Abstract
Systemic lupus erythematosus (SLE) is a CD4+ T cell–dependent, immune complex–mediated, autoimmune disease that primarily affects women of childbearing age. Generation of high-titer affinity-matured IgG autoantibodies, specific for double-stranded DNA and other nuclear antigens, coincides with disease progression. Current forms of treatment of SLE including glucocorticosteroids are often inadequate and induce severe side effects. Immunological approaches for treating SLE in mice using anti-CD4 mAb’s or CTLA4-Ig and anti-CD154 mAb’s have proven to be effective. However, like steroid treatment, these regimens induce global immunosuppression, and their withdrawal allows for disease progression. In this report we show that lupus-prone NZB × NZW F1 mice given three injections of anti-CD137 (4-1BB) mAb’s between 26 and 35 weeks of age reversed acute disease, blocked chronic disease, and extended the mice’s lifespan from 10 months to more than 2 years. Autoantibody production in recipients was rapidly suppressed without inducing immunosuppression. Successful treatment could be traced to the fact that NZB × NZW F1 mice, regardless of their age or disease status, could not maintain pathogenic IgG autoantibody production in the absence of continuous CD4+ T cell help. Our data support the hypothesis that CD137-mediated signaling anergized CD4+ T cells during priming at the DC interface.
Introduction
NZB × NZW F1 female mice develop disseminated multiorgan systemic lupus erythematosus–like (SLE-like) disease that closely resembles its human counterpart (1). The mice develop elevated levels of IgM anti–single-stranded DNA (anti-ssDNA) at a very early age (2) and soon thereafter begin to produce IgM anti-dsDNA antibodies. Between 3 and 4 months of age, B cells undergo class switch of anti–double-stranded DNA (anti-dsDNA)antibodies from IgM to IgG in an almost spontaneous fashion. Soon thereafter the mice develop lupus nephritis and renal pathology (3). SLE disease is B cell– and CD4+ Th cell–dependent (4, 5) and can be treated by immune intervention such as immunosuppressive drugs, T cell costimulatory blockade (6–8), or anti-CD4 mAb–mediated therapy (9). However, the regimens employed are recognized as being prophylactic rather than therapeutic and are burdened with significant undesirable side effects. In this report we show that minimal treatment of SLE-diseased NZB × NZW F1 female mice with anti-CD137 mAb’s reversed disease progression and prolonged survival of the mice to more than 2 years.
CD137 (4-1BB), an inducible T cell costimulatory receptor and member of the TNF receptor superfamily, is expressed on activated CD4+ and CD8+ T cells (10), activated NK cells (11), and DCs (12, 13). CD137 is not expressed on mouse B cells, including those from NZB × NZW F1 mice, at any stage of development. Its ligand, 4-1BBL, is expressed on resting B cells and can be upregulated on activated professional APCs and B cells (14, 15). Monoclonal anti-CD137 antibodies and CD137 ligand fusion proteins enhance antigen-specific and polyclonal T cell proliferation of both CD4+ and CD8+ subsets in vitro (16–20). Anti-CD137 mAb’s enhance proliferation of CD4+ T cells in vivo in normal and experimental autoimmune encephalomyelitic mice (21). However, CD4+ T cell proliferation in experimental autoimmune encephalomyelitic mice appears to end after several rounds of division. Anti-CD137 mAb’s preferentially activate CD8+ T cells in vitro and in vivo and induce the production of type 1 cytokines by CD8+ cells. They accelerate and exacerbate acute graft-versus-host disease, as well as cardiac and skin allograft rejection (16), and they induce CD8+ CTL–mediated antitumor immunity (22). Central to many of these activities may be the ability of CD137-mediated costimulation to block antigen-activated cell death of CD8+ T cells (23).
While anti-CD137 mAb’s enhance CD4+ and CD8+ T cell proliferation, they have been found to suppress CD4+ T cell help during T-dependent humoral immune responses through a mechanism involving the action of regulatory T cells (24). As a consequence, mice injected with rat anti-CD137 mAb’s fail to generate anti-rat IgG antibodies (our unpublished observations). Therefore, these animals can be injected repeatedly with anti-CD137 mAb’s without concern for the induction of immune responses against the rat antibody. We sought to determine whether anti-CD137 mAb’s would protect NZB × NZW F1 mice from SLE disease rather than exacerbating autoimmune reactions. We hypothesized that anti-CD137 mAb’s would anergize CD4+ autoantigen-reactive T cells and thus prevent the initiation of disease, but we questioned whether such treatment would be beneficial in mice with established disease. In this report we demonstrate that treatment of NZB × NZW F1 mice with anti-CD137 mAb’s at any stage in their life or in disease progression led to profound suppression and reversal of the disease process.
Methods
Mice.
Six-week-old female NZB × NZW F1 (H-2d/z) mice, hereafter referred to as NZB/W F1, and BALB/c and BALB/c Rag–/– (H-2d) mice were purchased from The Jackson Laboratory (Bar Harbor, Maine, USA) and maintained in the Yerkes National Primate Research Center vivarium following Institutional Animal Care and Use Committee procedures. Each experimental group contained ten mice unless otherwise stated.
Statistical analysis.
Analysis was carried out using repeated-measures ANOVA. Measurements were made using the generalized estimating equations method.
Antibodies and reagents.
3H3, a rat IgG2a anti–mouse CD137 mAb, was produced in our laboratory (16). Monoclonal antibody 9D6, also produced in our laboratory, is a rat IgG2a anti–human CD137 mAb. The 9D6 mAb does not cross-react with mouse CD137 receptors and was used as an isotype-matched control mAb. The mice were injected intraperitoneally with 200 μg anti-CD137 mAb’s or isotype-matched control mAb as described in Results. GK1.5, a rat anti–mouse CD4 mAb, and 53.6.7, a rat anti–mouse CD8 mAb, were kindly provided by Jeff Ledbetter (Pacific Northwest Research Institute, Seattle, Washington, USA).
Depletion of CD4+ and CD8+ T cells in vivo.
We depleted CD4+ or CD8+ T cells in vivo by giving the mice four intraperitoneal injections of 500 μg of GK1.5 or 53.6 purified mAb’s, respectively, spaced 3 days apart. The final injection was given 3 days prior to use of the mice for experimental purposes. These procedures depleted more than 95% of CD4+ or CD8+ T cells, as measured by FACS analysis using noncompetitive anti-CD4 or -CD8 mAb’s.
Measurement of serum anti-dsDNA autoantibodies.
Anti-ssDNA or anti-dsDNA antibody production was determined by a modified ELISA assay (6). Fifty microliters of 10 μg/ml methylated BSA (Sigma Chemical Co., St. Louis, Missouri, USA) was added to the wells of Immulon 2 plates (Dynatech Laboratories, Alexandria, Virginia, USA) and incubated overnight at 4°C. The plates were washed with PBS containing 0.05% Tween-20. Fifty microliters dsDNA or ssDNA (Sigma Chemical Co.) was added overnight at 4°C. Purity of the preparations of ssDNA or dsDNA was assessed by acridine orange staining of DNA preparations separated by agarose electrophoresis (25). The plates were washed and then blocked with 2% BSA in PBS overnight at 4°C. After washing with PBS plus 0.05% Tween-20 serum, samples that had been serially diluted with PBS containing 1% BSA and 0.05% Tween-20 were incubated at 0.05 ml/well for 3 hours at room temperature. The wells were then extensively washed and incubated with 0.05 ml (1:7,500 dilution) peroxidase-conjugated goat anti-mouse IgM or goat anti-mouse IgG γ chain–specific antibody (Caltag Laboratories Inc., Burlingame, California, USA). After incubation overnight at 4°C, wells were washed five times and treated in the dark for 10 minutes with 0.05 ml o-phenylenediamine (Sigma Chemical Co.). The reaction was stopped with 0.05 ml of 2N H2SO4, and absorbance was measured at 495 nm. Serum from MRLFas–/lpr– and C57BL/6 mice served as positive and negative controls, respectively. The data are expressed as the mean OD at 495 nm of triplicates from serial dilutions or from serum samples diluted 1:100.
Measurement of proteinuria.
The extent of proteinuria was determined colorimetrically by the use of Albustix (Miles Inc., Elkhart, Indiana, USA). In this semiquantitative method, 1+ corresponds to a range of values from 30 to 100 mg/dl; 2+ corresponds to 100–300 mg/dl; 3+ corresponds to 300–2,000 mg/dl; and 4+ corresponds to ≥2,000 mg/dl. Significant proteinuria in the mice was defined as ≥100 mg/dl in accordance with previous studies (6).
Histology and immunohistology.
Kidneys and spleens were fixed in 10% formalin. Paraffin-embedded tissues were cut into 5- to 6-μm-thick sections and stained with H&E for histological examination. Tissue sections were prepared and independently analyzed by two veterinary pathologists. Glomerular immune complex deposition was examined by immunofluorescence. Kidneys were snap-frozen in OCT compound (Miles Inc.) without prior fixation. Cryostat sections 6 μm thick were cut and mounted on glass slides for staining. After paraformaldehyde fixation, cryosections were incubated with 10 μg/ml fluorescein-conjugated horse anti-mouse IgG (Vector Laboratories Inc., Burlingame, California, USA) and washed extensively. Parallel sections incubated with mouse IgG (Sigma Chemical Co.) showed no immunofluorescence.
Disease severity score.
Anti-dsDNA antibody levels were measured as OD at 495 nm and placed on the following scale: 1 = 0.25–0.50, 2 = 0.51–1.0, 3 = 1.25–1.50, 4 = 1.51–2.0, 5 = >2.1. Proteinuria was measured colorimetrically by the Albustix dipstick method on the following scale: 1 = undetectable, 2 = 10–30 mg/dl, 3 = >30–100 mg/dl, 4 = >100–300 mg/dl, 5 = >300 mg/dl. Immune complex deposition was recorded as the percentage of glomeruli stained per field as described below and by the brightness of fluorescence according to the following scale: 1 = very dim and not uniform; 2 = dim and uniform; 3 = bright, uniform, and observable in >25% of the glomeruli; 4 = very bright, uniform, and observable in >50% of glomeruli; 5 = very bright, uniform, and observable in >80% of glomeruli. Pathological kidney structure was evaluated in each group of mice by measurement of mesangial thickening, mononuclear cell infiltration, occlusion of tubules with protein, and glomerular destruction, and the percentage of diseased glomeruli was counted in four separate slides from each kidney observed under ×200 magnification. The scores for each observation were summed and the mean and SD recorded.
Generation of bone marrow–derived DCs.
Bone marrow cells were aspirated from femurs of NZB/W F1 mice and cultured for 6 days in RPMI 1640, 10% FBS, 1% penicillin/streptomycin, 1% L-glutamine, 10 ng/ml GM-CSF, and 4 ng/ml IL-4. Cultures were phenotyped for CD11c, CD11b, MHC class II, CD40, CD80, and CD86 expression.
CFSE staining of T cells.
Cells were incubated in PBS containing 10 μM CFSE at 1 × 107 per milliliter for 10 minutes at 37°C, washed twice with PBS, counted, checked for viability, and resuspended in PBS for injection. For adoptive transfer of T cells, 1 × 107 cells were injected by tail vein in 0.25 ml of PBS. DCs were loaded with Cell Tracker Orange (Molecular Probes, Eugene, Oregon, USA), and 1 × 106 cells were injected i.v. as described above.
Results
Anti-CD137 mAb’s suppress production of IgG but not IgM anti-dsDNA.
Anti-CD137 mAb’s suppress the development of T-dependent humoral immunity in normal mice when administered during the period of antigen priming and appear to be CD8+ T cell–independent (24). This result led us to believe that anti-CD137 mAb treatment directly suppressed CD4+ T cell help. We hypothesized that this would also be true if anti-CD137 mAb’s were used to treat antibody-dependent autoimmune diseases. However, we questioned whether treatment of autoimmune-prone mice with anti-CD137 mAb’s would be effective once the disease process had been established. We first determined whether anti-CD137 mAb’s would prevent NZB/W F1 mice from lupus-like disease by measuring the ability of these antibodies to suppress the generation of T-dependent and T-independent humoral immunity against dsDNA.
At 14 weeks of age, a time prior to the manifestation of overt disease, female NZB/W F1 mice were injected intraperitoneally with 200 μg of rat anti–mouse CD137 mAb’s or with an equivalent amount of isotype-matched control mAb. The procedure was repeated three times at intervals of 3 weeks, after which the mice received no further treatment. At 41 weeks of age the treated groups of mice were bled and euthanized, and their organs were removed for tissue studies. Serum samples were analyzed for the presence of IgM and IgG anti-dsDNA Ig’s.
Treatment with anti-CD137 mAb’s suppressed the spontaneous development of anti-dsDNA IgG, whereas anti-dsDNA autoantibody production was unchanged after treatment with isotype-matched control mAb’s (Figure 1a). In contrast, the production of T-independent IgM anti-dsDNA was not suppressed by anti-CD137 (Figure 1b), a result consistent with our previously published studies showing that anti-CD137 mAb’s suppressed only T-dependent humoral immune responses in normal mice (24). Suppression of humoral immunity was observed when anti-CD137 mAb’s were administered before or during antigen priming, but not when they were administered thereafter.
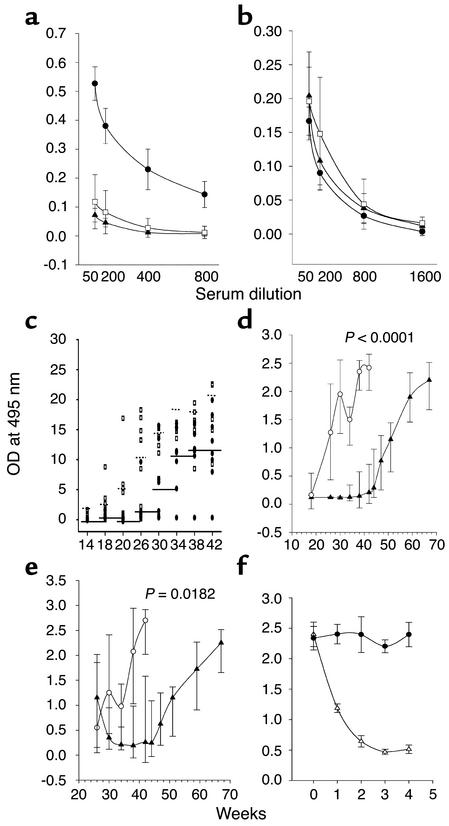
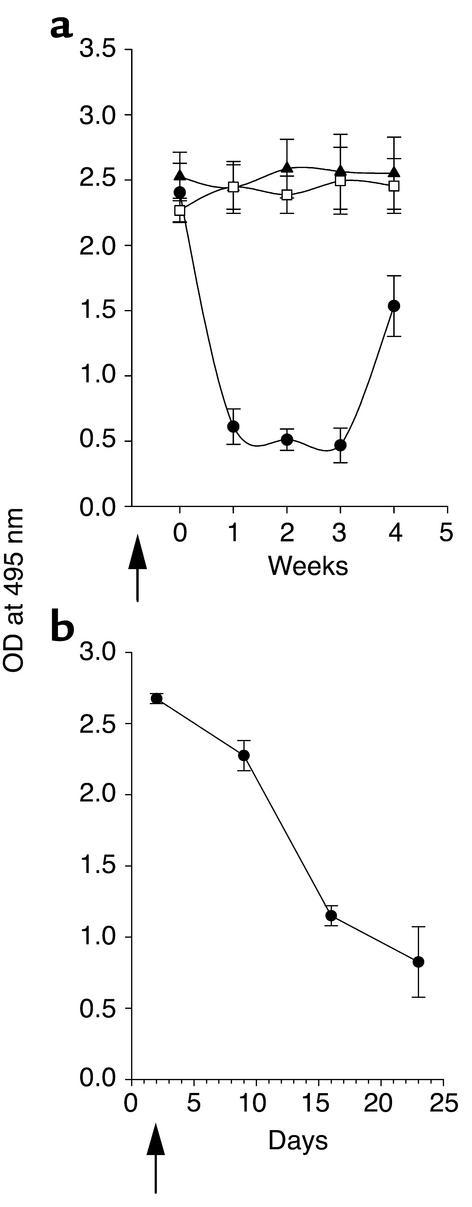
Figure 1.
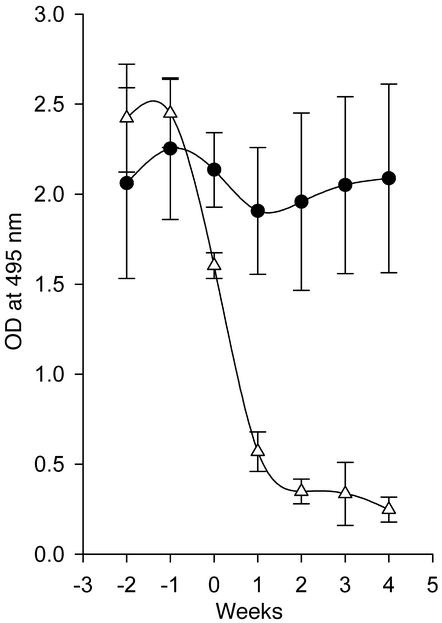
Inhibition of autoantibody production by anti-CD137 mAb’s. NZB/W F1 mice were injected intraperitoneally with anti-CD137 or control mAb’s, and serum samples were collected and assayed in triplicate by ELISA for anti-dsDNA antibodies. IgG or IgM anti-dsDNA autoantibody titers are expressed as the mean ± SD measuring the OD, at 495 nm, of triplicates for individual mice, using serially diluted serum samples. (a) Anti-dsDNA IgG serum titers of 41-week-old NZB/W F1 mice that were injected at weeks 14, 17, 20, 23, and 26 with anti-CD137 (open squares) or isotype-matched control mAb (filled circles), compared with serum from BALB/c mice (filled triangles). (b) Kinetics of IgM production in anti-CD137 mAb–treated mice (filled circles), mice treated with isotype-matched control mAb’s (open squares), and PBS-treated mice (filled triangles). (c) Kinetics of the appearance of IgG dsDNA-reactive autoantibodies in NZB/W F1 mice that received a single 200-μg intraperitoneal injection of anti-CD137 (filled squares) or control mAb (open squares) at 8 weeks of age. (d) Kinetics of IgG anti-dsDNA autoantibody production in mice in which anti-CD137 treatment (filled triangles) or isotype control mAb (open circles) 8-week-old were treated through 25 weeks of age (200 μg injected intraperitoneally every third week). (e) Kinetics of anti-dsDNA autoantibody production anti-CD137 treated (filled triangles) or control (open circles) mAb treated at 26 weeks of age after they had developed autoantibodies in their serum. (f) Clearance of serum anti-dsDNA IgG autoantibodies in proteinuric 40-week-old NZB/W F1 mice treated with a single injection of anti-CD137 (open triangles) or control mAb’s (filled circles).
We next initiated a series of experiments to determine whether there was a critical window during the development of disease when treatment would be efficacious, and what the minimal number of injections of anti-CD137 mAb’s would be in order to induce long-term suppression of anti-dsDNA antibody production in these mice. We began the study by injecting a single 200-μg dose of anti-CD137 mAb intraperitoneally into NZB/W F1 mice at 8 weeks of age. At this time point, the mice did not have detectable levels of anti-dsDNA IgG in their serum. From this experiment (representative of three conducted) we found that a single injection of anti-CD137 mAb significantly suppressed anti-dsDNA IgG production until the mice reached 30 weeks of age, despite the fact that the half-life of anti-CD137 mAb’s is 7 days. By week 30 the mice acquired the capacity to generate anti-dsDNA IgG autoantibodies. Nevertheless, they did not develop a mean anti-dsDNA antibody titer, as 26-week-old isotype-matched control mAb–treated mice did, until they reached 34 weeks of age; from this point their titers remained unchanged through week 42 of age (Figure 1c). In contrast, during this time frame the mean anti-dsDNA antibody titers more than doubled in the isotype-matched mAb control group (Figure 1c).
Seeing that a single treatment of anti-CD137 mAb’s significantly delayed the onset of disease, we increased the number of injections of anti-CD137 mAb from one to seven in the next set of experiments. Once again, the mice received the first injection at 8 weeks of age, and treatment was repeated once every third week until the mice reached 26 weeks of age. This treatment regimen suppressed the development of anti-dsDNA IgG antibodies until the mice reached 52 weeks of age. Anti-dsDNA titers in the mice did not approach those observed in 30-week-old control mAb–treated mice until they reached 70 weeks of age (Figure 1d), at which time only one of ten mice showed any sign of proteinuria. By 72 weeks of age, three of ten mice died, but only one mouse was proteinuric. The cause of death of the other two mice could not be determined, and neither had developed any signs of lupus disease.
Our earlier studies with anti-CD137 mAb’s in normal mice would predict that NZB/W F1 mice already producing T-dependent anti-dsDNA antibodies would be refractive to treatment. To determine whether this was the case, we injected 26-week-old mice three times with 200 μg of anti-CD137 or isotype-matched control mAb’s; the injections occurred once every third week until the mice reached 35 weeks of age. Contrary to our expectations, we found that anti-dsDNA antibody production in the anti-CD137 mAb–treated mice dropped rapidly (Figure 1e) and remained depressed until week 50. Furthermore, the majority of these mice did not begin to produce significant levels of anti-dsDNA until they reached 60–70 weeks of age. Here, too, only one of the ten mice presented with low-grade proteinuria by week 110 (data not shown). By this time, three mice had died, including the proteinuric mouse, a second mouse that died from a hepatic tumor, and a third that died from undetermined causes. At this point the experiment was terminated, and the remaining mice were euthanized. Thus, our studies showed that minimal treatment of SLE-diseased mice with anti-CD137 mAb’s reversed the disease process, protected 70% of the mice from relapse, and afforded the survivors a normal lifespan.
Recognizing that the time point of autoantigen priming is not of critical consequence for the suppressive action of anti-CD137 mAb in NZB/W F1 mice, we allowed the animals to develop moderate to severe disease, as determined by the development of high levels of anti-dsDNA antibodies and onset of proteinuria. Two groups of 36- to 41-week-old diseased mice were given a single 200-μg intraperitoneal injection of anti-CD137 or control mAb. Within 7–10 days after anti-CD137 mAb treatment, we observed a profound therapeutic effect manifested by the marked reduction of serum anti-dsDNA autoantibodies (Figure 1f). Within 3 weeks, the mice were nearly depleted of IgG anti-dsDNA autoantibody, and they remained so 6 weeks later when they were euthanized. The rapidity and extent of autoantibody clearance in these mice were unexpected, as the serum half-life of IgG in mice is approximately 3 weeks. Nevertheless, 80% of anti-dsDNA autoantibody was eliminated within 2 weeks after administration of anti-CD137 mAb’s. We found that the mice secreted substantial amounts of anti-dsDNA antibodies in their urine, but this could only partially account for the rapid and marked loss of serum autoantibody levels (data not shown). One may hypothesize that the reason why serum IgG anti-dsDNA autoantibody half-life is so short in the diseased mice is because of the formation and catabolism of immune complexes, as well as the loss of autoantibodies that have been bound to tissues throughout the body. Normally, this loss of serum autoantibodies goes unnoticed because of ongoing rapid de novo synthesis. However, when antibody production is suppressed, clearance of these antibodies in the serum becomes evident. This view is supported by the observation that anti-dsDNA–containing serum injected continuously i.v. into prediseased mice accumulates as immune complexes in their kidneys and is rapidly lost from the circulation in the absence of any treatment (data not shown).
Anti-CD137 mAb treatment prevents renal disease.
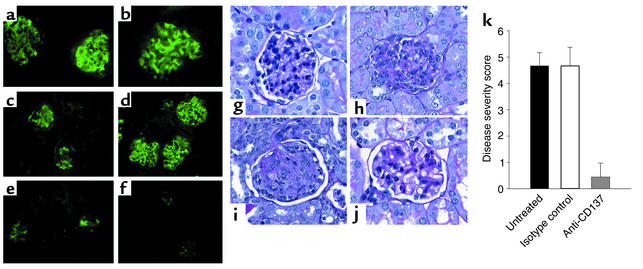
Approximately 95% of untreated NZB/W F1 mice develop glomerulonephritis and end-stage kidney disease and die within 12 months of age. Disease progression involves immune complex deposition in the glomeruli, inflammation within these sites, mesangial proliferation, and infiltration of mononuclear cells, including B and T cells. To determine whether anti-CD137 mAb’s prevented immune complex disease as well as interstitial glomerulonephritis, we analyzed frozen or formalin-fixed thin sections of kidneys obtained from anti-CD137 mAb–treated, untreated, and isotype-matched control mAb–treated mice. The frozen sections were stained with a FITC-conjugated goat anti-mouse IgG antiserum in order to detect immune complexes. We also examined fixed H&E-stained thin sections of kidneys from each group of mice to detect pathological changes in kidney architecture that typically accompany disease progression. Frozen kidney sections obtained from 35- and 45-week-old mice that received an isotype-matched control mAb were visually identical to sections from untreated animals with respect to immune complex deposition (Figure 2, a–d). However, mice that had been treated with anti-CD137 mAb’s had only minimal traces of immune complexes in their kidneys (Figure 2, e and f). We then compared the histology and architecture of normal glomeruli from age-matched BALB/c mice with those of tissue sections from kidneys of NZB/W F1 mice that had been treated with anti-CD137 mAb’s. In contrast to kidneys from 26-week-old BALB/c mice, those from age-matched NZB/W F1 mice were markedly abnormal (Figure 2h). In comparison, NZB/W F1 mice that received anti-CD137 mAb’s beginning at 14 weeks of age and then again every third week until they reached 23 weeks of age exhibited normal kidney architecture, albeit with some mesangial proliferation (Figure 2j), whereas the structural damage to kidneys in mice that were treated with isotype-matched control mAb’s was identical to that seen in the untreated mice (Figure 2i).
Figure 2.
(a–f) Anti-CD137 mAb’s block immune complex formation. Kidneys sections from NZB/W F1 mice at 35 (a, c, and e) and 45 (b, d, and f) weeks of age that had received no treatment (a and b), isotype-matched control mAb’s (c and d), or anti-CD137 mAb’s (e and f) were stained with FITC-goat anti-mouse IgG. (g–j) Anti-CD137 mAb’s block kidney disease. Shown are glomeruli from a BALB/c mouse (g), an untreated NZB/W F1 mouse (h), an isotype control-treated mouse (i), and an anti-CD137 mAb–treated mouse (j). (k) Disease severity following anti-CD137 mAb treatment. NZB/W F1 mice were untreated or treated with either isotype-matched control mAb’s or anti-CD137 mAb’s beginning at 14 weeks of age. Disease index was based upon kidney histopathology (see Methods).
We next assessed the overall level of disease progression in each group of mice enrolled in this experiment, based on levels of serum anti-dsDNA autoantibodies, proteinuria, immune complex deposition, and kidney architecture. In this study, mice in the untreated and isotype-matched control mAb–treated groups displayed evidence of advanced disease, whereas the anti-CD137 mAb treatment group showed minimal changes within the kidney that were confined to some mesangial proliferation (Figure 2k).
Anti-CD137 mAb’s blocked germinal center formation.
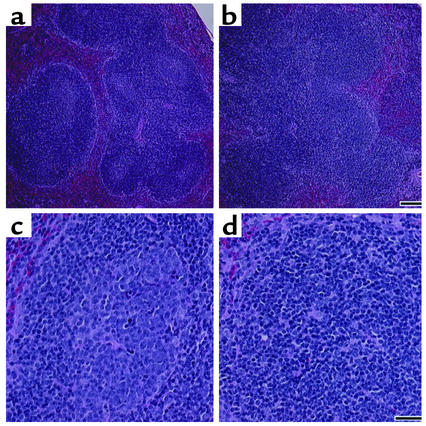
Germinal center reactions occur in secondary lymphoid tissues, where B cells receive maturation signals from Th cells, undergo isotype switching, and select high-affinity receptors for antigen (26). This process is central to the development of mature functional B cells. Cognate interactions between CD154 expressed on activated CD4+ T cells and its ligand, CD40 expressed on B cells, are essential for the development of germinal center reactions. Interruption of the germinal center reaction resulting from natural mutations in the CD154 receptor on human CD4+ T cells causes hyper-IgM syndrome, an immunodeficiency that arises because of the inability of IgM-bearing B cells to switch class and isotype and to develop into mature high-affinity IgG-secreting B cells (27). This phenomenon is also observed in CD154–/– mice and can be induced in normal mice by injecting them with anti-CD154 specific mAb’s (28–30). To determine whether T cells suppressed antibody production by mature B cells in anti-CD137 mAb–treated mice or blocked the development of B cell maturation at the stage of germinal center reactions, we treated mice, beginning at 14 weeks of age, with anti-CD137 mAb’s or isotype-matched control mAb’s once every 3 weeks until they reached 26 weeks of age. The mice were euthanized, and histological analysis of H&E-stained spleen sections was carried out. The outcome of our analysis revealed a complete absence of germinal center formation in the spleens of anti-CD137 mAb–treated mice, whereas mice treated with isotype-matched control mAb’s had multiple well-developed germinal centers (Figure 3, b and d).
Figure 3.
Anti-CD137 mAb treatment blocks germinal center reactions. NZB/W F1 mice were injected once every third week with anti-CD137 or isotype-matched control mAb’s beginning at 14 weeks of age and continuing through 26 weeks of age. The mice were euthanized, and spleens were removed for thin sectioning and H&E staining. Sections were viewed with ×20 and ×40 objectives and photographed. (a and c) Representative fields at ×20 and ×40, respectively, of mice injected with isotype-matched control mAb’s. (b and d) Representative fields at ×20 and ×40, respectively, of mice treated with anti-CD137 mAb’s. Bar, 100 μg.
Anti-CD137 mAb’s prevent development of proteinuria.
NZB/W F1 mice develop proteinuria as a consequence of glomerular pathologies that arise from the deposition of immune complexes and tissue specific antibodies in the kidneys, and the development of inflammation within these sites caused by the influx of mononuclear cells. These events lead to the destruction of glomerular structure and associated tubules. As a result of injury, selective filtration is compromised and protein is excreted in the urine.
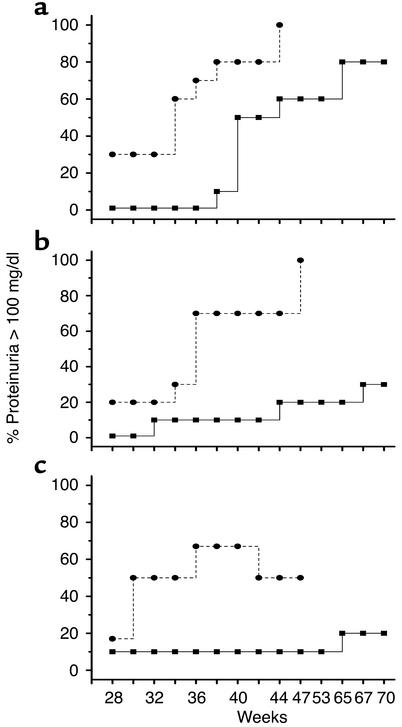
To complete our understanding of the effects of anti-CD137 mAb’s on the development or progression of disease in NZB/W F1 mice, we measured levels of proteinuria in anti-CD137 mAb–treated and control mAb–treated mice. We expected to find no disease in mice treated early in life and in mice just developing overt signs of disease that were treated with anti-CD137 mAb’s, since we knew that anti-dsDNA antibody production was suppressed. However, we did not know what to expect from mice that had substantial disease prior to treatment, or from mice that had last received treatment 12–18 months earlier. We measured urine protein levels of the mice whose serum was analyzed for the presence of anti-dsDNA antibodies as described above (Figure 1).
Mice that received a single injection of anti-CD137 exhibited a significant delay in the development of proteinuria as compared with the control mice. For example, 100% of the control mice became proteinuric by 44 weeks of age and died shortly thereafter. Only 60% of the mice that had received a single injection of anti-CD137 mAb’s at 8 weeks of age were proteinuric at 44 weeks, and this number remained fixed until they reached 65 weeks of age (Figure 4a). A time course of repeated anti-CD137 mAb injections beginning at week 8 and continuing every third week through 26 weeks of age blocked the development of proteinuria (Figure 4) until the mice reached 79 weeks of age, at which point three of the ten mice died. Of the three mice that had died, one had more than 300 mg/dl of urinary protein, whereas proteinuria in the two others was undetectable. A third group of mice received anti-CD137 mAb starting at 26 weeks of age and continuing to week 35, after production of anti-dsDNA autoantibodies had commenced but before severe proteinuria was established. One mouse died during injection for unknown reasons, and a second developed proteinuria at 65 weeks of age (Figure 4c). By 90 weeks of age, two of the remaining nine mice died. One of the mice that died was the proteinuric mouse, but the second mouse never developed proteinuria and its cause of death is unknown.
Figure 4.
Anti-CD137 mAb treatment protects mice from developing proteinuria. Urine was collected from mice in each of the three groups enrolled in the serum anti-dsDNA autoantibody studies described in Figure 1 and tested for protein content by the Albustix method as described in Methods. (a–c) Ovals represent untreated mice and squares represent mice treated with anti-CD137 mAb’s. (a) One injection of anti-CD137 mAb was given at 8 weeks of age. (b) Mice received seven injections of anti-CD137 mAb’s beginning at 8 weeks of age and continuing once every third week. (c) Mice received their first of three anti-CD137 mAb injections at 25 weeks of age and one thereafter every third week for a total of three injections. All injections were administered intraperitoneally at a dose of 200 μg.
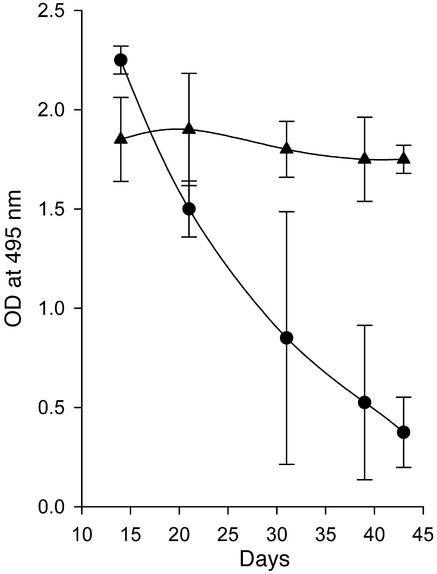
Deletion of CD4+ T cells blocks disease.
Wofsy and colleagues have shown that depletion of CD4+ T cells from NZB/W F1 mice (6–8) or injection of nondepleting anti-CD4 mAb’s into these mice blocked initiation of disease (9), demonstrating the critical role that CD4+ T cells play in the establishment of disease. We have shown that regulation of CD4+ Th cell function is a central event in anti-CD137–mediated suppression of T-dependent humoral immunity (24). Our current studies suggest that CD4+ Th cell activity is required for the maintenance of autoimmune responses throughout the lifespan of NZB/W F1 mice. To test this hypothesis, CD4+ T cells were deleted using a depleting anti-CD4 mAb after the mice had fully developed disease. A second group of mice was treated to deplete CD8+ T cells. Our data show that elimination of CD4+ T cells mimicked the effect of anti-CD137 mAb in that the mice demonstrated dramatically reduced serum titers of anti-dsDNA antibodies, albeit for a shorter period than that induced by anti-CD137 mAb–mediated suppression (Figure 5a). The differences in duration of the effects of anti-CD4 versus anti-CD137 mAb treatment may stem from the repopulation of CD4+ T cells by thymic emigrants in the CD4+ T cell–depleted group. In contrast, anti-CD137 mAb treatment is thought to induce the generation of a stable population of regulatory T cells (24). In comparison with anti-CD4 or anti-CD137 mAb treatment, elimination of CD8+ T cells produced no visible effect on autoantibody production. To demonstrate that anti-CD137 mAb’s directly affected CD4+ Th cell function in NZB/W F1 mice as we had reported for normal mice, the experiment was repeated, and CD8+ T cell–depleted mice were injected with anti-CD137 mAb’s. As with untreated NZB/W F1 mice, anti-CD137 mAb treatment of CD8+ T cell–depleted mice led to suppression of anti-dsDNA production (Figure 5b). The presented data are consistent with our earlier studies demonstrating that anti-CD137 mAb’s blocked T-dependent humoral immune responses in β2-microglobulin–deficient mice (24).
Figure 5.
(a) CD4+ T cell depletion decreases serum anti-dsDNA autoantibodies. Thirty-six- to 40-week-old NZB/W F1 mice with high titers of anti-dsDNA antibodies were depleted of CD4+ T cells or CD8+ T cells by four intraperitoneal injections of anti-CD4 mAb (filled circles) or anti-CD8 mAb (open squares) every 3 days, or left untreated (filled triangles). After the last injection of antibody (arrow, x axis), serum samples were collected as indicated and assayed in triplicate for anti-dsDNA reactive antibodies. IgG anti-dsDNA autoantibody titers as a function of time and treatment are expressed as the mean ± SD measured at an OD at 495 nm of serum from individual mice, using a 1:100 diluted serum sample. (b) Anti-CD137 mAb’s suppress anti-dsDNA production in the absence of CD8+ T cells. CD8+ T cells were depleted from NZB/W F1 mice prior to treatment with anti-CD137 mAb’s to determine whether CD8+ regulatory T cells were generated in response to CD137-mediated T cell costimulation. Mice depleted of CD8+ T cells as described above were injected with 200 μg anti-CD137 mAb and assayed for anti-dsDNA autoantibodies in their serum over a 23-day period.
Anti-CD137 does not induce T cell or B cell deletion.
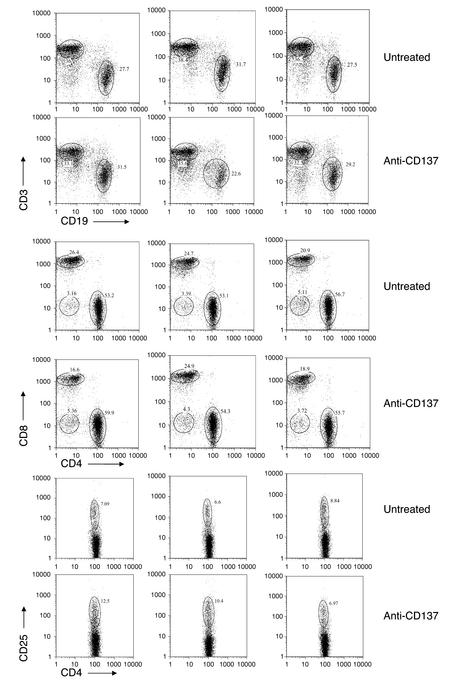
To determine whether anti-CD137 mAb’s affected the frequency or absolute numbers of spleen-derived or lymph node–derived T cells or B cells, we injected six 6-month-old female NZB/W F1 mice that displayed prominent proteinuria with 200 μg of anti-CD137 mAb’s intraperitoneally. An equal number of age- and disease-matched mice were left untreated. FACS analysis of T and B cells was carried out on each mouse 3 and 21 days after injection (day 21 data not shown). In all cases, no differences were observed with regard to light-scatter profiles, percent of lymphocytes within the gated areas, or frequencies or absolute numbers of T cells, T cell subsets, and B cells found within the spleen or lymph nodes. The only notable difference between the two groups was an increase in the number of CD4+CD25+ T cells, possibly regulatory T cells, in approximately two-thirds of the anti-CD137 mAb–treated group. We are currently conducting further experiments to explore the statistical significance of this observation. Data obtained from the spleens of three randomly chosen mice from each group are shown (Figure 6).
Figure 6.
Anti-CD137 mAb’s do not delete or alter the frequency of T or B cells. Six-month-old NZB/W F1 mice with prominent signs of proteinuria were injected intraperitoneally with 200 μg anti-CD137 mAb’s or left untreated. Three days or 21 days later (only day 3 data are shown, as there were no differences between the two data sets), the mice were euthanized, and spleen-derived lymphocytes were phenotyped using a dual-laser multiparameter FACSCalibur analytical flow cytometer (Becton Dickinson Immunocytometry Systems, San Jose, California, USA) for single-cell expression of the following cell surface receptors: CD3, CD4, CD8, CD25, and CD19.
Anti-CD137 mAb treatment fails to suppress established humoral immunity.
Anti-CD137 mAb’s fully suppressed established T-dependent autoantibody responses to dsDNA, as shown above. This was unexpected based on our earlier studies in normal mice, in which we found that T-dependent humoral immune responses to foreign antigens, once established, were no longer susceptible to anti-CD137 mAb–induced suppression (24). We therefore asked whether NZB/W F1 mice were unique in that T-dependent humoral immunity could be suppressed by anti-CD137 mAb treatment regardless of whether it was administered during antigen priming or at some distant time point. To test this possibility, 26-week-old NZB/W F1 mice that had developed disease were immunized and boosted to sheep red blood cell (SRBC) or human IgG (huIgG) and subsequently given a single injection of anti-CD137 mAb’s. Antibody production to SRBC and dsDNA was then measured at various time points following anti-CD137 mAb treatment (Figure 7).
Figure 7.
Anti-CD137 mAb’s do not suppress established anti-SRBC humoral immune responses. Five 26-week-old NZB/W F1 mice were immunized with SRBC and boosted at 28 weeks of age. At 30 weeks of age, the mice were injected with 200 μg anti-CD137 mAb’s intraperitoneally. Serum from the mice was collected at various time points after treatment and assayed by ELISA for anti-SRBC (triangles) or anti-dsDNA (circles) antibodies.
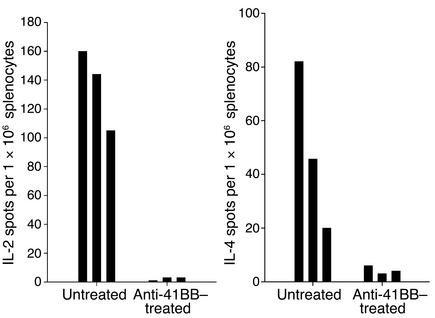
Inhibition of IL-2 and IL-4 production by CD4+ T cells.
Cytokines regulate the development of Th1-driven proinflammatory versus Th2-driven humoral immune responses initiated by CD4+ T cells. We had previously shown that in vitro costimulation of anti-CD3–activated CD8+ T cells with anti-CD137 mAb’s markedly enhanced production of Th1 cytokines (24). Others have shown that cross-linking CD137 on CD4+ T cells using 4-1BB ligand induced these T cells to produce IL-2 and IL-4 in vitro, two cytokines that are essential for the support of T cell proliferation and antibody production, respectively (20). We questioned whether anti-CD137 mAb’s suppressed the production of these two cytokines by CD4+ T cells in NZB/W F1 mice, as suppression of these cytokines could have a profound inhibitory effect on the development of humoral autoimmune responses in these mice. CD8+ T cells were virtually all at rest and therefore did not express CD137 on their surface, nor did they produce any measurable amount of cytokines either before or after anti-CD137 mAb treatment (data not shown). In contrast, a substantial fraction of CD4+ T cells were CD69+CD25+ and constitutively produced large quantities of IL-2 and IL-4. We observed that production of these cytokines was elevated in these mice by 8 weeks of age and that the elevation persisted during the first 9 months of age. Upon treatment of the mice with anti-CD137 mAb’s, CD4+ T cells, although positive for activation markers such as CD25 or CD69, ceased all production of IL-4 and IL-2 (Figure 8).
Figure 8.
Anti-CD137 mAb treatment suppresses IL-2 and IL-4 production. Twenty-week-old NZB/W F1 mice were injected intraperitoneally with 200 μg anti-CD137 or isotype-matched control mAb. The mice were euthanized 5 days later, and IL-4 and IL-2 production was measured over a 36-hour period by ELISpot assays using spleen- and lymph node–derived CD4+ T cells. Shown are the responses of individual mice treated or not treated with anti-CD137 mAb’s. The results are highly reproducible and typical of multiple experiments performed.
NZB/W F1 CD4+ T and B cells have shortened lifespans.
That anti-CD137 mAb’s could not suppress existing T-dependent humoral immune responses to SRBC or huIgG in NZB/W F1 mice suggested that immune regulation of normal antibody responses to foreign cells or proteins in these animals was likely to be no different from that seen in normal mice. Why, then, is it possible to suppress T-dependent humoral immunity against dsDNA in NZB/W F1 mice? One possible explanation is that autoimmune T and/or B cells have a rapid turnover rate or short lifespan. This could be the result of antigen-induced cell exhaustion, a phenomenon observed in mice suffering from chronic virus infections such as lymphocytic choriomeningitis virus (LCMV) (31). New CD4+ T cells emigrating from the thymus, having cross-reactivity with dsDNA, could provide cognate help to maturing B cells in germinal centers. These cells, unlike memory cells, would be susceptible to suppression mediated by anti-CD137 mAb’s.
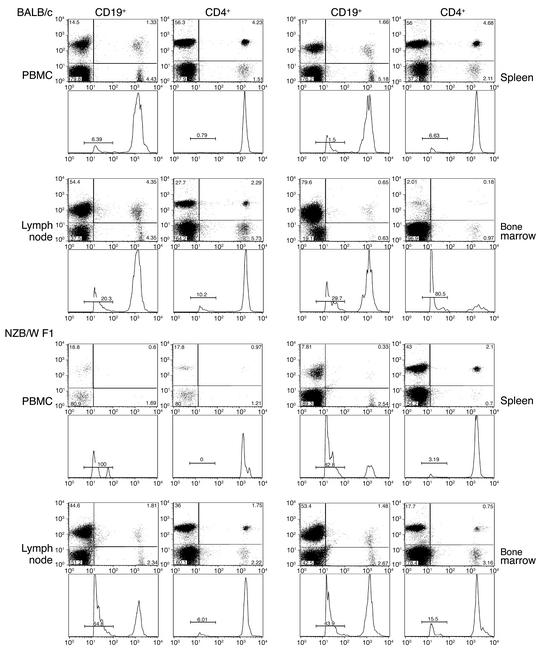
To study the survival and distribution of CD4+ T cells and B cells in NZB/W F1 mice, we performed adoptive cell transfer studies employing CFSE-labeled donor T or B cells from 26-week-old mice. Labeled cells were injected i.v. into age-matched recipients, retrieved from the blood, lymph nodes, spleen, and bone marrow 72 hours later, and analyzed by flow cytometry to determine the percentage of labeled cells in each organ and to measure the extent of their proliferation. We recovered similar numbers of cells from the spleen and lymph nodes of NZB/W F1 as compared with BALB/c mice. However, the combined total percentage of CD19+ CFSE-labeled B cells retrieved from the spleen, lymph nodes, bone marrow, and PBMCs of NZB/W F1 mice was 42% (4.22 × 106 B cells). In contrast, 68% (6.8 × 106 B cells) were recovered from BALB/c mice. Similar analysis of CD4+ T cells recovered from each strain 72 hours after adoptive cell transfer revealed 55.7% (5.7 × 106) CD4+ T cells in NZB/W F1 mice, versus 75% (7.54 × 106) CD4+ T cells in BALB/c mice (Figure 9). While we cannot rule out the possibility that some B and T cells have homed to other sites in the body, the kidneys in particular (see below), our analysis suggests that, like human SLE patients (31), NZB/W F1 mice are B-lymphocytopenic. With respect to cell redistribution within the organs studied, we found that within 72 hours after adoptive cell transfer there was a 77% reduction of CD4+ T cells in the PBMC fraction, a 9% decline in the spleen, a 64% decrease in the lymph nodes, and a fourfold increase in the bone marrow of NZB/W F1 mice, compared with BALB/c mice (Figure 9). Minimal T cell or B cell proliferation was observed. Peaks to the far left of the histograms represent unlabeled, weakly labeled, and probably dead or dying lymphocytes, as they were present even at 24 hours after cell transfer. Furthermore, the peak fluorescence of nondividing T or B cells was at channel 2,000 when analyzed by FACS. The peak fluorescence channel of the population at the far left of each histogram was 20. Thus, viable cells in this population would have had to undergo approximately eight divisions in 72 hours, or one every 9 hours.
Figure 9.
Survival and homing of CD4+ T cells and B cells. T and B cells were obtained from 26-week-old NZB/W F1 or BALB/c mice, labeled with CFSE, and injected i.v. into age-, sex-, and strain-matched recipients. At 72 hours, recipient mice were bled and euthanized. Single-cell suspensions of spleen, lymph node, bone marrow, and PBMCs were stained with phycoerythrin-conjugated anti-CD19 or -CD4 mAb’s, and their frequency was determined by flow cytometry. CFSE fluorescence intensity was measured, and proliferation of each cell population was determined based on the incremental loss of CFSE fluorescence. The far-left peaks in each histogram represent either dead or dying cells, which were present within 24 hours of culture; the extreme loss of fluorescence could not be attributed to cell division in so short a period of time.
To study B cell homing, we compared this distribution of CFSE-labeled B cells in several organs of age and sex-matched NZB/W F1 and BALB/c mice. Within 72 hours of injecting CFSE-labeled B cells into the mice we found that we could only recover from the peripheral blood 2% of the number of CFSE labeled CD19+ B cells compared to what was recovered from BALB/c mice. In a similar situation, we recovered only 15% and 7% of the number of B cells from the spleen and lymph nodes, respectively, of NZB/W F1 mice compared with the numbers recovered from BALB/c mice. However, there was a twofold increase in the percentage of B cells found in the bone marrow of NZB/W F1 mice compared with that found in BALB/c mice (Figure 9). Analysis of frozen kidney sections revealed infiltration of CFSE-labeled CD4+ T cells and B cells in the NZB/W F1 mice, but not in BALB/c mice. It has been previously shown that the kidneys of NZB/W F1 mice are a source of extramedullary hematopoiesis and a site of plasma cell development (32). Antibody-forming B cells and plasma cells that develop within or infiltrate the kidneys are not restricted to the autoreactive subset and have been found to be just as likely to react with foreign proteins as with autoantigens (32). Approximation of the number of CFSE-positive B cells in the kidneys based on kidney volume and fluorescence microscopy could not account for the substantial loss of B cells in the NZB/W F1 mice (data not shown).
Antigen-primed CD4+ T cells override anti-CD137–induced suppression.
We have previously shown that a subpopulation of DCs in the spleen express CD137 on their surface (12). Because DCs are instrumental in priming T cells with antigen, it occurred to us that anti-CD137 mAb–mediated suppression of autoimmune responses may be initiated during the critical period of T cell priming, and that signals delivered through CD137 to DCs, T cells, or both could account for this phenomenon. To address this question, we tested whether we could restore autoimmune reactions in asymptomatic anti-CD137 mAb treated mice. Here and in the next section we describe two sets of experiments that tested this possibility. First we determined whether primed CD4+ T cells could override anti-CD137 immunosuppression in 26-week-old mice that had been treated earlier with anti-CD137 mAb’s. We adoptively transferred CD4+ T cells from untreated 26-week-old mice into recipients that had received 200 μg of anti-CD137 mAb 2 weeks earlier. We then measured anti-dsDNA antibody levels in the serum of the recipient mice once a week for 4 weeks. The recipient mice rapidly resumed autoantibody production, whereas the control mice failed to produce anti-dsDNA autoantibodies (Figure 10). These data indicate that autoantigen-experienced CD4+ T cells were not susceptible to anti-CD137 mAb induced immune suppression or anergy in the treated mice and were able to rapidly initiate disease in these mice.
Figure 10.
Adoptive transfer of primed CD4+ T cells bypasses CD137-induced suppression. CD4+ T cells from 26-week-old NZB/W F1 untreated diseased mice were used as a source of dsDNA-primed Th cells. Age-matched mice (n = 10) that had been injected 2 weeks earlier with anti-CD137 were injected i.v. with 1 × 107 T cells (filled circles). A second group of mice received only anti-CD137 mAb’s (open triangles). The mice were subsequently bled on a weekly basis, and serum anti-dsDNA levels were determined by ELISA.
Adoptive transfer of DCs overrides anti-CD137–mediated protection.
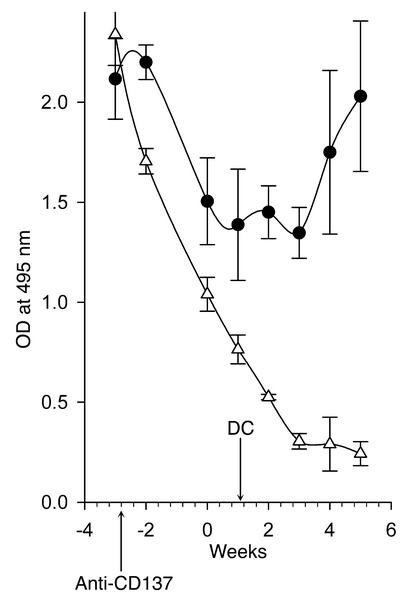
One could account for the ability of anti-CD137 mAb’s to suppress anti-dsDNA autoantibody responses in NZB/W F1 mice if they act directly on the DC during T cell priming. DCs treated with anti-CD137 mAb’s receive signals that prevent them from priming T cells. At the same time, they convey local signals that suppress this function in CD137– DCs. To approach this question we determined whether bone marrow–derived DCs could override anti-CD137 mAb–induced immunosuppression of humoral immunity in NZB/W F1 mice. Bone marrow–derived DCs generated in vitro (see Methods) were phenotyped for CD137 expression and then adoptively transferred into 26-week-old recipient mice that had received a single 200-μg injection of anti-CD137 mAb’s 4 weeks earlier. Anti-CD137 mAb–treated mice not reconstituted with DCs did not develop autoimmune disease, and detectable levels of serum anti-dsDNA autoantibodies all but disappeared. However, mice that received adoptively transferred DCs showed a decrease in anti-dsDNA production initially but resumed production of anti-dsDNA antibodies soon thereafter, indicating that CD4+ T cells in the anti-CD137 mAb–treated mice were not deleted and that, if they were anergized, anergy could be broken (Figure 11).
Figure 11.
Adoptive transfer of naive bone marrow–derived DCs bypasses CD137-induced suppression. One million NZB/W F1 bone marrow–derived CD11c+ DCs (15–30% CD137+) were injected i.v. into 26-week-old NZB/W F1 mice (n = 10) that had been injected with anti-CD137 mAb’s 4 weeks earlier. The mice were bled weekly, and anti-dsDNA titers were determined as above.
Discussion
SLE is a progressive multiorgan T cell– and B cell–dependent autoimmune disease for which there is no effective cure, and for which current therapy relies largely on long-term suppression of the immune system. Our work shows that it is possible to suppress the initiation or reverse the progression of established SLE-like disease in lupus-prone female NZB/W F1 mice in a manner that does not entail global immunosuppression, consists of a short-term treatment regimen, and extends their lifespan to that of normal mice.
Antibodies reactive with the CD137 T cell costimulatory receptor suppress the initiation of T-dependent humoral immune responses in normal mice (24). In this study we show that similar treatment of NZB/W F1 SLE-prone mice suppresses autoimmune reactions and reverses SLE disease, thus extending the mice’s lifespan from approximately 10 months to more than 2 years. Our observations raised two important questions: (a) Why is it that established autoreactive immune responses are susceptible to anti-CD137–mediated suppression, whereas immune responses to non-self antigens are not? (b) How does such treatment suppress autoantibody production and reverse the progression of disease in NZB/W F1 mice?
Autoimmune reactions may represent a state of chronic immune activation by self-antigens that results from their continuous presence. As a counterpoint, immune responses to foreign antigens are generally acute in nature and short in duration. We found that lymphocytes, particularly B cells from NZB/W F1 as compared with BALB/c mice, undergo rapid turnover, and, to a somewhat lesser extent, so do CD4+ T cells. We found no other fundamental physiological differences between autoimmune reactions and those specific for foreign antigens in NZB/W F1 mice. The suppression of autoimmunity by anti-CD137 mAb’s in NZB/W F1 mice may be attributed to the suppression of B cell maturation in germinal centers due to the lack of CD4+ T cell help. This view is supported by the fact that during and shortly after anti-CD137 mAb treatment, there is a virtually complete absence of germinal center formation within the spleen. It is predicted that, during this period, the mice are globally immunocompromised and will fail to respond to infectious agents that they encounter. This view has been recently confirmed in an LCMV infection model (J. Foell et al., manuscript submitted for publication). Surprisingly, in the LCMV model, immunosuppression is limited in duration to the first 48 hours of antigen priming. Therefore, it seems likely that anti-CD137 mAb’s suppress T cell–dependent humoral immunity in the same manner whether it is to a foreign antigen or a self-antigen, as it interferes with T cell immune responses at the level of cognate cellular interactions. A possible target of suppression appears to be the T cell–DC interface during antigen priming. The consequence of this event may be the induction of Vβ-restricted regulatory T cells and, at the very least, the failure to generate antigen-specific T cell help, thus accounting for the loss of germinal center reactions. Since anti-CD137 mAb’s that do not block receptor-ligand interactions can exert the same effect, and anti-CD137 mAb’s function in an identical fashion when injected into 4-1BB ligand–deficient mice, it is plausible that these mAb’s may interfere with or modify signaling pathways at a point in time when the immune response is first being initiated.
We have previously shown that while anti-CD137 mAb’s induce rapid allograft rejection (16) and highly effective CTL responses against poorly immunogenic tumors (22), they also suppress CD4+ T-dependent humoral immune responses in a CD8+ T cell–independent manner (24), despite the fact that they costimulate CD4+ T cells (20). How, then, does one reconcile these seemingly disparate observations? We suggest that anti-CD137 regulates CD4+ and CD8+ T cell responses alike, and that it does so at the T cell level by providing T cell costimulation and protection from activation-induced cell death (20, 23). However, immune responses by either T cell subset can be blocked as well. Timing is the critical issue. We have recently shown that DCs can express CD137 on their surface (12). We have further noted that splenic DCs constitutively expressed CD137, whereas T cells did not express CD137 in vivo until after 48 hours following LCMV infection (J. Foell et al., unpublished observations). The reason why anti-CD137 mAb’s suppress or enhance immune reactivity seems to have little to do with the T cell lineage or the source of antigenic stimulation. The determining factor is whether or not T cells have received priming. In the case of acute graft-versus-host disease responses, allograft rejection, or antitumor immunity, non-self antigens are already presented in the context of MHC prior to the administration of anti-CD137 mAb’s. Activated T cells express CD137 and can then be costimulated by CD137 signaling after engagement with 4-1BB ligand or anti-CD137 mAb’s.
T-dependent humoral immune responses to SRBC, human IgG, or LCMV can be suppressed by administration of anti-CD137 mAb’s during antigen priming by blocking Th cell activation at the DC interface. This could be accomplished in various ways such as blocking DC maturation, antigen processing, antigen presentation, cytokine production, etc. The end result is that the T cell fails to undergo activation and may become anergized in the process. The same is true for CD8+ T effectors in anti-LCMV immune responses. However, when given after priming, anti-CD137 mAb’s uniformly enhance the immune response or fail to affect it at all. Thus, autoimmune responses that require T cell help in order to drive maturation of antigen-specific B cells are interrupted. However, once priming has been successfully completed and T cells begin to activate and express CD137, administration of anti-CD137 mAb’s leads to amplification of proliferative responses and development of effector functions and provides survival signals that protect the cells from activation-induced cell death (23).
If our hypotheses of how anti-CD137 mAb’s regulate immune responses are correct and our interpretations of the data presented in this report are accurate, how can we account for the fact that autoimmune reactions established prior to injection of anti-CD137 mAb’s can be effectively terminated following injection of anti-CD137 mAb’s? From previous studies and our unpublished observations, we have learned that B cells do not express CD137, and that T cells and NK cells do not seem to kill antigen-specific B cells in NZB/W F1 mice (24). In this report, we show that significantly more CD4+ T cells and CD19+ B cells have a short lifespan in NZB/W F1 mice than in age- and sex-matched BALB/c mice (Figure 8). We suggest that one reason for this may be exhaustion of autoreactive T and B cells as a result of chronic stimulation. In contrast, immune lymphocytes reactive with foreign cells or proteins become acutely activated, clear the antigen, and undergo cell death, leaving behind a contracted population of resting memory cells. Anti-CD137 mAb treatment is immunosuppressive for autoimmune reactions in NZB/W F1 mice regardless of when in the disease process they are given to the mice. In this respect, their action differs from that observed in normal immune responses to foreign proteins whether they are measured in normal or NZB/W F1 mice. In autoimmune mice, cell exhaustion drives the homeostatic process to reach stabilization through the entry of naive T cells from the thymus, and B cells from bone marrow. These naive T cells require antigen-priming signals by DCs, and the B cells require maturation signals, a T cell–dependent process that occurs during germinal center reactions. Therefore, the situation is analogous to a primary immune response to antigen, and, as in the case of normal mice, these responses are susceptible to anti-CD137 mAb–induced suppression and/or anergy.
In a recent study, Sun et al. demonstrated that anti-CD137 mAb’s suppressed the development of SLE-like disease in MRLlpr–/– mice suffering from lymphoproliferative disease (33). The study of these mice has contributed greatly to our understanding of the process of CD95-mediated apoptosis and the role of apoptosis in eliminating autoreactive T cells that escape deletion in the thymus. However, these mice bear clinical manifestations that are quite distinct from those in NZB/W F1 female mice and humans afflicted with SLE. Rather than suffering from a lymphoproliferative disorder, NZB/W F1 mice and humans are lymphopenic, as is the case for most if not all autoimmune diseases. Secondly, NZB/W F1 mice and humans do not have any defects in maintaining peripheral tolerance, nor do they have a defect in CD95 function. Finally, we find, contrary to the observations of Sun et al., that anti-CD137 mAb’s induce transient splenomegaly and lymphadenopathy when injected into NZB/W F1 mice. NZB/W F1 mice have smaller spleens and lymph nodes than normal mice. In contrast, MRLlpr–/– mice naturally suffer from splenomegaly and lymphadenopathy, and upon treatment with anti-CD137 mAb’s these disorders disappear according to Sun et al. (33). It is also worth noting that in our studies of LCMV-infected mice (unpublished observations), injection of anti-CD137 mAb’s 1 day after infection leads to long-term tolerance and viremia without deleting LCMV T cell receptor transgenic P14 T cells. These results are in keeping with our earlier studies (24) and our observations described herein and support the notion that anti-CD137 signaling during antigen priming induces the generation of regulatory T cells that can suppress DC antigen processing or presentation to T cells.
In summary, we have found that minimal treatment of SLE-diseased NZB/W F1 mice with anti-CD137 mAb’s profoundly suppressed or reversed the disease process, and that as a consequence their lifespan could be routinely extended to greater than 2 years, essentially that observed in normal mice. We have further shown that existing immune responses to non-self antigens remained intact. These observations, while preliminary, provide a sense of optimism that more detailed studies will uncover the biochemical and genetic regulatory events that shape the behavior of DCs as they interact, or fail to interact, with T cells. Preliminary studies employing humanized anti-CD137 mAb’s generated in our laboratory have shown that their in vitro signaling effects on human, rhesus macaque, and sooty mangabey T cells are the same as the in vitro effects of mAb’s specific for mouse CD137. Furthermore, injection of humanized anti-CD137 mAb’s into nonhuman primates suppressed the development of T-dependent humoral immunity (34). Collectively, these and future studies will hopefully give rise to preclinical and clinical evaluation of the potential application of this form of treatment in human SLE patients.
Acknowledgments
This work was supported by NIH grants R21 AI48471-01, 5R0-1CA085860-01, the Carlos and Marguerite Mason Trust , and 1RO 1AI/RR49155-01A1 (to R.S. Mittler), and grant FO318/1-1 from the Deutsche Forschungsgemeinschaft (to J. Foell).
Footnotes
Shawn P. O’Neil’s present address is: New England National Primate Research Center, Harvard Medical School, One Pine Hill Drive, Southborough, Massachusetts, USA.
Juergan Foell’s present address is: Department of Pediatrics, Hematology, Oncology and Immunology, University Hospital, Martin-Luther University Halle-Wittenberg, Halle, Germany.
Conflict of interest: The authors have declared that no conflict of interest exists.
Nonstandard abbreviations used: systemic lupus erythematosus (SLE); NZB × NZW F1 (NZB/W F1); single-stranded DNA (ssDNA); double-stranded DNA(dsDNA); sheep red blood cell (SRBC); human IgG (huIgG); lymphocytic choriomeningitis virus (LCMV).
References
- 1.Wakeland EK, Liu K, Graham RR, Behrens TW. Delineating the genetic basis of systemic lupus erythematosus. Immunity. 2001;15:397–408. doi: 10.1016/s1074-7613(01)00201-1. [DOI] [PubMed] [Google Scholar]
- 2.Stott DI, Merino J, Schurmans S, Lambert PH. Expression of anti-DNA clonotypes and the role of helper T-lymphocytes during the autoimmune response in mice tolerant to alloantigens. Autoimmunity. 1988;1:253–266. doi: 10.3109/08916938809010679. [DOI] [PubMed] [Google Scholar]
- 3.Channing AA, Kasuga T, Horowitz RE, Dubois EL, Demopoulos HB. An ultrastructural study of spontaneous lupus nephritis in the NZB-BL-NZW mouse. Am. J. Pathol. 1965;47:677–694. [PMC free article] [PubMed] [Google Scholar]
- 4.Humbert M, Galanaud P. B-lymphocyte hyperreactivity and differentiation factors of T-lymphocytes in systemic lupus erythematosus. Ann. Med. Interne (Paris). 1990;141:213–216. [PubMed] [Google Scholar]
- 5.Sobel ES, et al. T-B collaboration for autoantibody production in lpr mice is cognate and MHC-restricted. J. Immunol. 1994;152:6011–6016. [PubMed] [Google Scholar]
- 6.Wofsy D, Seaman WE. Successful treatment of autoimmunity in NZB/NZW F1 mice with monoclonal antibody to L3T4. J. Exp. Med. 1985;161:378–391. doi: 10.1084/jem.161.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wofsy D, Chiang NY, Greenspan JS, Ermak TH. Treatment of murine lupus with monoclonal antibody to L3T4. I. Effects on the distribution and function of lymphocyte subsets and on the histopathology of autoimmune disease. J. Autoimmun. 1988;1:415–431. doi: 10.1016/0896-8411(88)90065-0. [DOI] [PubMed] [Google Scholar]
- 8.Wofsy D. Treatment of murine lupus with anti-CD4 monoclonal antibodies. Immunol. Ser. 1993;59:221–236. [PubMed] [Google Scholar]
- 9.Carteron NL, Schimenti CL, Wofsy D. Treatment of murine lupus with F(ab′)2 fragments of monoclonal antibody to L3T4. Suppression of autoimmunity does not depend on T helper cell depletion. J. Immunol. 1989;142:1470–1475. [PubMed] [Google Scholar]
- 10.Pollok KE, et al. Inducible T cell antigen 4-1BB. Analysis of expression and function. J. Immunol. 1993;150:771–781. [PubMed] [Google Scholar]
- 11.Melero I, Johnston JV, Shufford WW, Mittler RS, Chen L. NK1.1 cells express 4-1BB (CDw137) costimulatory molecule and are required for tumor immunity elicited by anti-4-1BB monoclonal antibodies. Cell. Immunol. 1998;190:167–172. doi: 10.1006/cimm.1998.1396. [DOI] [PubMed] [Google Scholar]
- 12.Wilcox RA, et al. Cutting edge: expression of functional CD137 receptor by dendritic cells. J. Immunol. 2002;168:4262–4267. doi: 10.4049/jimmunol.168.9.4262. [DOI] [PubMed] [Google Scholar]
- 13.Futagawa T, et al. Expression and function of 4-1BB and 4-1BB ligand on murine dendritic cells. Int. Immunol. 2002;14:275–286. doi: 10.1093/intimm/14.3.275. [DOI] [PubMed] [Google Scholar]
- 14.Goodwin RG, et al. Molecular cloning of a ligand for the inducible T cell gene 4-1BB: a member of an emerging family of cytokines with homology to tumor necrosis factor. Eur. J. Immunol. 1993;23:2631–2641. doi: 10.1002/eji.1830231037. [DOI] [PubMed] [Google Scholar]
- 15.Zhou Z, et al. Characterization of human homologue of 4-1BB and its ligand. Immunol. Lett. 1995;45:67–73. doi: 10.1016/0165-2478(94)00227-i. [DOI] [PubMed] [Google Scholar]
- 16.Shuford WW, et al. 4-1BB costimulatory signals preferentially induce CD8+ T cell proliferation and lead to the amplification in vivo of cytotoxic T cell responses. J. Exp. Med. 1997;186:47–55. doi: 10.1084/jem.186.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeBenedette MA, et al. Role of 4-1BB ligand in costimulation of T lymphocyte growth and its upregulation on M12 B lymphomas by cAMP. J. Exp. Med. 1995;181:985–992. doi: 10.1084/jem.181.3.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hurtado JC, Kim YJ, Kwon BS. Signals through 4-1BB are costimulatory to previously activated splenic T cells and inhibit activation-induced cell death. J. Immunol. 1997;158:2600–2609. [PubMed] [Google Scholar]
- 19.DeBenedette MA, Shahinian A, Mak TW, Watts TH. Costimulation of CD28- T lymphocytes by 4-1BB ligand. J. Immunol. 1997;158:551–559. [PubMed] [Google Scholar]
- 20.Cannons JL, et al. 4-1BB ligand induces cell division, sustains survival, and enhances effector function of CD4 and CD8 T cells with similar efficacy. J. Immunol. 2001;167:1313–1324. doi: 10.4049/jimmunol.167.3.1313. [DOI] [PubMed] [Google Scholar]
- 21.Sun Y, et al. Administration of agonistic anti-4-1BB monoclonal antibody leads to the amelioration of experimental autoimmune encephalomyelitis. J. Immunol. 2002;168:1457–1465. doi: 10.4049/jimmunol.168.3.1457. [DOI] [PubMed] [Google Scholar]
- 22.Melero I, et al. Monoclonal antibodies against the 4-1BB T-cell activation molecule eradicate established tumors. Nat. Med. 1997;3:682–685. doi: 10.1038/nm0697-682. [DOI] [PubMed] [Google Scholar]
- 23.Takahashi C, Mittler RS, Vella AT. Cutting edge: 4-1BB is a bona fide CD8 T cell survival signal. J. Immunol. 1999;162:5037–5040. [PubMed] [Google Scholar]
- 24.Mittler RS, Bailey TS, Klussman K, Trailsmith MD, Hoffmann MK. Anti-4-1BB monoclonal antibodies abrogate T cell-dependent humoral immune responses in vivo through the induction of helper T cell anergy. J. Exp. Med. 1999;190:1535–1540. doi: 10.1084/jem.190.10.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McMaster GK, Carmichael GG. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc. Natl. Acad. Sci. U. S. A. 1977;74:4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kelsoe G. The germinal center: a crucible for lymphocyte selection. Semin. Immunol. 1996;8:179–184. doi: 10.1006/smim.1996.0022. [DOI] [PubMed] [Google Scholar]
- 27.Aruffo A, Hollenbaugh D, Wu LH, Ochs HD. The molecular basis of X-linked agammaglobulinemia, hyper-IgM syndrome, and severe combined immunodeficiency in humans. Curr. Opin. Hematol. 1994;1:12–18. [PubMed] [Google Scholar]
- 28.Van den Eertwegh AJ, et al. In vivo CD40-gp39 interactions are essential for thymus-dependent humoral immunity. I. In vivo expression of CD40 ligand, cytokines, and antibody production delineates sites of cognate T-B cell interactions. J. Exp. Med. 1993;178:1555–1565. doi: 10.1084/jem.178.5.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Foy TM, et al. In vivo CD40-gp39 interactions are essential for thymus-dependent humoral immunity. II. Prolonged suppression of the humoral immune response by an antibody to the ligand for CD40, gp39. J. Exp. Med. 1993;178:1567–1575. doi: 10.1084/jem.178.5.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Foy TM, et al. gp39-CD40 interactions are essential for germinal center formation and the development of B cell memory. J. Exp. Med. 1994;180:157–163. doi: 10.1084/jem.180.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Odendahl M, et al. Disturbed peripheral B lymphocyte homeostasis in systemic lupus erythematosus. J. Immunol. 2000;165:5970–5979. doi: 10.4049/jimmunol.165.10.5970. [DOI] [PubMed] [Google Scholar]
- 32.Cassese G, et al. Inflamed kidneys of NZB / W mice are a major site for the homeostasis of plasma cells. Eur. J. Immunol. 2001;31:2726–2732. doi: 10.1002/1521-4141(200109)31:9<2726::aid-immu2726>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 33.Sun Y, et al. Costimulatory molecule-targeted antibody therapy of a spontaneous autoimmune disease. Nat. Med. 2002;8:1405–1413. doi: 10.1038/nm1202-796. [DOI] [PubMed] [Google Scholar]
- 34.Hong HJ, et al. A humanized anti–4-1BB monoclonal antibody suppresses antigen-induced humoral immune response in nonhuman primates. J. Immunother. 2000;23:613–621. doi: 10.1097/00002371-200011000-00002. [DOI] [PubMed] [Google Scholar]