Serum vitamin B-12 concentrations are measured to assess the presence of its deficiency in patients presenting with haematolological, neurological, and neuropsychiatric abnormalities. Replacement therapy is instituted promptly, particularly to prevent irreversible neurological and cognitive dysfunction.
I present two cases with paradoxical vitamin B-12 results, which highlight the fallacies of the serum vitamin B-12 assay and emphasise the importance of taking into account the overall clinical picture before prejudging the significance of the vitamin B-12 assay result.
Case reports
Case 1
A 59 year old white woman was seen urgently for assessment of a macrocytic anaemia. She had normal serum B-12 concentrations, confirmed on three occasions. She complained of progressively increasing lethargy, palpitations, and buzzing in the ears over about three months. She had a good, well balanced diet and was not a vegetarian. Apart from thyroxine, she was taking no regular medication. She said her father had had pernicious anaemia. On clinical examination the only clinically significant findings were a mild glossitis and pallor. A full blood count showed a substantial macrocytic anaemia and a mild reduction of the white cell count (figure). The blood film showed mild oval macrocytosis, occasional nucleated red cells, and some hypersegmented neutrophils. An urgent bone marrow examination showed megaloblastic haemopoiesis.
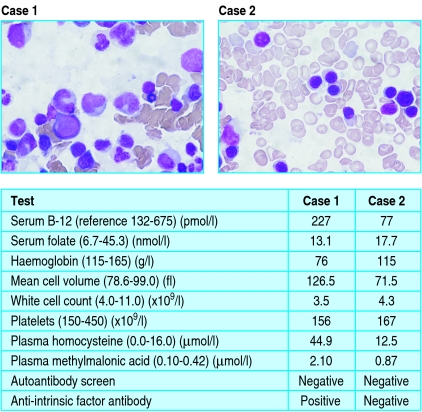
Figure 1.
Laboratory results and bone marrow appearances for the cases presented. Case 1 shows a normal serum vitamin B-12 concentration with biochemical features of B-12 deficiency (raised homocysteine and methylmalonic acid). The haematology indices (macrocytic anaemia) and bone marrow appearances are consistent with megaloblastic anaemia caused by classic pernicious anaemia, confirmed by a positive anti-intrinsic factor antibody. Case 2 shows a markedly reduced serum vitamin B-12 concentration but largely unremarkable biochemical features (normal homocysteine concentration and slightly raised methylmalonic acid) of vitamin B-12 deficiency. The mild anaemia and reduced mean cell volume are consistent with a β thalassaemia trait, and the bone marrow appearances are normal
The most likely diagnosis, in the face of normal serum vitamin B-12 concentration, would have been a myelodysplastic syndrome needing urgent blood transfusion and further haematology assessment. However, in view of her history of thyroid disease and family history of pernicious anaemia, an underlying functional vitamin B-12 deficiency could not be entirely discounted. A blood sample for autoantibody screen, anti-intrinsic factor antibody, and serum homocysteine and methylmalonic acid were taken and she was started immediately on replacement vitamin B-12 therapy pending these results.
She had a prompt response, with her full blood count returning to normal within eight weeks of therapy (haemoglobin 126 g/l; mean cell volume 87.5 fl) without the need for blood transfusion. The results of serum homocysteine and methylmalonic acid were substantially raised and consistent with functional vitamin B-12 deficiency. The anti-intrinsic factor antibody was positive, confirming the diagnosis of pernicious anaemia. Cytogenetic analysis of her bone marrow was normal.
Case 2
A 60 year old white woman with β thalassaemia trait had her serum vitamin B-12 concentration checked as she mentioned that she was a vegan. She had no symptoms of note and gave a history of avoiding meat and milk products for the past eight years. She had no family history of pernicious anaemia or other autoimmune conditions. She was not taking any medications. Her serum vitamin B-12 concentration was markedly reduced (figure)—confirmed on three occasions—with a haemoglobin concentration at the lower end of the normal range, consistent with her thalassaemia trait. On examination, there were no clinical features of note. Her anti-intrinsic factor antibody was negative and her serum ferritin concentration was within normal limits. Conventionally, she would have been given a course of vitamin B-12 injections immediately, and subsequently a Schilling test (or equivalent vitamin B-12 absorption study) would have been performed. In view of her general wellbeing, a bone marrow examination was done to evaluate any effect on erythropoiesis as a marker for functional vitamin B-12 deficiency. The bone marrow showed normal erythropoiesis, her plasma homocysteine was normal, and her methylmalonic acid was raised sufficiently to indicate possible subclinical deficiency.1
Discussion
The diagnosis of vitamin B-12 deficiency has always been problematic owing to the unavailability of a robust assay.2 Normal concentrations of vitamin B-12 have been reported in patients with overt deficiency,3 and the type of assay used may be relevant.4 We use the Access Immunoassay System (Beckman Coulter) which has shown a coefficient of variation of 6-10% under the United Kingdom National External Quality Assessment Scheme in the recent past. This implies that the results obtained in the cases presented are qualitatively and quantitatively in defined categories of “normal” in case 1 and “deficient” in case 2.
Case 1 highlights the dangers of a “false normal” vitamin B-12 result. The existence of a large “grey area” for vitamin B-12 assays with regard to interpreting deficiency is well recognised. However, the repeated (three) results showing vitamin B-12 values in the upper part of the normal range in this patient is unusual. There may be unique analytical reasons in this patient (for example, heterophil antibodies), although this remains speculative. Despite a normal result, a diagnosis of vitamin B-12 deficiency was considered possible, and so treatment started straightaway. The more “logical” diagnosis—of myelodysplasia—would have led to her having a much more aggressive and potentially detrimental therapy combining chemotherapy and bone marrow transplantation.
In case 2, the vitamin B-12 concentration was particularly low despite the absence of clinical symptoms and normal bone marrow appearances. Possibly the patient is developing vitamin B-12 deficiency.5
Last year about 8500 serum vitamin B-12 assays from primary and secondary care were done in our haematology laboratory, which serves a population of 200 000. The number of assays is increasing annually by an average of 9%. Testing may be done as part of an assessment of anaemia, which may be mild,6 or as part of a screening process to assess neuropsychiatric symptoms.7 Testing for these disorders is increasing as a result of an increasingly older population. Of the vitamin B-12 tests done in our laboratory, 8% were in the “deficient” range (< 107 pmol/l), 10% were in the “intermediate range” (107-132 pmol/l), and 42% were in the “normal” range (132-227 pmol/l). It is difficult to know how many of these may have true B-12 deficiency or functional B-12 deficiency despite normal B-12 levels, particularly if other coincident medical problems such as bleeding and liver or renal disease are present.
It is difficult to know how many patients worldwide are diagnosed with myelodysplasia influenced by the presence of a normal serum vitamin B-12 concentration. Use of serum homocysteine and methylmalonic acid concentrations have been reported to help in identifying elderly patients with vitamin B-12 deficiency in a screening situation8,9 and to demonstrate biochemical benefit in such patients given vitamin B-12 treatment empirically.10 However, a recent study of patients in an ambulatory setting also raised the question of whether homocysteine and methylmalonic acid were as reliable as generally believed.11 Measurement of holotranscobalamin II, another recent marker for functional vitamin B-12 concentrations, is claimed to be more sensitive than methylmalonic acid12 but its clinical utility is open to question.13
Ultimately, meticulous clinical assessment— including assessment of other autoimmune conditions and taking a family history—is important, given that a single ideal test is still not available. Testing for anti-intrinsic factor antibody, despite a normal serum vitamin B-12 concentration, can be particularly useful when underlying pernicious anaemia is strongly suspected, as in case 1. However, a reduced serum vitamin B-12 concentration associated with a negative anti-intrinsic factor antibody, as in case 2, may not help in arriving at a diagnosis as only half of patients with pernicious anaemia are positive for this. Empirical treatment, to assess any clinical response and to prevent neurological damage, may be pragmatically justifiable as the dangers of treatment are not as devastating as those of not treating.
The clinical picture is of utmost importance when interpreting a vitamin B-12 assay result
I thank the staff at the University Hospital of Wales, Cardiff, who carried out methylmalonic acid and homocysteine assays in the two patients described, and Gareth Davies for taking photographs of the bone marrow.
Contributors: VD is the sole contributor.
Competing interests: None declared.
References
- 1.Carmel R. Current concepts in cobalamin deficiency. Annu Rev Med 2000;51: 357-75. [DOI] [PubMed] [Google Scholar]
- 2.Ward PC. Modern approaches to the investigation of vitamin B12 deficiency. Clin Lab Med 2002;22: 435-45. [DOI] [PubMed] [Google Scholar]
- 3.Lindenbaum J, Savage DG, Stabler SP, Allen RH. Diagnosis of cobalamin deficiency: II. Relative sensitivities of serum cobalamin, methylmalonic acid, and total homocysteine concentrations. Am J Hematol 1990;34(2): 99-107. [DOI] [PubMed] [Google Scholar]
- 4.Carmel R, Brar S, Agrawal A, Penha PD. Failure of assay to identify low cobalamin concentrations. Clin Chem 2000;46: 2017-8. [PubMed] [Google Scholar]
- 5.Refsum H, Yajnik CS, Gadkari M, Schneede J, Vollset SE, Orning L, et al. Hyperhomocysteinemia and elevated methylmalonic acid indicate a high prevalence of cobalamin deficiency in Asian Indians. Am J Clin Nutr 2001;74: 233-41. [DOI] [PubMed] [Google Scholar]
- 6.Guralnik JM, Eisenstaedt RS, Ferrucci L, Klein HG, Woodman RC. Prevalence of anemia in persons 65 years and older in the United States: evidence for a high rate of unexplained anemia. Blood 2004;104: 2263-8. [DOI] [PubMed] [Google Scholar]
- 7.Lindenbaum J, Healton EB, Savage DG, Brust JC, Garrett TJ, Podell ER, et al. Neuropsychiatric disorders caused by cobalamin deficiency in the absence of anemia or macrocytosis. N Engl J Med 1988;318: 1720-8. [DOI] [PubMed] [Google Scholar]
- 8.Pennypacker LC, Allen RH, Kelly JP, Matthews LM, Grigsby J, Kaye K, et al. High prevalence of cobalamin deficiency in elderly outpatients. J Am Geriatr Soc 1992;40: 1197-204. [PubMed] [Google Scholar]
- 9.Clarke R, Refsum H, Birks J, Evans JG, Johnston C, Sherliker P, et al. Screening for vitamin B-12 and folate deficiency in older persons. Am J Clin Nutr 2003;77: 1241-7. [DOI] [PubMed] [Google Scholar]
- 10.Henning BF, Tepel M, Riezler R, Naurath HJ. Long-term effects of vitamin B(12), folate, and vitamin B(6) supplements in elderly people with normal serum vitamin B(12) concentrations. Gerontology 2001;47(1): 30-5. [DOI] [PubMed] [Google Scholar]
- 11.Solomon LR. Cobalamin-responsive disorders in the ambulatory care setting: unreliability of cobalamin, methylmalonic acid, and homocysteine testing. Blood 2005;105: 978-85. [DOI] [PubMed] [Google Scholar]
- 12.Herrmann W, Obeid R, Schorr H, Geisel J. Functional vitamin B12 deficiency and determination of holotranscobalamin in populations at risk. Clin Chem Lab Med 2003;41: 1478-88. [DOI] [PubMed] [Google Scholar]
- 13.Nilsson K, Isaksson A, Gustafson L, Hultberg B. Clinical utility of serum holotranscobalamin as a marker of cobalamin status in elderly patients with neuropsychiatric symptoms. Clin Chem Lab Med 2004;42: 637-43. [DOI] [PubMed] [Google Scholar]