and proliferative abilities over time change dramatically depending on which media is used. Pr-111 cells cultured in either media appear acinar and grow as aggregates (Figure 5A). However, these cells proliferate more rapidly in GM+2%, consistent with proliferation assay results (Figure 4). Pr-117 cells show acinar characteristics in DMEM+10% (Figure 5B), but form long tube-like ductal structures in the GM+2%, as previously described for the mouse prostate adenocarcinoma cell line, Pr-142 [14].
Figure 5.
Morphological characteristics of Pr-111 and Pr-117 cells grown on plastic and Matrigel. Cells (5x103) were plated on 30-mm2 plastic tissue culture dishes with and without 400 µl of the ECM, Matrigel. Both Pr-111 (A) and Pr-117 (B) cells exhibit cobblestone morphology when grown on plastic (phase contrast, x200). (A) Pr-111 demonstrates glandular-type growth characteristics on Matrigel in both GM+2% and DMEM+10%; (B) Pr-117 cells show ductal growth on Matrigel in GM+2% and glandular growth in DMEM+10% (varel, x200).
Androgen Regulation in Pr-111 and Pr-117 Cells
Normal prostate cell growth and function is dependent on the interaction of androgens with the AR. In prostate cancer, a correlation has been shown between tumor aggressiveness and androgen independence. The presence of AR by RT-PCR was carried out to determine if our potential PIN cell lines express AR transcripts. Analysis revealed that both Pr-111 and Pr-117 cell lines express AR, as well as the adenocarcinoma cell line, Pr-142 (Figure 6). The Ur-12 cell line, a mouse urethral tumor cell line derived from the C3(1)/Tag transgenic mouse model (unpublished data), shows no AR expression. GAPDH was used as an internal control.
Figure 6.
AR expression of Pr-111 and Pr-117 cell lines by RT-PCR. Total RNA was reverse transcribed into cDNA and amplified using specific mouse primers for AR. Both Pr-111 and Pr-117 cell lines express high levels of AR. Ur-12 (mouse urethral tumor cell line) does not exhibit AR expression by RTPCR (unpublished data). GAPDH was used as the internal control. The PCR products were separated on a 1% agarose gel and stained with ethidium bromide.
In addition, we wanted to characterize the functionality of the receptor by determining the ability of the Pr-111 and Pr-117 cells to grow with and without androgens in an in vitro androgen-ablation assay. Cells were plated on 96-well dishes in four types of media: GM+2% (standard medium, which includes DHT), GM+2% CS-FBS (charcoal stripping removes steroidal hormones such as androgen and estrogen), DMEM+10% (standard medium, which does not include additional DHT), and DMEM+10% CS-FBS. Every 4 days, cells were quantified using an MTT assay to measure cell viability.
In control media (GM+2% and DMEM+10%), Pr-111 and Pr-117 cells grew in a manner consistent with the proliferation assay (Figure 4). The Pr-111 cells showed a significantly reduced growth rate in the GM+2% CS-FBS and interestingly, were unable to survive in the DMEM+10% CS-FBS (Figure 7A). As expected of a more aggressive cell type, Pr-117 cells maintained a near-normal growth rate in the GM+2% CS-FBS, but like Pr-111 cells, Pr-117 cells stopped growing in the DMEM+10% CS-FBS (Figure 7B). On day 22, DHT was added back to the DMEM+10% CS-FBS and within 4 days, Pr-117 cells resumed proliferating as normal (Figure 7C). However, adding back DHT did not increase the number of Pr-111 cells (Figure 7C), indicating that these cells had not survived the extended time without androgens, and implying an androgen-responsive/dependent phenotype.
Figure 7.
Androgen regulation in Pr-111 and Pr-117 cells. Ninety-six-well plates were plated with 1x103 cells/well under various media conditions. Cells were quantified by an MTT assay every 4 days for cell growth. (A) Pr-111 cells show a significant response to androgen depletion (GM+2% CS-FBS and DMEM+10% CS-FBS) when compared to control conditions (GM+2% and DMEM+10%). (B) Pr-117 cells show androgen-independent growth in the GM+2% CS-FBS but not in the DMEM+10% CS-FBS. (C) When androgens are added back to the cells in DMEM+10% CS-FBS on day 22, Pr-117 cells resume replicating and Pr-111 cells did not. This is a representative of repeated experiments each showing similar results.
Tumorigenicity of Pr-111 and Pr-117 Cells in Nude Mice
To characterize the tumorigenicity of Pr-111 and Pr-117 cell lines in vivo, 1x106 cells were injected s.c. in the right flank of 8-week-old male athymic mice and tumor growth was observed. Utilizing early passage Pr-111 cells (passage 8), no tumors formed until 32 weeks after injection, and then in only three of five mice injected. With passage 12 cells, tumors developed by 14 weeks in four of five mice injected (see Table 1). In contrast, Pr-117 cells formed solid tumors in all mice injected within 6 weeks (passage 8) or 3 weeks (passage 12) postinjection. Interestingly, analyses of the lungs of mice injected with passage 12 Pr-117 cells were found to have numerous metastases (data not shown). Histologic analysis indicated that all growths were adenocarcinomas (Figure 8). The tumorigenicity of the Pr-111 cells in nude mice is significantly reduced compared to the Pr-117 cells.
Table 1.
Tumorigencity of Prostate Cells Lines Injected into Male, Athymic Mice.
| Cell Line | Number of Cells Injected/ 0.2 ml PBS | Number of Mice with Tumors | Time to Onset of Tumors (Weeks) | Average Size of Tumors at 6 Weeks (cm3) |
| Pr-111 (8)* | 1x106 | 3/5 | 32 | ND† |
| Pr-111 (12)* | 1x106 | 4/5‡ | 14 | 0 |
| Pr-111 (8)* | 1x106 | 8/8 | 6 | ND |
| Pr-111 (12)* | 1x106 | 5/5 | 3 | 4.1 |
| Pr-142§ | 1x106 | 1/1 | 2 | 0.18 |
Cell passage.
ND=not determined.
Average tumor volume at 23 weeks measured only 0.14 cm3.
In other experiments, 100% of mice injected with 2x106 cells resulted in tumor formation by 2 to 3 weeks 14.
Figure 8.
Histological analysis of tumors from nude mice studies. (A) H&E staining of a small growth (1.0 cm3 at 23 weeks) resulting from Pr-111 cells injected s.c. into nude mice. Cells exhibit variation in size of the nucleus, as well as sporadically stained chromatin (arrows). (B) Darkly stained cells demonstrating increased chromatin content (arrows) of a tumor (11.6 cm3 at 8 weeks) that developed at the injection site with Pr-117 cells (light, x400).
Discussion
Existing in vitro systems for the study of prostate cancer use cell lines developed from late-stage disease [18–22]. To understand the development and progression of prostate cancer at early stages, we have developed cell lines that appear to represent both early- and late-stage disease from the C3(1)/Tag transgenic mouse model. The C3(1)/Tag transgenic mouse model was developed using a transgene composed of the promoter region of the C3(1) rat steroid binding gene fused to the regulatory sequence of SV40 Tag [11]. In addition to exhibiting tumorigenesis in prostate and mammary tissue, C3(1)/Tag transgenic mice have evidence of SV40 Tag expression in several other tissues, but it has been shown that SV40 Tag is not sufficient in these tissues to establish malignancies [11]. In the C3(1)/Tag transgenic model for prostate cancer, PIN develops in 2 to 7 months and adenocarcinomas arise after 6 to 12 months [13]. The extended time needed for these transgenic mice to develop carcinomas make this model ideal for studying the various early genetic changes prostate cells undergo en route to acquiring an invasive phenotype in vivo [23]. In addition, the predictability of tumor progression in this model allows for the isolation of tissues and cells at different stages for in vitro studies.
Several studies of human patients strongly suggest that PIN is the precursor lesion to prostate adenocarcinoma. These studies compare PIN to both cancerous and normal tissues and fully demonstrate that characteristics of PIN lesions (e.g., proliferation rates, protein expression, etc.) fall between those of normal and cancerous prostate tissues [4,24–26]. To help in developing early-detection assays by studying early-stage or precancerous tissues, we have focused our efforts toward establishing a cell line from prostates with PIN lesions.
In this study, we isolated and characterized two mouse prostate cell lines, Pr-111 and Pr-117. These cell lines were established from the prostates of 3-and 5 1/2-month-old, male C3(1)/Tag transgenic mice, with the intent to develop cell lines representing low-grade PIN (3 months) and high-grade PIN (5 1/2 months). As we discovered, PIN tissue was difficult to grow in culture because like normal mouse prostate tissue, most cells do not survive their first passage with trypsin. Pr-111 and Pr-117 represent cell lines that were successfully passaged after nearly 50 individual attempts. Based on our results, the Pr-111 cell line appears to represent a PIN cell line, whereas the Pr-117 cell line has the characteristics of a more aggressive adenocarcinoma. Immunofluorescence studies demonstrate that Pr-111 and Pr-117 cell lines express cytokeratin, an epithelial cell marker, but not vimentin, a stromal cell marker. Immunofluorescence and Western blot analyses also show that both Pr-111 and Pr-117 cells express SV40 Tag. Three cell lines derived from the C3(1)/Tag transgenic model, Pr-111 (a PIN cell line), Pr-117, and Pr-142 (a mouse prostate adenocarcinoma cell line [14]), show an increasing level of SV40 Tag expression, a trend that has also been demonstrated by immunohistochemical staining of C3(1)/Tag mouse PIN and adenocarcinoma tissue [27]. This further suggests that the Pr-111 cell line represents an early stage of prostate cancer progression. SV40 Tag has been shown to bind and inactivate p53 and Rb function [28,29]. Due to the lower expression level of SV40 Tag, our data suggest that Pr-111 cells remain under some level of tumor suppression by p53 and/or Rb.
In addition to demonstrating the epithelial status and expression levels of SV40 Tag, we also looked at the growth rates of these two cell lines. Pr-111 cells show a significantly slower growth rate compared to Pr-117 cells in both media analyzed. Interestingly, the Pr-117 cells have a faster growth rate than all other adenocarcinoma cell lines established from the C3(1)/Tag transgenic mouse model (data not shown).
The growth characteristics of the Pr-111 and Pr-117 cell lines were also evaluated on Matrigel, an ECM. The growth of Pr-111 cells on Matrigel shows acinar/ glandular structure formation in both GM+2% and DMEM+10%, but as demonstrated in the proliferation assay, Pr-111 cells did not grow as well in DMEM+10%. The Pr-117 cells show acinar/glandular growth in DMEM+10%, but demonstrate ductal structures in GM+2%, a characteristic described by Jorcyk et al. [14] with Pr-142, the prostate adenocarcinoma cell line. GM+2% is a complex medium, containing many added factors, which has led to studies to determine which factors are contributing to the ductal formation of adenocarcinoma-like cell lines, such as Pr-117 and Pr-142.
In humans, as well as mice, the cells of the prostate are under androgen regulation. The progression and staging of prostate cancer can be determined by the status of AR expression and response to androgen ablation therapy. Pr-111 cells express AR and demonstrate androgen-responsive/dependent growth in culture. The androgen responsiveness, along with a limited tumorigenic ability in vivo, suggests that this cell line is in an early stage of tumor progression, that is, PIN. However, the Pr-117 cells express AR, but exhibit androgen-independent growth in the GM+2%, and have a high tumorigenic rate. This adenocarcinoma cell line, with further investigation, may contribute to an understanding of androgen-independent prostate cancer progression.
To further characterize the Pr-111 and Pr-117 cell lines, we evaluated nude mice injected with the cell lines for tumor growth. From our tumorigenicity assays, we determined that the passage number of the cells was related to the time required for tumor growth in nude mice. With the Pr-111 cell line, early passage cells did not form tumors for over 32 weeks, whereas later passage cells developed small, slow-growing cancerous growths by 14 weeks. All tumors were analyzed histologically and determined to be adenocarcinomas. This further supports the prostate epithelial origin of Pr-111 cells. As the precursor lesion to carcinoma, it is expected that over time, PIN will convert into the cancerous phenotype through additional mutations, which may be the case in our nude mice studies utilizing both early and later passages. The control cell line injections (Pr-142) developed tumors within 2 to 3 weeks, which is a significantly faster rate than the Pr-111 cell line. In contrast to the Pr-111 cells, all nude mice injected with Pr-117 cells developed adenocarcinomas within 3 to 6 weeks. Additionally, all mice injected with Pr-117 cells and analyzed had gross and histologic evidence of metastases in the lungs. These data may suggest that Pr-117 cells were derived from aggressive carcinoma cells not present in the histologic analysis of the high-grade PIN lesion, or had progressed dramatically in culture from the cells of the original high-grade PIN lesion.
The development and characterization of the androgen-dependent mouse PIN cell line, Pr-111, and the androgen-responsive aggressive adenocarcinoma cell line, Pr-117, allows for comparison studies between normal prostate cells, PIN cells (Pr-111), adenocarcinoma cells (Pr-142 [14], Pr-117), and metastatic adenocarcinoma cells (Pr-14C1, Pr-14C2) (unpublished data) in vitro. These cells can be transferred easily into the C3(1)/Tag transgenic mouse model system for in vivo analyses. The cell lines described here contribute to the C3(1)/Tag transgenic mouse model system and will be used for comparison analyses between all cell lines of the model system to investigate prostate-specific proteins that could potentially be used as markers for cancer progression.
Further in vitro and in vivo studies into the mechanisms involved in the changes of the cells from normal tissue to metastatic disease are underway to extend the characterization of the cell lines derived from the C3(1)/Tag transgenic model system.
Acknowledgements
We thank Marcelo Serpe, at Boise State University, for assistance with fluorescent microscopy; Randy Ryan and Donna MacDonald for support and maintenance of our nude mice; and the use of resources and facilities at the Boise VA Medical Center.
Footnotes
This study was supported in part by a grant to Mountain States Tumor Institute/ Mountain States Medical Research Institute from the J.A. and Kathryn Albertson Foundation. C.R.S. was supported by Boise State University and MSTI/MSMRI Fellowship Awards.
References
- 1.American Cancer Society, author. Cancer Facts and Figures 2001. Atlanta, GA: American Cancer Society; pp. 18–19. [Google Scholar]
- 2.Bostwick DG. Progression of prostatic intraepithelial neoplasia to early invasive adenocarcinoma. Eur Urol. 1996;30:145–152. doi: 10.1159/000474164. [DOI] [PubMed] [Google Scholar]
- 3.Bostwick DG. High grade prostatic intraepithelial neoplasia: the most likely precursor of prostate cancer. Cancer. 1995;75:1823–1836. [Google Scholar]
- 4.Bostwick DG, Pacelli A, Lopez-Beltran A. Molecular biology of prostatic intraepithelial neoplasia. Prostate. 1996;29:117–134. doi: 10.1002/(SICI)1097-0045(199608)29:2<117::AID-PROS7>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 5.Bostwick DG, Brawer MK. Prostatic intra-epithelial neoplasia and early invasion in prostate cancer. Cancer. 1987;59:788–794. doi: 10.1002/1097-0142(19870215)59:4<788::aid-cncr2820590421>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 6.Wang M, Liu A, Garcia FU, Rhim JS, Stearns ME. Growth of HPV-18 immortalized human prostatic intraepithelial neoplasia cell lines: influence of IL-10, follistatin, activin-A, and DHT. Int J Oncol. 1999;14:1185–1195. doi: 10.3892/ijo.14.6.1185. [DOI] [PubMed] [Google Scholar]
- 7.Garabedian EM, Humphrey PA, Gordon JI. A transgenic mouse model of metastatic prostate cancer originating from neuroendocrine cells. Proc Natl Acad Sci USA. 1998;95:15382–15387. doi: 10.1073/pnas.95.26.15382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greenberg NM, DeMayo G, Finegold MJ, Medina D, Tilley WD, Aspinall JO, Cunha GR, Donjacour AA, Matusik RJ, Rosen JM. Prostate cancer in a transgenic mouse. Proc Natl Acad Sci USA. 1995;92:3439–3443. doi: 10.1073/pnas.92.8.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DiGiovanni J, Kiguchi K, Frijhoff A, Wilker E, Bol DK, Beltrán L, Moats S, Ramirez A, Jorcano J, Conti C. Deregulated expression of insulin-like growth factor 1 in prostate epithelium leads to neoplasia in transgenic mice. Proc Natl Acad Sci USA. 2000;97:3455–3460. doi: 10.1073/pnas.97.7.3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perez-Stable C, Altman NH, Mehta PP, Deftos LJ, Roos BA. Prostate cancer progression, metastasis, and gene expression in transgenic mice. Cancer Res. 1997;57:900–906. [PubMed] [Google Scholar]
- 11.Maroulakou IG, Anver M, Garrett L, Green JE. Prostate and mammary adenocarcinoma in transgenic mice carrying a rat C3(1) simian virus 40 large tumor antigen fusion protein. Proc Natl Acad Sci USA. 1994;91:11236–11240. doi: 10.1073/pnas.91.23.11236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parker MGR, White H, Hurst M, Needham M, Tilly R. Prostatic steroid-binding protein: isolation and characterization of C3 genes. J Biol Chem. 1983;258:12–15. [PubMed] [Google Scholar]
- 13.Shibata M-A, Jorcyk CL, Liu M-L, Yoshidome K, Gold LG, Green JE. The C3(1)/SV40 T antigen transgenic mouse model of prostate and mammary cancer. Toxicol Pathol. 1998;26:177–182. doi: 10.1177/019262339802600121. [DOI] [PubMed] [Google Scholar]
- 14.Jorcyk CL, Liu M-L, Shibata M-A, Maroulakou IG, Komschlies KL, McPhaul MJ, Resau JH, Green JE. Development and characterization of a mouse prostate adenocarcinoma cell line: ductal formation determined by extracellular matrix. Prostate. 1998;34:10–22. doi: 10.1002/(sici)1097-0045(19980101)34:1<10::aid-pros2>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 15.Peehl DM. Culture of human prostatic epithelial cells. In: Freshney RI, editor. Culture of Epithelial Cells. New York: Wiley-Liss; 1992. pp. 159–180. [Google Scholar]
- 16.Fueyo J, Gomez-Manzano C, Yung WK, Liu TJ, Alemany R, McDonnell TJ, Shi X, Rao JS, Levin VA, Kyritsis AP. Overexpression of E2F-1 in glioma triggers apoptosis and suppresses tumor growth in vitro and in vivo. Nat Med. 1998;4:685–690. doi: 10.1038/nm0698-685. [DOI] [PubMed] [Google Scholar]
- 17.Holst-Hansen C, Brünner N. MTT-cell proliferation assay. In: Celis JE, editor. Cell Biology: a Laboratory Handbook. 2nd ed. San Diego: Academic Press; 1998. pp. 16–18. [Google Scholar]
- 18.Horoszewicz JS, Leong SS, Kawinski E, Karr JP, Rosenthal H, Chu TM, Mirand EA, Murphy GP. LNCaP model of human prostatic carcinoma. Cancer Res. 1983;43:1809–1818. [PubMed] [Google Scholar]
- 19.Isaacs JT, Issacs WB, Feitz WFJ, Scheres J. Establishment and characterization of seven Dunning rat prostatic cancer cell lines and their use in developing methods for predicting metastatic abilities of prostatic cancers. Prostate. 1986;9:261–281. doi: 10.1002/pros.2990090306. [DOI] [PubMed] [Google Scholar]
- 20.Kaighn ME, Narayan KS, Ohnuki Y, Lechner JF, Jones LW. Establishment and characterization of a human prostatic carcinoma cell line (PC-3) Invest Urol. 1979;17:16–23. [PubMed] [Google Scholar]
- 21.Stone KR, Mickey DD, Wunderli H, Mickey GH, Paulson DF. Isolation of a human prostate carcinoma cell line (DU-145) Int J Cancer. 1978;21:274–281. doi: 10.1002/ijc.2910210305. [DOI] [PubMed] [Google Scholar]
- 22.Bright RK, Vocke CD, Emmert-Buck MR, Duray PH, Solomon D, Fetsch P, Rhim JS, Linehan WM, Topalian SL. Generation and genetic characterization of immortal human prostate epithelial cell lines derived from primary cancer specimens. Cancer Res. 1997;57:995–1002. [PubMed] [Google Scholar]
- 23.Yoshidome K, Shibata M-A, Maroulakou IG, Liu M-L, Jorcyk CL, Gold LG, Welch VN, Green JE. Genetic alterations in the development of mammary and prostate cancer in the C3(1)/Tag transgenic mouse model. (Review) Int J Oncol. 1998;12:449–453. [PubMed] [Google Scholar]
- 24.Tsuji M, Kanda K, Murakami Y, Kurokawa Y, Kanayama H-O, Sano T, Kagawa S. Biologic markers in prostatic intraepithelial neoplasia: immunohistochemical and cytogenetic analyses. J Med Invest. 1999;46:35–41. [PubMed] [Google Scholar]
- 25.van der Kwast TH, Labrie F, Tetu B. Prostatic intraepithelial neoplasia and endocrine manipulation. Eur Urol. 1999;35:508–510. doi: 10.1159/000019889. [DOI] [PubMed] [Google Scholar]
- 26.Harper ME, Glynne-Jones E, Goddard L, Mathews P, Nicholson RI. Expression of androgen receptor and growth factors in premalignant lesions of the prostate. J Pathol. 1998;186:169–177. doi: 10.1002/(SICI)1096-9896(1998100)186:2<169::AID-PATH164>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 27.Shibata M-A, Ward JM, Devor DE, Liu M-L, Green JE. Progression of prostatic intraepithelial neoplasia to invasive carcinoma in C3(1)/SV40 large T antigen transgenic mice: histopathological and molecular biological alterations. Cancer Res. 1996;56:4894–4903. [PubMed] [Google Scholar]
- 28.Dyson N, Buchkovich K, Whyte P, Harlow E. The cellular 107K protein that binds to adenovirus E1A also associates with the large T antigens of SV40 and JC virus. Cell. 1989;58:249–255. doi: 10.1016/0092-8674(89)90839-8. [DOI] [PubMed] [Google Scholar]
- 29.Mietz JA, Unger T, Huibregtse JM, Howley PM. The transcriptional transactivation function of wild-type p53 is inhibited by SV40 large T-antigen and by HPV-16 E6 oncoprotein. EMBO J. 1992;11:5013–5020. doi: 10.1002/j.1460-2075.1992.tb05608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]