Abstract
The porcine reproductive and respiratory syndrome virus (PRRSV) has a very restricted tropism for well-differentiated cells of the monocyte-macrophage lineage, which is probably determined by specific receptors on these cells. In this study, the importance of heparinlike molecules on porcine alveolar macrophages (PAM) for PRRSV infection was determined. Heparin interacted with the virus and reduced infection of PAM up to 92 or 88% for the American and European types of PRRSV, respectively. Other glycosaminoglycans, similar to heparin, had no significant effect on infection while heparinase treatment of PAM resulted in a significant reduction of the infection. Analysis of infection kinetics showed that PRRSV attachment to heparan sulfate occurs early in infection. A heparin-sensitive binding step was observed which converted completely into a heparin-resistant binding after 120 min at 4°C. Using heparin-affinity chromatography and sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), it was observed that the structural matrix (M) and nucleocapsid (N) proteins attached to heparin. Nonreducing SDS-PAGE revealed that M bound to heparin mainly as a complex with glycoprotein GP5 and that the N protein bound to heparin as a homodimer. GP3, which was identified as a minor structural protein of European types of PRRSV, did not bind to heparin. Since the N protein is not exposed on the virion surface, it was concluded that the structural M protein and the M-GP5 complex contribute to PRRSV attachment on a heparinlike receptor on PAM. This is the first report that identifies a PRRSV ligand for a cell surface heparinlike receptor on PAM.
Porcine reproductive and respiratory syndrome (PRRS) was first described in 1987 in North America as a new syndrome in pigs, characterized by reproductive failure in sows and respiratory problems in pigs of all ages (5). The causative agent of the disease was identified in 1991 in The Netherlands as a virus (35) and is now designated PRRS virus (PRRSV). PRRSV has been classified as a member of the Arteriviridae, a family of enveloped, positive-strand RNA viruses which also includes Equine arteritis virus, Lactate dehydrogenase-elevating virus (LDV), and Simian hemorrhagic fever virus (4, 29).
PRRSV is 45 to 70 nm in diameter and consists of a nucleocapsid core surrounded by an envelope. The virus contains three major structural proteins which together represent approximately 90 to 95% of the structural protein content: the nucleocapsid protein N (15 kDa), which makes up the nucleocapsid; a nonglycosylated membrane protein M (18 to 19 kDa); and a glycosylated membrane protein GP5 (24.5 kDa) (21, 23, 34). The N protein is incorporated in virions mainly as a disulfide-linked dimer with an Mr of 28 kDa (23). The M and GP5 proteins are incorporated in virions mainly as a disulfide-linked heterodimer or as a disulfide-linked multimer with an approximate Mr of, respectively, 40 and 87 kDa (21, 23). The M and N proteins are also present as single proteins in purified virions (23). Also, minor N-glycosylated structural proteins have been described: GP2 (29 to 30 kDa), GP3 (45 to 50 kDa), and GP4 (31 to 35 kDa) (23, 24, 37).
PRRSV has a highly specific cell tropism. In vivo, the virus infects well-differentiated cells of the monocyte-macrophage lineage, in particular porcine alveolar macrophages (PAM), the primary target cells of the virus (6). For the propagation of the virus in vitro, only PAM and cells derived from African green monkey kidney cells, such as MA-104, Marc-145, and CL-2621, can be used (1, 16, 39, 40). Both in PAM and in the monkey kidney-derived cell lines, the virus enters through a mechanism of receptor-mediated endocytosis (17, 27). The first step in this entry process is the attachment to one or more cellular receptors. Until now, only one candidate PRRSV receptor has been described on PAM, a 210-kDa protein (7). Infection of PAM can be blocked completely using monoclonal antibodies (MAbs) that immunoprecipitate this 210-kDa protein from membranes of PAM (7, 8). Despite a complete block of infection, binding of the virus cannot be fully blocked (7), indicative of the involvement of another molecule. Jusa et al. (13) showed that heparin can reduce PRRSV infection of Marc-145 cells. In this study, we investigated whether heparan sulfate proteoglycans play a specific role in PRRSV infection of its natural target cell, the PAM, for both the European and the American type of PRRSV. Furthermore, we tried to identify which viral proteins were involved in PRRSV attachment to heparin.
MATERIALS AND METHODS
Cells and viruses.
PAM were obtained from 4- to 6-week-old conventional Belgian Landrace pigs from a PRRSV-negative herd as described by Wensvoort et al. (40). Cells were cultivated in Earle's modified Eagle medium supplemented with 10% fetal bovine serum, 2 mM l-glutamine (BDH Chemicals Ltd., Poole, England), 1% nonessential amino acids (100x, Gibco BRL), 1 mM sodium pyruvate, and a mixture of antibiotics in a humidified 5% CO2 atmosphere at 37°C.
A 13th and a 4th passage on PAM of, respectively, the European prototype PRRSV strain, Lelystad virus (LV) (kindly provided by G. Wensvoort), and the Belgian PRRSV strain, 94V360 (6), and a 2nd and a 4th passage on Marc-145 cells of, respectively, the American PRRSV strain US-5 (20) and the American prototype PRRSV strain, VR-2332 (5), were used. The Kaplan strain of pseudorabies virus (PrV) (14) and its deletion mutant Kaplan gCnull, which lacks the viral protein gC (33), were kindly provided by T. C. Mettenleiter and passaged twice on ST cells. For infection experiments, all viruses were used at a multiplicity of infection that resulted in an infection rate of PAM of 50% at 10 h postinoculation (hpi).
For some experiments, the PRRSV strain 94V360 was semipurified by ultracentrifugation at 100,000 × g for 3 h through a 30% sucrose cushion in an SW41Ti rotor (Beckman Coulter Inc., Palo Alto, Calif.). Virus pellets were resuspended in phosphate-buffered saline (PBS) in 1/100 of the original volume and kept at −70°C. Where needed, virus was labeled with biotin immediately after ultracentrifugation using a protein biotinylation kit (Amersham Pharmacia Biotech Ltd.) as described earlier (6) and stored at −70°C.
Incubation of viruses with heparin and other GAG.
Virus was incubated for 1 h at 37°C with different concentrations of glycosaminoglycans (GAG) (heparin, heparan sulfate, chondroitin sulfate A, or dermatan sulfate; Sigma Chemical Company, St. Louis, Mo.) and added to the PAM for 2 h. Cells were washed with medium without fetal bovine serum (FBS) to remove unbound virus and fixed 10 hpi by a 20-min treatment with acetone-methanol (50/50) at −20°C. The fixative was removed, and the plates were dried and kept at −70°C until staining.
Treatment of PAM with heparinase I or with heparin.
Heparinase I or heparin (Sigma Chemical Company) was diluted in medium without FBS and added to the PAM. After 1 h of incubation at 37°C, the cells were washed extensively and virus was added. The cells were washed 2 hpi, fixed 10 hpi as described above, and stored at −70°C. All wash steps were performed with medium without FBS.
Immunoperoxidase staining of infected PAM.
Fixed and frozen cells were thawed, washed once with PBS, and rinsed three times with water. The endogenous peroxidase activity was blocked by incubating the cells with PBS supplemented with 1% sodium azide and 0.5% H2O2 for 10 min. PRRSV-infected cells were incubated for 1 h at 37°C with MAb P3/27, directed against the PRRSV-nucleocapsid protein (41), and 1/100 diluted in PBS supplemented with 10% complement-inactivated goat serum (PBS-G), followed by an incubation for 1 h at 37°C with peroxidase labeled goat anti-mouse immunoglobulin (Ig) (Dako A/S, Glostrup, Denmark) diluted 1/100 in PBS-G. PrV-infected PAM were incubated for 1 h at 37°C with MAb 1C11, directed against the glycoprotein gB of PrV (26) diluted 1/500 in PBS-G, and then during 1 h at 37°C with peroxidase labeled rabbit anti-swine Ig (Dako A/S) diluted 1/100 in PBS-G. Before each incubation with antibodies, the cells were washed three times with PBS. Infected PAM were visualized with a substrate solution of 3-amino-9-ethylcarbazole in 0.05 M acetate buffer (pH 5) with 0.05% H2O2, and the reaction was blocked by washing with acetate buffer (pH 5). Viral antigen-positive cells and total cells were counted with a light microscope (Olympus Optical Co., Hamburg, Germany), and the percentage of infected cells was calculated. Three microscope fields, and a minimum of 300 cells per field, were counted for each well. The t test was used to determine if the percentage of infected cells was reduced significantly due to the different treatments compared to a control infection without treatment. To compare the effect of heparin on the different PRRSV strains, an analysis of variance test was used together with the least-significant-difference post hoc test. All statistical analysis was performed with SPSS (SPSS Inc., Chicago, Ill.).
Flow cytometric analysis of PRRSV attachment.
PAM were suspended by flushing, washed with cold PBS supplemented with 2% FBS (PBS-F), and finally resuspended in cold PBS-F. Cell suspensions were kept on ice during the experiment. In one experiment, biotinylated virus, mixed with PBS-F containing different concentrations of heparin, was added to the PAM. After 1 h of incubation with gentle rocking, the PAM were washed three times with cold PBS-F to remove unbound virus.
In another experiment, PAM were incubated for 5 min with PBS-F containing biotinylated PRRSV (multiplicity of infection, 5) and washed with PBS-F to remove unbound virus. At different time intervals after the PBS-F wash, one set of cells was washed with PBS-F and a second set of cells was washed with PBS-F containing heparin (2,500 μg/ml). All PAM were washed once more with PBS-F before analysis.
To detect PAM with attached PRRSV, cells were incubated for 1 h on ice with fluorescein isothiocyanate (FITC)-conjugated streptavidin (Molecular Probes, Eugene, Oreg.), diluted 1/100 in PBS-F. The PAM were washed with cold PBS, and the median fluorescence intensity (MFI) was determined by flow cytometric analysis, conducted with a Becton-Dickinson (San Jose, California) FACScalibur, equipped with a 15-mW air-cooled argon ion laser and interfaced to a Macintosh Quadra 650 computer (Apple Computer Inc., Cupertino, California) using Becton-Dickinson Cellquest software. Ten thousand cells were analyzed for each sample, and three parameters were stored for further analysis: forward light scattering, sideward light scattering, and green fluorescence. The relative MFI was calculated according the following formula: relative MFI = 100 × {1 − [(MFI when no heparin was added − MFI when heparin was added)/MFI when no heparin was added]}.
Binding of virus and viral proteins on heparin Sepharose.
Virus was mixed with different concentrations of lyophilized heparin Sepharose CL-6B (Amersham Pharmacia Biotech Ltd.) and incubated at 37°C during 1 h with agitation. The heparin Sepharose slurry was centrifuged at 15,000 × g, and the 50% tissue culture infective dose (TCID50) of the supernatant was determined (31).
Viral proteins were solubilized from semipurified PRRSV 94V360 by a 30-min incubation in Tris buffer (50 mM Tris-HCl [pH 7.4], 150 mM NaCl, 1 mM EDTA) containing 1% NP-40 (BDH Chemicals Ltd., Poole, England) and a mixture of protease inhibitors (Complete; Boehringer Mannheim GmbH, Mannheim, Germany). Heparin Sepharose CL-6B was packed in a column and washed with PBS containing 0.1% NP-40 (PBS-N). Solubilized viral proteins were added, and after 30 min of incubation at room temperature, the heparin Sepharose was washed with PBS-N to remove unbound material. Bound viral proteins were eluted from the column with PBS-N containing 4 mg of heparin per ml (PBS-N-H) or PBS-N-H adjusted to a final concentration of 1.5 or 2 M NaCl. Immunoprecipitation of PRRSV proteins from the different fractions obtained from the column was performed with an anti-PRRSV polyclonal serum from a hyperimmunized pig, covalently coupled to protein A-Sepharose (Amersham Pharmacia Biotech Ltd.) as described before (10). Immunoprecipitated viral proteins were eluted from the protein A-Sepharose-antibody complexes by boiling for 5 min in reducing or nonreducing Laemmli buffer.
In another experiment, the virus lysate was added to a heparin Sepharose slurry. After 30 min of incubation, the heparin Sepharose was washed with PBS-N, and bound proteins were eluted by direct addition of reducing Laemmli buffer and boiling for 5 min. To be able to detect the viral glycoprotein GP3, which is only present in very low amounts in virions, the original and the wash fractions were concentrated with Microcon centrifugal filter devices with a 10-kDa molecular mass cutoff (Millipore Corporation, Bedford, Mass.) prior to the addition of the reducing Laemmli buffer and boiling. Standard SDS-PAGE was performed on a 12.5% polyacrylamide gel with a mini-protean 3 system (Bio-Rad Laboratories, Hercules, Calif.), and separated proteins were blotted onto a polyvinylidene difluoride (PVDF) membrane (Amersham Pharmacia Biotech Ltd.) in a wet blot apparatus (Bio-Rad Laboratories). The PVDF membrane was incubated with 5% membrane blocking agent (Amersham Pharmacia Biotech Ltd.) in PBS containing 0.1% Tween 20 (PBS-T) for 1 h at room temperature. To detect the PRRSV M, GP3, or N protein, the PVDF membrane was incubated, respectively, with MAb 126.9, MAb 126.2 (37), or MAb P3/27 (41). The membrane was washed with PBS-T and incubated for 1 h with horseradish peroxidase-labeled goat anti-mouse Ig (Dako A/S), diluted 1/100 in PBS-T. After washing with PBS-T, the proteins were visualized by staining with 3,3′-diaminobenzidine.
RESULTS
Effect of heparin and heparinase I on PRRSV infection of PAM.
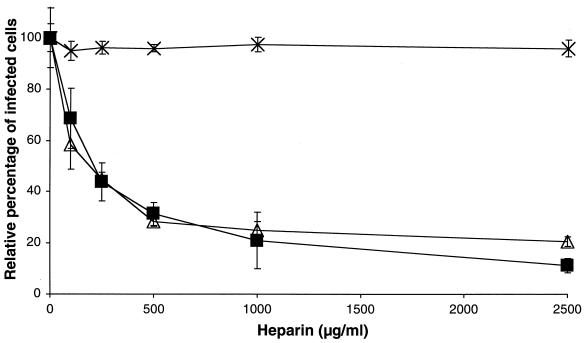
To evaluate the effect of heparin on PRRSV infection, PAM were incubated in the presence of heparin at 0, 125, 250, 500, 1,000, or 2,500 μg/ml with the Belgian PRRSV strain 94V360, which was used for all experiments in which no PRRSV strain is specified. Cells were fixed 10 hpi to allow only one cycle of virus replication, and infected cells were visualized by immunocytochemistry. As shown in Fig. 1, a dose-dependent reduction of PRRSV infection was observed, with a maximum reduction of 88% at a heparin concentration of 2,500 μg/ml. PrV and PrV gCnull, which are, respectively, sensitive and not sensitive to heparin (22), were used as a positive and a negative control. The reduction of PrV infection was similar to that of PRRSV, while PrV gCnull infection was not significantly reduced, an effect that was already described for PrV and PrV gCnull infection of MDBK cells (22).
FIG. 1.
Effect of heparin on PRRSV infection of PAM. PRRSV (▪), PrV (▵), and PrV gCnull (×) were incubated for 1 h at 37°C with different concentrations of heparin. PAM were inoculated with the mixtures, washed after 2 h of incubation at 37°C to remove unbound virus, and finally fixed after 10 h. The data represent the means ± standard deviations (error bars) of triplicate assays.
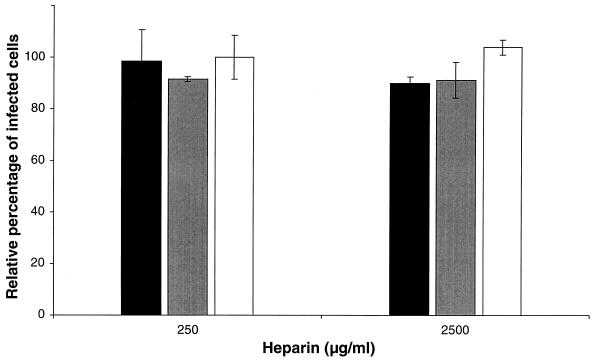
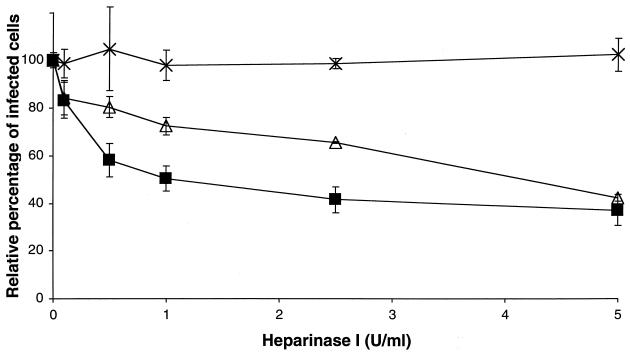
To determine if the observed effect was due to an interaction of heparin with the PAM or with the virus, PAM were incubated with heparin before infection. As shown in Fig. 2, pretreatment of the PAM with heparin had no significant effect on the PRRSV infection. To investigate if heparan sulfate proteoglycans, present on the cell surface, are involved in PRRSV infection, PAM were treated with different concentrations of heparinase I and infected with PRRSV or the PrV control viruses. As shown in Fig. 3, PRRSV infection showed a significant reduction after heparinase I treatment of the PAM, with a maximum of 63% at 5 U/ml. PrV infection was also reduced significantly with a maximum of 58% at 5 U/ml, while no significant effect on PrV gCnull infection was detected.
FIG. 2.
Effect of incubation of PAM with heparin before infection. PAM were incubated for 1 h at 37°C with heparin, washed extensively, and incubated with PRRSV (solid bars), PrV (shaded bars), and PrV gC− (open bars). The cells were washed after 2 h of incubation to remove unbound virus, and the cells were fixed after 10 h. The data represent the means ± standard deviations (error bars) of triplicate assays. No significant differences were observed in comparison to the control (no heparin added).
FIG. 3.
Effect of heparinase treatment of PAM on PRRSV infection. Heparinase-treated PAM were washed and incubated with PRRSV (▪), PrV (▵), and PrV gCnull (×). The PAM were washed after 2 h at 37°C and fixed after a total infection period of 10 h. The data represent the means ± standard deviations (error bars) of triplicate assays.
Effect of other glycosaminoglycans on PRRSV infection of PAM.
To investigate if the reduction of PRRSV infection was due to a specific interaction of the virus with heparin or due to a general interaction with negatively charged molecules, virus was incubated with three other GAG—heparan sulfate, chondroitin sulfate A, and dermatan sulfate—before inoculation. Heparan sulfate significantly reduced PRRSV infection at a concentration of 250 (50% ± 12% reduction) and 2,500 (63% ± 5% reduction) μg/ml. Neither of the two other GAG could significantly reduce PRRSV infection at a concentration of 2,500 μg/ml, nor was there an effect on infection with the parental PrV and the gCnull mutant (Fig. 4).
FIG. 4.
Effect of other GAG on PRRSV infection. Virus was incubated for 1 h at 37°C with a 2,500-μg/ml concentration of chondroitin sulfate A (▪) or dermatan sulfate (□). PAM were incubated with the virus-GAG mixtures, washed after 2 h, and fixed after a total infection time of 10 h. The data represent the means ± standard deviations of triplicate assays. No significant differences were observed in comparison to the control (no GAG added).
Effect of heparin on different PRRSV strains.
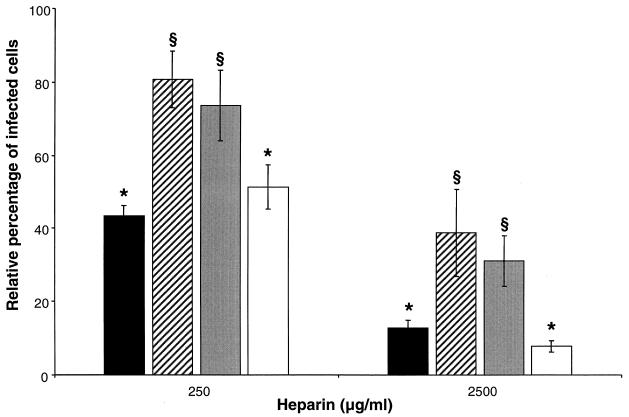
Two European and two American PRRSV strains were tested for their sensitivities to heparin to evaluate if the observed effect is a general feature for PRRSV. Virus was incubated with heparin at concentrations of 250 and 2,500 μg/ml prior to inoculation of the PAM. As shown in Fig. 5, the different PRRSV strains exhibited different sensitivities to heparin, with a maximum difference of 37% at the 250-μg/ml concentration of heparin and a maximum difference of 31% at the 2,500-μg/ml concentration of heparin, both being significant (P < 0.01). The European LV was the least heparin-sensitive PRRSV strain, whereas the Belgian strain 94V360 and the American strain VR-2332 were the most sensitive ones.
FIG. 5.
Effect of heparin on different PRRSV strains. PRRSV 94V360 (solid bars), LV (striped bars), US-5 (shaded bars), and VR-2332 (open bars) were incubated for 1 h at 37°C with different concentrations of heparin. PAM were inoculated with the mixtures, washed after 2 h of incubation at 37°C to remove unbound virus, and finally fixed after a total infection time of 10 h. The data represent the means ± standard deviations (error bars) of triplicate assays. The analysis of variance test and the least-significant-difference post hoc test were performed to compare the sensitivities of the different PRRSV strains to heparin, with heparin at both at 250 and 2,500 μg/ml. A different symbol on top of two bars indicates that the number of infected cells of these two PRRSV strains are significantly different at the given concentration of heparin (P < 0.01).
Effect of heparin on PRRSV attachment.
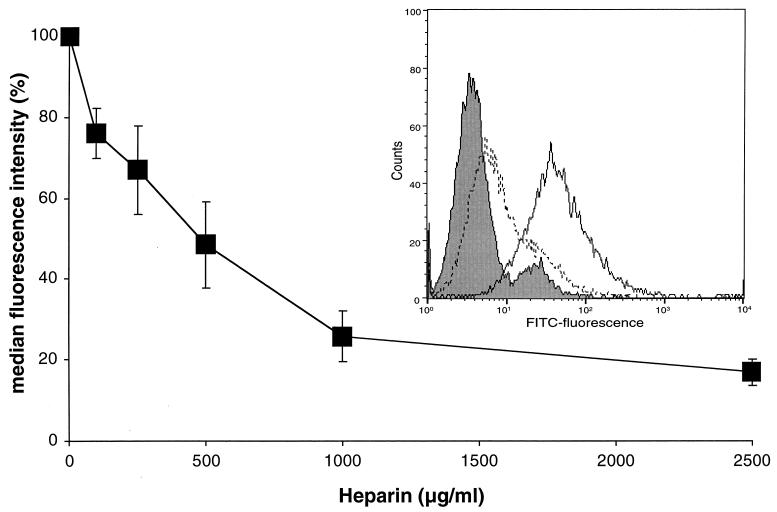
To check if the observed reduction in PRRSV infection was due to an interference of heparin with PRRSV binding or with a later step in the infection such as the uptake of the virus, PAM were inoculated with biotinylated PRRSV in the presence or in the absence of heparin and at 4°C to block endocytosis. After washing the PAM to remove unbound virus and incubation with streptavidin-FITC, the MFI was determined by flow cytometric analysis. The results from this experiment, shown in Fig. 6, clearly demonstrate that heparin reduces the virus adsorption in a dose-dependent manner, with a maximum reduction of 83% at a 2,500-μg/ml concentration of heparin.
FIG. 6.
Heparin inhibits PRRSV attachment to PAM in a dose-dependent manner. Biotinylated PRRSV was incubated with different concentrations of heparin, and PAM were inoculated with the mixtures at 4°C for 1 h. After washing, bound virus particles were stained by direct immunofluorescence, and fluorescence intensity was analyzed by flow cytometry. The data represent the means ± standard deviations (error bars) of triplicate assays. The histogram shows the green fluorescence intensity frequency when no virus was added (shaded histogram), when virus was added (solid-line histogram), and when virus and heparin was added (dotted-line histogram).
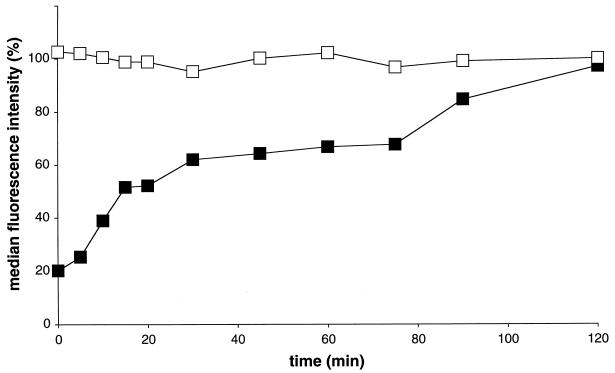
To investigate at which time after inoculation PRRSV attachment to a heparinlike molecule occurs, PAM were incubated with biotinylated PRRSV at 4°C for 5 min and then washed with PBS-F to remove unbound virus. At different time intervals afterwards, the cells were washed with PBS-F with or without heparin (2,500 μg/ml). Washing with heparin clearly reduced the amount of bound virus particles (4°C), with the strongest reduction immediately after inoculation (Fig. 7). The effect of heparin diminished continuously with increasing time after PRRSV inoculation. At 120 min after inoculation at 4°C, no more viral particles were removed by washing with heparin. Consequently, attachment of PRRSV to PAM is heparin sensitive and converts completely into a heparin-resistant binding.
FIG. 7.
Heparin-dependent kinetics of PRRSV attachment. PAM were inoculated with PRRSV at 4°C, unbound virus was removed by washing the cells after a 5-min incubation at 4°C, and at different time intervals PAM were washed with PBS-F with (▪) or without (□) heparin (2,500 μg/ml). Attachment was evaluated at 4°C by immunofluorescent staining of bound virus particles with streptavidin-FITC and flow cytometric analysis. The data represent the means of duplicate assays.
Binding of PRRSV to heparin Sepharose and identification of PRRSV heparin binding proteins.
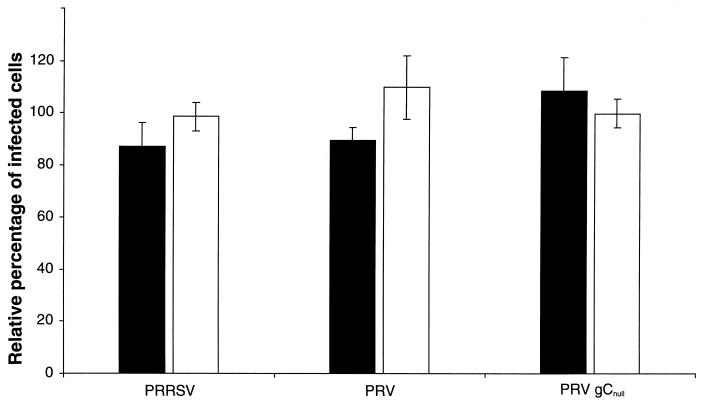
To identify the viral proteins binding to heparin, we first checked to see whether PRRSV binds to immobilized heparin. PRRSV was incubated with heparin Sepharose for 1 h at 37°C, and the TCID50 of the supernatant was determined. Incubation with heparin Sepharose reduced the virus titer of PRRSV and PrV by a maximum of 65 and 98%, respectively (Fig. 8). Incubation of PrV gCnull with heparin Sepharose had no significant effect on the virus titer.
FIG. 8.
PRRSV (▪), PrV (▵), and PrV gCnull (×) were incubated with different concentrations of heparin Sepharose for 1 h at 37°C. The mixtures were centrifuged and the supernatant was titrated. The data are shown relatively to the original TCID50 of each virus suspension and represent the mean values of duplicate experiments.
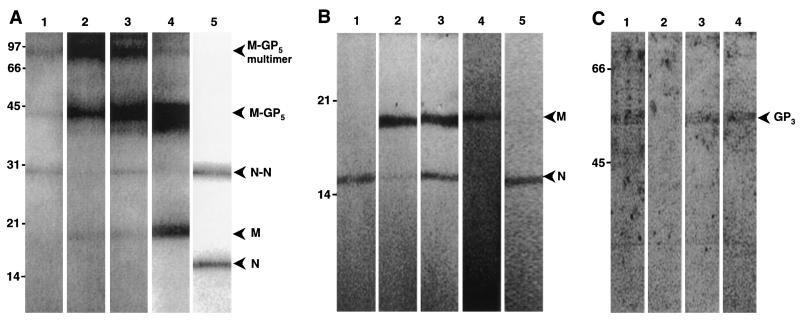
To identify the PRRSV proteins that bind to heparin, a lysate of semipurified PRRSV was incubated with heparin Sepharose, packed in a column. After extensive washing, the bound proteins were eluted with heparin in combination with increasing salt concentrations and immunoprecipitated with an anti-PRRSV hyperimmune serum. The proteins binding to heparin were identified by Western blotting (WB) using MAbs specific for the M and N protein. The results (Fig. 9A and B) show that the dimeric form of the N protein, the M protein, and the M-GP5 protein complexes bound to the heparin Sepharose. A large fraction of the dimeric N protein could be eluted by addition of free heparin only to the heparin Sepharose (Fig. 9A and B, lane 1), while elution of the M and M-GP5 proteins also required high salt concentrations (Fig. 9A and B, lanes 2 and 3), indicating that the latter proteins bound more strongly to the heparin Sepharose. In another experiment, solubilized viral proteins were incubated with a slurry of heparin Sepharose, the slurry was washed extensively, and bound proteins were eluted by direct addition of Laemmli buffer and boiling. The original lysate, the bound fraction, and the wash fraction were concentrated with Microcon centrifugal devices prior to SDS-PAGE and WB analysis to be able to detect the minor PRRSV glycoprotein GP3, which is only present in very low amounts in virions. The M and N proteins were clearly detected in the original lysate and in the bound fraction, while only faint bands were detected in the unbound and wash fractions (data not shown). The GP3 protein, however, did not bind to the heparin Sepharose (Fig. 9C, lane 2), but it could be detected in the original virus lysate (Fig. 9C, lane 1), in the unbound fraction (Fig. 9C, lane 3), and in the wash fraction (Fig. 9C, lane 4).
FIG. 9.
SDS-PAGE analysis of the binding of PRRSV proteins to heparin Sepharose. PRRSV proteins were solubilized with Tris buffer containing 1% NP-40. (A) Analysis of bound fractions and original lysates under nonreducing conditions. Solubilized proteins were added to a heparin Sepharose column, and bound fractions were eluted with PBS containing heparin at 4 mg/ml (PBS-H) (lane 1), with PBS-H containing 1.5 M NaCl (lane 2), or with PBS-H containing 2 M NaCl (lane 3). Proteins were detected on a WB with a mixture of MAbs directed against the nucleocapsid (N) and the matrix (M) protein. Original lysates were also analyzed with anti-M MAb (lane 4) and anti-N MAb (lane 5). (B) Lanes 1 to 5 contain the same samples as lanes 1 to 5 in panel A, except that they were analyzed under reducing conditions. (C) Detection of PRRSV GP3 in different fractions obtained. The original lysate (lane 1), the bound fraction (lane 2), the unbound fraction (lane 3), and the wash fractions (lane 4) were analyzed for the presence of GP3 under reducing conditions. Molecular weight standards (in thousands) are indicated on the left of each blot, and positions of different PRRSV proteins and protein complexes are indicated with an arrowhead.
DISCUSSION
In this study, it was shown that PRRSV infection of PAM could strongly be reduced by heparin. This effect was clearly dose dependent and was due to an interaction of heparin with the virus, since incubation of PAM with heparin prior to inoculation had no significant effect on PRRSV infection. It has already been demonstrated for several viruses, such as PrV (22), human cytomegalovirus (15, 28), and Sindbis virus (3), that heparan proteoglycans play a role in virus binding to their target cells. In our study, a wild-type PrV and a mutant PrV, which lacks the viral gC protein, responsible for PrV binding to heparin (22), were used as controls. Differentiated porcine monocytes and porcine macrophages can be infected with PrV (12, 25), and it was assumed that PrV would infect PAM in a similar heparin-sensitive way as observed in other cells, such as MDBK cells (22). Our results indicate that PrV infection of PAM is sensitive to heparin and that PrV binds to heparan sulfate GAG on PAM. However, the observed reduction of the infection was lower than for MDBK cells. This can possibly be explained by the different cells that were used, since it has been described that PrV exhibits different sensitivities to heparin, depending on the cell type used (22). The dose-dependent reduction of PRRSV infection that was obtained by addition of heparin to the inoculum was similar to the reduction we observed for PrV.
Proteoglycans consist of a core protein on which GAG are covalently bound (2). These GAG are a group of unbranched polysaccharide chains with repeating disaccharide subunits consisting of an amino sugar and hexuronic acid or galactose (30). Depending on the type of the disaccharide units, the bonds between the sugars and the modifications (pattern and extent of sulfation, epimerization of glucuronic acid to iduronic acid), the GAG can be classified into five types (for a review, see reference 30). The interaction between PRRSV and heparin or heparan sulfate was shown to be specific since two other GAG, chondroitin sulfate A and dermatan sulfate, had no significant effect on PRRSV infection of PAM. This means not only that the effect of heparin or heparan sulfate on PRRSV infection was not merely a matter of attraction between negatively charged sulfate groups and positively charged basic amino acids but also that the structure of the GAG backbone is important for this interaction. Addition of heparin or heparin sulfate, or heparinase treatment of PAM could clearly reduce PRRSV infection, which indicates that PRRSV binds to cell surface heparan sulfate GAG and that this binding is important for efficient infection of PAM.
Fluorescence-activated cell sorting analysis experiments with biotinylated PRRSV at 4°C confirmed that heparin interfered with the binding of PRRSV to PAM. The observed dose-dependent reduction of PRRSV binding was similar to the observed reduction of infection. Thus, the effect of heparin on infection is the consequence of a reduced PRRSV binding to PAM. Since infection with other PRRSV strains, both American and European types, was also reduced by heparin, it may be concluded that binding to heparan sulfate GAG is generally used by PRRSV to attach to PAM. Other studies have demonstrated that PRRSV can bind to different cell types, though these cells cannot be infected (18, 36). The presence of heparan sulfate GAG on these cells may be responsible for the binding of PRRSV.
Although cell surface heparan sulfate GAG appear to play an important role in PRRSV infection of PAM, their presence seems not to be an absolute prerequisite since addition of heparin did not result in a complete block of binding and infection. When the effect of the addition of heparin on PRRSV binding was studied at different time intervals after inoculation, it was observed that virus binding on PAM converted from being heparin sensitive to being heparin resistant, which is indicative of the existence of another receptor. A similar effect has been observed for PrV (22). The 210-kDa protein on PAM that was identified as a putative PRRSV receptor (7) could account for the heparin-resistant binding step.
The PRRSV virion contains several structural proteins that might be involved in the PRRSV attachment to heparin. By using a lysate of semipurified PRRSV and heparin-affinity chromatography, it was shown that the M and the disulfide-linked M-GP5 complexes bind to heparin. The N protein also bound to the heparin Sepharose but was eluted with less-stringent conditions, in comparison to the M-GP5 heterodimer. The relevance of the binding of the N protein to heparin for the binding of PRRSV to heparan sulfate GAG on the cell surface is also questionable, since it is generally assumed that the N protein makes up the nucleocapsid core, which is completely hidden inside the virus envelope (34, 38). The N protein interacts with the PRRSV genomic RNA, presumably through an interaction between the negatively charged RNA and regions of positively charged basic amino acid residues in the N protein (32). The N protein contains two clusters of positively charged basic amino acid residues (9K→K12 and 47K→K53) which may be responsible for the interaction with heparin. The M and GP5 proteins do not contain such clusters, suggesting that conformational epitopes may be involved in heparin binding. Hileman et al. (11) reported that conformational epitopes can be involved in interactions between proteins and heparin. Different sensitivities for the European type of PRRSV LV and the American type of PRRSV VR-2332 to heparin were observed. It is, however, unlikely that this difference could be explained by a difference in the M protein of the two strains since a comparison between the amino acid sequences of the M protein of both strains did not reveal major differences in the position and number of basic amino acid residues.
The M-GP5 complex is assumed to play a role in virus attachment. GP5 contains neutralizing epitopes (19, 38), and it has been shown for LDV, which is closely related to PRRSV, that disruption of the disulfide bond between the LDV equivalents of PRRSV M and GP5 results in loss of infectivity (9). Our data confirm the importance of the PRRSV M-GP5 complexes for attachment to and infection of PAM.
Acknowledgments
We thank P. Van Rijn (Institute for Animal Science and Health, Lelystad, The Netherlands) and Intervet International for the gifts of antibodies and T. C. Mettenleiter for the gift of the PrV strains. We also thank C. Vanmaercke, I. Pareyn, and V. Van Hoorde for excellent technical assistance.
Peter Delputte was supported by a grant from the Flemish Institute for the Promotion of Innovation by Science and Technology (I.W.T.-Flanders).
REFERENCES
- 1.Benfield, D. A., E. Nelson, J. E. Collins, L. Harris, S. M. Goyal, D. Robinson, W. T. Christianson, R. B. Morrison, D. Gorcyca, and D. Chladek. 1992. Characterisation of swine infertility and respiratory syndrome (SIRS) virus (isolate ATCC VR-2332). J. Vet. Diagn. Investig. 4:127-133. [DOI] [PubMed] [Google Scholar]
- 2.Bernfield, M., M. Götte, P. W. Park, O. Reizes, M. L. Fitzgerald, J. Lincecum, and M. Zako. 1999. Functions of cell surface heparan sulphate proteoglycans. Annu. Rev. Biochem. 68:729-777. [DOI] [PubMed] [Google Scholar]
- 3.Byrnes, A. P., and D. E. Griffin. 1998. Binding of Sindbis virus to cells surface heparan sulfate. J. Virol. 72:7349-7356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cavanagh, D. 1997. Nidovirales: a new order comprising Coronaviridae and Arteriviridae. Arch. Virol. 142:629-633. [PubMed] [Google Scholar]
- 5.Collins, J. E., D. A. Benfield, W. T. Christianson, L. Harris, J. C. Hennings, D. P. Shaw, S. M. Goyal, S. McCullough, R. B. Morrison, H. S. Joo, D. Gorcyca, and D. Chladek. 1992. Isolation of swine infertility and respiratory syndrome virus (isolate ATCC VR-2332) in North America and experimental reproduction of the disease in gnotobiotic pigs. J. Vet. Diagn. Investig. 4:117-126. [DOI] [PubMed] [Google Scholar]
- 6.Duan, X., H. J. Nauwynck, and M. B. Pensaert. 1997. Effects of origin and state of differentiation and activation of monocytes/macrophages on their susceptibility to PRRSV. Arch. Virol. 142:2483-2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duan, X., H. J. Nauwynck, H. W. Favoreel, and M. B. Pensaert. 1998. Porcine reproductive and respiratory syndrome virus infection of alveolar macrophages can be blocked by monoclonal antibodies against cell surface antigens. Adv. Exp. Med. Biol. 440:81-88. [DOI] [PubMed] [Google Scholar]
- 8.Duan, X., H. J. Nauwynck, H. W. Favoreel, and M. B. Pensaert. 1998. Identification of a putative receptor for porcine reproductive and respiratory syndrome virus on porcine alveolar macrophages. J. Virol. 72:4520-4523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Faaberg, K. S., C. Even, G. A. Palmer, and P. G. W. Plagemann. 1995. Disulfide bonds between two envelope proteins of lactate dehydrogenase-elevating virus are essential for viral infectivity. J. Virol. 69:613-617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harlow, E., and D. Lane. 1988. Antibodies: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 11.Hileman, R. E., J. R. Fromm, J. M. Weiler, and R. J. Linhardt. 1998. Glycosaminoglycan-protein interactions: definition of consensus sites in glycosaminoglycan binding proteins. Bioessays 20:156-167. [DOI] [PubMed] [Google Scholar]
- 12.Iglesias, G., C. Pijoan, and T. Molitor. 1989. Interactions of pseudorabies virus with swine alveolar macrophages. I. Virus replication. Arch. Virol. 104:107-115. [DOI] [PubMed] [Google Scholar]
- 13.Jusa, E. R., Y. Inaba, M. Kohno, and O. Hirose. 1997. Effect of heparin on infection of cells by porcine reproductive and respiratory syndrome virus. Am. J. Vet. Res. 58:488-491. [PubMed] [Google Scholar]
- 14.Kaplan, A. S., and A. E. Vatter. 1959. A comparison of herpes simplex and pseudorabies viruses. Virology 7:394-407. [DOI] [PubMed] [Google Scholar]
- 15.Kari, B., and R. Gehrz. 1992. A human cytomegalovirus glycoprotein complex designated gC-II is a major heparin binding component of the envelope. J. Virol. 66:1761-1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim, H. S., J. Kwang, I. J. Yoon, H. S. Joo, and M. L. Frey. 1993. Enhanced replication of porcine reproductive and respiratory syndrome (PRRS) virus in a homogeneous subpopulation of MA-104 cell line. Arch. Virol. 133:477-483. [DOI] [PubMed] [Google Scholar]
- 17.Kreutz, L. C., and M. R. Ackerman. 1996. Porcine reproductive and respiratory syndrome virus enters cells through a low pH-dependent endocytic pathway. Virus Res. 42:137-147. [DOI] [PubMed] [Google Scholar]
- 18.Kreutz, L. C. 1998. Cellular membrane factors are the major determinants of porcine reproductive and respiratory syndrome virus tropism. Virus Res. 53:121-128. [DOI] [PubMed] [Google Scholar]
- 19.Kwang, J., F. Zuckermann, G. Ross, S. Yang, F. Osorio, W. Liu, and S. Low. 1999. Antibody and cellular immune responses of swine following immunisation with plasmid DNA encoding the PRRS virus ORFs 4, 5, 6 and 7. Res. Vet. Sci. 67:199-201. [DOI] [PubMed] [Google Scholar]
- 20.Labarque, G. G., H. J. Nauwynck, P. A. M. van Woensel, N. Visser, and M. B. Pensaert. 2000. Efficacy of an American and a European serotype PRRSV vaccine after challenge with American and European wild-type strains of the virus. Vet. Res. 31:97. [Google Scholar]
- 21.Mardassi, H., B. Massie, and S. Dea. 1996. Intracellular synthesis, processing and transport of proteins encoded by ORFs 5 to 7 of porcine reproductive and respiratory syndrome virus. Virology 221:98-112. [DOI] [PubMed] [Google Scholar]
- 22.Mettenleiter, T. C., L. Zsak, F. Zuckermann, N. Sugg, H. Kern, and T. Ben-Porat. 1990. Interaction of glycoprotein gIII with a cellular heparinlike substance mediates adsorption of pseudorabies virus. J. Virol. 64:278-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meulenberg, J. J. M., A. Petersen-den Besten, E. P. De Kluyver, R. J. Moormann, W. M. Schaaper, and G. Wensvoort. 1995. Characterization of proteins encoded by ORFs 2 to 7 of Lelystad virus. Virology 206:155-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meulenberg, J. J. M., and A. P. Den Besten. 1996. Identification and characterization of a sixth structural protein of Lelystad virus: the glycoprotein GP2 encoded by ORF2 is incorporated in virus particles. Virology 225:44-51. [DOI] [PubMed] [Google Scholar]
- 25.Nauwynck, H. J., and M. B. Pensaert. 1994. Virus production and viral antigen expression in porcine blood monocytes inoculated with pseudorabies virus. Arch. Virol. 137:69-79. [DOI] [PubMed] [Google Scholar]
- 26.Nauwynck, H. J., and M. B. Pensaert. 1995. Effect of specific antibodies on the cell-associated spread of pseudorabies virus in monolayers of different cell types. Arch. Virol. 140:1137-1146. [DOI] [PubMed] [Google Scholar]
- 27.Nauwynck, H. J., X. Duan, H. W. Favoreel, P. Van Oostveldt, and M. B. Pensaert. 1999. Entry of porcine reproductive and respiratory syndrome virus into porcine alveolar macrophages via receptor-mediated endocytosis. J. Gen. Virol. 80:297-305. [DOI] [PubMed] [Google Scholar]
- 28.Neyts, J., R. Snoeck, D. Schols, J. Balzarini, J. D. Esko, A. Schepdael, and E. De Clercq. 1992. Sulfated polymers inhibit the interaction of human cytomegalovirus with cell surface heparan sulfate. Virology 189:48-58. [DOI] [PubMed] [Google Scholar]
- 29.Plagemann, P. G., and V. Moennig. 1992. Lactate dehydrogenase-elevating virus, equine arteritis virus, and simian hemorrhagic fever virus: a new group of positive-strand RNA viruses. Adv. Virus Res. 41:99-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prydz, K., and K. T. Dalen. 2000. Synthesis and sorting of proteoglycans. J. Cell Sci. 113:193-205. [DOI] [PubMed] [Google Scholar]
- 31.Reed, L. J., and H. Muench. 1938. A simple method of estimating fifty percent endpoints. Am. J. Hyg. 27:493-497. [Google Scholar]
- 32.Rowland, R. R., R. Kervin, C. Kuckleburg, A. Sperlich, and D. A. Benfield. 1999. The localisation of porcine reproductive and respiratory syndrome virus nucleocapsid protein to the nucleolus of infected cells and identification of a potential nucleolar localisation signal sequence. Virus Res. 64:1-12. [DOI] [PubMed] [Google Scholar]
- 33.Schreurs, C., T. C. Mettenleiter, F. Zuckerman, N. Sugg, and T. Ben-Porat. 1988. Glycoprotein gIII of pseudorabies virus is multifunctional. J. Virol. 62:2251-2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Snijder, E. J., and J. J. M. Meulenberg. 1998. The molecular biology of the arteriviruses. J. Gen. Virol. 79:961-979. [DOI] [PubMed] [Google Scholar]
- 35.Terpstra, C., G. Wensvoort, and J. M. A. Pol. 1991. Experimental reproduction of porcine epidemic abortion and respiratory syndrome (mystery swine disease) by infection with Lelystad virus: Koch's postulates fulfilled. Vet. Q. 13:131-136. [DOI] [PubMed] [Google Scholar]
- 36.Therrien, D., Y. St.-Pierre, and S. Dea. 2000. Preliminary characterization of protein binding factor for porcine reproductive and respiratory syndrome virus on the surface of permissive and non-permissive cells. Arch. Virol. 145:1099-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van Nieuwstadt, A. P., J. J. M. Meulenberg, A. Van Essen-Zandbergen, A. P. Den Besten, R. J. Bende, R. J. M. Moorman, and G. Wensvoort. 1996. Proteins encoded by open reading frames 3 and 4 of the genome of Lelystad virus (Arteriviridae) are structural proteins of the virion. J. Gen. Virol. 70:4767-4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weiland, E., M. Wieczorek-Krohmer, D. Kohl, K. K. Conzelmann, and F. Weiland. 1999. Monoclonal antibodies to the GP5 of porcine reproductive and respiratory syndrome virus are more effective in virus neutralization than monoclonal antibodies to the GP4. Vet. Microbiol. 66:171-186. [DOI] [PubMed] [Google Scholar]
- 39.Wensvoort, G., E. P. de Kluyver, J. M. A. Pol, R. J. M. Moorman, M. M. Hulst, R. Bloemraad, A. den Besten, T. Zetstra, and C. Terpstra. 1992. Lelystad virus, the cause of porcine epidemic abortion and respiratory syndrome: a review of mystery swine disease research at Lelystad. Vet. Microbiol. 33:185-193. [DOI] [PubMed] [Google Scholar]
- 40.Wensvoort, G., C. Terpstra, J. M. A. Pol, E. A. ter Laak, M. Bloemraad, E. P. de Kluyver, C. Kragten, L. van Buiten, A. den Besten, F. Wagenaar, J. M. Broekhuijsen, P. L. J. M. Moonen, T. Zetstra, E. A. de Boer, H. J. Tibben, M. F. de Jong, P. van 't Veld, G. J. R. Groenland, J. A. van Gennep, M. T. Voets, J. H. M. Verheijden, and J. Braamskamp. 1991. Mystery swine disease in the Netherlands: the isolation of the Lelystad virus. Vet. Q. 13:121-130. [DOI] [PubMed] [Google Scholar]
- 41.Wieczorek-Krohmer, M., F. Weiland, K. Conzelmann, D. Kohl, N. Visser, P. Van Woensel, H. J. Thiel, and E. Weiland. 1996. Porcine reproductive and respiratory syndrome virus (PRRSV): monoclonal antibodies detect common epitopes on two viral proteins of European and U.S. isolates. Vet. Microbiol. 51:257-266. [DOI] [PubMed] [Google Scholar]