Abstract
The aim of the present study was to establish whether in healthy human subjects the actions of group I muscle afferents arising from the same spinal segments as the soleus innervation (e.g., common peroneal nerve; CPN) or from more proximal spinal segments (femoral nerve; FN) on the soleus H-reflex are modified by changes in hip position. Control and conditioned soleus H-reflexes were elicited and recorded via conventional methods. In seated subjects, CPN and FN stimulation resulted in similar effects to the soleus H-reflex to that previously reported in healthy subjects. However, during hip angle changes, CPN stimulation at the C-T interval of 2 ms resulted in soleus H-reflex depression only when the hip was flexed at 30°, whereas with the hip flexed or extended at 10° the H-reflex was facilitated. CPN stimulation delivered at 100 ms also induced soleus H-reflex facilitation regardless of the hip angle tested. The heteronymous reflex facilitation (conditioned H-reflex with FN stimulation) did not vary systematically with hip angle changes. These findings indicate that hip proprioceptors interact with spinal inhibitory interneurons to enhance spinal reflex excitability under static conditions. This neural switch might constitute an important feature of movement regulation in humans.
Keywords: H-reflex, hip position, muscle afferents, presynaptic inhibition, reciprocal Ia inhibition, sensorimotor integration
INTRODUCTION
Activity in specific spinal reflex pathways can be indirectly assessed in humans using specific electrophysiological techniques (Pierrot-Deseilligny, 1997; Pierrot-Deseilligny & Mazevet, 2000), contributing to a better understanding of the neural control of movement in humans. For example, earlier studies have described a reciprocal short latency inhibition of the soleus H-reflex evoked by a conditioning, electrical low strength stimulus to the common peroneal nerve (CPN), mediated via the muscle spindles of the flexor group Ia afferents (Tanaka, 1974; Crone et al., 1987; Crone, 1993). Further, presynaptic inhibition, which controls the transmission from Ia afferents, gating in this way selectively the sensory feedback from the periphery (Rudomin & Schmidt, 1999), can also be indirectly assessed in humans. A conditioning volley to the CPN evokes in the H-reflex of the soleus muscle a long-lasting inhibition (Crone & Nielsen, 1994; Zehr & Stein, 1999). This reflex depression is attributed to presynaptic inhibition of Ia afferents mediating the test volley (reviewed in Katz, 1999). Moreover, presynaptic inhibition can be indirectly assessed by estimating changes in the femoral nerve-induced soleus H-reflex facilitation in healthy subjects at rest (Hultborn et al., 1987a).
Previous human studies have demonstrated diverse excitatory inputs onto Ia and presynaptic inhibitory interneurons in man, with their inhibitory actions to decrease or to increase depending on the direction of the movement (Petersen et al., 1999; Morita et al., 2001; Nielsen & Kagamihara, 1993; Pierrot-Deseilligny, 1997; Hultborn et al., 1987b), or on the type of excited afferents, for example, cutaneous afferents (Iles, 1996). Hip-mediated sensory feedback under static conditions modulates substantially the soleus H-reflex (Chapman et al., 1991; Knikou & Rymer, 2002a), possibly mediated at a spinal level (Knikou & Rymer, 2002b), as well as actions of putative Ib inhibitory pathways and cutaneous afferent input onto soleus α motoneurons in humans (Knikou & Rymer, 2002a).
To summarize, spinal reflex pathways and shifts in actions of spinal inhibitory interneurons can be indirectly assessed in humans, providing information about sensorimotor integration depending on the arrangement of the conditioning stimulus. In addition, hip-mediated sensory feedback modulates substantially soleus H-reflex and actions of synergistic muscle afferents and plantar cutaneous afferents in healthy subjects. However, changes in the pathway of reciprocal Ia inhibition or on actions of presynaptic inhibitory interneurons during imposed hip angle changes have not yet been reported in humans. Thus, the current study investigated whether actions of these inhibitory interneurons are influenced by hip-mediated sensory feedback. Some of the present results have been previously reported in abstract form (Knikou & Rymer, 2003b).
METHODS
All experiments were approved by the Institutional Review Board (IRB), Office for the Protection of Human Subjects at Northwestern University (Chicago IL, USA), and conducted according to the 1964 Declaration of Helsinki. Informed consent was obtained from each subject prior to testing. Twenty subjects participated in this study and none reported any sign or history of neurological deficit.
Soleus H-Reflex Elicitation and Recording Protocol
The soleus H-reflex was elicited by stimulating the right posterior tibial nerve in the popliteal fossa through a monopolar electrode using a 1-ms rectangular pulse generated by a constant current stimulator (DS7A; Digitimer Ltd., UK) and triggered once every 5 s. The indifferent electrode was placed just above the patella. The reflex responses were recorded with single differential bipolar electrodes (DelSys Inc., Boston, MA) placed over the soleus muscle. Light abrasion of the skin was performed before placement of surface electrodes, which were secured with surgical adhesive tape.
At the start of each test, a hand-held monopolar electrode was used as a probe to establish the correct site for stimulating the posterior tibial nerve. This site was identified as the one during which the soleus H-reflex was present without the M-wave being present. The probe electrode was then replaced by a permanent one (N-10-A, Ambu Inc., Denmark) and the evoked responses were observed on the oscilloscope screen. In case the surface electrode did not evoke the same response behavior the procedure was repeated. After this procedure was completed, the maximal M-wave was measured. Then, the stimulus strength was adjusted to give an H-reflex of 15–30% of the maximal M-wave. For each reflex recorded in this study (control or conditioned), 20 repeated reflex responses evoked every 5 s were acquired and saved for further analysis.
The recorded electromyographic (EMG) signals were amplified 1,000 times and band pass filtered (10 Hz–1 kHz), and subjected to an analogue to digital conversion (PCI-MIO 16E, National Instruments Co., Austin, TX). The digitized EMG signals were rectified and the size of the evoked M-waves and H-reflexes was measured as the area under the full-wave rectified waveforms (Matlab vs. 5.3., Mathworks).
The susceptibility of the reflex to inhibition and to facilitation and the amount of reciprocal Ia inhibition depend on the size of the test H-reflex (Crone et al., 1985, 1990), whereas imposed hip angle changes influence substantially the magnitude of the soleus H-reflex in healthy human subjects (Knikou & Rymer, 2002a). Accordingly, at each hip angle tested (30° flexion, 10° extension) the stimulus strength was carefully adjusted to evoke a control H-reflex (Hohomonymous) that had the same amplitude as the control reflex recorded with hip flexed at 10°. These control reflexes were selected to have a size of 15–30% of the maximal M-wave, elicited only on the ascending part of the recruitment curve, because H-reflexes within this range have a minimal sensitivity to inhibition and facilitation (Crone et al., 1990). This allowed a valid comparison to be made on the amount of facilitation/inhibition due to the conditioning stimulation across hip angles and subjects tested.
The M-wave amplitude was used as a screening factor for accepting control H-reflexes across hip angles and conditioned reflexes at different C-T intervals at each hip angle tested. By keeping the M-wave amplitude stable, the number of group-Ia afferent fibers recruited by the stimulus can be well controlled (Boorman et al., 1996). This resulted in a constant Ia afferent test volley across hip angles tested. Control and conditioned soleus H-reflexes were recorded with the subjects seated and supine with the ipsilateral hip positioned randomly at 10°, 30° of flexion and at 10° of extension.
Conditioning Stimulation of the Soleus H-Reflex
Common Peroneal Nerve (CPN) Stimulation
Twelve subjects (aged 22–32 years, 4 females and 8 males) participated in this test. Methods for CPN stimulation used in the present study were similar to those described in several studies conducted in healthy subjects (Crone et al., 1987; Crone & Nielsen, 1994). The CPN was stimulated with a single shock of 1-ms in duration through a bipolar electrode placed distal to the head of the fibula. The conditioning stimulus was adjusted to 1 × motor threshold (MT) in the tibialis anterior (TA) muscle (Petersen et al., 1999). The MT was determined by the appearance of EMG activity recorded by a differential bipolar surface electrode (DelSys, Boston, MA) placed over the TA muscle. In all subjects, TA M-wave amplitude was monitored on-line to ensure stability of the conditioning afferent volley. Selective CPN stimulation was ensured on that activity in the peroneal muscles was not present, and a pure ankle dorsiflexion without eversion was evoked with increases in the stimulation intensity.
Ipsilateral CPN stimulation always preceded the soleus H-reflex at conditioning test (C-T) intervals of short (2,4, and 6 ms) and long (60, 80, 100, and 120 ms) duration. Control and conditioned H-reflexes at these intervals were recorded with the subjects’ seated. The effects of CPN stimulation on the soleus H-reflex were then investigated using C-T intervals of 2, 80, and 100 ms with the hip positioned in different angles of flexion and extension. These C-T intervals were selected on the basis that in quiescent healthy subjects the reciprocal Ia inhibitory pathway has the shortest latency with 2 to 3 ms between activation of the agonist and inhibition of die antagonist (Crone et al., 1987; Crone & Nielsen, 1989), whereas the reflex inhibition produced by this weak stimulation at such long C-T intervals (80 and 100 ms) is predominantly presynaptic (Iles, 1996; Zehr & Stein, 1999).
Femoral Nerve (FN) Stimulation
Eight subjects (aged 22–52 years, 3 females and 5 males) participated in this test. The methods employed to condition the soleus H-reflex with FN stimulation were similar to those developed by Hultborn and colleagues (Hultborn et al., 1987a). The stimulus to the FN was a single shock of 1-ms duration adjusted at MT in the vastus lateralis muscle. The conditioning stimulation strength was maintained stable at MT level during the experiments, which was monitored on-line by estimating the peak-to-peak amplitude of the M-wave in the vastus lateralis muscle. The stimulus was generated by a constant current stimulator and delivered through a monopolar ball electrode placed in the femoral triangle. The indifferent electrode was placed on the gluteus maximus muscle. Due to the more proximal position of the conditioning electrode, tibial nerve stimulation occurred before FN stimulation and these intervals were considered to be negative.
Earlier human studies have demonstrated that FN stimulation induces soleus H-reflex facilitation (Hultborn et al., 1987a; Pierrot-Deseilligny, 1997) that is monosynaptic and largely dependent on the size of the conditioning Ia monosynaptic excitatory postsynaptic potential (Fournier et al., 1986; Hultborn et al., 1987a). Thus, in the present study changes in the FN-induced soleus H-reflex facilitation were regarded as indicators of modifications in the ongoing presynaptic inhibition of heteronymous Ia afferent terminals.
Experimental Procedures
With subjects seated (hip at 120° flexion, knee at 40° flexion, and ankle at 20° dorsiflexion), the optimal stimulating site of the posterior tibial nerve was first established as described previously. Then, the maximal M-wave was observed and adjustments on the stimulus intensity were made to evoke a test soleus H-reflex that ranged between 15–30% of the maximal M-wave. This H-reflex was then conditioned with CPN stimulation at short (2, 4, 6 ms) and at long (60–120 ms) C-T intervals. Conditioned H-reflexes were recorded randomly with the control reflexes.
For conditioning the soleus H-reflex with FN stimulation, the onset of the reflex facilitation was initially established with subjects seated. The reflex facilitation was identified in increments of .2 ms for all subjects. A representative example of FN-induced soleus H-reflex facilitation is illustrated in Figures 1A & B. The heteronymous H-reflex facilitation for one subject is presented in Figure 1A as the difference between the conditioned and the control H-reflex expressed as a percentage of the maximal M-wave across C-T intervals. This reflex facilitation is further illustrated in Figure 1B as EMG recordings of the control and conditioned H-reflex. In the example shown in Figure 1A, the onset of reflex facilitation occurred at the C-T interval of —8.0 ms. Changes in the FN-induced H-reflex facilitation for this subject with hip positioned at different angles were investigated at the C-T interval of —7.8 ms (see arrow in Figure 1A), for example, within the initial .4 ms after the onset of reflex facilitation. At this short latency the reflex facilitation is monosynaptic and remains unchanged when the alpha motoneurons responsible for the soleus H-reflex receive postsynaptic inhibition (Fournier et al., 1986; Hultborn et al., 1987a). The onset of reflex facilitation was established in a similar way with subjects supine.
Figure 1.
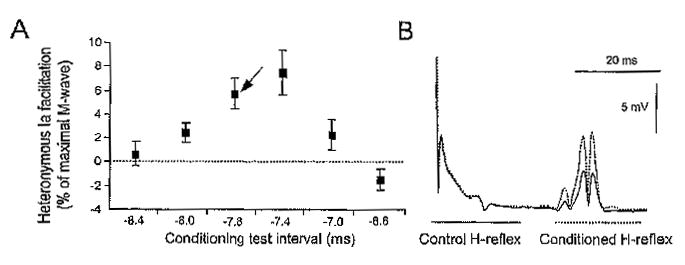
(A) Representative example of the time course of the effects of femoral nerve stimulation on the soleus H-reflex in one seated subject. The arrow indicates the conditioning test interval (—7.8 ms) during which the heteronymous reflex facilitation during hip angle changes in this particular subject was established. The heteronymous H-reflex facilitation at each conditioning test interval is shown as the mean difference (±SEM) between the conditioned and the control reflex expressed as a percentage of the maximal M-wave. (B) The full-wave waveform rectified averages (n = 20) of the control and conditioned H-reflex following femoral nerve stimulation at —7.8 ms are shown. Note that the reflex facilitation occurred without significant changes in the size of the M-wave.
Each subject was then transferred to the supine position and a knee ankle foot orthosis (KAFO) was attached to the right lower limb. Hip angle changes were imposed according to procedures previously described in detail (Knikou & Rymer, 2002a). In the tests where the soleus H-reflex was conditioned with CPN stimulation, the hip was positioned at 10° of flexion and conditioned H-reflexes at C-T intervals of 2, 80, and 100 ms were recorded randomly with the control H-reflexes at this hip angle. Then the hip was positioned at a new angle and control and conditioned reflexes were recorded again.
In the tests where the soleus H-reflex was conditioned with FN stimulation and having established the onset of reflex facilitation with subjects' supine as described previously, the hip was positioned at 10° of flexion by the experimenter. At this hip angle, the control and the conditioned H-reflex were recorded. Conditioned H-reflexes with FN stimulation were recorded with hip positioned in different angles only at the C-T interval that was within .4 ms from the onset of reflex facilitation. Across subjects, conditioned H-reflexes with CPN and FN stimulation were recorded with the hip positioned in different angles (10°, 30° flexion; 10° extension).
Statistical Analysis
For each trial, the sizes of the M-waves of the control and conditioned H-reflexes were expressed as a percentage of the maximal M-wave. A one-way analysis of variance (ANOVA) was used to test for differences between the M-waves of the reflexes recorded under control conditions and following sensorimotor conditioning. Statistical analysis was also performed between the sizes of the M-waves in the Hohomonymous reflexes. When significant differences were identified in the aforementioned tests the trial was rejected.
For all tests in which the effects of CPN stimulation on the soleus H-reflex were investigated with subjects seated, the conditioned H-reflexes were expressed as a percentage of the mean size of the control H-reflex (Ho). A one-way analysis of variance (ANOVA) along with post hoc Bonferroni tests was applied to the data to establish significant differences between control and conditioned reflexes across C-T intervals. This analysis was done for each subject separately but also for the pool data grouped according to the C-T interval tested. In the tests utilizing CPN reflex conditioning during imposed hip angles, the conditioned H-reflexes were expressed as a percentage of the mean size of the Hohomonymous. A one-way ANOVA was used to determine significant differences between the control and the conditioned H-reflexes at each hip angle. The mean size of the conditioned H-reflex from each subject was then grouped by the hip angle and C-T interval tested. A two-way ANOVA with repeated measures was applied to the experimental data sets in order to establish changes in the magnitude of the conditioned H-reflex across hip angles and C-T intervals investigated.
In the tests utilizing FN reflex conditioning during hip angle changes, the conditioned H-reflex at each hip angle was expressed as a percentage of the mean size of the Hohomonymous. A paired t-test was used to establish significant differences between the control and the conditioned H-reflex recorded at each hip angle. A one-way ANOVA was used to determine significant differences between the conditioned H-reflexes across hip angles.
For all tests, alpha was set at .05 to signify a statistically significant difference at 95% of confidence interval. Results are presented as mean values along with the standard error of the mean (SEM).
RESULTS
Effects of CPN and FN Stimulation on the Soleus H-Reflex with Subjects Seated
CPN stimulation resulted in the suppression of the soleus H-reflex across subjects, in line with previous studies (Crone et al., 1987; Crone, 1993). A representative example of the reflex depression is shown in the EMG records of Figure 2A. Note that the M-wave was stable during reflex conditioning, which signifies stable stimulation and recording procedures. A summary of the changes (pool data) in the conditioned H-reflex size following CPN stimulation at short and at long C-T intervals is illustrated in Figure 2B. The soleus H-reflex was equally inhibited at the C-T intervals of 2 and 4 ms (p = .4), whereas the reflex depression did not differ across the long C-T intervals (60–120 ms) tested (F(3,21) = 0.22, p = .88). FN conditioning stimulation resulted in a significant facilitation in the soleus H-reflex with subjects seated. The overall magnitude of the conditioned H-reflex ranged from 118 to 140% of control reflex values across subjects (data not shown graphically).
Figure 2.
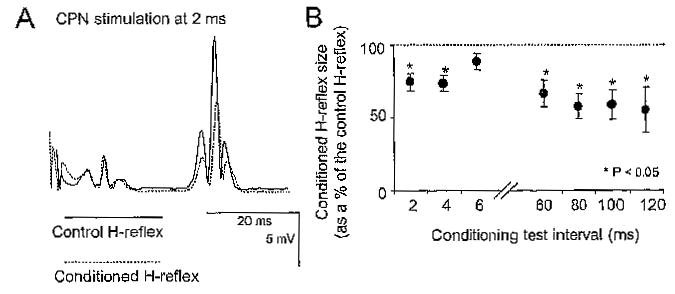
(A) The full-rectified H-reflex averages (n = 20) recorded under control conditions and following common peroneal nerve (CPN) stimulation at a conditioning test (C-T) interval of 2 ms are shown. Note the significant reflex depression without changes in the M-waves of the control and of the conditioned H-reflex. (B) Time course of the effects of CPN stimulation on the soleus H-reflex for all subjects (pool data) seated at short (2–6 ms) and at long (60–120 ms) C-T intervals. Asterisks indicate cases of statistically significant differences between the control and the conditioned H-reflex (p < .05). Error bars illustrate the standard error of the mean.
Effects of CPN Stimulation on the Soleus H-Reflex at Different Hip Angles
CPN stimulation delivered at the C-T interval of 2 ms induced a significant soleus H-reflex depression only when the hip was flexed at 30° (88.7 ± 5.5% of Hohomonymous, p < .05) (Figure 3A). No significant changes in the size of the conditioned H-reflex were recorded when the hip was flexed or extended at 10°, suggesting that at these angles the reciprocal Ia inhibition was depressed.
Figure 3.
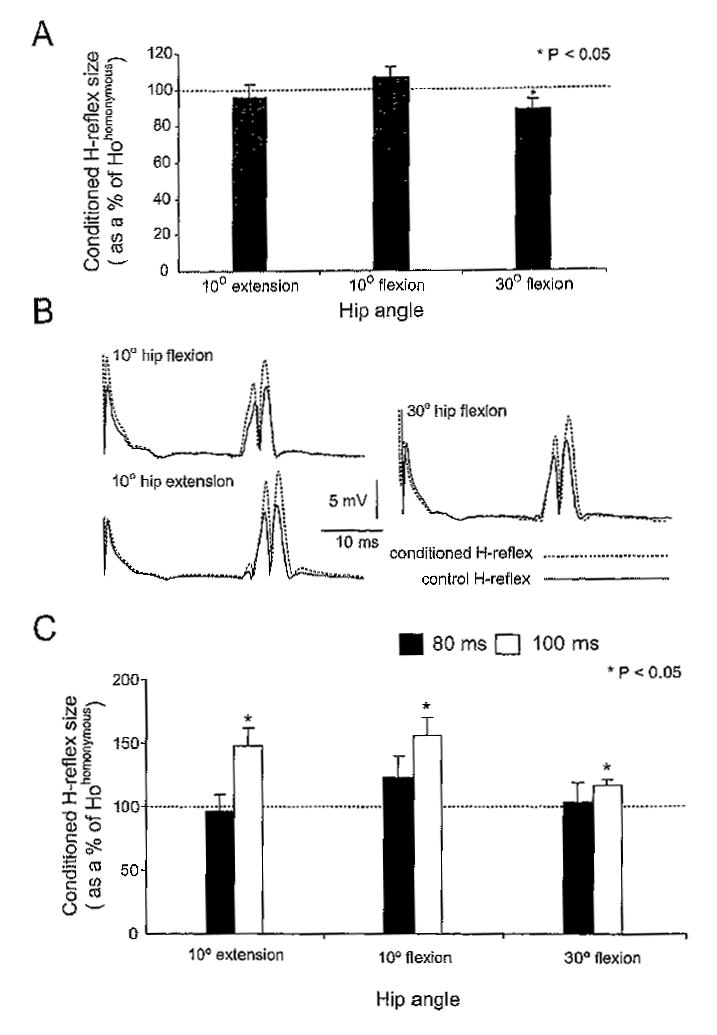
(A) Effects of common peroneal nerve (CPN) stimulation at a conditioning-test (C-T) interval of 2 ms on the soleus H-reflex with hip positioned at different angles. Only at 30° of hip flexion was the conditioned H-reflex significantly different from the control reflex. (B) The average H-reflex (20 responses) recorded under control conditions (solid lines) and following CPN stimulation at 100 ms with the hip positioned at 10° of flexion, 30° of flexion, and at 10° of extension (dashed lines) for one subject is shown, (C) Pool data showing the effects of CPN stimulation delivered at 80 and at 100 ms on the soleus H-reflex with the hip positioned at 10°, 30° of flexion, and at 10° of extension. In both bar charts the conditioned reflexes are presented as a percentage of the mean size of the Hohomonymous whereas the abscissa identifies the hip angle. Asterisks indicate cases of statistically significant differences between the conditioned and the control (Hohomonymous) reflexes (p < .05). Error bars illustrate the standard error of the mean.
CPN stimulation delivered at 100 ms induced a significant facilitation of the soleus H-reflex compared to control reflex values across hip angles tested. This reflex facilitation can be clearly seen in the full-wave rectified waveform averages (n = 20) of the control and conditioned H-reflexes with hip positioned in different angles shown in Figure 3B (data are from a single subject). Note that M-wave was stable during conditioning stimulation across hip angles tested. Figure 3C shows the effects of CPN stimulation on the overall (pool data) size of the soleus H-reflex for the C-T interval of 80 and 100 ms when the hip was positioned at 10° of flexion and extension and at 30° of flexion. At the C-T interval of 100 ms, the reflex facilitation was of equal strength with hip either flexed or extended (p > .05). For the C-T interval of 80 ms, the amplitude of the conditioned reflex was not statistically significant different from control reflex values and did not vary across hip angles tested (p = .21).
Effects of FN Stimulation on the Soleus H-Reflex at Different Hip Angles
The effect of FN stimulation on the soleus H-reflex at different hip angles is shown in the EMG waveform averages for two subjects in Figure 4A. The conditioned H-reflexes (dashed lines) are shown superimposed on the control H-reflexes (solid lines). Note that M-wave stability is demonstrated by nearly identical M-responses in the control and conditioned reflexes of both subjects. A summary of changes on the soleus H-reflex following FN stimulation with hip positioned at different angles of flexion and extension is presented in Figure 4B. FN stimulation with hip extended at 10°, or flexed at 10° and at 30° resulted in a significant increment in the H-reflex size, reaching overall amplitudes of 127 ± 10.91%, 120 ± 7.3%, and 140 ± 10.9% of Hohomonymous, respectively. FN-induced soleus H-reflex facilitation did not vary with changes in hip angles (p = .28).
Figure 4.

(A) Full-wave rectified EMG records showing the control (solid line) and the conditioned H-reflexes (dashed lines) for two subjects and hip positioned in different angles. (B) Pool data indicate the effects of FN stimulation on the soleus H-reflex with the hip positioned in different angles. Asterisks indicate cases of statistically significant differences between the Hohomonymous reflex and the conditioned reflex (p < .05). Error bars designate the standard error of the mean.
DISCUSSION
The primary new finding of this study is that hip-mediated sensory input interacts with spinal inhibitory interneurons exerting either pre- or post-synaptic inhibition on soleus Ia afferents and motoneurons to alter spinal reflex excitability during imposed hip angle changes. These findings contribute to an understanding of the behavior of interneuronal circuits that might be important for the regulation of movement in normal and in pathological states.
Earlier studies have reported diverse excitatory inputs onto Ia inhibitory interneurons in humans (Jankowska, 1992; Baldissera et al., 1981). For example, the inhibitory actions of these interneurons were shown to decrease during voluntary plantar flexion (Petersen et al., 1999; Morita et al., 2001), and during co-contractions of antagonist muscles (Nielsen & Kagamihara, 1993). Similarly, presynaptic inhibition is depressed during ankle plantar flexion (Morita et al., 2001), and at the onset of soleus muscle contraction (Pierrot-Deseilligny, 1997), whereas it is increased during isolated dorsi flexion (Nielsen & Kagamihara, 1993). These findings suggest that the strength of the inhibition is related not only to the stretch of the homologous muscle fibers that mediate the inhibition but also to the direction of the movement (e.g., flexion or extension).
Consistent with the earlier observations, the short latency CPN-induced reflex inhibition, involving principally the pathway of Ia reciprocal inhibition acting at a postsynaptic level (Crone et al., 1987; Crone, 1993), was only active when the hip was positioned at 30° of flexion (Figure 3A). This finding suggests that in quiescent healthy human subjects the number of active Ia inhibitory interneurons is smaller at angles of 10° flexion and extension, whereas they are more active when the leg adopts a more flexed position. In this respect, placing the hip into flexion angles decreases the amount of postsynaptic inhibition exerted by medial gastrocnemius group Ib inhibitory interneurons onto soleus motoneurons in humans (Knikou & Rymer, 2002a). Compared to the current findings, a different modulation pattern of postsynaptic inhibition during hip flexion is evident when Ia or Ib afferents are involved in mediating the reflex inhibition.
The CPN long latency-induced reflex depression, attributed mostly to presynaptic inhibition acting on soleus Ia afferent terminals (Iles, 1996; Crone et al, 1987), was abolished during hip angle changes, suggesting that hip angle is critical for modulating actions of presynaptic inhibitory interneurons, in agreement to studies proposing a premotoneuronal contribution to the reflex modulation during imposed leg movements (Brooke et al., 1997). Thus, hip proprioceptors appear to interact with spinal inhibitory interneurons intercalated in spinal reflex pathways that involve distal muscle afferents. Similarly, the FN-induced reflex facilitation, with its amount to reflect the ongoing presynaptic inhibition (Hultborn et al., 1987a), did not vary across hip angles tested, suggesting of a differential control in the strength of presynaptic inhibition exerted by distal or by proximal muscle afferents.
It is apparent that hip proprioceptors interact with spinal inhibitory interneurons to modulate soleus H-reflex excitability. The current findings switch from net inhibitory to net facilitatory reflex actions, might result from recruitment of excitatory interneurons or via depression of intermediary inhibitory interneurons projecting to excitatory interneurons in group I reflex pathways and triggered when hip-mediated sensory feedback is present. This possibility might be correlated to the complex heteronymous connections between the ankle and thigh muscles postulated in humans (Meunier et al., 1990, 1993), and that imposed hip angle changes modulate not only the size of the H-reflex but also its latency and duration suggesting recruitment of additional interneurons (Knikou & Rymer, 2002b).
Although the involved receptors are uncertain, at this point one should consider the most prevalent ones. It has been reported that denervation of hip joint afferents does not affect the ability of the hip position to entrain the fictive locomotor rhythm in the spinal cat (Kriellaars et al., 1994), which signifies the importance of hip muscle afferents registering stretch and position. In a similar way, static ankle angle changes in humans produce soleus H-reflex modulation (Pinniger et al., 2001; Robinson et al., 1982), attributed mostly to muscle afferents responding to stretch. It is worth noting that muscle spindle afferents are able to detect limb position (Prochazka et al., 1989), with their static discharges to depend on the amount of the muscle shortening or lengthening (Ribot-Ciscar et al., 2003). Further, in the absence of any background force, it is unlikely that group Ib afferents were activated during static hip angle changes. Thus, it is possible that muscle spindle afferents responding to stretch contributed to the observed effects. This is further supported by the connections postulated between thigh and ankle muscles in humans (Meunier et al., 1990, 1993).
It is possible that the observed effects on the soleus H-reflex induced by CPN and by FN stimulation might also be related to the positions of the body (seated vs. supine). Changing the body position from sitting to supine could induce a change in composition of the afferent volley generated by inputs from several joint, muscle, and skin receptors. It is known that descending pathways excite spinal interneurons, which in turn influence the reciprocal inhibition of α motoneurons (Crone & Nielsen, 1994), whereas changes in body position modulate substantially soleus H-reflex excitability (Knikou & Rymer, 2003a). However, it has been reported that the reciprocal Ia inhibition is increased after backward inclination of the body (Rossi et al., 1988), whereas the current authors observed that the reciprocal Ia inhibition was decreased during hip angle changes with subjects supine signifying further that the main source that influenced the amount of reciprocal inhibition it was the hip proprioceptors and not the body position. In addition, because the body position was not a tested variable in the current study, throughout the experiment head and trunk were kept stable, whereas the reflexes were recorded after the lower limb was positioned to a new hip angle by the experimenter. In this respect, transcranial magnetic stimulation induces two separate phases of H-reflex facilitation with subjects in supine and in seated position (Goulart et al., 2000), suggesting that these body positions do not influence differently soleus H-reflex excitability. Nonetheless, it is possible that descending influences might have contributed to the reflex modulation observed at different body positions following both types of conditioning electrical stimuli.
To conclude, the present study provided evidence for modulation of heteronymous group I inhibition and facilitation during hip angle changes. Hip proprioceptors have the potential to change motor output of distant muscles, such as the soleus, and in addition to influence neuronal pathways, such as group Ia reciprocal inhibition and heteronymous group I reflex facilitation, interacting with interneuronal circuits acting at a post- or presynaptic level. These neural switches might constitute an important feature of movement regulation in humans and warrant further investigation using techniques that allow more precise measurements.
Footnotes
This study was supported by the National Institute of Child Health and Human Development (NICHD), National Institutes of Health (NIH), Grant No. 5R03HD043951-01.
References
- Baldissera, F., Hultborn, H., & Illert, M. (1981). Integration in spinal neuronal systems: The nervous system. In V. Brooks (Ed.), Handbook of physiology, motor control. (pp. 509–595). Bethesda, MD: American Physiological Society.
- Boorman GI, Hoffer JA, Kallesoe K, Viberg D, Mali C. A measure of peripheral nerve stimulation efficacy applicable to H-refiex studies. Can J Neurolog Sci. 1996;23:264–270. doi: 10.1017/s0317167100038208. [DOI] [PubMed] [Google Scholar]
- Brooke JD, Cheng J, Collins DF, McIllroy WE, Misiaszek JE, Staines WR. Sensori-sensory afferent conditioning with leg movement: Gain control in spinal reflex and ascending paths. Progr Neurobiol. 1997;51:393–421. doi: 10.1016/s0301-0082(96)00061-5. [DOI] [PubMed] [Google Scholar]
- Chapman CE, Sullivan SJ, Pompura J, Arsenault AB. Changes in hip position modulate soleus H-reflex excitability in man. Electromyogr Clin Neurophysiol. 1991;31:131–143. [PubMed] [Google Scholar]
- Crone C, Hultborn H, Jespersen B. Reciprocal Ia inhibition from the peroneal nerve to soleus motoneurones with special reference to the size of the test reflex. Exp Brain Res. 1985;59:418–422. doi: 10.1007/BF00230924. [DOI] [PubMed] [Google Scholar]
- Crone C, Hultborn H, Jespersen B, Nielsen J. Reciprocal Ia inhibition between ankle flexors and extensors in man. J Physiol. 1987;389:163–185. doi: 10.1113/jphysiol.1987.sp016652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone C, Hultborn H, Mazieres L, Morin C, Nielsen J, Pierrot-Deseilligny E. Sensitivity of monosynaptic test reflexes to facilitation and inhibition as a function of the test reflex: A study in man and the cat. Exp Brain Res. 1990;81:35–45. doi: 10.1007/BF00230098. [DOI] [PubMed] [Google Scholar]
- Crone C, Nielsen J. Spinal mechanisms in man contributing to reciprocal inhibition during voluntary dorsifiexion of the foot. J Physiol. 1989;416:255–272. doi: 10.1113/jphysiol.1989.sp017759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone C, Nielsen J. Central control of disynaptic reciprocal inhibition in humans. Acta Physiol Scand. 1994;152:351–363. doi: 10.1111/j.1748-1716.1994.tb09817.x. [DOI] [PubMed] [Google Scholar]
- Crone C. Reciprocal inhibition in man. Dan Med Bulletin. 1993;40:571–581. [PubMed] [Google Scholar]
- Fournier E, Meunier S, Pierrot-Deseilligny E, Shindo M. Evidence for interneuronally mediated Ia excitatory effects to human quadriceps motoneurones. J Physiol. 1986;377:143–169. doi: 10.1113/jphysiol.1986.sp016179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulart P, Valls-Sole J, Alvarez R. Posture-related changes of soleus H-reflex excitability. Muscle Nerve. 2000;23:925–932. doi: 10.1002/(sici)1097-4598(200006)23:6<925::aid-mus13>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Hultborn H, Meunier S, Morin C, Pierrot-Deseilligny E. Assessing changes in presynaptic inhibition of Ia fibres: A study in man and the cat. J Physiol. 1987a;389:729–756. doi: 10.1113/jphysiol.1987.sp016680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultborn H, Meunier S, Morin C, Pierrot-Deseilligny E. Changes in presynaptic inhibition of Ia fibres at the onset of voluntary contraction in man. J Physiol. 1987b;389:757–772. doi: 10.1113/jphysiol.1987.sp016681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iles JF. Evidence for cutaneous and corticospinal modulation of presynaptic inhibition of Ia afferents from the human lower limb. J Physiol. 1996;491:197–207. doi: 10.1113/jphysiol.1996.sp021207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E. Interneuronal relay in spinal pathways from proprioceptors. Prog NeurobioL. 1992;38:335–378. doi: 10.1016/0301-0082(92)90024-9. [DOI] [PubMed] [Google Scholar]
- Katz R. Presynaptic inhibition in humans: A comparison between normal and spastic patients. J Physiol (Paris) 1999;93:379–385. doi: 10.1016/s0928-4257(00)80065-0. [DOI] [PubMed] [Google Scholar]
- Knikou M, Rymer WZ. Effects of changes in hip joint angle on H-reflex excitability in humans. Exp Brain Res. 2002a;143:149–159. doi: 10.1007/s00221-001-0978-4. [DOI] [PubMed] [Google Scholar]
- Knikou M, Rymer WZ. Hip angle induced modulation of H reflex amplitude, latency and duration in spinal cord injured humans. Clin Neurophysiol. 2002b;113:1698–1708. doi: 10.1016/s1388-2457(02)00285-7. [DOI] [PubMed] [Google Scholar]
- Knikou M, Rymer WZ. Static and dynamic changes in body orientation modulate spinal reflex excitability in humans. Exp Brain Res. 2003a;152:466–475. doi: 10.1007/s00221-003-1577-3. [DOI] [PubMed] [Google Scholar]
- Knikou, M., & Rymer, W. Z. (2003b). Possible mechanisms associated with hip-motion induced H reflex modulation in humans. Program No. 186.1. Abstract Viewer/Itinerary Planner Online. Washington DC: Society for Neuroscience.
- Kriellaars DJ, Brownstone RM, Noga BR, Jordan LM. Mechanical entrainment of fictive locomotion in the decerebrate cat. J Neurophysiol. 1994;71:2074–2086. doi: 10.1152/jn.1994.71.6.2074. [DOI] [PubMed] [Google Scholar]
- Meunier S, Penicaud A, Pierrot-Deseilligny E, Rossi A. Monosynaptic Ia excitation and recurrent inhibition from quadriceps to ankle flexors and extensors in man. J Physiol. 1990;423:661–675. doi: 10.1113/jphysiol.1990.sp018046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier S, Pierrot-Deseilligny E, Simonetta M. Pattern of monosynaptic heteronymous Ia connection in the human lower limb. Exp Brain Res. 1993;96:534–544. doi: 10.1007/BF00234121. [DOI] [PubMed] [Google Scholar]
- Morita H, Crone C, Christenhuis D, Petersen NT, Nielsen JB. Modulation of presynaptic inhibition and disynaptic reciprocal Ia inhibition during voluntary movement in spasticity. Brain. 2001;124:826–837. doi: 10.1093/brain/124.4.826. [DOI] [PubMed] [Google Scholar]
- Nielsen J, Kagamihara Y. The regulation of presynaptic inhibition during co-contraction of antagonistic muscles in man. J Physiol. 1993;464:575–593. doi: 10.1113/jphysiol.1993.sp019652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen N, Morita H, Nielsen J. Modulation of reciprocal inhibition between ankle extensors and flexors during walking in man. J Physiol. 1999;520:605–619. doi: 10.1111/j.1469-7793.1999.00605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierrot-Deseilligny E. Assessing changes in presynaptic inhibition of Ia afferents during movement in humans. J Neurosci Methods. 1997;74:189–199. doi: 10.1016/s0165-0270(97)02249-8. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny E, Mazevet D. The monosynaptic reflex: A tool to investigate motor control in humans. Interests and limits. Neurophysiol Cl. 2000;30:67–80. doi: 10.1016/s0987-7053(00)00062-9. [DOI] [PubMed] [Google Scholar]
- Pinniger GJ, Nordlund MM, Steele JR, Cresswell AG. H-reflex modulation during passive lengthening and shortening of the human triceps surae. J Physiol. 2001;534:913–923. doi: 10.1111/j.1469-7793.2001.00913.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochazka A, Trend PSJ, Hulliger M, Vincent S. Ensemble proprioceptive activity in the cat step cycle: Towards a representative look-up chart. Prog Brain Res. 1989;80:61–74. doi: 10.1016/s0079-6123(08)62200-1. [DOI] [PubMed] [Google Scholar]
- Ribot-Ciscar E, Bergenheim M, Albert F, Roll JP. Proprioceptive population coding of limb position in humans. Exp Brain Res. 2003;149:512–519. doi: 10.1007/s00221-003-1384-x. [DOI] [PubMed] [Google Scholar]
- Robinson KL, McComas AJ, Belanger AY. Control of soleus motoneuron excitability during muscle stretch in man. J Neurol Neurosurg Psychiatry. 1982;45:699–704. doi: 10.1136/jnnp.45.8.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi A, Mazzochio R, Scarpini C. Changes in Ia reciprocal inhibition from the peroneal nerve to the soleus alpha-motoneurons with different static body positions in man. Neurosci Lett. 1988;84(3):283–286. doi: 10.1016/0304-3940(88)90521-6. [DOI] [PubMed] [Google Scholar]
- Rudomin P, Schmidt RF. Presynaptic inhibition in the vertebrate spinal cord revisited. Exp Brain Res. 1999;129:1–37. doi: 10.1007/s002210050933. [DOI] [PubMed] [Google Scholar]
- Tanaka R. Reciprocal Ia inhibition during voluntary movements in man. Exp Brain Res. 1974;21:529–540. doi: 10.1007/BF00237171. [DOI] [PubMed] [Google Scholar]
- Zehr PE, Stein RB. Interaction of the Jendrassik maneuver with segmental presynaptic inhibition. Exp Brain Res. 1999;124:474–480. doi: 10.1007/s002210050643. [DOI] [PubMed] [Google Scholar]


