Abstract
Compromised white matter (WM) integrity in inferior frontal WM has been related to impulsivity in men with schizophrenia. However, these relationships may be more widespread. Fractional anisotropy (FA) derived from diffusion tensor imaging of 25 men with schizophrenia was transformed into Talairach space. Correlations between FA and impulsiveness were examined on a voxelwise basis. We found negative correlations between FA and impulsivity in inferior frontal WM, anterior cingulate, caudate, insula, and inferior parietal lobule. Positive correlations were obtained in the left postcentral gyrus, right superior/middle temporal gyrus, and bilateral fusiform gyrus. These areas may comprise a fronto-temporo-limbic circuit that modulates impulsivity. The voxelwise correlation method can serve as a hypothesis-generation method for relating target behaviors to their underlying neural networks.
Keywords: Diffusion tensor imaging, Impulsivity, Schizophrenia
INTRODUCTION
The neural basis of impulsivity is poorly understood, although it is clear that a number of regions, particularly inferior frontal cortical regions, are involved [1]. An impulsive behavioral style is associated with a variety of poor outcomes, including aggression, substance abuse, and high-risk sexual behaviors. Thus, a better understanding of the neural underpinnings of impulsivity is of great importance. Impulsivity can be particularly problematic in patients with schizophrenia, who already have the burdens of psychiatric symptomatology and cognitive dysfunction. Patients with schizophrenia who also abuse substances are at particularly high risk for impulsive and aggressive behavior. Unfortunately, little is known about the neural basis of impulsivity.
Magnetic resonance diffusion tensor imaging (DTI) is a method that examines the directionality and magnitude of water diffusion in vivo. When there are no boundaries to diffusion, water moves in all directions equally (isotropic diffusion). However, when diffusion boundaries are present, as in the case of axonal membranes, diffusion tends to follow the long axis of those boundaries (anisotropic diffusion). DTI allows the examination of diffusion anisotropy through the application of diffusion-sensitized gradients in at least six nonparallel directions. From these images, scalar metrics of diffusion anisotropy such as fractional anisotropy (FA) can be computed. FA ranges from 0 (perfectly isotropic diffusion) to 1 (perfectly anisotropic diffusion), and has been found to be reduced in a number of populations with white matter pathology, such as multiple sclerosis [2]. FA reductions also have been reported in patients with schizophrenia [3–5]. Analytic approaches for evaluating DTI metrics have varied from region of interest (ROI) based analyses to voxelwise analyses of group differences. Some of these approaches have examined specific white matter tracts [6].
We previously reported [7] that reduced white matter (WM) integrity in inferior frontal regions and as measured by DTI, was associated with higher levels of impulsivity. This finding is consistent with a considerable body of literature that relates damage or pathology in inferior frontal regions to increased impulsivity and impaired response inhibition [8]. Thus, DTI appears to have relevance for the understanding of the neural basis of impulsivity.
Our previous study [7] used an approach in which specific ROIs were placed in brain, FA was measured in those regions. However, a limitation of that study was that the regional specificity of the effects was not examined. In addition, the sample size in that study was modest (14 patients). Moreover, the DTI sequence reported in that paper covered only a limited region of the brain compared to the one used in the current paper. The present report addresses these issues by studying 25 men with schizophrenia using scans that provided nearly whole head coverage. We report, for the first time, the results of a voxelwise correlational analysis (VCA) examining relationships impulsivity and FA in schizophrenia.
MATERIALS AND METHODS
Participants
Participants were 25 men with a DSM-IV diagnosis of schizophrenia or schizoaffective disorder. Diagnosis was confirmed using the Structured Clinical Interview for DSM-IV – Patient Version (SCID-P) [9]. The participants had a mean age of 38.6±7.4 years (range 24–51 years) and a mean education of 11.0±2.2 years (range 8–16 years). One of the patients overlapped with those described in our prior report [7], but we felt that including him in the current sample was justified because the DTI scans (he received two of them) and analytical methods differed. All participants signed written informed consent and all procedures were approved by the local Institutional Review Boards.
Measures
The Barratt Impulsiveness Scale – Version 11 (BIS) [10] has 30 items answered on a four point scale. For each item, the subject indicated the degree to which he endorsed a statement, with 1=rarely/never, and 4=almost always/always. Higher scores on the BIS reflect higher levels of impulsivity. The BIS has three higher order factors. Of these, the Motor Impulsiveness factor, which consists of items regarding acting on the spur of the moment, has been found to correlate with violent behavior in the mentally ill [11]. Thus, as in our previous work [7], the Motor Impulsiveness score was used as the dependent measure from this scale.
Neuroimaging
Scanning was performed on a 1.5 T Siemens Vision System (Erlangen, Germany). Three main sequences were acquired: a magnetization prepared gradient echo scan (MPRAGE; TR/TE=11.4/4.9 ms, matrix=256 × 256, FOV=300 mm, NEX=1, 1.17 mm slice thickness, 172 slices, no gap), a turbo spin echo scan (TSE; TR/TE=5000/22,90 ms, matrix=256 × 256, FOV=224 mm, NEX=1, 5 mm slice thickness, 26 slices, no gap), and a DTI sequence. The DTI sequence has been described elsewhere [12] (TR/TE=6000/100 ms, matrix=128 × 128, FOV=240 mm, 5 mm slice thickness, 20 slices, no gap); it employed a double echo pulse to minimize eddy current effects [13]. The sequence differs from that used in our previous report [7] in that it used a slice thickness of 5 mm, rather than 2.5 mm, and it used an in-plane resolution of 1.875 × 1.875 mm2, rather than 2.5 × 2.5 mm2. The sequence entailed four acquisitions of six diffusion-weighted images (b=1000 s2/mm) for 20 slices. In addition, two acquisitions without diffusion weighting (b=0 s2/mm) were acquired.
Image processing
FA was calculated using software written in the C + + programming language by one of the authors (BAA). The b=0 images were corrected for susceptibility induced distortion and were transformed into Talairach space using methods described elsewhere [3]. Briefly, MPRAGE images were registered to the b=0 images using the automated registration toolbox (ART) [14]. The MPRAGE images were skull stripped using 3D slicer (http://www.slicer.org) by manually tracing the outline of the skull on every other slice. The resulting skull-stripped brain was saved as a binary mask, which was then applied to the original MPRAGE. The MPRAGE also was registered to the dual echo images using ART. This transformation was also applied to the skull strip mask created above, which was then used to strip the dual echo images. The MPRAGE images were then registered to a template brain that had already been placed into Talairach space using ART. Next, the b=0 images were matched to the raw T2 weighted images and were corrected for distortion using ART. Finally, the distortion-corrected b=0 images were transformed into Talairach space. The same transformations were then applied to the FA maps. All images ended up with a final voxel size of 1 × 1 × 1 mm3.
Following transformation into Talairach space, images were masked such that only voxels with data present for all participants were included in the analyses. This ensured that missing data, which would have zero values, would not drive correlations.
A major problem in voxelwise analyses is that of multiple comparisons. Nominally, the scans used in our study would result in 161 × 191 × 151=4 643 401 tests. In reality, the number of tests was closer to 1 500 000 due to the elimination of extracranial voxels using the masking procedures described above. Such a large number of comparisons results in a high risk of Type I error. A Bonferroni approach to deal with this inflation is inappropriate, because the voxel values (and thus the correlations performed) are not statistically independent.
To address this problem, we selected an approach based on [15]. This approach finds seed voxels with highly significant correlations and then applies a region growing approach such that adjacent voxels with correlations significant at some lower level are included in the cluster of interest. This strategy results in the identification of highly significant correlational clusters.
Correlations between the Motor Impulsivity subscale of the BIS and FA were computed on a voxelwise basis using a program written in IDL 6.0 (Research Systems, Boulder, CO, USA) by KOL. First, we found correlations that exceeded given threshold levels for our seed and our cluster and applied a minimum cluster size. Thus, only correlations that met these criteria were deemed statistically significant. In this case, we selected a seed level of p<0.005, a seed cluster size of 200 voxels, and a cluster level p<0.05. Finally, the correlation map was superimposed onto an MPRAGE image in Talairach space using AFNI (Fig. 1).
Fig. 1.
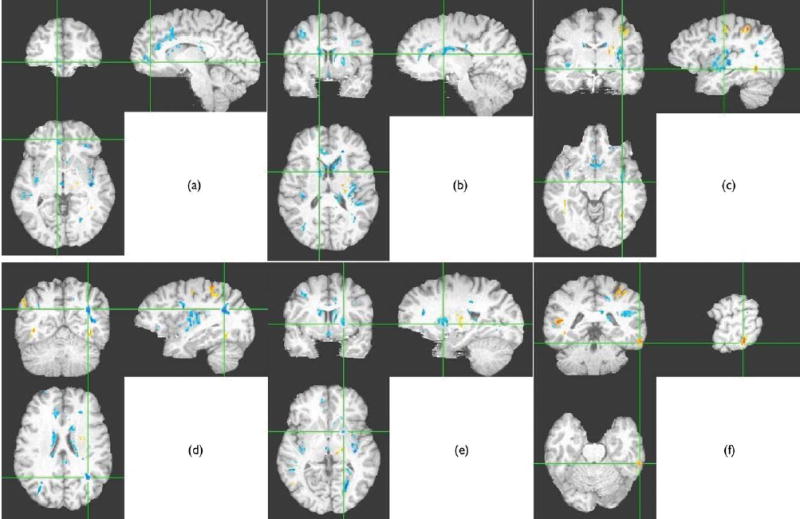
Significant correlations between fractional anisotropy (FA) and motor impulsivity overlaid on target T1 image. Negative correlations are in blue, positive correlations are in red. The thresholds were: seed threshold of p<0.005, cluster threshold of p<0.05, minimum cluster size=200. (a) Negative correlations in right inferior frontal regions and anterior cingulate, (b) negative correlations in caudate, (c) negative correlations in insula, (d) negative correlations in inferior parietal lobule, (e) negative correlations in left putamen, and (f) positive correlations in inferior temporal gyrus. Although each slice shows many areas in which correlations were significant, the crosshairs are focused on the named region.
FA decreases occur across adulthood [16]. For this reason, we residualized the FA data for age prior to computing the correlations. This greatly reduced the likelihood that the observed effects were due to the influence of age.
RESULTS
As in our previous study, we found inverse correlations between age-corrected FA and motor impulsivity in right ventromedial prefrontal WM. In addition we also observed significant bilateral inverse correlations in anterior cingulate white matter, as well as in the caudate, insula, caudal inferior parietal lobule, middle temporal gyrus, and the inferior frontal gyrus. Lateralized inverse correlations also were observed in the left putamen, lingual gyrus and posterior cingulate, and right medial dorsal thalamus. Positive correlations were observed in the left postcentral gyrus, left supplementary motor area, left superior frontal gyrus, right superior temporal gyrus, right middle temporal gyrus (more posteriorly than the negative correlations), and in left fusiform gyrus. These patterns are shown in Fig. 1.
DISCUSSION
The current study examined, for the first time, correlations between FA and behavior on a voxelwise basis. We replicated our previous finding [7] of an inverse association between right inferior frontal WM FA and motor impulsiveness. However, we also found a number of additional areas whose FA were inversely associated with motor impulsiveness. These included the dorsal anterior cingulate, the insula, the putamen, the caudate, the right middle temporal gyrus, and the left inferior parietal lobule.
The present findings are consistent with the notion that multiple, possibly interconnected, structures are involved in the neurobiology of impulsive behavior. Our findings also are consistent with the suggestion [17] that dysfunction in the circuitry of emotional regulation may play a role in the genesis of impulsive/aggressive behavior. Thus, reduced white matter integrity in such a circuit may be associated with dysregulation of impulsivity and aggression. It is of relevance here that our prior work [7] showed a positive association between trace, a measure of overall diffusion, and aggression in men with schizophrenia. This increase may suggest that there is more space in which diffusion can occur. This could be the result of fiber shrinkage/loss, membrane damage, or dysmyelination in these white matter regions [18].
The results also are consistent with studies in healthy controls showing that performance on a go/no-go task (a task requiring response inhibition) is associated with functional magnetic resonance imaging (fMRI) activation in a number of regions, including the right orbitofrontal cortex [19]. In addition, go/no-go performance also has been associated with activation in a set of structures almost identical to those in which negative correlations between FA and impulsivity were found in the current study [20].
The findings of positive correlations between impulsivity and FA in left postcentral gyrus, left supplementary motor area, left superior frontal gyrus, right middle and superior temporal gyrus, and left fusiform gyrus bear some discussion. The regions in which these were found are disparate, and do not share clear connections with the regions that showed negative correlations. It may be that, whereas the regions in which negative correlations were found subserve response inhibition functions, these regions are involved in response preparation or execution mechanisms. Consistent with this notion are findings from an fMRI study using the go/no-go task in which activation in the fusiform gyrus was associated with response preparation [21]. Moreover, the left supplementary motor area, superior frontal gyrus, and postcentral gyrus are areas involved in response preparation and sensorimotor function. The preponderance of left-sided positive correlations may be consistent with the left hemisphere’s putative role in response planning and execution [22]. Thus, positive correlations may indicate greater white matter integrity in a system of brain areas that subserve response preparation or execution.
Type I errors can be controlled in part by setting a more rigorous entry magnitude and extent thresholds, thereby addressing one of the main critiques of voxelwise methods. A number of different approaches could have been applied to resolve this problem. For instance, SPM99 (Wellcome Department of Neurology) uses Gaussian random field theory to correct for test multiplicity. This approach is undesirable because it is necessary to used smoothed data, which results in loss of anatomical information. More conservative approaches, including permutation methods [23] and false discovery rate analyses [24] can provide a real correction for test multiplicity, albeit at greatly reduced statistical power.
CONCLUSION
The examination of voxelwise correlations between behavioral (or other) measures and DTI measures provides a rich way to examine the functional significance of variations in white matter integrity. This method is particularly useful in evaluating the specificity of findings obtained using ROI-based methods and in hypothesis generation. In combination with fMRI studies, electrophysiological approaches, and fiber tractography approaches [25], the voxelwise correlational methods may provide a novel approach for describing the neural networks involved in particular behavioral or other kinds of functions.
Acknowledgments
This research was supported by Biomedical Engineering Research Grant RG-00-0350 from the Whitaker Foundation to BAA, grant RO1 MH60662 (K.O.L.), grant RO1 MH66374 (P.D.B.), R37 MH49334 and K02 MH01439 (D.C.J.), a Burroughs Wellcome Translational Science Award (D.C.J.), and a NARSAD Young Investigator Award and grant RO1 MH064783 to M.J.H. This work was presented in part at the 59th Annual Meeting of the Society of Biological Psychiatry, NewYork, NY, May, 2004. We thank Lynda R. Lipatas and Michael L. Radosta for their assistance in data collection and processing.
References
- 1.Hoptman MJ. Neuroimaging studies of violence and antisocial behavior. J Psychiatric Pract. 2003;9:265–278. doi: 10.1097/00131746-200307000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Horsfield MA, Larsson HB, Jones DK, Gass A. Diffusion magnetic resonance imaging in multiple sclerosis. . J Neurol Neurosurg Psychiatry. 1998:S80–S84. [PubMed] [Google Scholar]
- 3.Ardekani BA, Nierenberg J, Hoptman MJ, Javitt DC, Lim KO. MRI study of white matter diffusion anisotropy in schizophrenia. Neuroreport. 2003;14:2025–2029. doi: 10.1097/00001756-200311140-00004. [DOI] [PubMed] [Google Scholar]
- 4.Lim KO, Hedehus M, Moseley M, de Crespigny A, Sullivan EV, Pfefferbaum A. Compromised white matter tract integrity in schizophrenia inferred from diffusion tensor imaging. Arch Gen Psychiatry. 1999;56:367–374. doi: 10.1001/archpsyc.56.4.367. [DOI] [PubMed] [Google Scholar]
- 5.Buchsbaum MS, Tang CY, Peled S, Gudbjartsson H, Lu D, Hazlett EA, et al. MRI white matter diffusion anisotropy and PET metabolic rate in schizophrenia. Neuroreport. 1998;9:425–430. doi: 10.1097/00001756-199802160-00013. [DOI] [PubMed] [Google Scholar]
- 6.Kubicki M, Westin C-F, Maier SE, Frumin M, Nestor PG, Salisbury DF, et al. Uncinate fasciculus findings in schizophrenia: a magnetic resonance diffusion tensor imaging study. Am J Psychiatry. 2002;159:813–820. doi: 10.1176/appi.ajp.159.5.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoptman MJ, Volavka J, Johnson G, Weiss E, Bilder RM, Lim KO. Frontal white matter, aggression and impulsivity in men with schizophrenia. Biol Psychiatry. 2002;52:9–14. doi: 10.1016/s0006-3223(02)01311-2. [DOI] [PubMed] [Google Scholar]
- 8.Grafman J, Schwab K, Warden D, Pridgen A, Brown HR, Salazar AM. Frontal lobe injuries, violence, and aggression: a report of the Vietnam Head Injury study. Neurology. 1996;46:1231–1238. doi: 10.1212/wnl.46.5.1231. [DOI] [PubMed] [Google Scholar]
- 9.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders, Research Version, Patient Edition (SCID-I/P) New York: Biometrics Research, New York State Psychiatric Institute; 1996. [Google Scholar]
- 10.Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt Impulsiveness Scale. J Clin Psychol. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 11.Monahan J, Steadman HJ, Appelbaum PS, Robbins PC, Mulvey EP, Silver E, et al. Developing a clinically useful actuarial tool for assessing violence risk. Br J Psychiatry. 2000;176:312–319. doi: 10.1192/bjp.176.4.312. [DOI] [PubMed] [Google Scholar]
- 12.Lim KO, Choi SJ, Pomara N, Wolkin A, Rotrosen JP. Reduced frontal white matter integrity in cocaine dependence: a controlled diffusion tensor imaging study. Biol Psychiatry. 2002;51:890–895. doi: 10.1016/s0006-3223(01)01355-5. [DOI] [PubMed] [Google Scholar]
- 13.Reese TG, Heid O, Weisskopf RM, Wedeen VJ. Reduction of eddy-current-induced distortion in diffusion MRI using a twice-refocused spin echo. Magn Reson Med. 2003;49:177–182. doi: 10.1002/mrm.10308. [DOI] [PubMed] [Google Scholar]
- 14.Ardekani BA, Braun M, Hutton BF, Kanno I, Iida H. A fully automatic multimodality image registration algorithm. J Comp Assist Tomogr. 1995;19:615–623. doi: 10.1097/00004728-199507000-00022. [DOI] [PubMed] [Google Scholar]
- 15.Baudewig J, Dechent P, Merboldt KD, Frahm J. Thresholding in correlational analyses of magnetic resonance functional neuroimaging. Magn Reson Imag. 2003;21:1121–1130. doi: 10.1016/j.mri.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 16.Pfefferbaum A, Sullivan EV, Hedehus M, Lim KO, Adalsteinsson E, Moseley M. Age-related decline in brain white matter anisotropy measured with spatially corrected echo-planar diffusion tensor imaging. Magn Reson Med. 2000;44:259–268. doi: 10.1002/1522-2594(200008)44:2<259::aid-mrm13>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 17.Davidson RJ, Putnam KM, Larson CL. Dysfunction in the neural circuitry of emotion regulation – a possible prelude to violence. Science. 2000;289:591–594. doi: 10.1126/science.289.5479.591. [DOI] [PubMed] [Google Scholar]
- 18.Beaulieu C. The basis of anisotropic water diffusion in the nervous system – a technical review. NMR Biomed. 2002;15:435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- 19.Garavan H, Ross TJ, Stein EA. Right hemispheric dominance of inhibitory control: an event-related functional MRI study. Proc Natl Acad Sci USA. 1999;96:8301–8306. doi: 10.1073/pnas.96.14.8301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garavan H, Ross TJ, Murphy K, Roche RA, Stein EA. Dissociable executive functions in the dynamic control of behavior: inhibition, error detection, and correction. Neuroimage. 2002;17:1820–1829. doi: 10.1006/nimg.2002.1326. [DOI] [PubMed] [Google Scholar]
- 21.Watanabe J, Sugiura M, Sato K, Maeda Y, Matsue Y, Fakuda H, et al. The human prefrontal and parietal association cortices are involved in no-go performances: an event-related fMRI study. Neuroimage. 2002;17:1207–1216. doi: 10.1006/nimg.2002.1198. [DOI] [PubMed] [Google Scholar]
- 22.Tucker DM, Williamson PA. Asymmetric neural control systems in human self-regulation. Psychol Rev. 1984;91:185–215. [PubMed] [Google Scholar]
- 23.Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp. 2001;15:1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Benjamini Y, Drai D, Elmer G, Kafkafi N, Golani I. Controlling the false discovery rate in behavior genetics research. Behav Brain Res. 2001;125:279–284. doi: 10.1016/s0166-4328(01)00297-2. [DOI] [PubMed] [Google Scholar]
- 25.Conturo TE, Lori NF, Cull TS, Akbudak E, Snyder AZ, Shimony JS, et al. Tracking neuronal fiber pathways in the human brain. Proc Natl Acad Sci USA. 1999;96:10422–10427. doi: 10.1073/pnas.96.18.10422. [DOI] [PMC free article] [PubMed] [Google Scholar]


