Abstract
The present study examined the hypothesis that hypertonic saline dextran (HSD), given after an initial insult, attenuates exaggerated inflammation that occurs with a second insult. Adult rats (n = 15 per group) were divided into groups 1 (sham burn), 2 [40% total body surface area burn + 4 ml/kg isotonic saline (IS) + 4 ml·kg−1 ·% burn−1 lactated Ringer solution (LR)], and 3 (burn + 4 ml/kg HSD + LR), all studied 24 h after burns. Groups 4 (sham burn), 5 (burn + IS + LR), and 6 (burns + HSD + LR) received intratracheal (IT) vehicle 7 days after burns; groups 7 (burn + IS + LR) and 8 (burn + HSD + LR) received IT Streptococcus pneumoniae (4 × 106 colony-forming units) 7 days after burn. Groups 4–8 were studied 8 days after burn and 24 h after IT septic challenge. When compared with sham burn, contractile defects occurred 24 h after burn in IS-treated but not HSD-treated burns. Cardiac inflammatory responses (pg/ml TNF-α) were evident with IS (170 ± 10) but not HSD (45 ± 5) treatment vs. sham treatment (80 ± 15). Pneumonia-related sepsis 8 days after IS-treated burns (group 7) exacerbated TNF-α responses/contractile dysfunction vs. IS-treated burns in the absence of sepsis (P < 0.05). Sepsis that occurred after HSD-treated burns (group 8) had less myocyte TNF-α secretion/better contractile function than IS-treated burns given septic challenge (group 7, P < 0.05). We conclude that an initial burn injury exacerbates myocardial inflammation/dysfunction occurring with a second insult; giving HSD after the initial insult attenuates myocardial inflammation/dysfunction associated with a second hit, suggesting that HSD reduces postinjury risk for infectious complications.
Keywords: rat model, Langendorff perfusion, burn complicated by sepsis, cardiac myocytes, tumor necrosis factor-α, interleukin-1β, interleukin-6
large-volume infusion of lactated Ringer solution, early excision of burned eschar, and wound grafting have improved survival in the immediate postburn period (6-8, 11, 12, 15). However, major burn alters immune function, producing an imbalance between pro- and anti-inflammatory cytokine synthesis and increasing susceptibility to postburn infection and sepsis (5, 30, 33, 49, 50). Recent attention has focused on the observation that conventional crystalloid fluid resuscitation exaggerates injury-related neutrophil activation and adhesion (2, 24, 36-38), suggesting that the type of fluid used for resuscitation may aggravate immunologic derangements and predispose the injured subject to infectious complications (13, 21, 23).
Hypertonic saline has been shown to prevent trauma-related T cell suppression (20, 22) and to provide immunologic protection (20). Our previous studies (17, 19) described that hypertonic saline 6% dextran 70 (HSD) attenuated TNF-α, IL-1β, and IL-6 secretion by several cell populations, and reduced inflammatory cytokine secretion was paralleled by improved organ function. The role of inflammatory cytokines in myocardial injury and dysfunction has been explored in a variety of injury/disease states including hemorrhagic shock, ischemia-reperfusion, myocardial failure, and thermal injury (9, 10, 14, 19, 25-27, 29, 31). In this regard, overexpression of TNF-α in the heart produced ventricular dilation, cardiomyopathy, and heart failure (14). Addition of TNF-α, IL-1β, or IL-6 alone or in combination to isolated, perfused hearts, ventricular myocyte preparations, or cardiomyocyte cultures depressed contraction and relaxation (9, 10, 25-27, 29, 31). Finally, therapeutic strategies that specifically neutralize or eliminate proinflammatory cytokines have been shown to improve myocardial function (14).
The present study was designed to examine the hypothesis that HSD administration, after an initial insult such as burn injury, would attenuate the exaggerated proinflammatory cytokine response that has been shown to occur with a second insult. We further hypothesized that attenuation of myocardial inflammatory cytokine secretion would be paralleled by improved myocardial contraction and relaxation.
MATERIALS AND METHODS
Experimental Model
Adult Wistar-Furth rats (325–350 g) were used in the present study. Animals obtained from Harlan Laboratories (Houston, TX) were conditioned in-house for 5–6 days after arrival, with commercial rat chow and tap water available at will. All studies performed in this study were reviewed and approved by the University of Texas Southwestern Medical Center's Institutional Review Board for the care and handling of laboratory animals and conformed to all guidelines for animal care as outlined by the American Physiological Society and the National Institutes of Health.
Catheter Placement and Burn Procedure
Rats were anesthetized lightly with isoflurane 12–13 h before the burn experiment, and body hair on the side, back, and neck was closely clipped. The neck region was treated with a surgical scrub, the left carotid artery was exposed, and a polyethylene catheter (PE-50) inserted into the artery was advanced retrogradely to the level of the aortic arch. In addition, a polyethylene catheter (PE-50) placed in the right external jugular vein was used to administer fluids and drugs. All catheters were filled with heparinized saline, exteriorized, and secured. Twelve hours after catheter placement, animals were deeply anesthetized (isoflurane) and secured in a constructed template device, and the surface of the skin was exposed through the aperture in the template was immersed in 100°C water for 10 s on the back and upper sides. Use of the template produced a well-circumscribed burned area, avoided injury to the abdominal organs, and accomplished full-thickness dermal burns over 40% of the total body surface area (TBSA). Exposure to this water temperature in adult rats was shown previously by our laboratory (17, 18) to destroy all underlying nerves and to avoid injury to underlying organs. Sham-burned rats were subjected to identical preparation, except that they were immersed in room temperature water and served as controls. Immediately after immersion, rats were dried and returned to individual cages, and each external jugular catheter was connected to a swivel device (BSP99 Syringe Pump, Braintree Scientific, Braintree, MA) for fluid administration (lactated Ringer solution, 4 ml·kg−1 ·% burn−1, with one-half of the calculated volume given during the first 8 h after burn and the remaining volume given over the next 16 h after burn). Buprenorphine (0.5 mg/kg) was given every 12 h during the postburn period. Burned rats did not display discomfort or pain, moved freely about the cage, and consumed food and water at will. In the sham burn group, the external jugular vein was cannulated, lactated Ringer solution was given to maintain catheter patency (0.2 ml·kg−1 ·h−1), and an identical regimen of analgesics (buprenorphine) was given throughout the study period. Twenty-four hours after burn injury, systemic blood pressure was measured with a Gould-Statham pressure transducer (model IDP23, Gould Instruments, Oxnard, CA), connected to a Grass medical recorder (model 7D Polygraph, Grass Instruments, Quincy, MA). A Grass tachycardiograph (model 7P4F) was used to monitor heart rate. A Grass Poly VIEW data acquisition system was used to convert acquired data into digital form (1, 18).
Experimental Groups
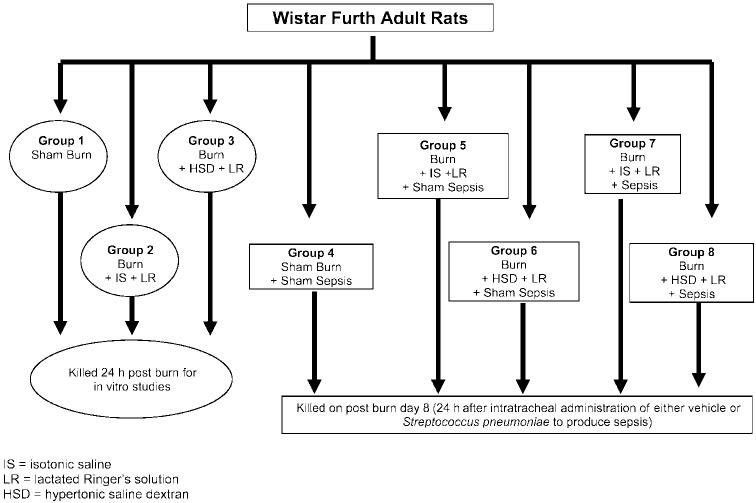
Rats were randomly divided into sham burn and burn groups. In the rats designated for the burn group, a full-thickness burn was accomplished over 40% TBSA and lactated Ringer solution was initiated as described above. Forty-five minutes after initiation of lactated Ringer solution infusion, burned rats were randomized to receive a bolus of either isotonic saline (IS) or HSD (4 ml/kg body wt). The IS or HSD infusion was accomplished over 45 min; lactated Ringer solution resuscitation was then resumed. The IS or HSD infusion was repeated on day 1 (24 h after burn), day 3 (72 h after burn), and day 5 (120 h after burn) in those animals included for postburn septic challenge (groups 4–8). HSD was given as a slow infusion over 45 min (4 ml/kg), as described for the initial treatment. The following experimental groups were included: group 1 (sham burn, n = 12), group 2 [burn over 40% TBSA given a bolus of IS (4 ml/kg) + lactated Ringer solution resuscitation (4 ml·kg−1 ·% burn−1), n = 12], and group 3 [burn over 40% TBSA treated with HSD (4 ml/mg) + lactated Ringer solution (4 ml·kg−1 ·% burn−1), n = 12]. Groups 1–3 were killed 24 h after burn, and cardiac myocyte secretion of inflammatory cytokines (n = 3–4 rats/group) and left ventricular (LV) function (n = 7–9 rats/group) were studied in vitro. Group 4 [sham burns, n = 12], group 5 (burns given IS + lactated Ringer solution as described above, n = 12), and group 6 (burns given HSD + lactated Ringer solution as described above, n = 12) received 0.3 ml of intratracheal vehicle (PBS) on postburn day 7. Group 7 (burns given IS + lactated Ringer solution resuscitation, n = 12) and group 8 (burns given HSD + lactated Ringer solution, n = 12) received intratracheal Streptococcus pneumoniae challenge [4 × 106 colony-forming units (CFU) in 0.4 ml of PBS] on postburn day 7 to produce sepsis. Animals in groups 4–8 were studied 24 h after intratracheal S. pneumoniae or vehicle challenge (groups are described in Fig. 1).
Fig. 1.
Experimental groups.
Sepsis Protocol
Preparation of inoculum
S. pneumoniae type 3 was obtained from the American Type Culture Collection (ATCC 6303, Rockville, MD) in lyophilized form. Bacteria were reconstituted and then injected into the lungs of a rat to increase their virulence; lung lavage was plated and purified, and aliquots were prepared and stored at −80°C. Before each experiment, individual aliquots were thawed, inoculated onto trypticase soy agar with 5% sheep blood agar plates, and incubated overnight at 37°C. The plates were washed with sterile endotoxin-free PBS, and a concentration of 1 × 107 CFU/ml was determined by absorbance at 540 nm. The bacteria were agitated and drawn up into sterile tuberculin syringes in 0.4-ml aliquots, producing a final inoculum of 4 × 106 CFU in 0.4 ml. Bacterial CFU were determined by plating 10 μl of the 1 × 104 CFU/ml bacterial suspension onto blood agar plates and incubating the plates overnight at 37°C. The number of viable bacteria inoculated into animals in either the pneumonia-alone group or the burn + pneumonia groups was ∼4 × 106 CFU. Additional previous studies showed that the transtracheal injection of nonviable bacteria, to produce additional controls, produced no ill effects and no cardiac dysfunction (41).
Induction of aspiration pneumonia
To produce sepsis, animals were anesthetized with isoflurane and placed in a supine position, and the area over the trachea was prepped with a surgical scrub (povidone-iodine, Betadine). A midline incision was made over the trachea; the trachea was identified and isolated via blunt dissection. An aliquot of bacterial suspension (4 × 106 CFU in 0.4 ml) or sterile endotoxin-free PBS was injected directly into the trachea with a 30-gauge needle; the wound was then closed with surgical staples. Animals were placed on a 30° incline, with the head up, to ensure that the injected fluid entered the lungs. All rats were given 10 ml of lactated Ringer solution (ip) while anesthetized to ensure hydration. All animals given intratracheal S. pneumonia had a blood sample collected 24 h after septic challenge. Hearts were then collected for in vitro perfusion (cardiac function or myocyte secretion of cytokines), and lungs were collected to determine pulmonary inflammation and morphology (16, 41, 49).
Cardiomyocyte Isolation
To isolate cardiac myocytes, rats (n = 4–6 rats from each experimental group) received an intraperitoneal injection of heparin (2,000 U) 20–30 min before death. The rats were decapitated, and hearts were harvested and placed in a petri dish containing room temperature heart medium [in mM: 113 NaCl, 4.7 KCl, 0.6 KH2PO4, 0.6 Na2HPO4, 1.2 MgSO4, 12 NaHCO3, 10 KHCO3, 20 d-glucose, 10 HEPES, 30 taurine, 2.0 carnitine, and 2.0 creatine, with 0.5× MEM and amino acids (50×, GIBCO-BRL 11130-051)]. Hearts were cannulated via the aorta and perfused with heart medium at a rate of 12 ml/min for a total of 5 min in a nonrecirculating mode. Enzymatic digestion was initiated by perfusing the heart with digestion solution, which contained 34.5 ml of heart medium described above, plus 50 mg of collagenase II (Worthington 4177, lot no. MOB3771), 50 mg of BSA (fraction V, GIBCO-BRL 11018-025), 0.5 ml of trypsin (2.5%, 10×, GIBCO-BRL 15090-046), and 15 μM CaCl2. Enzymatic digestion was accomplished by recirculating this solution through the heart at a flow rate of 12 ml/min for 20 min. All solutions perfusing the heart were maintained at a constant temperature of 37°C. At the end of the enzymatic digestion, the ventricles were removed and mechanically dissociated in 6 ml of enzymatic digestion solution containing a 6-ml aliquot of 2× BSA solution [2 mg of BSA (fraction V) to 100 ml of heart media]. After mechanical dissociation with fine forceps, the tissue homogenate was filtered through a mesh filter into a conical tube. The cells adhering to the filter were collected by washing with an additional 10-ml aliquot of 1× BSA solution, 100 ml of heart medium described above, and 1 g of BSA (fraction V)]. Cells were then allowed to pellet in the conical tube for 10 min. The supernatant was removed, and the pellet was resuspended in 10 ml of 1× BSA. The cells were washed and pelleted further in BSA buffer with increasing increments of calcium (100, 200, 500 μM, to a final concentration of 1,000 μM). After the final pelleting step, the supernatant was removed, and the pellet was resuspended in MEM [prepared by adding 10.8 g of 1× MEM (Sigma M-1018), 11.9 mM NaHCO3, 10 mM HEPES, and 10 ml of penicillin-streptomycin (100×, GIBCO-BRL 1540-122) with 950 ml of MilliQ water]; total volume was adjusted to 1 liter. At the time of MEM preparation, the medium was bubbled with 95% O2-5% CO2 for 15 min and the pH was adjusted to 7.1 with 1 M NaOH. The solution was then filter sterilized and stored at 4°C until use. At the final concentration of calcium cell viability was measured, and cell suspensions with >85% viability were used for subsequent studies. Myocytes with a rodlike shape, clear, defined edges, and sharp striations were prepared with a final cell count of 5 × 104 cells·ml−1 ·well−1 (16).
Measurement of Cardiac Myocyte Cytokine Secretion
Primary cardiac myocytes were pipetted into microtiter wells (5 × 104 cells/well), and cells were incubated in a CO2 incubator at 37°C for 18 h. At the end of the incubation period, supernatants were collected to measure secreted inflammatory cytokines (ELISA). Cell viability and morphology were examined (17). Additional aliquots of myocytes were loaded with either fura-2 AM or sodium-binding benzofuran isophthalate (SBFI) to measure myocyte calcium and sodium, respectively.
Cardiac Myocyte Calcium and Sodium Measurement
Myocyte loading with fura-2 AM was accomplished over 45 min and myocyte loading with SBFI over 1 h at room temperature in the dark. Myocytes were then suspended in 1.0 mM calcium-containing MEM and washed to remove extracellular dye; myocytes were placed on a glass slide on the stage of a Nikon inverted microscope. The microscope was interfaced with Grooney optics for epi-illumination, a triocular head, phase optics, and ×30 phase-contrast objective and mechanical stage. The excitation illumination source (300-W compact xenon arc illuminator) was equipped with a power supply. In addition, this InCyt Im2 Fluorescence Imaging System (Intracellular Imaging, Cincinnati, OH) included an imaging workstation and an Intel Pentium Pro200 MHZ-based personal computer. The computer-controlled filter changer allowed alternation between the 340- and 380-nm excitation wavelengths. Images were captured by a monochrome charge-coupled device camera equipped with a TV relay lens. InCyt Im2 Image software allowed measurement of intracellular calcium and sodium concentrations from the ratio of the two fluorescent signals generated from the two excitation wavelengths (340 nm/380 nm); background was removed by the InCyt Im2 software. The calibration procedure included measuring fluorescence ratio with buffers containing different concentrations of either calcium or sodium. At each wavelength, the fluorescence emissions were collected for 1-min intervals and the time between data collection was 1–2 min. Because quiescent or noncontracting myocytes were used in these studies, the calcium levels measured reflect diastolic levels (45, 49).
Isolated Coronary Perfused Heart
Additional rats from each experimental group (n = 9–10 rats/group) were heparinized, and hearts were removed and placed in ice-cold (4°C) Krebs-Henseleit bicarbonate-buffered solution (in mM: 118 NaCl, 4.7 KCl, 21 NaHCO3, 1.25 CaCl2, 1.2 MgSO4, 1.2 KH2PO4, and 11 glucose). All solutions were prepared each day with demineralized, deionized water and bubbled with 95% O2-5% CO2 (pH 7.4, Po2 550 mmHg, and Pco2 38 mmHg). A 17-gauge cannula placed in the ascending aorta and connected via glass tubing to a buffer-filled reservoir (Ismatec model 7335-30, Cole-Parmer Instrument, Chicago, IL) was used to maintain perfusion of the coronary arteries by retrograde perfusion of the aortic stump cannula. Coronary perfusion pressure was measured, and effluent was collected to confirm coronary flow rate. Contractile function was assessed by measuring intraventricular pressure with a H2O-filled latex balloon attached to a polyethylene tube and threaded through the apex of the LV chamber. Peak LV systolic pressure and LV end-diastolic pressure were measured with a Statham pressure transducer (model P23 ID, Gould Instruments, Oxnard, CA) attached to the balloon cannula, and the rates of LV developed pressure (LVP) rise (+dP/dt) and fall (−dP/dt) were obtained with an electronic differentiator (model 7P20C, Grass Instruments) and recorded (Grass model 7DWL8P). LVP was calculated by subtracting end-diastolic from peak systolic pressure. A Grass PolyVIEW data acquisition system was used to convert acquired data into digital form (16, 17, 49).
Statistical Analysis
All values are expressed as means ± SE. ANOVA was used to assess an overall difference among the groups for each of the variables. Levene's test for equality of variance was used to suggest the multiple-comparison procedure to be used. If equality of variance among the four groups was suggested, multiple-comparison procedures were performed (Bonferroni); if inequality of variance was suggested by Levene's test, Tamhane multiple comparisons (which do not assume equal variance in each group) were performed. Probability values <0.05 were considered statistically significant (analysis was performed with SPSS for Windows, version 7.5.1).
RESULTS
There were no deaths in groups 1, 2, or 3 (Fig. 1) studied 24 h after sham burn or burn injury in the absence of sepsis. When compared with sham burn, mean arterial blood pressure was lower 24 h after burn injury, regardless of the type of fluid resuscitation administered (Table 1). In addition, hematocrit was lower in all burned animals at this time, likely because of the hemodilutional effects of volume resuscitation. Base excess and whole blood lactate were altered in burns given IS (group 2) compared with values measured in sham burns (group 1); in contrast, there was a modest burn-related change in base excess in HSD-treated burns (group 3), whereas blood lactate levels were not significantly different in HSD-treated burns compared with values measured in sham burns (group 1) (Table 1). A burn-related fall in serum ionized calcium was evident in all burned animals regardless of IS or HSD administration. Systemic inflammatory responses to burn injury were evident in burns given IS (group 2), as indicated by the rise in circulating TNF-α, IL-1β, and IL-6 (Table 1). Administration of HSD (group 3) attenuated the systemic inflammatory response to burn injury, as indicated by lower plasma TNF-α, IL-1β, and IL-6, measured 24 h after burn injury treated with IS (P < 0.05).
Table 1.
Experimental groups killed 24 h after burn in absence of sepsis
| Group 1 | Group 2 | Group 3 | |
|---|---|---|---|
| Sham Burn | Burn + IS | Burn + HSD | |
| In Vivo | |||
| MAP, mmHg | 124±2 | 95±5* | 107±3*† |
| HR, beats/min | 475±16 | 440±15* | 490±9† |
| pH | 7.46±.01 | 7.47±.02 | 7.46±.02 |
| PCO2, mmHg | 30±2 | 25±3* | 25±3* |
| Hct, % | 38±2 | 27±2* | 28±2* |
| Base excess, mM | 1.64±0.04 | −2.17±0.68* | −0.23±0.06* |
| Lactate, mM | 2.0±0.31 | 3.33±0.28* | 2.80±0.24 |
| Ca2+, mM | 1.30±0.01 | 0.84±0.0* | 0.75±0.04* |
| Plasma TNF-α, pg/ml | 3.6±0.3 | 26±4* | 7.1±0.4*† |
| Plasma IL-1β, pg/ml | 3±1 | 8.3±0.6* | 2.3±0.1† |
| Plasma IL-6, pg/ml | 57±3 | 149±40* | 20±3*† |
| In Vitro | |||
| LVP, mmHg | 90±4 | 73±3* | 83±5† |
| +dP/dtmax, mmHg/s | 1,990±80 | 1,350±75* | 1,970±75† |
| −dP/dtmax, mmHg/s | 1,650±85 | 1,210±80* | 1,640±84† |
All values are means ± SE. IS, isotonic saline; HSD, hypertonic saline dextran 70; MAP, mean arterial blood pressure; HR, heart rate; LVP, left ventricular developed pressure; +dP/dtmax, maximal rate of LVP rise; −dP/dtmax, maximal rate of LVP fall.
Significant difference from sham burn (P < 0.05)
significant difference between IS- and HSD-treated burns (P < 0.05).
In the groups studied 8 days after burn injury and 24 h after S. pneumoniae challenge (groups 7 and 8, Fig. 1), the presence of sepsis was confirmed by positive blood cultures (1 × 107 CFU/ml blood). Mortality rate 24 h after intratracheal bacterial challenge was higher in the rats given IS after burn injury (17% mortality in group 7) compared with rats given HSD after burn injury (0% mortality in group 8) despite the fact that the number of S. pneumoniae CFU per milliliter of blood (1 × 107) was identical in groups 7 and 8. There were no bacteria in the blood of animals given intratracheal vehicle (groups 4–6), and there were no deaths in these experimental groups in the absence of sepsis.
Mean arterial blood pressure, measured 8 days after burns treated with either IS (group 5) or HSD (group 6) and given intratracheal vehicle on postburn day 7 (in the absence of an infectious challenge), returned toward values measured in sham-burned animals (group 1) regardless of IS or HSD administration in the early postburn period (Table 2). Heart rates were similar in group 4 (sham burns given intratracheal vehicle and studied postburn day 8), group 5 (burns given IS + intratracheal vehicle on postburn day 7), and group 6 (burns given HSD + intratracheal vehicle on postburn day 7). Base excess was similar in groups 4–6 measured 8 days after burn injury, whereas arterial lactate remained higher in the burn group given IS in the early postburn period (group 5). Animals given HSD during the postburn period and before S. pneumoniae challenge on postburn day 7 (group 8) had significantly higher mean arterial blood pressure than animals with burns given IS followed by septic challenge on postburn day 7 (group 7). All S. pneumoniae-challenged animals (groups 7 and 8) had metabolic acidosis, as indicated by changes in base excess. In addition, both burn groups given septic challenge had significantly lower serum calcium levels than sham burn values (P < 0.05; Table 2).
Table 2.
Hemodynamic and metabolic response to injury and resuscitation measured 24 h after septic (or vehicle) challenge and on postburn day 8
| Group 4 | Group 5 | Group 6 | Group 7 | Group 8 | |
|---|---|---|---|---|---|
| Sham Burn + IT Vehicle | Burn + IS + IT Vehicle | Burn + HSD + IT Vehicle | Burn + IS + Sepsis | Burn + HSD + Sepsis | |
| MAP, mmHg | 150.6±4.9 | 145.1±4.1 | 145.0±5.6 | 100.9±4.8* | 131.9±6.8* |
| HR, beats/min | 523±12 | 505±21 | 489±16 | 421±29* | 503±8+ |
| pH | 7.47±0.04 | 7.51±0.01 | 7.49±0.01 | 7.51±0.01 | 7.52±0.01 |
| PCO2, mmHg | 23.5±1.2 | 28.4±1.1 | 26.3±1.1 | 26.1±1.2 | 25.3±0.5 |
| Base excess, mM | 1.64±0.4 | 0.52±0.58 | −1.2±0.3 | −1.7±0.3* | −1.27±0.4* |
| Lactate, mM | 2.20±0.3 | 4.14±0.31* | 2.25±0.14 | 3.11±0.22* | 2.3±0.2+ |
| Serum , meq/l | 19.5±0.7 | 22.4±0.6 | 20.4±0.8 | 20.1±0.5 | 20.6±0.5 |
| Hct | 42.8±3.0 | 37.3±1.5 | 34.9±1.4* | 35.6±1.6* | 34.7±1.7* |
| Ca2+, mM | 1.29±0.01 | 0.82±0.02* | 0.70±0.04* | 0.85±0.02* | 0.71±0.02* |
| Na+, mM | 141.2±0.6 | 144.8±0.7 | 146.3±0.8* | 143.4±0.4 | 145.7±0.5* |
| Plasma TNF-α, pg/ml | 4.7±0.3 | 19±0.2* | 8.4±0.6*† | 190±0.2* | 10.2±0.9*† |
| Plasma IL-1β, pg/ml | 2.3±0.3 | 14±0.9* | 2.2±0.4† | 140±1* | 3.3±0.7† |
| Plasma IL-6, pg/ml | 60±4.4 | 61±0.6 | 24±1.2*† | 337±10.2* | 30.1±2.3*† |
All values are means ± SE. IT, intratracheal; Ca2+, serum ionized calcium; Na+, serum ionized sodium.
Significant difference from sham burns (P < 0.05)
significant difference between IS- and HSD-treated burns (P < 0.05).
Cardiac Myocyte Secretion of Inflammatory Cytokines in Burn and in Burn Complicated by Sepsis
To determine whether HSD administration during the early postburn period alters myocardial inflammatory cytokine responses to a second challenge, cardiomyocytes were prepared 24 h after septic challenge or intratracheal vehicle (postburn day 8) from experimental groups 4–8. Myocytes prepared from burns given IS and studied 8 days after burn in the absence of sepsis (group 5) secreted significantly more TNF-α, IL-1β, IL-6, and IL-10 compared with sham burn myocytes (group 4) (Fig. 2). Animals studied 8 days after burn plus HSD and no sepsis (group 6) had significantly less cardiomyocyte secretion of pro- and anti-inflammatory cytokines compared with values measured in IS-treated burns given no sepsis (group 5, P < 0.05). Myocytes prepared from burns given IS in the early postburn period followed by septic challenge on postburn day 7 and studied 24 h after intratracheal S. pneumoniae challenge (group 7) had robust myocardial inflammatory responses, as indicated by significant increase in TNF-α (Fig. 2A), IL-1β (Fig. 2B), IL-6 (Fig. 2C), and IL-10 (Fig. 2D) secretion. In contrast, cardiomyocytes prepared from burned rats given HSD in the early postburn period followed by septic challenge on postburn day 7 and studied 24 h after intratracheal S. pneumoniae challenge (group 8) secreted significantly less pro- and anti-inflammatory cytokines compared with myocytes prepared from burns given IS plus sepsis (group 7, P < 0.05).
Fig. 2.
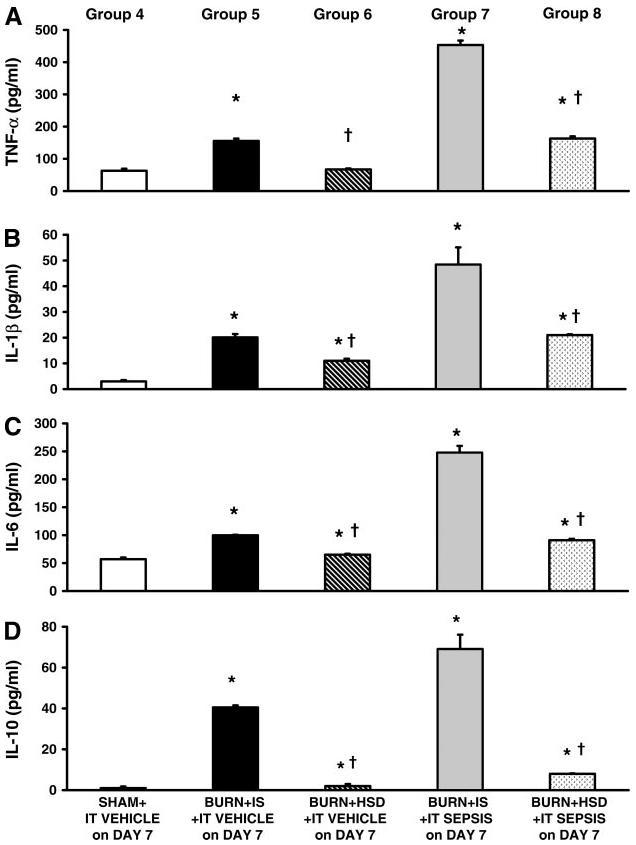
Cardiomyocyte secretion of inflammatory cytokines measured 24 h after either intratracheal (IT) vehicle or septic challenge on postburn day 8. Burns given IS and then septic challenge had significant increases in TNF-α (A), IL-1β (B), IL-6 (C), and IL-10 (D) secretion by cardiomyocytes compared with cytokines secreted by myocytes (pg · 10−4 cells · ml−1) prepared from either burns given IS and IT vehicle on postburn day 7 (group 5) or burn + HSD + IT vehicle on day 7 (group 6). Administration of hypertonic saline during the early postburn period followed by septic challenge on postburn day 7 (group 8) had significant lower TNF-α, IL-1β, IL-6, and IL-10 secretion by cardiomyocytes compared with those measured in burns given IS + an identical septic challenge (group 7). All values are means ± SE. *Significant burn-related differences compared with values measured in sham burn given IT vehicle on postburn day 7 (group 4) (ANOVA and multiple-comparison procedure); †significant differences between HSD and IS (P < 0.05).
Cardiomyocyte Sodium and Calcium Responses to Burn Injury and Sepsis
Burned animals given either IS (group 5) or HSD (group 6) plus intratracheal vehicle on postburn day 7 (no sepsis) and studied on postburn day 8 had persistent elevations in cardiac myocyte calcium (Fig. 3A) and sodium (Fig. 3B). Although calcium accumulation persisted 8 days after burn plus HSD, these values were less (P < 0.05) than calcium levels measured 8 days after burn plus IS. Septic challenge on postburn day 7 in burns given IS (group 7) produced robust calcium and sodium accumulation by cardiomyocytes; cardiomyocyte sodium and calcium loading was attenuated in burns given HSD followed by septic challenge (group 8) despite intratracheal inoculation with an identical number of S. pneumoniae CFU.
Fig. 3.
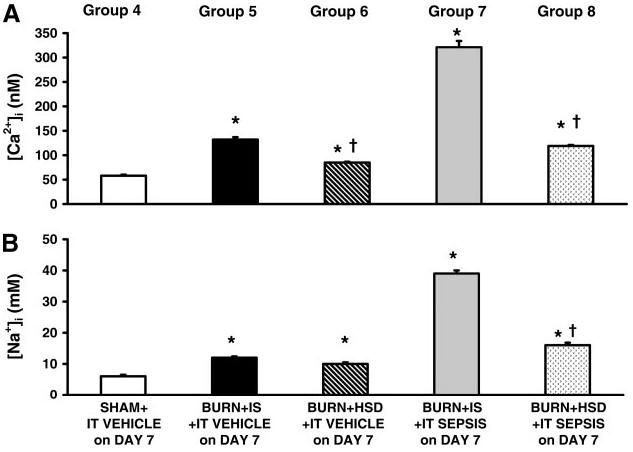
Intracellular calcium ([Ca2+]i, A) and sodium ([Na2+]i, B) concentration accumulation persisted at day 8 after burn injury in animals given IS (group 5) as well as animals given HSD (group 6) compared with animals with sham burns (group 4). Septic challenge on day 7 in burns given IS produced significant cardiomyocyte accumulation of calcium and sodium, and this septic-related effect was attenuated by the administration of HSD during the postburn period. All values are means ± SE. *Significant burn-related differences compared with values measured in sham burns (ANOVA and multiple-comparison procedure); †significant differences between HSD and IS (P < 0.05).
Cardiac Contractile Function
Cardiac function was studied in vitro during stabilization of the perfused heart at constant preload, constant coronary flow rate, and constant heart rate (Table 3). LVP and ±dP/dt measured 8 days after burn injury in the absence of sepsis (groups 5 and 6) confirmed a significant recovery in myocardial contraction and relaxation performance compared with values measured in burns studied 24 h after burn over 40% TBSA and resuscitated with IS plus conventional lactated Ringer solution (group 2). Eight days after burn injury, there was no significant difference in LVP and ±dP/dt in groups 5 and 6 compared with values measured in sham burns (group 4).
Table 3.
Stabilization data collected 24 h after septic challenge and 8 days after burn during perfusion of hearts at constant preload, constant coronary flow rate, and constant heart rate
| Group 4 | Group 5 | Group 6 | Group 7 | Group 8 | |
|---|---|---|---|---|---|
| Sham Burn + IT Vehicle | Burn + IS + IT Vehicle | Burn + HSD + IT Vehicle | Burn + IS + Sepsis | Burn + HSD + Sepsis | |
| LVP, mmHg | 93±5 | 88±2 | 91±4 | 65±4* | 80±3*† |
| +dP/dtmax, mmHg/s | 2,021±122 | 1,965±45 | 1,938±78 | 1,320±131* | 1,704±69*† |
| −dP/dtmax, mmHg/s | 1,725±126 | 1,710±52 | 1,654±72 | 1,180±142* | 1,393±63*† |
| dP40, mmHg/s | 1,792±123 | 1,775±44 | 1,706±61 | 1,140±116* | 1,486±56*† |
| TPP, ms | 85.7±1.9 | 84.7±2.6 | 94.0±2.0* | 91.4±5.8 | 98.3±3.1 |
| RT90, ms | 83.7±1.3 | 79.3±2.1 | 90.3±2.7* | 88.0±6.0 | 104.0±3.4 |
| Time to +dP/dtmax, ms | 56.3±1.0 | 56.7±4.9 | 54.5±1.2 | 52.6±2.1 | 57.6±2.1 |
| Time to −dP/dtmax, ms | 56.8±1.6 | 55.2±5.0 | 54.8±1.2 | 51.6±2.1 | 57.1±1.3 |
| CPP, mmHg | 55.0±2.8 | 51.3±5.0 | 44.3±4.6 | 44.2±5.9 | 49.4±4.7 |
| CVR, mmHg/ml | 11.0±1.0 | 10.4±1.0 | 8.9±0.9 | 8.84±1.19 | 9.89±0.95 |
| HR, beats/min | 262±15 | 265±4 | 250±6 | 257±14 | 247±11 |
All values are means ± SE; dP40, developed pressure at 40 mmHg; TPP, time to peak pressure; RT90, time to 90% relaxation; CPP, coronary perfusion pressure; CVR, coronary vascular resistance.
Significant difference from sham burns (P < 0.05)
significant difference between IS- and HSD-treated burns (P < 0.05).
Septic challenge on postburn day 7 in rats given IS plus lactated Ringer solution (group 7) produced significant myocardial contraction and relaxation defects, as indicated by lower LVP and ±dP/dt in these groups compared with values measured in either sham-burned rats (group 4) or in rats studied 8 days after burn without sepsis and given intratracheal vehicle on postburn day 7 (groups 5 and 6). However, LVP and ±dP/dt measured 24 h after septic challenge in the burns given HSD in the early postburn period (group 8) were significantly better (P < 0.05) compared with LVP and ±dP/dt measured in rats given IS plus sepsis (group 7).
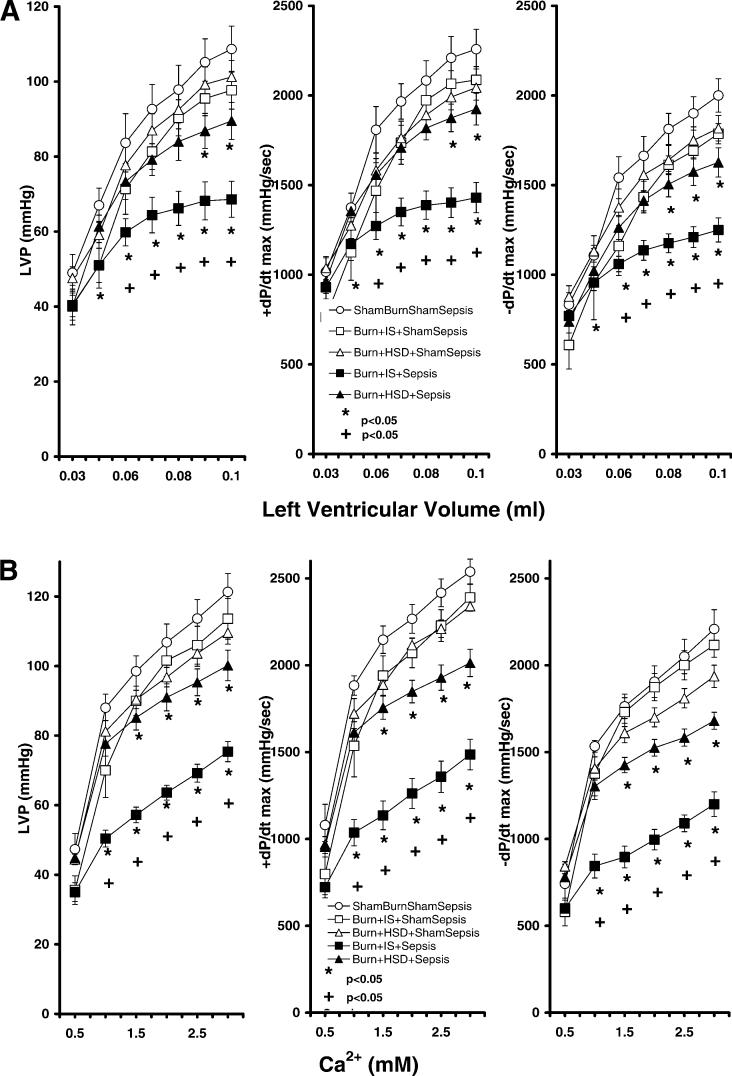
We then determined whether burn complicated by sepsis altered cardiac responsiveness to inotropic challenge. As seen in Fig. 4, hearts from burned animals given either IS or HSD plus intratracheal vehicle (no sepsis) had similar maximum systolic dP/dt (+dP/dtmax) and maximum diastolic dP/dt (−dP/dtmax) responses to increases in LV volume (Fig. 4A) and similar responses to increases in perfusate calcium (Fig. 4B) when studied on postburn day 8. Hearts from burn animals given IS plus septic challenge (group 7) had significant decreases in systolic performance as indicated by lower LVP as well as a reduced rate of maximal pressure generation (+dP/dtmax) compared with hearts from sham-burned animals (group 4, P < 0.05). These hearts also had impaired diastolic function as indicated by lower rates of LV relaxation (−dP/dtmax) compared with values measured in sham-burned animals. Ventricular defects in this group were evident from reduced responses to increases in preload (Fig. 4A) or decreased responses to increases in perfusate calcium concentration (Fig. 4B). Systolic and diastolic function in burns treated initially with HSD followed by septic challenge on postburn day 7 were significantly improved over function measured in burns given IS plus septic challenge (P < 0.05, ANOVA and multiple-comparison procedure). This improved contractile performance was evident from improved LVP and ±dP/dt responses to increases in either preload or perfusate calcium levels (Fig. 4).
Fig. 4.
Burn injury treated with IS followed by septic challenge on postburn day 7 significantly reduced left ventricular developed pressure (LVP), maximal rate of LVP rise (+dP/dtmax), and maximal rate of LVP fall (−dP/dtmax) responses to either increases in left ventricular volume (A) or increases in perfusate calcium (B) compared with values generated by hearts from sham-burned animals. HSD administration in burned animals attenuated sepsis-related myocardial contractile defects. All values are means ± SE. *Significant differences from sham burn (P < 0.05, ANOVA and Student-Newman-Keuls); +significant differences in burn + HSD + sepsis compared with burn + IS followed by septic challenge (P < 0.05, ANOVA and Student-Newman-Keuls).
DISCUSSION
We described previously (17) that HSD administration in the early postburn period attenuated cardiomyocyte inflammatory cytokine secretion and provided significant cardioprotection when animals were studied 24 h after burn. On the basis of this previous work, we had hypothesized that pharmacological interventions that attenuate the inflammatory responses to an initial injury would decrease susceptibility to a subsequent infection, reducing cardiac myocyte cytokine responses to a second challenge such as sepsis and improving myocardial performance. Data from the present study confirmed that postburn administration of HSD attenuates cardiomyocyte secretion of TNF-α, IL-1β, and IL-6 and attenuates the profound myocardial contractile defects. Furthermore, HSD treatment in the early postburn period improved survival after a subsequent septic challenge. Our findings suggest that therapeutic interventions in the early postburn period that limit inflammatory signaling cascades in downstream organs such as the heart provide significant protection from a second hit such as septic challenge.
Burn injury and aggressive fluid resuscitation to correct intravascular volume deficits alter several aspects of immune function, priming the burn subject for increased responsiveness to a second insult such as infection (5, 30, 49). Infection and severe sepsis after major burn injury are significant causes of postburn mortality (4, 39, 40).
In the burn unit, the need for intubation and ventilation contributes to a significant incidence of pneumonia-related sepsis (4, 40). On the basis of these clinical findings, we developed a model of burn complicated by aspiration pneumonia-related sepsis that closely mimics the acidosis, exaggerated inflammation, and increases in mortality that have been shown to occur in clinical postburn sepsis (16, 28, 41, 49). Data in the present study confirm previous work describing that burn injury primes adult subjects for increased responsiveness to a second infectious challenge (16, 28, 30, 34, 50). Myocytes prepared from adult rats given aspiration pneumonia-related sepsis on postburn day 7 secreted significantly more TNF-α, IL-1β, IL-6, and IL-10 compared with myocyte cytokine levels measured after either sepsis alone or burn alone (16, 28, 49). This cardiac inflammatory response was paralleled by an exaggerated systemic inflammatory response measured in burn complicated by sepsis (group 7). Numerous reports have described that the magnitude and duration of the initial inflammatory response to a first injury such as burn trauma not only determine the degree of multiple organ failure and mortality but also determine susceptibility to subsequent infection (16, 28, 30, 34, 49). These data would suggest that initial therapies that downregulate the magnitude as well as the duration of the initial systemic inflammatory response to burn injury could not only limit the initial injury to downstream organs but provide significant protection in the face of a subsequent septic challenge.
That hypertonicity alters immune responses is not a new concept. Junger and colleagues (21-23) described that lactated Ringer solution resuscitation from injury and trauma produced detrimental effects on several aspects of immune function, whereas these effects were not observed with hypertonic resuscitation (17). Similarly, Rhee and colleagues (2, 36, 38) showed that although lactated Ringers solution altered several aspects of cellular immune response, hypertonic saline solution had no detrimental effect. Others have shown a protective effect of hypertonic fluids in resuscitation of the injury patient. As early as 1973, Monafo and colleagues (32) described the benefits of concentrated sodium solutions in the resuscitation of patients with severe burns. Small-volume hypertonic saline has also been shown to restore blood pressure and cardiac output after blood loss and penetrating trauma (3, 35, 43, 44, 46, 47).
Previous studies have suggested that isotonic crystalloids exacerbate the cellular inflammatory signaling cascade as well as cellular injury produced by an initial injury such as burn trauma. Thus interest has developed in the use of hypertonic saline as a pharmacological tool to reduce injury-related inflammation and reduce the overall fluid requirements after injury, including burn injury. The use of hypertonic solution may also alleviate the need to transport and administer large volumes of lactated Ringer solution in the prehospital phase. Finally, strategies that have less effect on immune responses to burn injury would be expected to have beneficial effects in the face of a subsequent insult, such as sepsis or septic shock.
The limits of this animal study must be considered. Pneumonia represents only one cause of postburn infection; however, the incidence of nosocomial pneumonia has been described as ∼50% in burn patients (40). In addition, Shirani and colleagues (42) studied more than 1,000 burn patients, reporting that pneumonia increased the predicted burn-related mortality regardless of whether inhalation injury was present. Although this model does not consider sepsis that occurs as a result of wound infection, aggressive wound care, the use of antimicrobials, and early wound excision and grafting have reduced the incidence of wound-related sepsis in US burn units (6-8, 11, 12, 15). Because of these factors, we developed a model of aspiration pneumonia-induced sepsis in adult rats given a full-thickness burn over 40% of TBSA (41, 49). Another limitation of our study was the administration of hypertonic saline in combination with dextran. Although hypertonic saline alone has been shown to exert beneficial effects with regard to suppressing inflammation while improving cardiodynamic performance, dextran may have exerted immunomodulatory effects, contributing to the decreased cytokine response to sepsis that occurred after burn injury. However, we have accumulated considerable data with the experimental model of HSD administration in burned animals. We (17) showed previously that this regimen was well tolerated in adult rats, and our previous experience indicated that this regimen improved cardiac function after burn injury alone. However, subsequent studies should be directed to examine the role of hypertonic saline in the absence of dextran with regard to both immunomodulation and cardiac contractile performance.
In summary, data from the present study confirm that administration of HSD after an initial burn injury reduced myocardial inflammatory responses and reduced myocardial contraction and relaxation defects associated with a subsequent bacterial challenge. HSD suppressed myocardial TNF-α, IL-1β, and IL-6 secretion compared with values measured after IS administration, and downregulation of the myocardial inflammatory signaling was associated with improved ventricular performance after a subsequent septic challenge. These data suggest that attenuation of inflammation after burn injury abrogated the significant inflammation and myocardial contrac-tile depression that were associated with subsequent sepsis.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
GRANTS
This work was supported by National Institute of General Medical Sciences Grant 5P50-GM-21681-39.
REFERENCES
- 1.Adams HR, Baxter CR, Izsenberg SD. Decreased contractility and compliance of the left ventricle as complications of thermal trauma. Am Heart J. 1984;108:1477–1487. doi: 10.1016/0002-8703(84)90695-1. [DOI] [PubMed] [Google Scholar]
- 2.Alam HB, Stanton K, Koustova E, Burris D, Rich N, Rhee P. Effect of different resuscitation strategies on neutrophil activation in a swine model of hemorrhagic shock. Resuscitation. 2004;60:91–99. doi: 10.1016/j.resuscitation.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 3.Alpar EK, Killampalli VV. Effects of hypertonic dextran in hypovolaemic shock: a prospective clinical trial. Injury. 2004;35:500–506. doi: 10.1016/S0020-1383(03)00196-7. [DOI] [PubMed] [Google Scholar]
- 4.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology and severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Ayala A, Chung CS, Grutkoski PS, Song GY. Mechanisms of immune resolution. Crit Care Med. 2003;31:S558–S571. doi: 10.1097/01.CCM.0000081438.04801.D9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barret JP, Herndon DN. Modulation of inflammatory and catabolic responses in severely burned children by early burn wound excision in the first 24 hours. Arch Surg. 2003;138:127–132. doi: 10.1001/archsurg.138.2.127. [DOI] [PubMed] [Google Scholar]
- 7.Barret JP, Herndon DN. Effects of burn wound excision on bacterial colonization and invasion. Plast Reconstr Surg. 2003;111:744–750. doi: 10.1097/01.PRS.0000041445.76730.23. [DOI] [PubMed] [Google Scholar]
- 8.Boldt J. Fluid choice for resuscitation of the trauma patient: a review of the physiological, pharmacological, and clinical evidence. Can J Anaesth. 2004;51:500–513. doi: 10.1007/BF03018316. [DOI] [PubMed] [Google Scholar]
- 9.Bryant D, Becker L, Richardson J, Shelton J, Franco F, Peshock R, Thompson M, Giroir BP. Cardiac failure in transgenic mice with myocardial expression of tumor necrosis factor-α. Circulation. 1998;97:175–183. doi: 10.1161/01.cir.97.14.1375. [DOI] [PubMed] [Google Scholar]
- 10.Cain BS, Meldrum DR, Dinarello CA, Meng X, Joo KA, Banerjee A, Harken AH. Tumor necrosis factor-α and interleukin-1β synergistically depress human myocardial function. Crit Care Med. 1999;27:1309–1318. doi: 10.1097/00003246-199907000-00018. [DOI] [PubMed] [Google Scholar]
- 11.Cancio LC, Mozingo DW, Pruitt BA., Jr. The technique of fluid resuscitation for patients with severe thermal injuries. J Crit Illn. 1997;12:183–190. [Google Scholar]
- 12.Carlson RG, Miller SF, Finley RK, Jr, Billett JM, Fegelman E, Jones LM, Alkire S. Fluid retention and burn survival. J Trauma. 1967;27:127–135. doi: 10.1097/00005373-198702000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Coimbra R, Hoyt DB, Junger WG, Angle N, Wolf P, Loomis W, Evers MP. Hypertonic saline resuscitation decreases susceptibility to sepsis following hemorrhagic shock. J Trauma. 1997;42:602–607. doi: 10.1097/00005373-199704000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Giroir B, Horton JW, White DJ, McIntyre KL, Lin CQ. Inhibition of tumor necrosis factor prevents myocardial dysfunction during burn shock. Am J Physiol Heart Circ Physiol. 1994;267:H118–H124. doi: 10.1152/ajpheart.1994.267.1.H118. [DOI] [PubMed] [Google Scholar]
- 15.Hart DW, Wolf SE, Chinkes DL, Beauford RB, Mlcak RP, Heggers JP, Wolfe RR, Herndon DN. Effects of early excision and aggressive enteral feeding on hypermetabolism, catabolism, and sepsis after severe burn. J Trauma. 2003;54:755–764. doi: 10.1097/01.TA.0000060260.61478.A7. [DOI] [PubMed] [Google Scholar]
- 16.Horton JW, Maass DL, White DJ, Sanders B. Myocardial inflammatory responses to sepsis complicated by previous burn injury. Surg Infect (Larchmt) 2003;4:363–377. doi: 10.1089/109629603322761427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horton JW, Maass DL, White DJ, Sanders B. Hypertonic saline dextran suppresses burn-related cytokine synthesis by cardiomyocytes. Am J Physiol Heart Circ Physiol. 2001;280:H1591–H1601. doi: 10.1152/ajpheart.2001.280.4.H1591. [DOI] [PubMed] [Google Scholar]
- 18.Horton JW, White J, Maass D, Sanders B. Arginine in burn injury improves cardiac performance and prevents bacterial translocation. J Appl Physiol. 1998;84:695–702. doi: 10.1152/jappl.1998.84.2.695. [DOI] [PubMed] [Google Scholar]
- 19.Horton JW. Bacterial translocation from the GI tract and Toll/IL-1 signaling in the myocardium (Abstract) Shock. 2005;23:84. [Google Scholar]
- 20.Junger WG, Coimbra R, Liu FC, Herdon-Remelius C, Junger W, Junger H, Loomis W, Hoyt DB, Altman A. Hypertonic saline resuscitation: a tool to modulate immune function in trauma patients? Shock. 1997;8:235–241. [PubMed] [Google Scholar]
- 21.Junger WG, Hoyt DB, Davis RE, Herndon-Remelius C, Namiki S, Junger H, Loomis WH, Altman A. Hypertonicity regulates the function of human neutrophils by modulating chemoattractant receptor signaling and activating mitogen-activated protein kinase p38. J Clin Invest. 1998;101:2768–2779. doi: 10.1172/JCI1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Junger WG, Hoyt DB, Hamreus M, Liu FC, Herndon-Remelius C, Junger W, Altman A. Hypertonic saline activates protein tyrosine kinases and mitogen-activated protein kinase p38 in T-cells. J Trauma. 1997;42:437–445. doi: 10.1097/00005373-199703000-00011. [DOI] [PubMed] [Google Scholar]
- 23.Junger WG, Liu FC, Loomis WH, Hoyt DG. Hypertonic saline enhances cellular immune function. Circ Shock. 1994;42:190–196. [PubMed] [Google Scholar]
- 24.Kolsen-Petersen JA, Nielsen JO, Tonnesen EM. Effect of hypertonic saline infusion on postoperative cellular immune function: a randomized controlled clinical trial. Anesthesiology. 2004;100:1108–1118. doi: 10.1097/00000542-200405000-00012. [DOI] [PubMed] [Google Scholar]
- 25.Kumar A, Thota V, Dee L, Olson J, Uretz E, Parillo JE. Tumor necrosis factor α and interleukin 1β are responsible for in vitro myocardial cell depression induced by human septic shock serum. J Exp Med. 1996;183:949–958. doi: 10.1084/jem.183.3.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leeper-Woodford SK, Carey D, Byrne K, Walsh C, Fisher B, Sugerman HJ, Fowler AA. Histamine receptor antagonists, cyclooxygenase blockade, and tumor necrosis factor during acute septic insult. Shock. 1998;9:89–94. doi: 10.1097/00024382-199802000-00003. [DOI] [PubMed] [Google Scholar]
- 27.Maass DL, Hybki DP, White J, Horton JW. The time course of cardiac NF-κB activation and TNF-α secretion by cardiac myocytes after burn injury: contribution to burn-related cardiac contractile dysfunction. Shock. 2002;17:293–299. doi: 10.1097/00024382-200204000-00009. [DOI] [PubMed] [Google Scholar]
- 28.Maass DL, White DJ, Horton JW. Burn trauma complicated by infection in adult mice exacerbates myocardial inflammation seen in burn alone (Abstract) Surg Infect Soc. 2004;5:118. [Google Scholar]
- 29.Maass DL, White J, Horton JW. IL-6 and IL-1β act synergistically with TNF-α to alter cardiac contractile function after burn trauma. Shock. 2002;18:360–366. doi: 10.1097/00024382-200210000-00012. [DOI] [PubMed] [Google Scholar]
- 30.Mannick JA, Rodrick ML, Lederer JA. The immunologic response to injury. J Am Coll Surg. 2001;193:237–244. doi: 10.1016/s1072-7515(01)01011-0. [DOI] [PubMed] [Google Scholar]
- 31.Meldrum DR. Tumor necrosis factor in the heart. Am J Physiol Regul Integr Comp Physiol. 1998;274:R577–R595. doi: 10.1152/ajpregu.1998.274.3.R577. [DOI] [PubMed] [Google Scholar]
- 32.Monafo WW, Chuntrasakul C, Ayvazian VH. Hypertonic sodium solutions in the treatment of burn shock. Am J Surg. 1973;126:778–783. doi: 10.1016/s0002-9610(73)80070-4. [DOI] [PubMed] [Google Scholar]
- 33.Moss NM, Gough DB, Jordan AL, Grbic JT, Wood JJ, Rodrick ML, Mannick JA. Temporal correlation of impaired immune response after thermal injury with susceptibility to infection in a murine model. Surgery. 1988;105:882–887. [PubMed] [Google Scholar]
- 34.Murphy TJ, Paterson HM, Kriynovich S, Zang Y, Kurt-Jones EA, Mannick JA, Lederer JA. Linking the “two-hit” response following injury to enhanced TLR4 reactivity. J Leukoc Biol. 2005;77:16–23. doi: 10.1189/jlb.0704382. [DOI] [PubMed] [Google Scholar]
- 35.Peters RM, Shackford SR, Hogan JS, Cologne JB. Comparison of isotonic and hypertonic fluids in resuscitation from hypovolemic shock. Surg Gynecol Obstet. 1986;163:219–224. [PubMed] [Google Scholar]
- 36.Rhee P, Burris D, Kaufmann C, Pekooulis M, Austin B, Ling G, Harviel D, Waxman K. Lactated Ringer's solution resuscitation causes neutrophil activation after hemorrhagic shock. J Trauma. 1998;44:313–319. doi: 10.1097/00005373-199802000-00014. [DOI] [PubMed] [Google Scholar]
- 37.Rhee P, Koustova E, Alam HB. Searching for the optimal resuscitation method: recommendations for the initial fluid resuscitation of combat casualties. J Trauma. 2003;54:S52–S62. doi: 10.1097/01.TA.0000064507.80390.10. [DOI] [PubMed] [Google Scholar]
- 38.Rhee P, Wang D, Ruff P, Austin B, DeBraux S, Wolcott K, Burris D, Ling G, Sun L. Human neutrophil activation and increased adhesion by various resuscitation fluids. Crit Care Med. 2000;28:74–78. doi: 10.1097/00003246-200001000-00012. [DOI] [PubMed] [Google Scholar]
- 39.Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, Peterson E, Tomlanovich M. Early goal directed therapy in the treatment of burn sepsis and shock. N Engl J Med. 2001;345:1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 40.Rue IW, Cioffi WG, Mason AD, McManus WF, Pruitt BA. The risk of pneumonia in thermally injured patients requiring ventilatory support. J Burn Care Rehabil. 1995;16:262–268. doi: 10.1097/00004630-199505000-00008. [DOI] [PubMed] [Google Scholar]
- 41.Sheeran P, Maass DL, White DJ, Turbeville TD, Giroir BP, Horton J. Aspiration pneumonia-induced sepsis increases cardiac dysfunction after burn trauma. J Surg Res. 1998;76:192–199. doi: 10.1006/jsre.1998.5352. [DOI] [PubMed] [Google Scholar]
- 42.Shirani KZ, Pruitt BA, Jr, Mason AD., Jr The influence of inhalation injury and pneumonia on burn mortality. Ann Surg. 1987;205:82–87. doi: 10.1097/00000658-198701000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shukla A, Hashiguchi N, Chen Y, Coimbra R, Hoyt DB, Junger WG. Osmotic regulation of cell function and possible clinical applications. Shock. 2004;21:391–400. doi: 10.1097/00024382-200405000-00001. [DOI] [PubMed] [Google Scholar]
- 44.Smith GJ, Kramer GC, Perron P, Nakayama S, Gunther RA, Holcroft JW. A comparison of several hypertonic solutions for resuscitation of bled sheep. J Surg Res. 1985;39:517–528. doi: 10.1016/0022-4804(85)90120-9. [DOI] [PubMed] [Google Scholar]
- 45.Tepikin AV. Calcium Signaling. 2nd ed. Oxford Univ. Press; Oxford, UK: 2001. [Google Scholar]
- 46.Vassar MJ, Fischer RP, O'Brien PE, Bachulis BL, Chambers JA, Hoyt DB, Holcroft JW. A multicenter trial for resuscitation of injured patients with 7.5% sodium chloride. The effect of added dextran 70. The Multicenter Group for the Study of Hypertonic Saline in Trauma Patients. Arch Surg. 1993;128:1003–1013. doi: 10.1001/archsurg.1993.01420210067009. [DOI] [PubMed] [Google Scholar]
- 47.Vassar MJ, Perry CA, Gannaway WL, Holcroft JW. 7.5% Sodium chloride/dextran for resuscitation of trauma patients undergoing helicopter transport. Arch Surg. 1991;126:1065–1072. doi: 10.1001/archsurg.1991.01410330019002. [DOI] [PubMed] [Google Scholar]
- 49.White J, Thomas J, Maass DL, Horton JW. The cardiac effects of burn injury complicated by aspiration-pneumonia-induced sepsis. Am J Physiol Heart Circ Physiol. 2003;285:H47–H58. doi: 10.1152/ajpheart.00833.2002. [DOI] [PubMed] [Google Scholar]
- 50.Zang Y, Dolan SM, Choileain NN, Kriynovich SJ, Murphy TJ, Sayles P, Mannick JA, Lederer JA. Burn injury initiates a shift in superantigen-induced T cell responses and host survival. J Immunol. 2004;15:4883–4892. doi: 10.4049/jimmunol.172.8.4883. [DOI] [PubMed] [Google Scholar]