Abstract
The absence of immune defects that occurs in the syndrome of long-term nonprogressive (LTNP) HIV infection offers insights into the pathophysiology of HIV-induced immune disease. The (H[F/S]RIG)2 domain of viral protein R (Vpr) induces apoptosis and may contribute to HIV-induced T cell depletion. We demonstrate a higher frequency of R77Q Vpr mutations in patients with LTNP than in patients with progressive disease. In addition, T cell infections using vesicular stomatitis virus G (VSV-G) pseudotyped HIV-1 Vpr R77Q result in less (P = 0.01) T cell death than infections using wild-type Vpr, despite similar levels of viral replication. Wild-type Vpr-associated events, including procaspase-8 and -3 cleavage, loss of mitochondrial transmembrane potential (Δψm), and DNA fragmentation factor activation are attenuated by R77Q Vpr. These data highlight the pathophysiologic role of Vpr in HIV-induced immune disease and suggest a novel mechanism of LTNP.
Introduction
Infection with HIV typically results in continual loss of CD4+ T lymphocytes, which ultimately leads to immunodeficiency and enhanced susceptibility to opportunistic infections and malignancies. Interestingly, however, some HIV-infected individuals have detectable HIV replication yet do not experience progressive immunosuppression even in the absence of therapy (1, 2). In such long-term nonprogressors (LTNPs), as many as 25–30% may have specific homozygous mutations in the HIV-1 coreceptors CCR5 and CCR2, which are associated with an impairment of viral attachment and thus infectivity (3). Other LTNPs may have mutations in viral genes that influence replication, such as Nef (4). Certain MHC haplotypes (B27, B57, and B51) are strongly associated with long-term nonprogression, perhaps due to an efficient presentation of immunodominant epitopes for cell-mediated immune responses (5). Finally, accumulating evidence suggests that HIV-specific CD8 CTLs may affect disease progression and are associated with long-term nonprogression (6). As these mechanisms do not fully account for all cases of LTNP, other mechanisms of LTNP must exist, yet they remain undefined and most likely include both host and viral factors.
HIV-1 viral protein R (Vpr) is a 96-amino-acid accessory protein that is dispensable for viral replication in CD4+ T cells but is required for infection of primary macrophages (7). Vpr assists nuclear localization of viral preintegration complexes and blocks p34cdc2 cyclin B complex formation, resulting in G2/M cell cycle arrest. Extracellular soluble Vpr is present in serum and cerebrospinal fluid from HIV-infected patients (8) and is cell permeable. Treatment of cells with soluble Vpr induces caspase-dependent (9) apoptosis in CD4+ T cell lines, neurons, hepatocytes, fibroblasts, and primary peripheral blood lymphocytes (10). Thus, Vpr has been postulated to play a causal role in depleting both HIV-infected and uninfected CD4+ T cells from infected patients.
The apoptotic function of Vpr localizes to a C-terminal (H(F/S)RIG)2 domain (11) and induces apoptosis by binding to the adenine nucleotide translocator (ANT) (12) component of the mitochondrial permeability transition pore complex (PTPC). The interaction of Vpr with ANT is specific, with an affinity in the nanomolar range, and is inhibited by Bcl-2 (12). Mutations in the (H(F/S)RIG)2 domain of Vpr (e.g., R73A, R77A, and R80A) abrogate the ability of Vpr to induce apoptosis in T lymphocytes (13). We have therefore assessed whether naturally occurring mutations in Vpr may also modulate T cell death and/or survival in HIV-infected persons and thereby affect disease progression.
Methods
Vesicular stomatitis virus G pseudotyped virus infections.
To evaluate the effects of Vpr mutations on cell killing in the context of viral infection, a single-cycle HIV-1 superinfection was used. Vesicular stomatitis virus G (VSV-G) pseudotyped virus particles were prepared as previously described (14). Briefly, envelope-defective proviral plasmid HxBRUR+/Env–, HxBRUR–/Env–, or HxBRUR+/Env– with an introduced R77Q (CGA to CAA) mutation was transfected into 293 T cells. The VSV-G expressor (SVCMV-VSV-G) was cotransfected, and 48 hours after infection culture supernatants were collected, preclarified, and ultracentrifuged to pellet pseudotyped virus. The resultant virus preparations (VSV-G Vpr+, VSV-G Vpr–, or VSV-G Vpr R77Q, respectively) were titered using a multinuclear activation of galactosidase-indicator (MAGI) assay (14). Jurkat cells (2.5 × 106) were infected with 0.01 MOI in the presence of 10 ng/ml polybrene (Sigma-Aldrich, St. Louis, Missouri, USA) for 2 hours followed by culture in complete media. Cells were collected every day for annexin V staining as described below.
Vpr peptides.
C-terminal (52–96) Vpr wild-type and mutant R77Q peptides were synthesized (Genemed Synthesis Inc., San Francisco, California, USA) by automated solid-phase synthesis using 9-fluorenylmethoxycarbonyl, and purification was performed using reverse-phase HPLC. The peptides were analyzed by electrospray mass spectrometry, and the purity of both peptides was greater than or equal to 95%.
Cell culture and flow cytometry.
Jurkat T cells (ATCC, Rockville, Maryland, USA) were cultured in RPMI 1640 (all cell culture products were purchased from Canadian Life Technology, Montreal, Quebec, Canada, unless otherwise stated) with 10% heat inactivated fetal bovine serum (Sigma-Aldrich), penicillin, streptomycin, and L-glutamine. Cells were plated at 106 cells per well and incubated with varying concentrations of Vpr wild-type or Vpr R77Q peptides in isotonic buffer for 30 minutes followed by culture in complete media. Cells were collected at 8–20 hours and stained either with annexin V FITC (BD Pharmingen, San Diego, California, USA) and 1 mg/ml propidium iodide (Sigma-Aldrich) or by terminal deoxyuridine nucleotide end labeling (TUNEL; Boehringer Mannheim, Indianapolis, Indiana, USA) according to the manufacturers’ instructions. Mitochondrial transmembrane potential (Δψm) was quantified using 40 μM DiOC6 (Molecular Probes, Eugene, Oregon, USA) according to the manufacturer’s instructions, and 4 μM dihydroethidine (Molecular Probes) was used to determine superoxide anion generation as a measure of reactive oxygen species (ROS) production. For inhibition studies, 100 μM of the caspase inhibitor zVAD-fmk (Bachem Bioscience Inc., King of Prussia, Pennsylvania, USA) was used. All flow cytometry analysis was performed using Coulter Epics Altra XL with 10,000 acquired events (Coulter Immunology, Miami, Florida, USA). Cell cycle analysis was performed by resuspending cells in 80% ethanol on ice for 30 minutes. After washing, cells were incubated with 180 U/ml RNase H (Sigma-Aldrich) and subsequently stained with 30 μg/ml propidium iodide for 30 minutes at 37°C. DNA content was then analyzed using Consort 30 software (Becton Dickinson, Franklin Lakes, New Jersey, USA).
Immunoblotting.
Cytosolic extracts from treated Jurkat cells were prepared by resuspending cell pellets in caspase extraction buffer (100 mM HEPES [pH 7.5], 10% sucrose [wt/vol], 0.5 mM EDTA, 10 mM DTT) (15) followed by 40–60 strokes using dounce homogenizer. Cells were spun at 720 g for 5 minutes, and the cytosolic supernatant fraction was frozen at –80°C until use. Cytosolic proteins were separated by (4–15% gradient) SDS PAGE and transferred to Immobilon-P (Millipore, Bedford, Massachusetts, USA) membrane. Membranes were blocked with 5% skim milk and incubated with caspase-8 (a generous gift from P. Krammer), caspase-9 (MBL International Corp., Watertown, Massachusetts, USA), caspase-3 (Transduction Laboratories, Lexington, Kentucky, USA), and DFF (DNA fragmentation factor) (Santa Cruz Biotechnology, Santa Cruz, California, USA) primary antibodies followed by the appropriate secondary antibody (anti-mouse IgG HRP, Amersham Bioscience, Piscataway, New Jersey, USA; anti-rabbit HRP, Amersham; and anti-goat IgG, Santa Cruz Biotechnology). Detection of proteins was performed by supersignal enhanced chemiluminescence (Pierce, Rockford, Illinois, USA). Equal loading of proteins was ensured using proliferating cell nuclear antigen (Santa Cruz Biotechnology).
Isolation of mitochondria from mouse liver.
Mouse livers were maintained on ice-cold 0.9% NaCl, cut into small pieces, and homogenized in 2 ml of homogenizing medium (0.25 M sucrose, 3 mM HEPES buffer, 1 mM EDTA [pH 7.2]). Homogenates were spun for 10 minutes at 500 g, supernatants were spun for 10 minutes at 500 g, and nuclear pellets were discarded. Supernatants were then collected and spun at 13,000 g for 10 minutes to pellet the mitochondria, which were resuspended in homogenizing medium. Mitochondrial fractions were treated with R77Q or wild-type Vpr at 37°C for 6 hours and stained with DiOC6.
Vpr sequencing.
The clinical characteristics of LTNP used in this study have been previously published (3). Patients attending the immunodeficiency clinic at the Ottawa Hospital were used as controls. Eligible patients were naive to antiretroviral therapy (so as not to allow selection of mutations due to drug pressure) and had evidence of immunodeficiency (absolute CD4 count of less than 500 cells and viral load of greater than 1,000 copies per milliliter). This study was reviewed and approved by the Ottawa Hospital Research Ethics Board. Viral RNA was extracted from plasma samples (0.5–1.0 ml) using an automated nucleic acid extraction instrument (NucliSens, Organon Teknika, Toronto, Ontario, Canada). One fifth of the isolated RNA was reverse transcribed and amplified by polymerase chain reaction using a single-tube system (QiaGen One-Step RT-PCR, QiaGen, Toronto, Ontario, Canada) using primers Vpr1F (GAGACTGGCATTTGGGTCA) and Vpr1R (TTTGTAAAGGTTGCATTACAT). The reaction conditions were 1 cycle at 50°C for 30 minutes; 1 cycle at 95°C for 15 minutes; 30 cycles at 95°C for 1 minute, at 50°C for 1 minute, and at 72°C for 1.5 minutes; and 1 cycle at 70°C for 1.5 minutes. Three microliters of the primary RT-PCR reaction were added to a secondary PCR mixture (QiaGen Hot-Start with primers Vpr2F [GCAGGACATAACAAGGTAGGA] and Vpr2R [GTCGCTGTCTCCGCTTC]) and amplified. Reaction conditions were 1 cycle at 95°C for 15 minutes; 30 cycles at 95°C for 1 minute, at 50°C for 1 minute, and at 72°C for 1.5 minutes; and 1 cycle at 70°C for 10 minutes. The secondary PCR product was cloned into the pCR2.1-TOPO vector (Invitrogen, Carlsbad, California, USA) as recommended by the manufacturer. Recombinant clones were identified by blue-white screening. Isolation of cloned DNA (4–8 clones per patient) was used in simultaneous bidirectional sequencing on a Li-Cor 4200L automated sequencer with dye-labeled primers complementary to plasmid vector sequences. Sequences were assembled, aligned, and translated using Sequencher software (Gene Code, Ann Arbor, Michigan, USA).
Database search for Vpr sequences.
All sequences were found in the Los Alamos database (http://hiv-web.lanl.gov/seq-db.html). LTNP versus progressor status was determined by specific notation within the Los Alamos database or by cross-referencing the sequence with the original citation. If no mention was made either in the database or in the original citation as to the patient’s clinical status (i.e., LTNP versus progressor), the sequence was excluded from the analysis. Sequences were aligned and compared using Sequencher software (Gene Code).
Mouse studies.
Balb-c mice (21–28 days old) received tail-vein injections of 200 μl of H2O (control) alone or containing 10 mg/kg R77Q or wild-type Vpr peptide (residues 52–96). Immediately before injection and again 24 hours later, 200 μl of blood was collected by eye-vein venipuncture. Forty-eight hours after injection, blood was removed by cardiac puncture, the mice were euthanized, and autopsies were performed. Absolute CD3+, CD4+, and CD8+ T cell numbers were determined by single-platform flow cytometry (16, 17). Before mouse experiments, the performance characteristics of this assay were validated by dilution experiments using whole blood from mice (data not shown). Alignment and calibration was performed using Coulter flow check, and absolute lymphocyte count was calculated using Coulter Flow Count Fluorospheres (Coulter Immunology). Peripheral blood from mice was stained with monoclonal antibody panel CD3-FITC, CD4-PE, and CD8-PC5 (BD Pharmingen). Before analysis of whole-blood samples, 100 μl of Coulter Flow Count beads were added to each sample (Coulter Immunology). Absolute count was determined using the following formula: total number of cells counted/total number of Fluorospheres × Flow Count Fluorospheres assayed. All studies involving mice were reviewed and approved by the Animal Care Committee of the University of Ottawa.
Flow cytometric detection of intracellular p24 and apoptosis.
Uninfected and infected cells were harvested, washed, and stained by TUNEL for detection of apoptosis according to the manufacturer’s protocol (Roche, Nutley, New Jersey, USA). Before the fixation step, 5 μl of p24-PE antibody per 106 cells (Coulter Immunology) was added for 15 minutes at 4°C. Cells were then washed, fixed with 2% paraformaldehyde, and analyzed by flow cytometry. TUNEL-negative controls prepared by omitting the terminal deoxyuridine transferase step and TUNEL-positive controls prepared with 10 U of DNase were both used to determine the TUNEL gates, whereas uninfected cells were used to set p24 gates, and 30,000 events were acquired per sample.
Statistics.
For comparisons of the frequency of R77Q mutations between LTNP and progressor cohorts, a two-tailed Fisher’s exact test was used. For comparisons between treatment groups, a Wilcoxon rank test was used.
Results
Frequency of the Vpr R77Q mutation in patients with progressive HIV infection and in LNTPs.
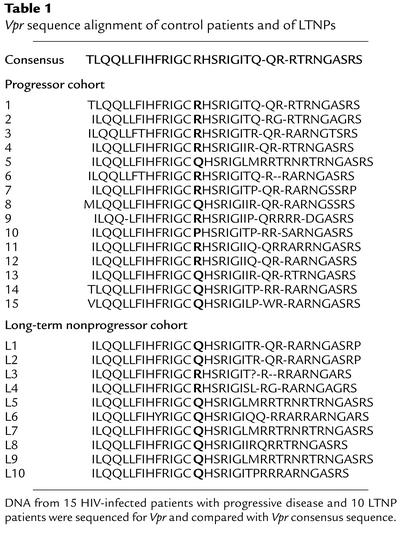
The LTNPs used in this study have been previously described (3); all met strict criteria for LTNP status, and no patients were homozygous for CCR5 mutations. Sequence analysis from our cohort of 10 LTNPs found that 80% of patients had the mutation R77Q in Vpr, whereas the remaining 20% had wild-type Vpr (Table 1). In parallel, viruses from 15 antiretroviral naive HIV-infected patients with CD4 counts below 500 were sequenced for Vpr, demonstrating that only 5 out of 15 individuals (33%) had the R77Q mutation (P = 0.041) (Table1).
Table 1.
Vpr sequence alignment of control patients and of LTNPs
We next assessed whether a similar association was present in viral sequences contained within the Los Alamos database. Sequences were only included in this analysis if the patient’s clinical status was defined (i.e., progressor versus LTNP). R77Q was present in 20 of 55 progressors (36%), as compared with 109 of 146 of LTNPs (75%, P < 0.001) (data not shown).
HIV infection R77Q Vpr results in less apoptosis than infections with wild-type virus.
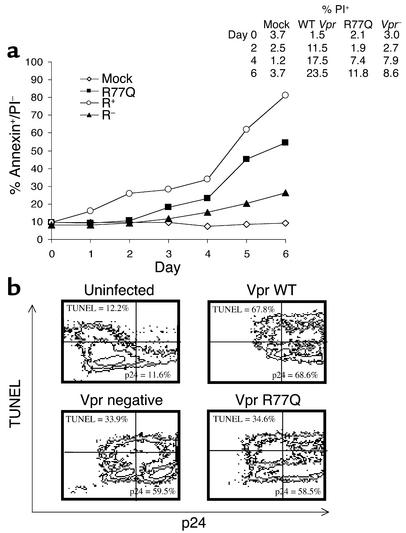
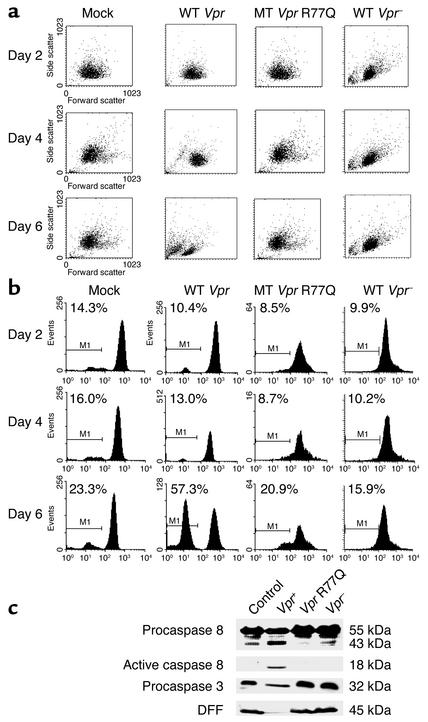
Jurkat T cell death was assessed in viral infections that differ only by virtue of a mutation at position 77 of Vpr (CGA to CAA). VSV-G pseudotyped virus was prepared by cotransfecting an envelope-deficient HIV-1 provirus with VSV-G envelope-expressing plasmid in 293T cells. Progeny virions were normalized by MAGI assay or RT activity and were then used to infect Jurkat T cells. In comparison with cells infected with wild-type virus, cells infected with R77Q Vpr showed less apoptosis (P = 0.01) (Figure 1), as determined by annexin V/propidium iodide staining. Similar results were obtained when infected cells were stained by TUNEL (data not shown). In parallel, cell cycle analysis was performed on infected cells, demonstrating no differences in cell cycle arrest between infections (data not shown).
Figure 1.
R77Q Vpr induces less apoptosis in single-cycle infections using VSV-G pseudotype HIV-1 virus. (a) Jurkat T cells were infected at an MOI of 0.01 with VSV-G pseudotyped virus expressing wild-type Vpr, Vpr-negative (Vpr–), or R77Q Vpr. Cells were analyzed by annexin V FITC/propidium iodide (PI) staining for apoptotic cells at the indicated time points after infection. Values are representative of three independent experiments. The inset denotes the number of propidium iodide–positive cells at each time point (% annextin+/PI– refers to the percentage of cells which stain with annexin V-FITC, but not PI). (b) Cells were isolated on day 6 after infection and stained with TUNEL for apoptosis measurement and with p24 antibody for measurement of viral replication. Data are representative of two independent infections.
To ensure that differences in apoptosis were not due to differences in viral replication, independent experiments were analyzed by flow cytometry for intracellular p24 and TUNEL staining. At days 2 and 4 after infection, minimal differences in TUNEL staining were observed, consistent with the results in Figure 1. On day 6 after infection, uninfected cells had low levels of TUNEL positivity (12.2%) and low levels of background intracellular p24 staining (11.6%). By contrast, cells infected with VSV-G expressing wild-type Vpr, Vpr-negative, or Vpr R77Q had 68.6%, 59.5%, and 58.5% intracellular p24, respectively. Despite the minimal differences in intracellular p24 staining, VSV-G wild-type Vpr infections had 67.8% TUNEL-positive cells, yet Vpr-negative infections had 33.9% and Vpr R77Q infections had 34.6%. Overall, therefore, VSV-G pseudotyped virus infections demonstrate minimal differences in viral replication as assessed by intracellular p24 staining (range, 58.5–68.6%), but wild-type infections had nearly double the degree of TUNEL-positive cells as compared with either Vpr-negative or Vpr R77Q infections.
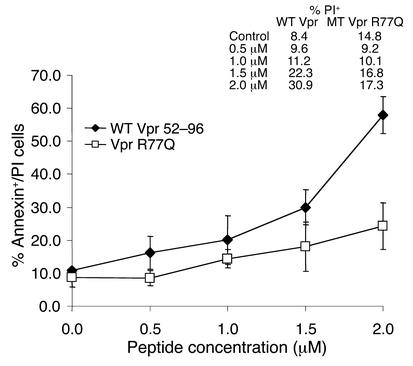
Decreased apoptosis induced by Vpr (52–96) R77Q peptide.
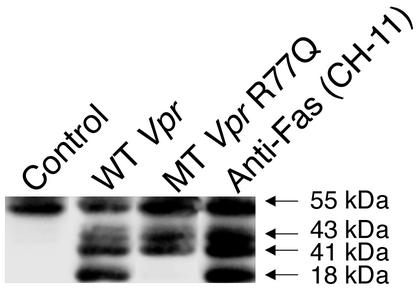
The C-terminal peptide 52–96 of Vpr, which contains the (H(F/S)RIG)2 domain, is responsible for Vpr-mediated apoptosis (11). Jurkat cells were incubated with increasing doses of Vpr wild-type peptide or R77Q peptide (Figure 2) and analyzed for apoptosis. Similar results were seen using either annexin V and propidium iodide staining (wild-type Vpr, 56.0%; R77Q Vpr, 27.3%; n = 3, P = 0.02) (Figure 2) or TUNEL staining (wild-type Vpr, 30.0%; R77Q Vpr, 9.2%; n = 3, P < 0.01) (data not shown). The induction of apoptosis by both wild-type and R77Q Vpr was blocked by the pancaspase inhibitor zVAD-fmk (as assessed by both TUNEL and annexin V/propidium iodide staining) (data not shown), and activation of procaspase-8 was greater in cells treated with wild-type Vpr peptides than in cells treated with Vpr R77Q (Figure 3).
Figure 2.
Vpr R77Q induces less apoptosis than wild-type Vpr. Jurkat cells were treated with synthetic C-terminal wild-type or R77Q Vpr peptides for 16 hours followed by annexin V FITC/propidium iodide staining. Data represent the average of three independent experiments. The inset denotes the number of propidium iodide–positive cells at each time point.
Figure 3.
Decreased activation of caspase-8 in cells treated with Vpr R77Q. Wild-type Vpr causes complete cleavage of caspase-8 into the intermediate and active p18 fragments in a manner similar to that seen after Fas ligation, using the agonistic anti-Fas antibody CH-11. Treatment with R77Q peptide has decreased caspase-8 cleavage and undetectable p18 fragments.
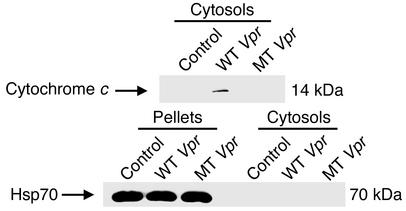
Vpr R77Q induces less mitochondrial release of cytochrome c than wild type.
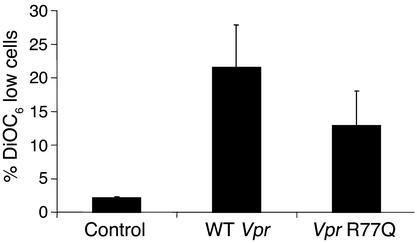
Vpr directly binds to ANT (12) and opens mitochondrial PTPC, resulting in loss of Δψm and the release of apoptogenic factors into the cytoplasm. Although 16-hour treatment with wild-type Vpr caused 21.6% of Jurkat-treated cells to lose Δψm, treatment with R77Q resulted in loss of Δψm in only 12.6% of Jurkat cells (P = 0.02, n = 2) (Figure 4). Similarly, the production of ROSs was attenuated with R77Q peptide when compared with wild-type peptide (R77Q Vpr, 10.8%; wild-type Vpr, 14.4%; n = 3, P = 0.05) (data not shown), and cytochrome c release into the cytoplasm was reduced in cells treated with R77Q Vpr peptide as compared with wild type (Figure 5, upper panel). We also observed comparable levels of cytochrome c in mitochondria pellets (data not shown). Immunoblots for heat-shock protein 70 (Hsp70) were performed in parallel as a mitochondria-specific marker (Figure 5, lower panel).
Figure 4.
Effects of wild-type or R77Q Vpr on mitochondria in Jurkat cells. Jurkat cells were treated with wild-type or R77Q peptide and assayed for transmembrane potential loss using DiOC6. Data represent the average of three experiments.
Figure 5.
Cytochrome c release from mitochondria treated with wild-type or R77Q Vpr. Jurkat T cells were treated with wild-type or R77Q (mutant) Vpr, lysed, and fractionated into mitochondrial and cytosolic fractions. The cytosolic fraction was blotted for cytochrome c (top), and each fraction was then blotted for the mitochondria-specific protein Hsp70 (bottom) to confirm no mitochondrial contamination within the cytosols.
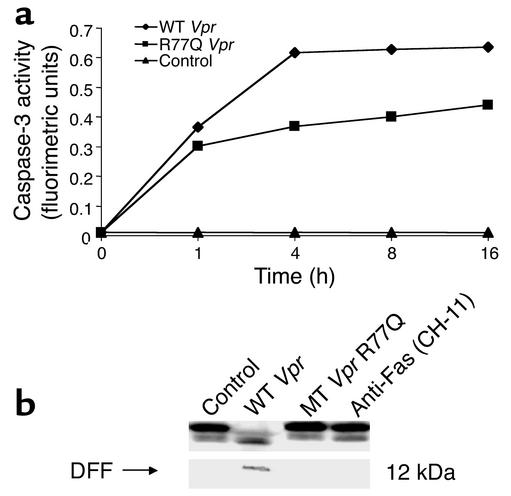
Vpr R77Q induces less caspase activation than wild-type Vpr.
After release of cytochrome c from mitochondria, sequential procaspase activation ultimately leads to apoptosis. Procaspase-9 and procaspase-3 levels were both reduced only in the cells treated with wild-type Vpr peptide (data not shown), indicating cleavage, yet in cells treated with R77Q, less loss of procaspases-9 and -3 was apparent. In parallel, caspase-3 activity was assayed in cells treated with either wild-type or R77Q Vpr peptide, demonstrating less caspase-3 activation in cells treated with R77Q than in cells treated with wild-type peptide (Figure 6a). Similarly, DFF cleavage was detected only in cells treated with the wild-type peptide (Figure 6b).
Figure 6.
Effect of wild-type or R77Q Vpr on caspase-3 and DFF. Jurkat T cells were treated overnight with wild-type or R77Q (mutant) Vpr and analyzed for (a) caspase-3 activity and (b) DFF cleavage. Blots were stripped and reprobed for proliferating cell nuclear antigen (PCNA) to ensure equal loading (data not shown).
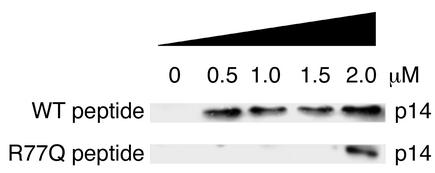
Effects of R77Q or wild-type Vpr on isolated mitochondria.
To assess the possibility that differences in Vpr permeability might explain the differences in induction of apoptosis, we incubated freshly isolated mitochondria from mouse liver and measured Δψm using DiOC6. Within 6 hours of incubation at the lowest concentration of peptides, wild-type Vpr caused 62.7% of mitochondria to lose Δψm, whereas only 26.6% of isolated mitochondria treated with R77Q Vpr lost Δψm (P = 0.02). In these experiments, cytochrome c release was detected at all concentrations of wild-type Vpr but only at the highest concentration of R77Q Vpr (Figure 7).
Figure 7.
Effect of wild-type or R77Q Vpr on isolated mitochondria. Mitochondria were isolated and treated with the indicated concentration of wild-type Vpr or R77Q Vpr, and supernatants were analyzed for cytochrome c release. Equal loading of protein was confirmed using PCNA, and purity was assessed by Hsp70 immunoblots (data not shown).
HIV infection R77Q Vpr results in less mitochondrial Δψm, caspase activation and DFF cleavage than wild-type virus.
Our data demonstrate that infection with VSV-G pseudotype virus containing R77Q is associated with less apoptosis than wild-type infections. To assess the impact of viral infections containing R77Q Vpr on the apoptotic cascade, we performed infections using VSV-G pseudotype virus prepared with Vpr negative, wild-type Vpr, or R77Q Vpr, and we examined changes in light scatter, mitochondrial Δψm, and cleavage of procaspase-8, procaspase-3, and DFF (Figure 8). VSV-G–infected cells were analyzed on days 2, 4, and 6 in comparison with mock-infected controls. Light scatter profiles demonstrate a significant shift toward an apoptotic morphology in cells infected with wild-type Vpr that is not as apparent in cells infected with R77Q Vpr (Figure 8a). Similarly, assessment of mitochondrial Δψm using DiOC6 demonstrates Δψm loss in 57.3% of cells infected with wild-type Vpr on day 6, which is attenuated in the cells infected with R77Q Vpr virus (Figure 8b). When procaspase-8 cleavage in the cells infected with wild-type virus was examined, cleavage was undetectable in cells infected with VSV-G pseudotype virus containing R77Q Vpr, whereas infections with wild-type virus resulted in significant procaspase-8 processing. Similarly, procaspase-3 and full-length DFF levels are reduced in the wild-type infected cells, yet disappearance of these proteins is not apparent in the R77Q Vpr infections (Figure 8c).
Figure 8.
Apoptosis signaling in Jurkat cells infected with VSV-G pseudotype virus containing Vpr R77Q. Jurkat cells were mock infected (control) or infected with 0.01 MOI of VSV-G pseudotyped virus containing wild-type Vpr, Vpr R77Q, or VSV-G with Vpr negative (Vpr–). Cells were collected on days 2, 4, and 6 and analyzed for apoptosis by (a) light scatter (leftward shift) and (b) mitochondrial Δψm using DIOC6. In parallel, cytosolic extracts from day-6 infections were immunoblotted for procaspase-8, procaspase-3, and DFF (c). Equal protein loading was confirmed by PCNA (data not shown).
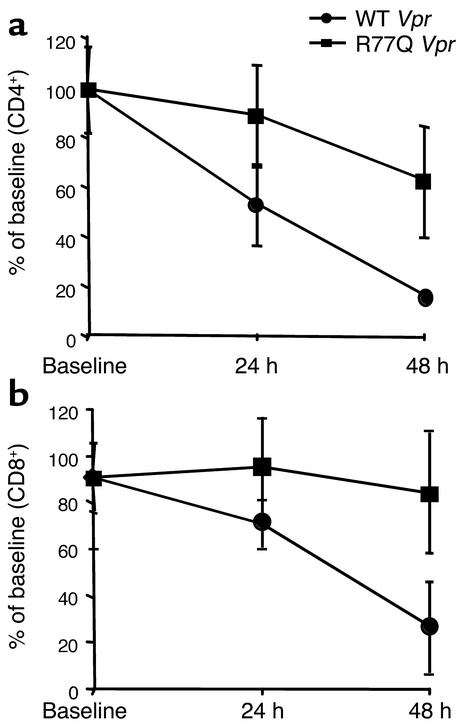
R77Q Vpr is less toxic than wild type in vivo.
Balb-c mice (21–28 days old) were treated with intravenous tail-vein injections of 200 μl of diluent alone or with either wild-type or R77Q Vpr peptide (10 mg/kg). Control mice had stable CD4+ and CD8+ T cell counts over the 48-hour period of observation (data not shown), whereas mice treated with wild-type Vpr experienced a dramatic reduction of both CD4 and CD8 T cell numbers by 48 hours. In mice injected with R77Q Vpr, less T cell depletion occurred as compared with mice receiving wild-type Vpr (Figure 9) (n = 5; CD4, P < 0.001; CD8, P = 0.02). Furthermore, the magnitude of CD4 T cell depletion with wild-type peptide was greater than that of CD8 T cells (CD4 percentage of baseline at 48 hours, 15.6%; CD8 percentage of baseline at 48 hours, 26.3%; P = 0.0035).
Figure 9.
Effects of wild-type or R77Q Vpr on T cell depletion in vivo. Balb-c mice (21–28 days old) were intravenously injected with vehicle control alone (data not shown) or vehicle containing wild-type Vpr or mutant Vpr. The absolute CD4+ or CD8+ T cell number at baseline, 24, and 48 hours after injection was determined by FACS analysis. Data are presented as the percent decrease from baseline normalized to control mice for CD4+ T cells (a) or CD8+ T cells (b).
Discussion
Only recently has the link between Vpr and apoptosis been established and, more importantly, the pivotal role of the (H(F/S)RIG)2 domain. Thus, although Vpr sequences from LTNP have been previously analyzed, the importance of point mutations within this domain has not been fully investigated. Indeed, all of the relevant previous literature confirms the association of R77Q Vpr with LTNP: (a) there is an approximately 80% incidence of R77Q mutations in LTNP (1, 18); (b) an analysis of the Los Alamos database demonstrates that 75% of 146 LTNPs contain R77Q, as opposed to 36% of 55 patients with progressive HIV disease; and (c) a longitudinal analysis of an HIV-infected mother-child pair (18), both of whom had LTNP, the disease was associated with an R77Q mutation of Vpr, yet spontaneous reversion to wild-type genotype at position 77 was temporally associated with progressive disease. In our analysis of Vpr mutations, we examined both a cohort of 10 LTNPs, all of whom had detectable levels of viral replication, and 15 HIV-infected patients with progressive HIV immune disease. According to this analysis, 80% of LTNPs but only 33% of progressors have the Vpr mutation R77Q, providing a fourth piece of evidence for this association.
Since early in the HIV epidemic, elevated levels of T cell apoptosis have been observed in the peripheral blood of infected patients (19). Although considerable debate exists concerning the signals that predominate in vivo, HIV infection leads to apoptosis of both infected and uninfected cells induced by activation signals, enhanced expression of apoptosis-inducing ligands, and apoptotic signals produced by viral proteins, including gp120, Nef, Tat, protease, and Vpr (20). A causal role for apoptosis as the mediator of CD4 T cell depletion in HIV infection is suggested by the following. First, animal models of lentivirus infection in which enhanced apoptosis is observed are associated with immunodeficiency, whereas those without apoptosis are not (21). Second, the disease course of humans infected with HIV demonstrates an inverse correlation between apoptosis and disease progression rates in both cross-sectional and longitudinal studies (22, 23). Third, initiation of antiretroviral therapy that results in immune reconstitution causes a dramatic reduction in T cell apoptosis in lymphoid tissues (24, 25). Finally, whereas patients with rapidly progressive HIV disease have high levels of apoptosis, patients with LTNP HIV disease have levels of apoptosis similar to those of HIV-uninfected patients (26–28). Intriguingly, mechanisms of LTNP defined thus far have identified host and viral means of impaired viral infectivity or replication, yet no mechanism has been defined that impairs the ability of HIV to induce T cell death.
Since the (H(F/S)RIG)2 domain of Vpr is responsible for inducing apoptosis and mutations within this domain are associated with LTNP, we compared the apoptotic effects of R77Q Vpr and wild-type Vpr. First, infections using pseudotyped VSV-G HIV-1 particles were performed. Despite no differences in cell cycle arrest or viral replication, a significant reduction in apoptosis occurred in the R77Q mutant as compared with wild type, suggesting that the attenuation in apoptosis is not due to changes in cell cycle or replication but due to the apoptotic effect of Vpr on mitochondria. Thus, the biochemical events of apoptosis were characterized using synthetic peptides of wild-type or R77Q Vpr (amino acids 52–96) and infections using VSV-G pseudotyped virus without Vpr or containing either wild-type or R77Q Vpr. Jurkat cells treated with R77Q peptides and/or infected with R77Q VSV-G pseudotyped virus underwent less apoptosis, mitochondrial Δψm, caspase activation, and cytochrome c release than cells treated with the respective wild-type Vpr controls. Further, these differential effects were also seen when isolated mitochondria were treated with wild-type or R77Q peptide. Thus, R77Q mutations of Vpr are both associated with an LTNP phenotype and have a decreased ability to induce apoptosis after viral infection or after addition of recombinant Vpr peptide to intact cells or to isolated mitochondria in vitro.
Transgenic mice that overexpress Vpr display CD4 and CD8 T cell depletion and thymic atrophy (29), suggesting that Vpr influences T cell survival in vivo. Therefore, we assessed the in vivo effects of R77Q versus wild-type Vpr in mice after tail-vein injection. Although significant levels of both CD4 and CD8 T cell depletion are seen, the magnitude of CD4 T cell depletion is statistically greater than that seen for CD8 T cells. These changes very closely mimic changes in CD4 and CD8 T cell number seen in patients during the course of HIV disease, suggesting that Vpr may affect the CD4 and CD8 T cell decline in vivo. Whereas significant CD4 and CD8 T cell depletion occurs after injection of wild-type Vpr, these effects are attenuated after injection of R77Q Vpr. Thus, both in vitro and in vivo, in experimental systems and in HIV-infected patients, mutation within the (H(S/F)RIG)2 apoptosis-inducing domain of Vpr is associated with attenuated T cell depletion.
Our cumulative data thus demonstrate an impaired ability of R77Q mutations of Vpr to induce apoptosis in vitro and in vivo, and this mutation is seen in a high frequency of LTNPs as opposed to patients with progressive HIV disease. These observations suggest that Vpr plays a significant role in CD4 T cell depletion in individuals infected with HIV. Moreover, it suggests a therapeutic opportunity for the development of Vpr inhibitors to reduce T cell death during HIV infection.
Acknowledgments
A.D. Badley is supported by grants from the Positive Action Fund of the Ontario Ministry of Health, the Canadian Institute of Health Research (CIHR), the Canadian Foundation for AIDS Research (CANFAR), and the Doris Duke Charitable Foundation. E.A. Cohen is supported by grants from CIHR. A.D. Badley is a recipient of a Career Scientist Award from the Ontario HIV Treatment Network (OHTN), J.J. Lum has received a Studentship Award from the OHTN, and A.A. Pilon has received a Postdoctoral Fellowship Award from the OHTN. X.-J. Yao is a recipient of a Médecine Relève 2000-Messenger Foundation award from the Faculté de Médecine, Université de Montréal. E.A. Cohen is the recipient of the Canada Research Chair in Human Retrovirology. The authors also greatly appreciate the expert administrative assistance of A. Carisse and T. Hoff as well as the editorial review of B.W.D. Badley.
Footnotes
See the related Commentary beginning on page 1455.
Conflict of interest: The authors have declared that no conflict of interest exists.
Nonstandard abbreviations used: long-term nonprogressive/nonprogressor (LTNP); viral protein R (Vpr); vesicular stomatitis virus G (VSV-G); multinuclear activation of galactosidase-indicator (MAGI); mitochondrial transmembrane potential (Δψm); adenine nucleotide translocator (ANT); permeability transition pore complex (PTPC); reactive oxygen species (ROS); DNA fragmentation factor (DFF); heat-shock protein 70 (Hsp70).
References
- 1.Zhang L, Huang Y, Yuan H, Tuttleton S, Ho DD. Genetic characterization of vif, vpr, and vpu sequences from long-term survivors of human immunodeficiency virus type 1 infection. Virology. 1997;228:340–349. doi: 10.1006/viro.1996.8378. [DOI] [PubMed] [Google Scholar]
- 2.Buchbinder SP, Katz MH, Hessol NA, O’Malley PM, Holmberg SD. Long-term HIV-1 infection without immunologic progression. AIDS. 1994;8:1123–1128. doi: 10.1097/00002030-199408000-00014. [DOI] [PubMed] [Google Scholar]
- 3.Cohen OJ, et al. Heterozygosity for a defective gene for CC chemokine receptor 5 is not the sole determinant for the immunologic and virologic phenotype of HIV-infected long-term nonprogressors. J. Clin. Invest. 1997;100:1581–1589. doi: 10.1172/JCI119682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deacon NJ, et al. Genomic structure of an attenuated quasi species of HIV-1 from a blood transfusion donor and recipients. Science. 1995;270:988–991. doi: 10.1126/science.270.5238.988. [DOI] [PubMed] [Google Scholar]
- 5.Kaslow RA, et al. Influence of combinations of human major histocompatibility complex genes on the course of HIV-1 infection. Nat. Med. 1996;2:405–411. doi: 10.1038/nm0496-405. [DOI] [PubMed] [Google Scholar]
- 6.Chun TW, et al. Suppression of HIV replication in the resting CD4+ T cell reservoir by autologous CD8+ T cells: implications for the development of therapeutic strategies. Proc. Natl. Acad. Sci. U. S. A. 2001;98:253–258. doi: 10.1073/pnas.98.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matsuda Z, Yu X, Yu QC, Lee TH, Essex M. A virion-specific inhibitory molecule with therapeutic potential for human immunodeficiency virus type 1. Proc. Natl. Acad. Sci. U. S. A. 1993;90:3544–3548. doi: 10.1073/pnas.90.8.3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levy DN, Refaeli Y, MacGregor RR, Weiner DB. Serum Vpr regulates productive infection and latency of human immunodeficiency virus type 1. Proc. Natl. Acad. Sci. U. S. A. 1994;91:10873–10877. doi: 10.1073/pnas.91.23.10873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stewart SA, Poon B, Jowett JB, Chen IS. Human immunodeficiency virus type 1 Vpr induces apoptosis following cell cycle arrest. J. Virol. 1997;71:5579–5592. doi: 10.1128/jvi.71.7.5579-5592.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel CA, Mukhtar M, Pomerantz RJ. Human immunodeficiency virus type 1 Vpr induces apoptosis in human neuronal cells. J. Virol. 2000;74:9717–9726. doi: 10.1128/jvi.74.20.9717-9726.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Macreadie IG, et al. A domain of human immunodeficiency virus type 1 Vpr containing repeated H(S/F)RIG amino acid motifs causes cell growth arrest and structural defects. Proc. Natl. Acad. Sci. U. S. A. 1995;92:2770–2774. doi: 10.1073/pnas.92.7.2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jacotot E, et al. Control of mitochondrial membrane permeabilization by adenine nucleotide translocator interacting with HIV-1 viral protein rR and Bcl-2. J. Exp. Med. 2001;193:509–519. doi: 10.1084/jem.193.4.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacotot E, et al. The HIV-1 viral protein R induces apoptosis via a direct effect on the mitochondrial permeability transition pore. J. Exp. Med. 2000;191:33–46. doi: 10.1084/jem.191.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yao XJ, et al. Vpr stimulates viral expression and induces cell killing in human immunodeficiency virus type 1-infected dividing Jurkat T cells. J. Virol. 1998;72:4686–4693. doi: 10.1128/jvi.72.6.4686-4693.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Phenix BN, Lum JJ, Nie Z, Sanchez-Dardon J, Badley AD. Antiapoptotic mechanism of HIV protease inhibitors: preventing mitochondrial transmembrane potential loss. Blood. 2001;98:1078–1085. doi: 10.1182/blood.v98.4.1078. [DOI] [PubMed] [Google Scholar]
- 16.Baudouin F, Sarda MN, Goguel A, Bene MC. Multicenter study of reference stabilized human blood for lymphocyte immunophenotyping quality control in flow cytometry. GEIL. Cytometry. 1999;38:127–132. [PubMed] [Google Scholar]
- 17.Keeney M, et al. Single platform flow cytometric absolute CD34+ cell counts based on the ISHAGE guidelines. International Society of Hematotherapy and Graft Engineering. Cytometry. 1998;34:61–70. [PubMed] [Google Scholar]
- 18.Wang B, et al. Gene defects clustered at the C-terminus of the vpr gene of HIV-1 in long-term nonprogressing mother and child pair: in vivo evolution of vpr quasispecies in blood and plasma. Virology. 1996;223:224–232. doi: 10.1006/viro.1996.0471. [DOI] [PubMed] [Google Scholar]
- 19.Kobayashi N, et al. Anti-Fas monoclonal antibody is cytocidal to human immunodeficiency virus-infected cells without augmenting viral replication. Proc. Natl. Acad. Sci. U. S. A. 1990;87:9620–9624. doi: 10.1073/pnas.87.24.9620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Badley AD, Pilon AA, Landay A, Lynch DH. Mechanisms of HIV-associated lymphocyte apoptosis. Blood. 2000;96:2951–2964. [PubMed] [Google Scholar]
- 21.Estaquier J, et al. Programmed cell death and AIDS: significance of T-cell apoptosis in pathogenic and nonpathogenic primate lentiviral infections. Proc. Natl. Acad. Sci. U. S. A. 1994;91:9431–9435. doi: 10.1073/pnas.91.20.9431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patki AH, Georges DL, Lederman MM. CD4+-T-cell counts, spontaneous apoptosis, and Fas expression in peripheral blood mononuclear cells obtained from human immunodeficiency virus type 1-infected subjects. Clin. Diagn. Lab. Immunol. 1997;4:736–741. doi: 10.1128/cdli.4.6.736-741.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Samuelsson A, Brostrom C, van Dijk N, Sonnerborg A, Chiodi F. Apoptosis of CD4+ and CD19+ cells during human immunodeficiency virus type 1 infection — correlation with clinical progression, viral load, and loss of humoral immunity. Virology. 1997;238:180–188. doi: 10.1006/viro.1997.8790. [DOI] [PubMed] [Google Scholar]
- 24.Badley AD, et al. Dynamic correlation of apoptosis and immune activation during treatment of HIV infection. Cell Death Differ. 1999;6:420–432. doi: 10.1038/sj.cdd.4400509. [DOI] [PubMed] [Google Scholar]
- 25.Badley AD, et al. In vivo analysis of Fas/FasL interactions in HIV-infected patients. J. Clin. Invest. 1998;102:79–87. doi: 10.1172/JCI2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liegler TJ, et al. Diminished spontaneous apoptosis in lymphocytes from human immunodeficiency virus-infected long-term nonprogressors. J. Infect. Dis. 1998;178:669–679. doi: 10.1086/515378. [DOI] [PubMed] [Google Scholar]
- 27.Wasmuth JC, et al. Prediction of imminent complications in HIV-1-infected patients by markers of lymphocyte apoptosis. J. Acquir. Immune Defic. Syndr. 2000;23:44–51. doi: 10.1097/00126334-200001010-00006. [DOI] [PubMed] [Google Scholar]
- 28.Franceschi C, et al. Phenotypic characteristics and tendency to apoptosis of peripheral blood mononuclear cells from HIV+ long term non progressors. Cell Death Differ. 1997;4:815–823. doi: 10.1038/sj.cdd.4400305. [DOI] [PubMed] [Google Scholar]
- 29.Yasuda J, Miyao T, Kamata M, Aida Y, Iwakura Y. T cell apoptosis causes peripheral T cell depletion in mice transgenic for the HIV-1 vpr gene. Virology. 2001;285:181–192. doi: 10.1006/viro.2001.0964. [DOI] [PubMed] [Google Scholar]