Abstract
A large number of bacterial toxins, viruses and bacteria target carbohydrate derivatives on the cell surface to attach to and gain entry into the cell. We report here the use of a monosaccharide-based array to detect protein toxins. The array-based technique provides the capability to perform simultaneous multianalyte analyses. Arrays of N-acetyl galactosamine (GalNAc) and N-acetylneuraminic acid (Neu5Ac) derivatives were immobilized on the surface of a planar waveguide and were used as receptors for protein toxins. These arrays were probed with fluorescently labeled bacterial cells and protein toxins. While Salmonella typhimurium, Listeria monocytogenes, Escherichia coli and staphylococcal enterotoxin B (SEB) did not bind to either of the monosaccharides, both cholera toxin and tetanus toxin bound to GalNAc and Neu5Ac. The results show that the binding of the toxins to the carbohydrates is density dependent and semi-selective. Both toxins were detectable at 100 ng/ml.
Keywords: monosaccharides, bacterial cells, cholera toxin, tetanus toxin, array biosensor, fluorescence
1. Introduction
Carbohydrate-based detection of protein toxins and pathogens presents an exciting alternative to standard immunoassay for screening and detecting targets for clinical applications and defense purposes. Although recent approaches for developing carbohydrate-based sensors have employed gangliosides as receptors for protein toxins (Charych et al., 1996; Song et al., 1999; Singh et al., 2000; Rowe-Taitt et al., 2000a; Fang et al., 2003; Ahn-Yoon et al., 2004), this approach has been less than fully successful at providing information about the specific carbohydrate moieties responsible for protein-carbohydrate interactions. As a result, there is limited understanding of these interactions and use in sensor development as well as toxicity and drug discovery studies. An important feature of utilizing carbohydrate arrays as receptors is that various combinations of the specific monosaccharide units associated with protein-carbohydrate binding can be investigated. This screening together with kinetic studies may help in identifying and elucidating the specific residues of oligosaccharides that bind to proteins. Our approach in this study is to employ an array-based technique that provides the capability to perform multiple analyses simultaneously.
A large number of bacterial toxins target carbohydrate derivatives on cell surfaces by which they attach and gain entry into the cell. These protein toxins include cholera toxin, E. coli heat-labile enterotoxin, shiga-like toxins, pertussis toxin, botulinum toxin, and tetanus toxin. Studies have shown that a number of these toxins use one of their chains or subunits to bind to the carbohydrates while the other fragment is responsible for toxicity (Zang et al., 1995; Emsley et al., 2000; Fotinou et al., 2001). A number of methods have been employed to study such protein-carbohydrate interactions. These techniques employ crystallography and molecular modeling (Emsley et al., 2000; Fotinou et al., 2001), surface plasmon resonance on self-assembled monolayers (Horan et al., 1999), microarrays (MacBeath et al., 1999; Fukui et al., 2002; Park et al., 2004; Ratner et al., 2004) and quartz crystal microbalance gravimetry (Zhang et al., 2003). However, identification and elucidation of the specific residues of oligosaccharide derivatives that bind to proteins still remain major challenges in studying protein-carbohydrate interactions.
We report here a novel technique of immobilizing sugars onto planar waveguides and employing the patterned arrays to analyze carbohydrate-binding protein toxins. For this study, we used an array biosensor which has demonstrated the ability to detect multiple analytes simultaneously (Rowe-Taitt et al., 2000b,c; Sapsford et al., 2004). We employed two monosaccharide derivatives: N-acetylneuraminic acid (Neu5Ac), and N-acetylgalactosamine (GalNAc) as receptors for protein toxins. The presence of both of these sugars has been demonstrated to be essential for the binding of some toxins to ganglioside receptors (e.g., cholera toxin to GM1, tetanus toxin to GT1b and GQ1b) (Angstrom, et al., 1994; Kitov et al., 2003; Turnbull et al., 2004). Based on these studies, it is possible to select the residues that are necessary for toxin binding.
2. Materials and Methods
2.1 Materials
Unless otherwise specified, all chemicals were reagent grade and used as received. Heat-killed Salmonella typhimurium, E. coli O157:H7 and Listeria monocytogenes ( KPL, Gaithersburg, MD), SEB (Toxin Technology, Inc., Sarasota, FL), and cholera and tetanus toxins (Calbiochem, La Jolla, CA) were labeled with Cy5 N-hydroxysuccinimidyl ester bisfunctional dye (Amersham Bioscience Corp., Arlington Heights, IL) according to the manufacturer’s instructions. Labeled toxins were separated from unincorporated dye using size-exclusion chromatography using BioGel P10, while cells were dialyzed against PBS. Toxin concentrations and protein-to-dye ratios were determined using UV-visible spectroscopy. All analytes were stored at 4 °C.
Appropriate personal protective equipment was worn at all times while working with the toxins. All surfaces, glassware and other containers used were treated with 20% bleach before cleaning with water. Disposables were placed in biohazard bags and later incinerated. Analyte solutions were treated with bleach (20% final concentration) before disposal.
2.2. Synthesis of monosaccharide-derivatives
The recognition molecules used in this study, Neu5Ac and GalNAc, were synthesized to possess a thiol-terminated linker (20 long) on the anomeric carbon (Fig. 1). Synthesis of these compounds was performed in D. Kahne’s laboratory and will be described in detail elsewhere. Briefly, sugars were converted to anomeric thiophenyl glycosides containing para-hydroxyl thiophenol. An acid linker was added and was subsequently coupled to a linker terminating in thioacetate. After deprotection, the ligands were characterized using electrospray ionization mass spectrometry. The GalNAc derivative showed peaks at m/z 518 and 540, corresponding to thiol-terminated derivatives: C21H32N3O8S2, [M+H]+ and C21H31N3O8S2Na, [M+Na]+, respectively. Likewise, the Neu5Ac derivative had two [M+H]+ peaks at m/z 1209 and 606 which correspond to the disulfide form, C48H69N6O22S4, and the thiol-terminated form, C24H35N3O11S2, respectively. Both ligands were supplied in the disulfide form.
Fig. 1.
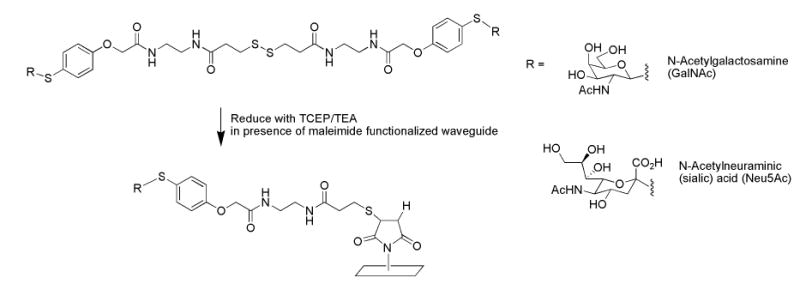
Immobilization of monosaccharides onto planar waveguide.
2.3. Immobilization of capture species
Borosilicate microscope glass used as waveguides were cleaned by immersion for 30 minutes in 10% KOH (w/v) in methanol followed by copious rinsing with deionized water and drying under nitrogen (Cras et al., 1999). The cleaned slides were then treated under nitrogen with 2% 3-aminopropyltriethoxysilane in 90% methanol/water (with one drop of acetic acid per 100 ml) for 1 hour. The slides were washed three times with methanol, followed by 3 washes with deionized water. Slides were dried under a stream of nitrogen and then baked at 120°C for approximately 6 minutes. The aminosilane-modified slides were used immediately or stored at room temperature under nitrogen. Immediately prior to attaching monosaccharides onto the waveguides, the aminosilane-derivatized slides were incubated for 30 min at room temperature in 1 mM N-succinimidyl-4-maleimidobutyrate (GMBS), a heterobifuctional crosslinker, in absolute ethanol. The primary amines on the surface of the modified waveguides react with the crosslinker to form amide bonds, resulting in surfaces that are densely functionalized with maleimide groups. After treatment with the crosslinker, the slides were washed three times with deionized water, dried with a stream of nitrogen and patterned immediately.
Patterning of the capture species onto the maleimide-modified slides was carried out using 12-channel poly(dimethylsiloxane) (PDMS) patterning templates. The flow cell was first clamped onto the maleimide-modified slide and a solution containing the monosaccharide derivative (10–50 μg/ml), tris(2-carboxyethy)phosphine hydrochloride (TCEP, 0.8 molar equivalent), and triethylamine (TEA, 1.8 molar equivalent) in 70% DMSO/H2O was injected into each channel. Negative controls consisted of 10 mM sodium phosphate buffer, pH 7.5, DMSO/H20 alone, and DMSO/H2O with TCEP and/or TEA. TCEP reduces the disulfide bond on the crosslinker between sugar moieties thereby generating two monosaccharides, each possessing a thiol-terminated long chain linker. Thiol-moieties were generated on control antibodies by reacting 50 μg/ml antibody with 2 μg/ml TCEP in 10 mM sodium phosphate buffer, pH 7.5 prior to addition of antibodies to the channels. The thiol termini react with the maleimide moiety of GMBS on the waveguide, thereby covalently immobilizing the sugars and anti-cholera toxin antibody on the surface. After overnight incubation at room temperature, the flow cell channels were then emptied and rinsed sequentially with either 0.5 ml deionized water, 0.2 ml DMSO and 1 ml deionized water (lanes containing sugars) or 1 ml of PBS containing 0.05% Tween 20 and 1 mg/ml BSA (PBSTB), (lanes containing control antibodies). After removing the PDMS patterning templates, patterned slides were first rinsed with deionized water and then immersed for 30 minutes in a blocking solution of 10 mg/ml BSA in PBS containing 0.05% Tween 20. The slides were briefly rinsed with deionized water, dried under a stream of nitrogen and assembled for assay or stored at 4°C.
2.4. Assay protocol and signal processing
For static assays, patterned waveguides were placed in contact with PDMS assay templates possessing 12 channels oriented orthogonal to the patterned stripes of immobilized sugars. The assembled slides were hooked up to a multichannel peristaltic pump by connecting one end (outlet) of each flow channel to the pump tubing via syringe needles. The inlet end of each flow channel was connected to a 1 ml syringe barrel used as reservoir. Each channel was washed with 1 ml of PBSTB at a flow rate of 0.5 ml/min, which also served as a check for leaks within the channels. Next, assays were performed either under flow whereby 0.8 ml of Cy5-labeled analyte in PBSTB was flowed through the channels at 0.1 ml/min, or under static conditions whereby 0.1 ml of Cy5-labeled analyte in PBSTB was applied to each channel and the slides were allowed to sit static (without flow) for 1 hr at room temperature. Each channel was then washed with 1 ml of PBSTB at a flow rate of 0.5 ml/min. After removing the PDMS, the slides were washed with deionized water, dried under nitrogen and imaged using the array biosensor (Feldstein et al., 1999; Golden and Ligler, 2002). The overall assay time was therefore approx. 65 minutes. Fluorescence imaging, data acquisition and analysis were performed as previously described by Ngundi at el. (2005). Limits of detection (LOD) were defined as the lowest concentration of toxin tested at which the net fluorescence was at least 3 standard deviations above both negative control (buffer blank) and localized background values.
3. Results and Discussion
3.1 Immobilization of monosaccharides
GalNAc and Neu5Ac derivatives were immobilized onto the surface of planar waveguides by coupling a maleimide-activated surface to a thiol-terminated linker on the carbohydrate derivative as shown in Fig. 1. The thiol termini on the monosaccharide derivatives were generated in situ and reacted with the maleimide functionalized waveguide giving rise to patterns of immobilized monosaccharides. Likewise, capture antibodies were immobilized through thiol termini generated by the reduction of disulfide bonds. This immobilization chemistry is similar to that used by Ratner et al. (2004), except that in this study, maleimide-derivatized surfaces were produced by incubation of amino silane-treated slides with the heterobifunctional crosslinker, GMBS; treatment of cleaned slides with amino silane could be performed up to several weeks in advance, with GMBS treatment performed immediately before immobilization of the sugar and antibody capture species. Reduction of the dithiols on the monosaccharide dimers and consequent generation of thiol termini on the sugars (and antibodies) was accomplished during the immobilization procedure, rather than in a prior incubation step as per Ratner et al. (2004). Surprisingly, the potentially harsh reducing conditions used to immobilize the antibodies did not significantly affect the antibodies’ ability to capture antigen (data not shown). The major advantage of this procedure is that the reactions can be performed inside channels of a PDMS patterning template placed in contact with the surface of functionalized waveguide, reducing the potential for air oxidation of thiol moieties. Furthermore, the need for any purification step is eliminated.
3.2 Binding of monosaccharides to toxins and bacteria
The immobilized monosaccharides were tested for direct binding to several Cy5-labeled toxins and bacteria: cholera toxin, tetanus toxin, SEB, S. typhimurium, L. monocytogenes and E. coli O157:H7. Monoclonal anti-cholera toxin B subunit antibody and goat anti-tetanus antibody were used as additional controls for binding of cholera and tetanus toxins, respectively. Cy5-labeled targets that bound to immobilized sugar derivatives were observed as bright spots in the CCD image of the assayed waveguide (Fig.2).
Figure 2.
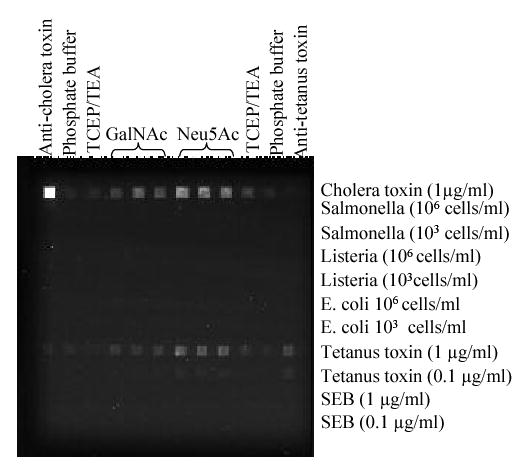
Specificity of binding of 10 μg/ml GalNAc and Neu5Ac to Cy5-labeled bacterial cells and toxins. Analytes were assayed for 15 minutes flowing at 0.1ml/min.
Although the three bacterial species tested have been shown to bind to glycoprotein receptors in host tissues, none of these targets showed binding to the immobilized monosaccharides above background levels (P < 0.05). Likewise, SEB showed no significant binding to the Neu5Ac and GalNAc; these results were not unexpected, SEB binds to its target cells via MHC Class II molecules. On the other hand, both, cholera and tetanus toxins bound to both immobilized monosaccharides (Fig. 2). These results illustrate the semi-selective binding behavior of the Neu5Ac and GalNAc derivatives towards cholera and tetanus toxin.
3.3 Dose-response studies
The density-dependence for binding of cholera and tetanus toxins to the immobilized monosaccharides was investigated (Fig. 3). Controls for various components of the surface chemistry - TCEP and TEA in the absence of sugar – showed that the binding of cholera toxin and tetanus toxin to patterned spots was due to the sugar moiety attached to the surface; the average net fluorescent intensity for the negative controls, 15.8 ± 17.8 fluorescence units, was not significantly above background (P > 0.05).
Fig. 3.
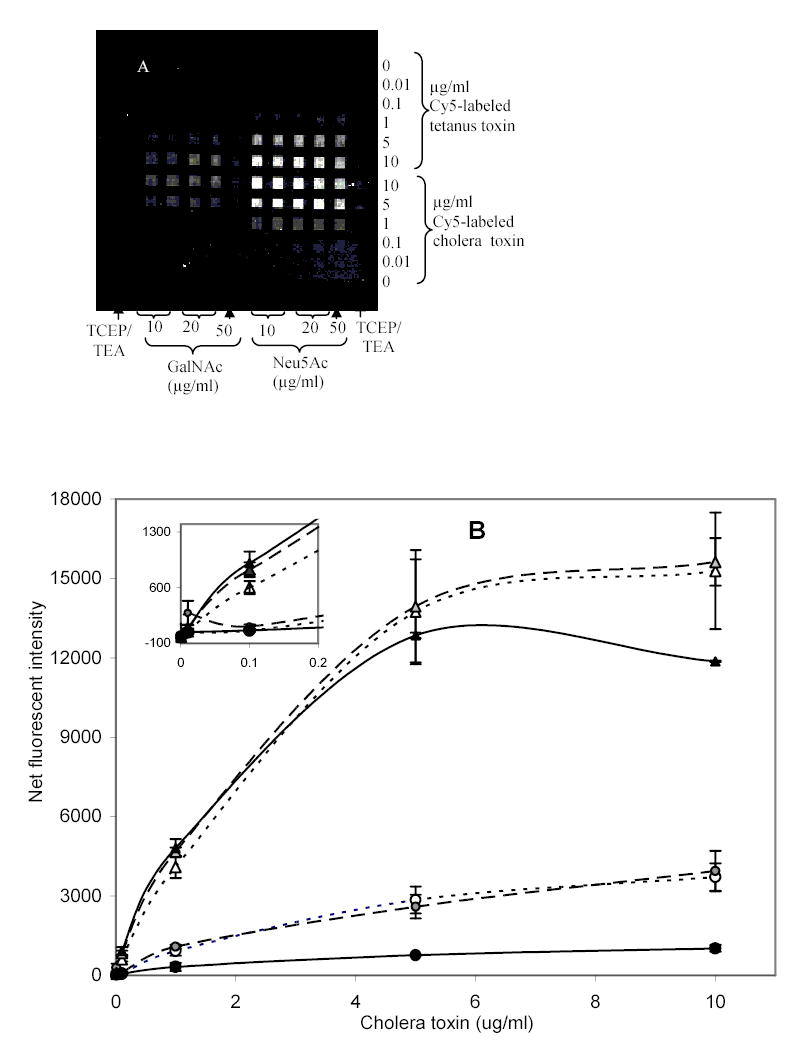
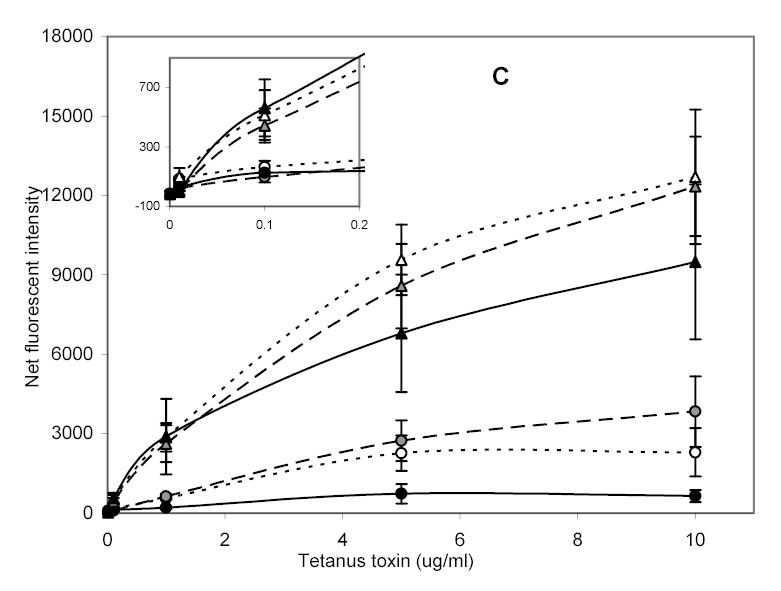
Detection of cholera and tetanus toxins with various concentrations of immobilized GalNAc and Neu5Ac derivatives. (a) CCD image of fluorescently-bound toxins assayed over immobilized sugar derivatives for 1 hour under static conditions. Dose-response curves for (b) Cholera toxin, and (c) Tetanus toxin each with: ···▵·· · 10, —◭— 20, —▴— 50 μg/ml patterned Neu5Ac and ···○··· 10, —◍— 20, —•— 50 μg/ml patterned GalNAc. Error bars are standard error of the mean for 2 slides with n=4 total.
Dose-response curves for cholera toxin and tetanus toxin binding to the two immobilized monosaccharides were generated (Fig. 3b and 3c); each data point shown is an average of two slides, with n = 2 array loci per slide. Higher signals were obtained for binding of both toxins to immobilized Neu5Ac versus GalNAc, as determined by Student’s t-test (P<0.05). These results were consistent with some previous reports describing the contribution of individual monosaccharide units from GM1 binding to cholera toxin (Park and Shin, 2002; Turnbull et al. 2004), although it has been demonstrated that some derivatives of Neu5Ac possess lower binding affinities to cholera toxin B subunit than corresponding GalNAc derivatives (Turnbull et al., 2004). The difference in binding between the two carbohydrate species was more marked in the case of cholera toxin than with tetanus toxin (compare Figs. 3b & 3c). Interestingly, cholera toxin has shown an absolute requirement for both a terminal galactose and an internal NeuAc for binding to its ganglioside receptor, whereas tetanus toxin requires two internal Neu5Ac residues (McKenzie et al., 1997). One might therefore expect the binding of tetanus toxin to be significantly more selective for Neu5Ac than cholera toxin.
For GalNAc, no significant difference in binding of cholera and tetanus toxins was observed (P > 0.05) on both a weight and per mole basis. Likewise, for Neu5Ac, and at toxin concentrations above 5 μg/ml, there was no significant difference in binding intensities for cholera and tetanus toxins, as determined by Student’s t-test (P>0.05). However, at low levels of toxins (less than 5 μg/ml) and for the same toxin concentration, cholera toxin bound to Neu5Ac gave higher fluorescent intensities than tetanus toxin (P < 0.05, Student’s t-test). The difference in binding may be due to the affinity of the binding site for monosaccharide or may be related to avidity. One means of distinguishing between the two is through kinetic analyses, which we have initiated. The current study has utilized intact cholera toxin, which belongs to the AB5-type toxins whose structure consists of a catalytic or toxic subunit A surrounded by five identical receptor-binding B-subunits. The B subunits provide pentavalent-binding to ligand per molecule of the intact toxin. On the other hand, tetanus toxin contains only a single binding site, the c-terminal fragment of the heavy (H)-chain (MW 47,000). This means that unlike cholera toxin, tetanus toxin does not exhibit polyvalent binding. It has been demonstrated that polyvalency increases protein-binding avidities (Kanai et al., 1997; Mammen et al., 1998; Mortell et al., 1996), which may be responsible for the high sensitivity observed with cholera toxin.
Binding of toxins to immobilized sugars, particularly GalNAc was dependent on the concentration of GalNAc in the patterning solution. When 50 μg/ml GalNAc was used, binding of the toxins was low (Fig. 3). This decrease in binding intensity may be caused by too great a surface density of immobilized sugars, resulting from steric hindrance from additional (proximal) sugars. Binding effects associated with surface density have been observed previously for binding of a lectin to immobilized carbohydrates (Horan et al., 1999). We are working on determining the actual number of sugar units on the surface of the waveguide and the optimum concentration of carbohydrates needed for best sensitivity of each target.
Limits of detection for both toxins were 100 ng/ml. Although these detection limits are higher than those obtained with antibody-based assays using the same instrument (Rowe-Taitt et al., 2000c), they are similar to those previously observed using immobilized GM1 for detection (Rowe-Taitt et al., 2000a). Other biosensor systems have also incorporated the natural receptors of cholera toxin and tetanus toxin, GM1 and GT1b, for detection purposes. Detection limits in the range of 10–20 nM (approx. 850 ng/ml – 1.5 μg/ml) have been obtained using electrochemical (Cheng et al., 2004), liposome-based (Singh et al., 2000), and resonant mirror biosensors (Puu, 2001). Song and Swanson (Song and Swanson, 1999; Song et al., 2000) were able to detect 50–100 pM cholera toxin using FRET-based flow cytometry and biomimetic membrane sensors. Zayats and coworkers (2002) were able to detect cholera toxin in the 10−11 M range with GM1 for target recognition using both SPR and an impedance-based system. Although the detection limits obtained in the current study with immobilized monosaccharides are significantly higher, we anticipate that our limits of detections will improve with the use of di- and trisaccharides that are closer mimics to the natural receptors. Furthermore, they compare favorably with results from a number of other studies using microarrays for characterization of binding reactions of lectins or antibodies to mono- and oligosaccharides (MacBeath et al., 1999; Fukui et al. 2002; Park et al., 2004). To our knowledge, no analogous studies using toxins have been reported. The vast majority of these reports (Fukui et al., 2002; Park et al., 2004; Ratner et al., 2004) use significantly higher concentrations of lectins and/or antibodies to probe the microarrays (>1 μg/ml to mg/ml range). As the purpose of these other studies was the demonstration of specific binding patterns, a constant concentration of each target was used and therefore, no dose–dependence curves were generated.
5. Conclusion
This is the first report of a new methodology for immobilizing several tethered monosaccharides in arrays whereby dose-dependent binding of toxins has been measured. During the immobilization procedure, the monosaccharide derivatives existing as dimers with a disulfide bond were reduced and thiol termini of the monosaccharide derivatives generated in situ inside the channels of PDMS patterning template. In addition, the study demonstrated the use of arrays of various immobilized monosaccharides to explore binding interactions between the sugars and protein toxins as well as bacterial cells. Fluorescent intensity values showed that only cholera and tetanus toxins bound to the immobilized sugars while there was no binding between the sugars and either SEB, Salmonella, Listeria or E. coli. Both cholera and tetanus toxins bound tighter to immobilized Neu5Ac than to GalNAc, with cholera toxin exhibiting the largest difference. At low levels of toxins and for the same toxin concentration, cholera toxin bound to Neu5Ac gave higher intensities than tetanus toxin.
Acknowledgments
M.M.N. thanks the American Society for Engineering Education for her postdoctoral fellowship. The authors thank the US Department of Defense Chem Bio Defense program and NIH grant EB000680 for support. The views expressed here are those of the authors and do not represent those of the U.S. Navy, the U.S. Department of Defense or the U.S. Government.
References
- Ahn-Yoon S, DeCory TR, Durst RA. Ganglioside-liposome immunoassay for the detection of botulinum toxin. Anal Bioanal Chem. 2002;378:68–75. doi: 10.1007/s00216-003-2365-4. [DOI] [PubMed] [Google Scholar]
- Angstrom J, Teneberg S, Karlsson KA. Delineation and comparison of ganglioside-binding epitopes for the toxins of Vibrio cholerae, Escherichia coli, and Clostridium tetani: Evidence for overlapping epitopes. Proc Natl Acad Sci. 1994;91:11859–11863. doi: 10.1073/pnas.91.25.11859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charych D, Cheng Q, Reichert A, Kuziemko G, Stroh M, Nagy JO, Spevak W, Stevens RC. A “litmus test” for molecular recognition using artificial membranes. Chem Biol. 1996;3:113–120. doi: 10.1016/s1074-5521(96)90287-2. [DOI] [PubMed] [Google Scholar]
- Cheng Q, Zhu S, Song J, Zhang N. Functional lipid microstructures immobilized on a gold electrode for voltammetric biosensing of cholera toxin. Analyst. 2004;129:309–314. doi: 10.1039/b315656g. [DOI] [PubMed] [Google Scholar]
- Cras JJ, Rowe-Taitt CA, Nivens D, Ligler FS. Comparison of chemical cleaning methods of glass in preparation of silanization. Biosens Bioelectron. 1999;14:683–688. [Google Scholar]
- Emsley P, Fotinou C, Black I, Fairweathers NF, Charles IG, Watts C, Hewitt E, Isaacs NW. The structure of the Hc fragment of tetanus toxin with carbohydrate subunit complexes provide insight into ganglioside binding. J Biol Chem. 2000;275:8889–8894. doi: 10.1074/jbc.275.12.8889. [DOI] [PubMed] [Google Scholar]
- Fang Y, Frutos AG, Lahiri J. Ganglioside microarray for toxin detection. Langmuir. 2003;19:1500–1505. [Google Scholar]
- Feldstein MJ, Golden JP, Rowe CA, MacCraith BD, Ligler FS. Array biosensor: Optical and fluidics systems. J Biomed Microdevices. 1999;2:139–153. doi: 10.1023/A:1009900608757. [DOI] [PubMed] [Google Scholar]
- Fotinou C, Emsley P, Black I, Ando H, Ishida H, Kiso M, Sinha KA, Fairweathers NF, Isaacs NW. The crystal structure of tetanus toxin Hc fragment complexed with a synthetic GT1b analogue suggests cross-linking between ganglioside receptors and the toxin. J Biol Chem. 2001;276:32274–32281. doi: 10.1074/jbc.M103285200. [DOI] [PubMed] [Google Scholar]
- Fukui S, Feizi T, Galustian C, Lawson AM, Chai W. Oligosaccharide microarray for high-throughput detection and specificity assignments of carbohydrate-protein interactions. Nature Biotech. 2002;20:1011–1017. doi: 10.1038/nbt735. [DOI] [PubMed] [Google Scholar]
- Golden JP, Ligler FS. A comparison of imaging methods for use in an array biosensor. Biosens Bioelectron. 2002;17:719–725. doi: 10.1016/s0956-5663(02)00060-x. [DOI] [PubMed] [Google Scholar]
- Horan N, Yan L, Isobe H, Whitesides GM, Kahne D. Nonstatistical binding of a protein clustered carbohydrates. Proc Natl Acad Sci. 1999;96:11782–11786. doi: 10.1073/pnas.96.21.11782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai M, Mortell KH, Kiessling LL. Varying the size of multivalent ligands: The dependence of concavalin A binding on neoglycopolymer length. J Am Chem Soc. 1997;119:9931–9932. [Google Scholar]
- Kitov PI, Shimizu H, Homans SW, Bundle DR. Optimization of tether length in nonglycosidically linked bivalent ligands that target sites 2 and 1 of a Shiga-like toxin. J Am Chem Soc. 2003;125:3284–3294. doi: 10.1021/ja0258529. [DOI] [PubMed] [Google Scholar]
- MacBeath G, Koehler AN, Schreiber SL. Printing small molecules as microarrays and detecting protein-ligand interactions en masse. J Am Chem Soc. 1999;121:7967–7968. [Google Scholar]
- Mammen M, Choi SK, Whitesides GM. Polyvalent interactions in biological systems: Implications for design and use of multivalent ligands and inhibitors. Angew Chem Int Ed. 1998;37:2754–2794. doi: 10.1002/(SICI)1521-3773(19981102)37:20<2754::AID-ANIE2754>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- McKenzie CR, Hirama T, Lee KK, Altman E, Young NM. Quantitative analysis of bacterial toxin affinity and specificity for glycolipid receptors by surface plasmon resonance. J Biol Chem. 1997;272:5533–5538. doi: 10.1074/jbc.272.9.5533. [DOI] [PubMed] [Google Scholar]
- Mortell KH, Weatherman RV, Kiessling LL. Recognition specificity of neoglycopolymers prepared by ring-opening metathesis polymerization. J Am Chem Soc. 1996;118:2297–2298. [Google Scholar]
- Ngundi MM, Shriver-Lake LC, Moore MH, Lassman ME, Taitt CR, Ligler FS. Array biosensor for detection of ochratoxin A in cereals and beverages. Anal Chem. 2005;77:148–154. doi: 10.1021/ac048957y. [DOI] [PubMed] [Google Scholar]
- Park S, Lee M, Soon-Jin P, Shin I. Carbohydrate chips for studying high-throughput carbohydrate-protein interactions. J Am Chem Soc. 2004;126:4812–4819. doi: 10.1021/ja0391661. [DOI] [PubMed] [Google Scholar]
- Puu G. An approach for analysis of protein toxins based on thin films of lipid mixtures in an optical biosensor. Anal Chem. 2001;73:72–79. doi: 10.1021/ac000619j. [DOI] [PubMed] [Google Scholar]
- Ratner DM, Adams EW, Su J, O’Keefe BR, Mrksich M, Seeberger PH. Probing protein-carbohydrate interactions with microarrays of synthetic oligosaccharides. ChemBioChem. 2004;5:379–383. doi: 10.1002/cbic.200300804. [DOI] [PubMed] [Google Scholar]
- Rowe-Taitt CA, Cras JJ, Patterson CH, Golden JP, Ligler FS. A ganglioside-based assay fro cholera toxin using an array biosensor. Anal Biochem. 2000a;281:123–133. doi: 10.1006/abio.2000.4571. [DOI] [PubMed] [Google Scholar]
- Rowe-Taitt CA, Hazzard JW, Hoffman KE, Cras JJ, Golden JP, Ligler FS. Simultaneous detection of six biohazardous agents using a planar waveguide array biosensor. Biosens Bioelectron. 2000b;15:579–589. doi: 10.1016/s0956-5663(00)00122-6. [DOI] [PubMed] [Google Scholar]
- Rowe-Taitt CA, Golden JP, Feldstein MJ, Cras JJ, Hoffman KE, Ligler FS. Array biosensor for detection of biohazards. Biosens Bioelectron. 2000c;14:785–794. doi: 10.1016/s0956-5663(99)00052-4. [DOI] [PubMed] [Google Scholar]
- Sapsford KE, Rasooly A, Taitt CR, Ligler FS. Detection of Campylobacter and Shigella species in food samples using an array biosensor. Anal Chem. 2004;76(2004):433–440. doi: 10.1021/ac035122z. [DOI] [PubMed] [Google Scholar]
- Singh AK, Harrison SH, Schoeniger JS. Ganglioside as a receptor for biological toxins: Development of sensitive fluoroimmunoassays using ganglioside-bearing liposomes. Anal Chem. 2000;72:6019–6024. doi: 10.1021/ac000846l. [DOI] [PubMed] [Google Scholar]
- Song X, Swanson BI. Direct, ultrasensitive, and selective optical detection of protein toxins using multivalent interactions. Anal Chem. 1999;71:2097–2107. doi: 10.1021/ac981145f. [DOI] [PubMed] [Google Scholar]
- Song X, Shi S, Swanson B. Flow cytometry-based biosensor for detection of multivalent proteins. Anal Biochem. 2000;284:35–41. doi: 10.1006/abio.2000.4664. [DOI] [PubMed] [Google Scholar]
- Turnbull WB, Precious BL, Homans SW. Dissecting the cholera toxin-ganglioside GM1 interaction by isothermal titration calorimetry. J Am Chem Soc. 2004;126:1047–1054. doi: 10.1021/ja0378207. [DOI] [PubMed] [Google Scholar]
- Zang RG, Westbrook ML, Westbrook EM, Scott DL, Otwinowski Z, Maulik PR, Reed RA, Shipley GG. The 2.4 Å crystal structure of cholera toxin B subunit pentamer: Choleragenoid. J Mol Biol. 1995;251:550–562. doi: 10.1006/jmbi.1995.0455. [DOI] [PubMed] [Google Scholar]
- Zayats M, Raitman OA, Chegel VI, Kharitonov AB, Willner I. Probing antigen-antibody binding processes by impedance measurements on ion-selective field-effect transistor devices and complementary surface plasmon resonance analyses: Development of cholera toxin sensors. Anal Chem. 2002;74:4763–4777. doi: 10.1021/ac020312f. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Telyatnikov V, Sathe M, Zeng X, Wang PG. Studying the interaction of α-gal carbohydrate antigen and proteins by quartz-crystal microbalance. J Am Chem Soc. 2003;125:9292–9293. doi: 10.1021/ja035350a. [DOI] [PMC free article] [PubMed] [Google Scholar]


