Abstract
Induction of cytotoxic T-cell-mediated virus-clearing responses by influenza virus T cell determinant-containing peptide immunogens was examined. The most potent synthetic immunogens for eliciting pulmonary viral-clearing responses contained peptides representing determinants for CD4 and CD8 T cells (TH and CTL peptides, respectively) together with two or four palmitic acid (Pal) groups. Inoculated in adjuvant, these Pal2- or Pal4-CTL-TH lipopeptides and the nonlipidated CTL peptide induced equivalent levels of cytolytic activity in the primary effector phase of the response. The ability to recall lytic responses, however, diminished much more rapidly in CTL peptide-primed than in lipopeptide-primed mice. By 15 months postpriming, the recalled lytic activity in lipopeptide-inoculated mice remained potent, but the response induced by the CTL peptide was weak. Enumeration of specific gamma interferon-secreting CD8 T cells revealed that a greater number of these T cells had entered or remained in the memory pool in lipopeptide-primed mice, arguing for a quantitative rather than qualitative enhancement of the response on recall. Addition of either the lipid or the TH peptide to the CTL peptide was not sufficient to provide these long-lived antiviral responses, but inclusion of both components augmented the response. CD4 T cells elicited by the lipopeptides did not influence the rate of viral clearance upon challenge and most likely had a role in induction or maintenance of the memory response. It therefore appears that the lipopeptide immunogens, although not significantly superior at inducing primary effector CD8 T cells, elicit a much more effective memory population, the recall of which may account for their superiority in inducing pulmonary protection after viral challenge.
CD8+ cytotoxic T lymphocytes are thought to be important effectors responsible for the clearance of viral infections and are consequently a valuable population to induce by vaccination. In contrast to live virus, conventional vaccines consisting of either inactivated virus or soluble proteins are not potent inducers of cytotoxic responses, presumably because the viral antigens are inefficient at entering the cytosol of the cell to gain access to the endogenous class I processing pathway (24). Strategies that can potentially overcome this problem include the use of live viral vectors that are able to express target proteins or individual determinants within the cell (2, 17, 49); lipid-based vaccine delivery systems, including ISCOMs (15, 25, 40) and liposomes (27, 50), which are thought to transport antigen across cell membranes into the cytosol; and DNA vaccines (44) from which antigen is synthesized directly within the cell.
An alternate approach involves the use of synthetic peptides representing minimal CD8 T-cell determinants. In general, induction of cytotoxic T-cell responses with peptides requires the use of an adjuvant such as the oil-based incomplete Freund’s adjuvant (1, 13, 19, 36, 51). Nevertheless, induction of CD8 T cells can be achieved with peptides in the absence of an adjuvant by the addition of a lipid to the peptide (10, 16, 23) or by utilizing an admixture of peptide and lipid molecules (7, 16). Cytolytic responses in nonhuman primates (8), as well as in human volunteers (21, 46), have been reported following vaccination with lipopeptides.
Considering the wealth of data addressing methods for cytotoxic T-cell induction using determinant-based strategies, it is surprising how few reports (19, 35, 36, 45) exist describing effective T-cell-mediated viral clearing responses. Other workers describe either no benefit or only partial enhancement of viral clearance after immunization (13, 29, 32). This dissociation of cytotoxic T-cell induction and effective viral clearing responses has been noted previously by Lawson et al. (20), who showed that the presence of pulmonary cytotoxic T cells induced by vaccinia virus recombinants expressing the dominant H-2d-restricted CTL determinant from influenza virus nucleoprotein (NP; residues 147 to 155) did not protect BALB/c mice against challenge with influenza virus. This was in direct contrast to adoptive transfer of NP-specific CD8 T cells, which led to significant reduction in lung virus titers and prevention of death from challenge with a lethal dose of virus (3, 42).
In the present study we assembled synthetic peptide-based immunogens incorporating the NP 147-155 determinant and examined their ability to induce both CD8 T cells and viral clearing responses. We show that CTL determinant-based vaccines can have a significant impact on the rate of viral clearance in a challenge model. The best reduction in lung virus titer was obtained with immunogens containing, in addition to the CTL determinant, a CD4 T-cell determinant from the virus, as well as two or four lipid groups. A lack of correlation between measurable CD8 T-cell activity and protection was also observed during the effector phase of the response; despite equivalent cytolytic and gamma interferon (IFN-γ)-secreting CD8 T-cell responses in both lipopeptide and nonlipidated peptide-primed mice, lipopeptides induced a greater degree of viral clearance. More remarkable, however, was the longevity of this lipopeptide-induced immunity, which contrasted sharply with the short-lived responses induced by nonlipidated peptides. Here we examine some of the properties of the lipopeptide vaccine that are important for its activity.
MATERIALS AND METHODS
Viruses.
The type A influenza viruses used in this study were an H3N1 subtype virus referred to as Mem 71, which was derived by genetic reassortment of A/Memphis/1/71 (H3N2) × A/Bellamy/42 (H1N1) and A/Puerto Rico/8/34 (PR8; H1N1). Virus was grown for 2 days in the allantoic cavity of 10-day embryonated hen's eggs. Allantoic fluid containing virus was stored in aliquots at −70°C. Purified virus, prepared by rate zonal centrifugation, was obtained from CSL Ltd., Parkville, Victoria, Australia. Virus titers were determined by the hemagglutination assay (12) and expressed as hemagglutinating units per milliliter. Infectious virus titers were obtained by assay of plaque formation in monolayers of Madin-Darby canine kidney (MDCK) cells (41) and are expressed as PFU/milliliter.
Preparation and purification of synthetic immunogens.
A panel of immunogens was synthesized that incorporated peptides representing a minimal determinant for CD8 T cells and/or a determinant for CD4 T cells, both from influenza virus. The peptide NP (147-155) with the sequence TYQRTRALV (a CTL determinant present in the NP of PR8 virus) is the dominant CD8 T-cell determinant recognized by BALB/c mice and is common to all type A influenza virus strains (6, 38). The peptide HA2 (166-180), with the sequence ALNNRFQIKGVELKS, is a TH determinant present within the HA2 chain of Mem 71 influenza virus hemagglutinin and elicits CD4 T cells that are cross-reactive with all viruses of the H3 subtype (18).
The synthetic immunogens were assembled by conventional solid-phase methodology using Fmoc (9-fluorenylmethoxy carbonyl) chemistry throughout in a Milligen 9050 Plus automatic peptide synthesizer. Peptides were synthesized on Novasyn KA 100 resin (Novabiochem). Side chain protecting groups were as follows: Arg, PMC; Thr, tBu; Ser, tBu; Gln, Trt; Asn, Trt; Lys, Boc or Dde; Glu, Otbu; and Tyr, tBu. All amino acids were incorporated as the free acid in the presence of equimolar amounts of o-benzotriazole-N,N,N′,N′-tetramethyl-uronium-hexafluorophosphate (HBTU) and 1-hydroxybenzotriazole (HOBt) and 1.5 eq of diisopropylethylamine (DIPEA). Acylation was done for 30 min and removal of the Fmoc group was carried out by using 2.5% 1,8-diazabicyclo-[5.4.0]undec-7-ene (DBU) in N,N′-dimethyl formamide (DMF).
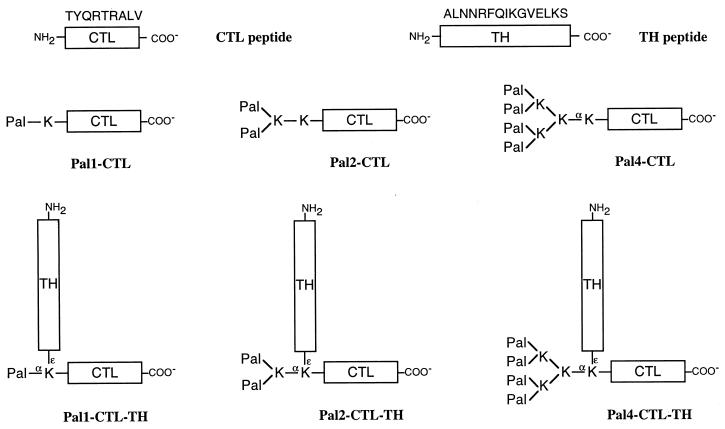
A schematic diagram of each of the structures is shown in Fig. 1.
FIG. 1.
Schematic representation of the synthetic peptide immunogens used in this study. The CTL peptide represents the CD8 T-cell determinant NP (147-155) present in the NP of influenza A viruses of different strains and subtypes. The TH peptide represents the CD4 determinant HA2 (166-180) present in the light chain of the HA molecule of A/Memphis/1/71 virus and highly conserved within the H3 influenza virus subtype. The amino acid sequence of these determinants is shown in single-letter code above the monomer peptides. Pal, palmitic acid; K, lysine. The α- and ɛ-amino groups of certain lysine residues are indicated for clarity, as well as the C and N termini of the CTL and TH peptides. Within the text, peptide immunogens are referred to by the abbreviated term shown next to each schematic diagram.
CTL peptide and TH peptide.
These were synthesized as single 9- and 15-residue peptides, respectively.
Pal1-CTL.
A lysine residue was added to the N terminus of the CTL peptide and a single palmitic acid (Pal) residue added to the α-amino group of this N-terminal lysine.
Pal2-CTL.
Two lysine residues were added to the N terminus of the CTL peptide, the N-terminal lysine of these was Fmoc-Lys(Fmoc)-OH, permitting the incorporation of two Pal residues after removal of the Fmoc groups.
Pal4-CTL.
Three lysine residues were added to the N terminus of the CTL peptide; the last two lysine residues added were both Fmoc-Lys(Fmoc)-OH, which allowed the formation of a fan-like structure with four amino groups available for the incorporation of Pal residues.
CTL-TH.
A residue of Fmoc-Lys(Dde)-OH was added to the N terminus of the CTL peptide. The α-amino group of this lysine was acetylated with N-acetylimidazole and the Dde group at the ɛ-position was then removed with 2.5% hydrazine in DMF. Exposure of this ɛ-amino group then allowed assembly of the TH determinant sequence from this point.
Pal1-CTL-TH.
A residue of Fmoc-Lys(Dde)-OH was added to the N terminus of the CTL peptide and a single Pal residue added to the α-amino group of this N-terminal lysine. The Dde group was then removed by using 2.5% hydrazine in DMF. Exposure of the ɛ-amino group in this way then allowed assembly of the TH epitope sequence from this point.
Pal2-CTL-TH.
A residue of Fmoc-Lys(Dde)-OH was added to the N terminus of the CTL peptide followed by a single residue of Fmoc-Lys(Fmoc)-OH to provide two amino groups, α and ɛ, to which two Pal residues were added. The Dde group was then removed from the penultimate lysine residue, and the TH determinant was assembled from this point.
Pal4-TH-CTL.
A residue of Fmoc-Lys(Dde)-OH was added to the N terminus of the CTL epitope peptide, followed by two residues of Fmoc-Lys(Fmoc)-OH to provide four amino groups in a branched fan-like structure. To these were added four Pal residues. The Dde group was then removed from the penultimate lysine residue, and the TH determinant was assembled from this point.
Immunization protocols.
To examine the ability of peptide immunogens to elicit viral clearing responses, mice were immunized subcutaneously (s.c.) in the scruff of the neck with 9 nmol of peptide antigens either emulsified at a 1:1 (vol/vol) ratio in complete Freund adjuvant (CFA; Sigma Chemical Co., St. Louis, Mo.) or in phosphate-buffered saline (PBS). Mice given one dose of the immunogens were challenged either 7 or 28 days after priming, and mice receiving two doses (days 0 and 21) were challenged 21 days after their final immunization.
Challenge of mice i.n. and preparation of mouse lung extracts.
Penthrane-anesthetized mice were challenged intranasally (i.n.) with 104.5 PFU of infectious Mem 71 influenza virus. Each mouse received 50 μl of virus in the form of allantoic fluid diluted in PBS. At 5 days after challenge, the mice were killed by cervical dislocation, and the lungs were removed and transferred aseptically to bottles containing 1.5 ml of Hank’s balanced salt solution supplemented with 100 U of penicillin, 100 μg of streptomycin, and 30 μg of gentamicin per ml. Lung homogenates were prepared by using a tissue homogenizer, and the cell material was pelleted by centrifugation at 300 × g for 5 min. The supernatants were removed, divided into aliquots, and stored at −70°C until use. Titers of infectious virus in the lung supernatants were determined by plaque assay on monolayers of MDCK cells (41).
Cell culture medium.
T-cell culture medium consisted of RPMI 1640 (CSL Ltd.) supplemented with 10% (vol/vol) heat-inactivated fetal calf serum, 2 mM l-glutamine, 2 mM sodium pyruvate, 30 μg of gentamicin/ml, 100 μg of streptomycin/ml, 100 IU of penicillin/ml, and 10−4 M 2-mercaptoethanol.
Cytotoxic T-cell assays.
Secondary effector cells were generated either from inguinal and popliteal lymph nodes of mice that had been immunized s.c. 7 days previously with peptide immunogens emulsified in CFA or from spleen cells of mice primed at least 28 days previously with the peptide immunogens. Briefly, 4 × 107 lymph node cells or spleen cells, depleted of erythrocytes by treatment with Tris-buffered ammonium chloride (0.15 M NH4Cl in 17 mM Tris-HCl at pH 7.2), were cultured with 107 irradiated (2,200 rads, 60Co source) virus-infected or peptide-pulsed syngeneic spleen cells in 25-cm2 tissue culture flasks (Falcon) containing 15 ml of T-cell culture medium. The virus-infected spleen cells had been preincubated at 37°C for 30 min with 3,000 hemagglutinating units of either infectious Mem 71 or PR8 virus in 1 ml of serum-free RPMI and washed once prior to addition to the flask. The peptide-pulsed spleen cells had been preincubated at 37°C for 60 min with 100 μg of the CTL peptide/ml and also washed once prior to addition to the flask. After 5 days of culture at 37°C in a humidified atmosphere containing 5% CO2, the cells were washed three times and used in 51Cr-release assays. The 51Cr-release assays were performed in triplicate as described previously (14) by using P815 mastocytoma cells (H-2d, DBA/2) as targets.
ELISPOT assay for IFN-γ-secreting cells.
CTL peptide-specific IFN-γ-secreting cells were enumerated by an ELISPOT assay modified from that of Murali-Krishna et al. (26). Flat-bottom polyvinyl chloride microtiter plates (96-well; Dynatech) were coated overnight with 50 μl of rat anti-(mouse IFN-γ) antibody (clone R4-6A2) at 5 μg/ml in PBS. Unoccupied sites on the wells were then blocked by incubation for 1 h with 10 mg of bovine serum albumin/ml in PBS, and the plates were washed three times with PBS containing 0.05% Tween 20 (PBST). Twofold dilutions of spleen or lymph node cells in T-cell medium were then added to the wells, together with 5 × 105 irradiated (2,200 rads, 60Co source) syngeneic spleen cells from unimmunized mice and 10 U of recombinant human interleukin-2 (Pharmingen, San Diego, Calif.)/well. Cells were incubated at 37°C in 5% CO2 for 18 h in the presence or absence of the CTL peptide at a concentration of 1 μg of peptide/ml. Cells were then lysed and removed by rinsing the plates, initially with distilled water and then PBST. Then, 50 μl of a 1/500 dilution of biotinylated anti-(mouse IFN-γ) antibody (clone XMG 1.2; Pharmingen) was added, and the plates were incubated at room temperature for 2 h. Plates were again washed, and 50 μl of streptavidin-alkaline phosphatase (Pharmingen; 1/400 dilution in 5 mg of bovine serum albumin/ml of PBST) was added to each well; the mixtures were then incubated for a further 2 h. The plates were washed, and 100 μl of ELISPOT substrate (37) containing 1 mg of BCIP (5-bromo-4-chloro-3-indolylphosphate) per ml of 2-amino-2-methyl-1-propynol buffer (Sigma) was added to each well. When blue-green spots had developed, the plates were washed with water and dried, and the spots were counted with the aid of an inverted microscope.
In vivo depletion of CD4+ T cells.
Rat GK1-5 hybridoma cells, specific for CD4, were cultured in T-cell medium and immunoglobulin G (IgG) isolated from tissue culture supernatant by affinity chromatography on protein G-Sepharose. BALB/c mice were depleted of CD4 T cells by intraperitoneal injection of 400 μg of purified GK1-5 antibody in PBS; control mice received 400 μg of normal rat IgG. Treatments were performed 8 days prior to viral challenge, and depletion was confirmed by flow cytometric analysis of cells from blood collected 5 to 7 days after antibody treatment.
RESULTS
Synthetic immunogens containing a viral CTL determinant can elicit virus-specific cytotoxic T cells.
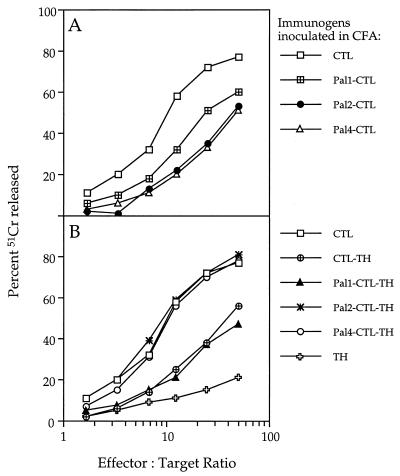
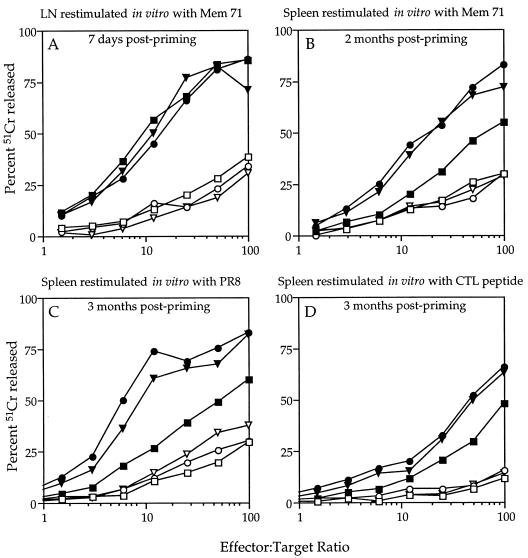
The peptide immunogens shown in Fig. 1 were compared for their ability to induce anti-influenza virus cytotoxic responses. Lymph node-derived effector cells from mice that had been primed 7 days previously with the immunogens emulsified in CFA were amplified in culture with Mem 71 virus-infected autologous spleen cells and tested in a 51Cr release assay for their ability to lyse virus-infected target cells. As shown in Fig. 2, all synthetic immunogens containing the CTL peptide induced a virus-specific cytotoxic response, suggesting that the CTL determinant, even within the most complex of these immunogens, could be appropriately processed and presented in vivo.
FIG. 2.
Cytotoxic T-cell response induced by the synthetic immunogens based on the CTL peptide alone (A) or the CTL-TH construct (B). A 51Cr release assay was performed with 104 uninfected or 104 Mem 71 virus-infected 51Cr-labeled P815 target cells and various numbers of effector cells. Secondary effectors were generated from lymph node cells of groups of five BALB/c mice that had been primed 7 days previously with 45 nmol of a peptide immunogen emulsified in CFA. The lymph node cell suspensions were cultured for 5 days with Mem 71 virus-infected autologous spleen cells and then tested in the 51Cr release assay. Each point on every curve represents the mean of triplicate cultures. For all effector populations, background lysis measured on uninfected P815 targets was <20% at an effector/target ratio of 100:1.
When the relative efficiency of the response was compared, it was seen that the nonlipidated CTL peptide induced a stronger response than when it was associated with one, two, or four Pal groups (Fig. 2A). In contrast, in the case of immunogens incorporating both CTL and TH determinants (Fig. 2B), the presence of Pal was found to augment the level of virus-specific cytotoxic activity induced; lipopeptides incorporating the CTL and TH determinants, together with either two or four Pal groups, induced a more potent cytotoxic response than those containing no Pal group or one Pal group. The level of cytotoxic activity elicited by the two lipopeptides Pal2-CTL-TH and Pal4-CTL-TH was comparable to that induced by the CTL peptide.
Further, effector cells generated from lymphocytes of mice primed with the TH peptide and restimulated in vitro with virus-infected stimulators did not lyse virus-infected targets (Fig. 2B). This result implies that there is no cytotoxic-T-cell determinant recognized by BALB/c mice within the TH peptide. This experiment also provides a control to illustrate that in vitro amplification of effector cells with virus-infected stimulators does not itself induce primary cytotoxic T cells in culture.
It should also be pointed out that no antibodies to influenza virus or to the TH peptide itself were detected in sera taken from mice primed with any of the constructs as assessed by enzyme-linked immunosorbent assay (data not shown). This indicates that, although the TH sequence was isolated from the viral hemagglutinin and contains a potent TH determinant, it does not contain a B-cell determinant.
Synthetic lipopeptide immunogens induce potent viral clearing responses.
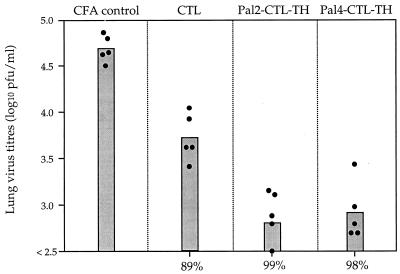
Since immunization with the synthetic lipopeptides leads to the induction of strong cytotoxic T-cell responses, it was of interest to determine whether the responses induced could aid in the clearance of a pulmonary influenza virus infection. Initially, only immunogens eliciting the strongest cytolytic responses were examined. Groups of five BALB/c mice were immunized with either the CTL peptide or the lipopeptides Pal2-CTL-TH or Pal4-CTL-TH emulsified in CFA. During the primary effector phase of the response (7 days after priming) mice were challenged i.n. with a nonlethal dose of Mem 71 virus, and 5 days later the lungs were removed and assayed for the presence of infectious virus. Despite the fact that the three immunogens could elicit T cells that exhibited similar lytic activity (Fig. 2), the lipopeptides elicited a higher level of viral clearance than did the nonlipidated CTL peptide (Fig. 3). The viral load in the lungs of lipopeptide-primed mice on day 5 after challenge was reduced from 104.8 PFU/ml by 99% (Pal2-CTL-TH) and by 98% (Pal4-CTL-TH). Virus load in the lungs of CTL peptide-primed mice was also significantly reduced but by only 89% relative to the CFA control group.
FIG. 3.
Clearance of pulmonary viral infection by primary effector responses induced by synthetic immunogens. Groups of five mice were immunized s.c. with 9 nmol of the specified immunogens emulsified in CFA. Seven days later mice were challenged i.n. with 104.5 PFU of Mem 71 influenza virus and, 5 days postchallenge, mice were sacrificed, the lungs were removed, and lung homogenates were prepared. Homogenates were assayed for infectious virus by plaque formation on MDCK cell monolayers. Closed circles represent the lung virus titers of individual mice, and the bar represents the geometric mean titer for the group of mice.
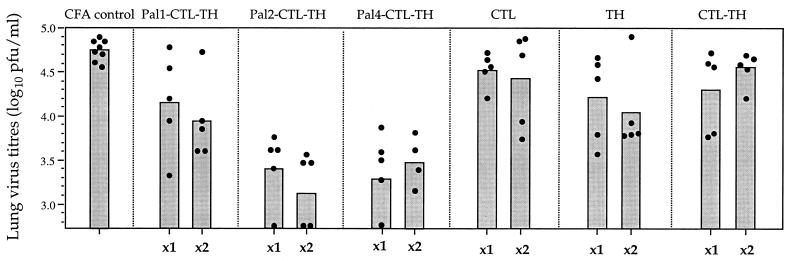
On the basis of these results a wider range of the synthetic immunogens were evaluated for their ability to induce viral clearing responses. In this experiment, mice were challenged at a later time point to assess the ability of the response induced by the immunogens to be recalled upon viral infection, a model more relevant to a vaccine situation. Mice were inoculated with either one or two doses of synthetic immunogen and challenged 3 to 4 weeks after the final dose. The results (Fig. 4) show that Pal2-CTL-TH and Pal4-CTL-TH elicited the highest level of viral clearance. After one dose of either immunogen, the virus load in the lungs after challenge was reduced from 104.8 PFU/ml by 96% (Pal2-CTL-TH) and by 97% (Pal4-CTL-TH). In the absence of Pal or with only a single Pal group the reduction in lung virus titer was significantly less. Clearance was therefore greatly augmented by the addition of two or four Pal groups. The level of clearance, however, was very similar after one or two doses of immunogen. The CTL peptide, despite eliciting significant levels of viral clearance when virus challenge was performed during the effector phase of the response, was not able to induce a response that significantly reduced the lung virus titers in mice challenged 1 month after priming.
FIG. 4.
Recall of pulmonary viral clearing responses in mice inoculated with synthetic immunogens. Groups of five mice were immunized s.c. with 9 nmol of the specified peptide immunogens emulsified in CFA. Mice receiving one dose of the immunogens (x1) were challenged 28 days after priming; mice receiving two doses (x2) were primed on day 0, boosted in an identical manner on day 21, and then challenged i.n. with 104.5 PFU of Mem 71 influenza virus on day 42. Titers of infectious virus in lung homogenates sampled 5 days after challenge were determined by plaque formation. Closed circles represent the lung virus titers of individual mice, and the bar represents the geometric mean titer of the group of mice.
The ability to recall CD8 T-cell responses diminishes more rapidly in CTL-peptide-primed than in lipopeptide-primed mice.
The ability of synthetic peptide immunogens to elicit a viral clearing response does not appear to be directly related to the magnitude of the lytic response measured during the effector phase. To investigate the relationship between viral clearance and cytotoxicity further, the relative levels of lytic activity of spleen cells taken from CTL peptide- and lipopeptide-primed mice just prior to viral challenge were compared when the challenge was given at either 7 days or several months after priming (Fig. 5). Unlike the primary effector population, the lytic activity of the memory population, after culture with Mem 71-infected stimulators in vitro, was greater with effectors derived from lipopeptide-primed mice than with those from CTL peptide-primed mice. However, it could be argued that the apparently more active lytic response of the effector population from lipopeptide-primed mice may have actually been due to enhanced expansion of the CD8 T cells during the in vitro culture phase by cytokines produced by simultaneously activated CD4 T cells specific for the TH determinant. To ensure that this was not the case, responder cells were restimulated in culture with PR8 virus (Fig. 5C) or the CTL peptide (Fig. 5D). In contrast to restimulation with Mem 71 virus, which expresses both CTL and TH determinants, restimulation with the CTL peptide or with PR8 virus, which only expresses the CTL determinant in common with the lipopeptides, would only expand the CD8 T-cell population. It was shown that, irrespective of which antigen was used in the in vitro restimulation phase, the lytic activity was always greater in the lipopeptide-vaccinated mice than in CTL peptide-primed mice. This result indicates that the drop in effectiveness of the viral clearing responses induced by the CTL peptide between one week and several months does follow a reduction in the lytic responses measured in vitro by Cr release. This decay in effective immunity is very much less pronounced when the response is induced by lipopeptide immunization.
FIG. 5.
Comparison of the lytic activity of cytotoxic T cells from CTL peptide-primed mice versus lipopeptide-primed mice at 7 days and at several months postpriming. A 51Cr release assay was performed by using uninfected P815 targets (open symbols) or Mem 71 virus-infected P815 targets (closed symbols) and various numbers of effector cells. Lymph node effectors (A) or spleen cell effectors (B, C, and D) were generated from mice that had been primed either 7 days (A) or at least 2 months previously (B, C, and D) with 9 nmol of CTL peptide (squares), Pal2-CTL-TH (circles), or Pal4-CTL-TH (triangles) emulsified in CFA. Lymph node or spleen cells were then cultured for 5 days with either virus-infected or peptide-pulsed autologous spleen cells, as indicated above each panel, and then tested in a 51Cr release assay.
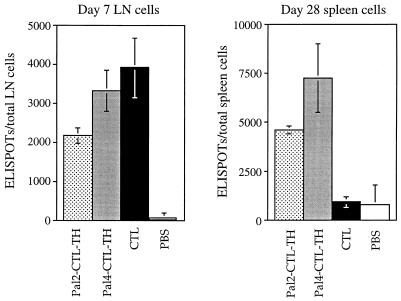
These results were supported by an IFN-γ ELISPOT assay used to enumerate CTL determinant-specific CD8 T cells induced by the different immunogens in the effector and memory phases of the response. Figure 6 shows that at 7 days after priming the number of specific CD8 T cells detected in the draining lymph nodes of CTL peptide- and lipopeptide-primed mice was similar, whereas at 28 days after priming there were significantly fewer of these cells detected in the spleens of CTL peptide-primed mice than of lipopeptide-primed mice. Clearly, Pal2- and Pal4-CTL-TH elicit a numerically greater and more effective memory CD8 T-cell population than does the CTL peptide alone.
FIG. 6.
Comparison of the number of CTL determinant-specific IFN-γ-secreting cells in lipopeptide- and CTL peptide-primed mice. ELISPOT assays were performed on cells from the inguinal and popliteal lymph nodes of mice primed 7 days previously (left panel) or from the spleens of mice primed 28 days previously (right panel) with the indicated immunogens, emulsified in CFA. The data represent the number of ELISPOTs per total cells recovered, with backgrounds in cultures lacking antigen subtracted. The results are expressed as the mean value obtained from three individual mice, and the error bars represent one standard deviation of the mean.
Lipid is a critical component for the induction of long-term memory CTL by lipopeptides.
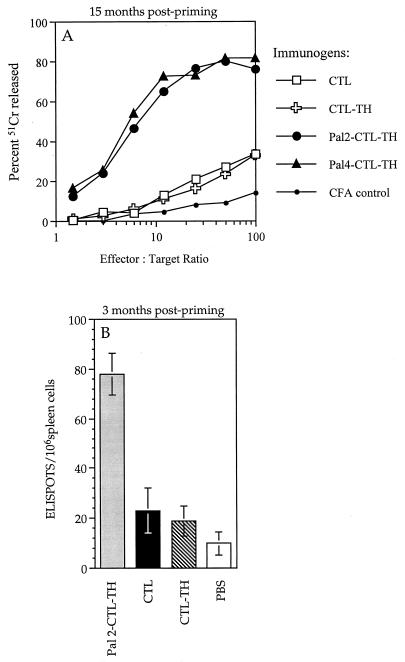
To determine which components of the Pal2- and Pal4-CTL-TH immunogens are responsible for the induction of long-term memory responses, virus-specific cytotoxic activity was measured by using the standard Cr release assay 15 months after priming. As can be seen in Fig. 7A, only Pal2-CTL-TH and Pal4-CTL-TH and not the nonlipidated CTL peptide or the CTL-TH immunogen induced potent memory cytolytic responses. The number of CTL determinant-specific CD8 T cells present in the spleens of mice 3 months after priming with the CTL peptide, CTL-TH, or Pal2-CTL-TH constructs in CFA were also determined by the IFN-γ ELISPOT assay (Fig. 7B). Overall, these results confirmed that the lipid is a critical component for the induction of long-term memory cytotoxic-T-cell responses because the addition of the TH peptide to the CTL peptide in the absence of lipid did not confer this property.
FIG. 7.
Lipopeptides elicit potent CD8 T-cell memory responses. (A) Cytolytic effector cells were generated in vitro from spleen cells of mice that had been primed 15 months previously with 9 nmol of the indicated constructs emulsified in CFA. The cells were cultured for 5 days with Mem 71 virus-infected autologous spleen cells and then tested in the 51Cr release assay. For each immunogen, the background lysis measured on uninfected P815 targets was <15% at an effector/target ratio of 100:1. (B) CTL determinant-specific IFN-γ-secreting cells were enumerated in the spleens of mice inoculated 3 months previously with 9 nmol of the indicated immunogens emulsified in CFA. The data are presented as the number of ELISPOTS per 106 spleen cells, with backgrounds in cultures lacking antigen subtracted. The results are expressed as the mean value obtained from three individual mice, and the error bars represent one standard deviation of the mean.
The level of viral clearance seen in Pal2-CTL-TH-immunized mice is not affected by the depletion of CD4 T cells prior to viral challenge.
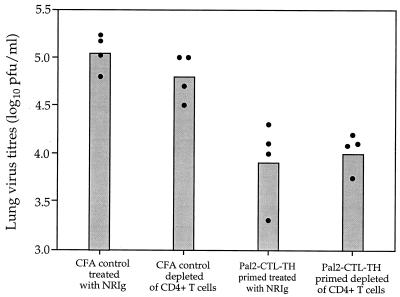
The role of the TH determinant in establishment of viral clearing responses was investigated by examining the effect of depleting CD4 T cells in the mouse after lipopeptide inoculation but prior to viral challenge. CD4 T-cell depletion was accomplished by using anti-CD4 monoclonal antibody GK1.5. Flow cytometric analysis showed that 6 days after mice were treated with 400 μg of GK1.5 IgG they had no detectable CD4 T cells in the periphery, whereas the levels of CD4 T cells in control mice treated with the same amount of normal rat IgG were unaffected (data not shown). Two groups of eight mice were inoculated s.c. with either the Pal2-CTL-TH immunogen emulsified in CFA or with adjuvant alone. After 28 days, four mice from each group were depleted of CD4 T cells by treatment with MAb GK1.5, and the other four mice received normal rat IgG. Successful depletion of CD4 T cells was verified by flow cytometric analysis of blood samples and all mice were subsequently challenged with Mem 71 virus on day 8 posttreatment. At 5 days after challenge, mice were sacrificed, their lungs were removed, and homogenates were assayed for infectious virus. Figure 8 shows that the level of viral clearance was not affected by the depletion of CD4 T cells prior to challenge, indicating that the helper cell population initially primed with the synthetic immunogen does not need to be recalled at the time of viral challenge for optimal viral clearance.
FIG. 8.
CD4 T-cell depletion of mice after lipopeptide immunization but prior to viral challenge. Two groups of eight BALB/c mice were immunized s.c. with either Pal2-CTL-TH emulsified in CFA or adjuvant alone. At 28 days postpriming, four mice from each group were depleted of CD4 T cells by treatment with 400 μg of MAb GK1.5 and the other four mice received 400 μg of normal rat IgG. Successful depletion of CD4 T cells was verified by flow cytometric analysis, and all mice were subsequently challenged with Mem 71 virus 8 days after treatment. Five days later, the mice were sacrificed, and the lung viral titers were determined by plaque assay. Closed circles represent the lung virus titers of individual mice, and the bar represents the geometric mean titer of the group of mice.
Exogenous adjuvant is not required for the generation of enhanced viral clearing responses by Pal2-CTL-TH.
The data generated in all of the above experiments were obtained with peptide immunogens emulsified in CFA so that comparisons could be made with nonlipidated peptide immunogens which are known to elicit only weak, if any, responses in the absence of adjuvant. To determine the requirement for adjuvant in the generation of cytolytic and viral clearing responses by these synthetic lipopeptide immunogens, mice were primed with 9 nmol of the CTL peptide, CTL-TH, Pal2-CTL, or Pal2-CTL-TH in PBS. Splenic effector cells were generated from these mice 28 days postpriming by culture with Mem 71 virus-infected autologous spleen cells and then tested in a 51Cr release assay for their ability to lyse virus-infected targets. In addition, similarly vaccinated mice were challenged on day 28 postpriming to assess the viral clearing responses.
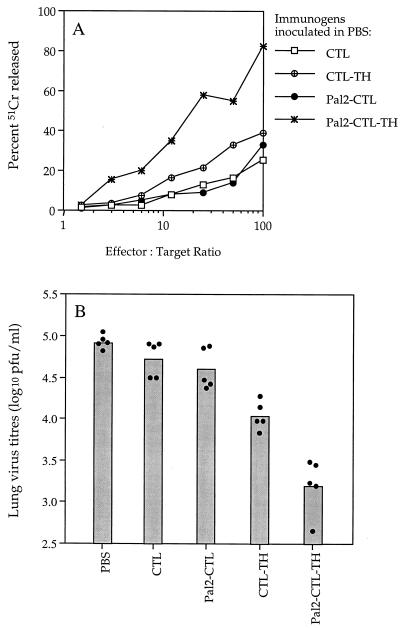
As shown in Fig. 9A, the CTL peptide in the absence of adjuvant elicited only a weak virus-specific lytic response, and this was not enhanced by the addition of two Pal groups. However, in the absence of any other source of stimulus for CD4 T cells (such as the mycobacterial antigen present in CFA), the addition of the TH peptide to the CTL peptide was found to slightly augment the level of virus-specific lytic activity induced. The Pal2-CTL-TH immunogen elicited the strongest lytic response and the same hierarchy was observed when the viral clearing responses were compared (Fig. 9B). Relative to the PBS control group, mice primed with Pal2-CTL-TH reduced the load of virus on day 5 after challenge by 98%. This was significantly greater than the level of reduction afforded by mice primed with CTL-TH (87%), Pal2-CTL (53%), or CTL peptide (37%).
FIG. 9.
Cytotoxic T-cell activity and viral clearance elicited by immunogens administered in the absence of adjuvant. (A) Spleen effectors were generated from mice that had been primed s.c. 28 days previously with 9 nmol of the indicated immunogens in PBS. Spleen cells were cultured for 5 days with Mem 71 virus-infected spleen cells and then tested in the 51Cr release assay. For all immunogens, background lysis measured on uninfected P815 targets was <15% at an effector/target ratio of 100:1. (B) Groups of five mice were immunized s.c. with 9 nmol of the peptide constructs in PBS and then challenged i.n. 29 days later with Mem 71 virus. At 5 days after challenge, mice were sacrificed and the lung viral titers determined. Closed circles represent the lung virus titers of individual mice, and the bar represents the geometric mean titer of the group of mice.
DISCUSSION
The data presented here represent the first demonstration of cytotoxic T-cell-mediated immunity induced by a synthetic lipopeptide immunogen that is capable of enhanced clearance of pulmonary influenza virus following challenge with infectious virus. In addition, it represents one of the few reports describing protective responses induced by a T-cell-determinant-based vaccine of any type for a viral disease.
Many previous studies have shown that lipid groups can confer self-adjuvanting properties on peptide immunogens (5, 9, 10, 16, 23, 28), and we confirm this in the experiment shown in Fig. 9, wherein Pal2-CTL-TH, unlike CTL-TH, elicited a significant response when delivered in the absence of external adjuvant. In the present study, however, we wanted to compare additional properties of these lipidated and nonlipidated immunogens beyond that of self-adjuvanticity. Delivery of the immunogens in CFA allowed the relative quality of the responses induced to be examined in a situation in which a source of stimulus for helper T-cell induction (from the mycobacterial antigen in the adjuvant) was not limiting and the nonlipidated peptides could induce immunity.
When delivered under these circumstances, the most effective lipopeptide immunogens incorporated a minimal determinant for CD8 T cells and CD4 T cells and two or four Pal groups as the source of lipid. Despite the considerable complexity of these immunogens, the CTL determinant and the TH determinant could be presented appropriately in vivo to generate cytotoxic T-cell (Fig. 2) and proliferative T-cell (not shown) responses, respectively, to virus. There were, however, some differences in the magnitude of the primary effector lytic response measured at 7 days postimmunization. The addition of Pal groups to the CTL peptide decreased its ability to elicit a lytic response, which may correlate with the corresponding decrease in solubility of the immunogen. Linking the TH determinant to the CTL determinant also led to a reduction in the lytic response, probably by introducing the requirement for processing before binding to class I molecules (43). Immunogens based on the CTL-TH peptides remained relatively soluble on addition of Pal groups and in this instance attachment of lipid increased the ability to elicit lytic responses. The CTL-TH constructs containing either two or four Pal groups induced a more potent lytic response than those with one or no Pal groups. Overall, the level of cytolytic activity elicited by the two lipopeptides, Pal2-CTL-TH and Pal4-CTL-TH, was comparable to that induced by the CTL peptide alone when each was given in CFA.
However, despite the ability of the CTL peptide and the Pal2- or Pal4-CTL-TH immunogens to elicit similar levels of lytic activity, their ability to induce a viral clearing response in the primary effector phase was different. Mice inoculated with either Pal2- or Pal4-CTL-TH emulsified in CFA and challenged 7 days after priming were able to reduce pulmonary virus titers on day 5 postchallenge by 99 and 98%, respectively. In contrast, in mice inoculated with the CTL peptide in CFA, ca. 10-fold more infectious virus remained in the lungs on day 5 postchallenge. These results show that the ability to induce a lytic response by in vivo immunization with synthetic peptides may in itself be insufficient to provide substantial viral clearance. Indeed, there have been several reports describing the effective generation of pulmonary influenza virus-specific cytotoxic T cells by inoculation of either short synthetic peptides (13, 32) or after vaccination with vaccinia virus recombinants expressing NP or peptides of NP (2, 3, 20), which fail to display substantial antiviral activity against influenza virus infection.
The difference between the lipidated and nonlipidated immunogens was even more marked when immunized animals were examined at later time points for their ability to recall cytolytic or viral clearing responses. Despite being similar in the primary effector phase, lytic responses recalled after at least 1 month were far greater in Pal2- or Pal4-CTL-TH-primed mice than in mice primed with CTL or CTL-TH peptides, indicating that CD8 memory T-cell responses diminished more rapidly in nonlipidated peptide-primed than in lipopeptide-primed mice. By 15 months the recalled lytic response in mice primed with the lipopeptides was still vigorous, while that in mice given nonlipidated immunogens was only slightly greater than that of the adjuvant control group. Further, the relative decline in lytic responses was mirrored by a numerical decline in specific IFN-γ-secreting CD8 T cells, indicating that the superior responses induced by the lipopeptides may be attributable to a quantitatively greater rather than qualitatively superior memory population. The long-lasting CD8 T-cell response may account for the observed superiority of the lipopeptides in inducing pulmonary protection upon challenge in the memory phase of the response.
Our data comparing lipidated and nonlipidated CTL-TH, as well as varying the number of lipid groups, indicated that the lipid moiety was crucial to obtaining effective responses, but the importance of inducing a CD4 T-cell response was less clear. As noted above, interpretation of any direct comparisons between responses induced by Pal2-CTL-TH and Pal2-CTL may not be valid because of solubility differences between these two immunogens, but in the several instances for which we have tested the Pal2-CTL peptide (not shown except in Fig. 2 and 9) its activity did not approach that of the equivalent immunogen containing the helper determinant. Potentially, induction of CD4 T cells may impact in determining the magnitude of the primary CD8 effector T-cell response and in the establishment or maintenance of the memory CD8 T-cell response, and there are several reports that provide data supporting this view (11, 21, 28, 33, 34, 39, 46, 47). Alternatively, the CD4 T cells may act as effectors to augment cytotoxic T-cell clearance of the virus or they may be involved in the rapid expansion of recalled cytotoxic T-cell effectors in the challenge phase of the response. To distinguish whether CD4 T cells are necessary in the induction or maintenance phase or in the recall effector phase, these cells were depleted from Pal2-CTL-TH-primed mice shortly prior to viral challenge. The observation that the level of clearance of pulmonary influenza virus in CD4 T-cell-depleted and intact mice was no different suggested that, once an effective memory CD8 response had been established, the presence of memory helper T cells did not act to augment viral clearance after challenge, either as direct antiviral effectors or for enhancing the recalled cytotoxic T-cell response. This is not to say that these lipopeptide-induced memory CD4 T cells did not allow a greater and more rapid expansion of the memory CD8 T cells upon exposure to virus (31) but that in terms of viral clearance there was no functional benefit due to additional T-cell help at this stage.
The mechanism by which lipopeptides can induce potent, long-lasting, and self-adjuvanting responses is still unclear. An early hypothesis was that the lipid allowed the peptide to translocate across the plasma membrane into the cytoplasm (48), thereby efficiently gaining access to the class I processing pathway. More recently, Andrieu et al. (4), using fluorescently labeled lipopeptides, demonstrated that these peptides enter dendritic cells (DC) by endocytosis, but the means of endosome escape is not understood. The lipopeptides require processing before they are presented to the immune system and cannot bind directly to class I or class II molecules (43), and advantages in the affinity and stability of the processed lipopeptide-class I complexes compared to complexes with nonlipidated peptides have been proposed (22). Our own hypothesis is that the lipopeptides can induce the triggering and maturation of DC and that the priming of T cells by DC matured in this manner is necessary for their continued longevity as memory cells. We already have preliminary data to support the notion that Pal2- and Pal4-CTL-TH, but not CTL-TH or CTL peptides, can trigger maturation of immature murine DC. This hypothesis would be in line with the recent finding that T cells induced by peptide immunization are relatively short-lived but, when administered with bacterial lipopolysaccharide, a known DC maturation stimulus, the persistence of antigen-specific T cells was dramatically enhanced (30).
For many viral diseases, effective resolution of infection depends on the presence of specific cytotoxic T cells. To date, methods for reliably generating long-lived antiviral CD8 T-cell responses by vaccination, in a manner compatible with delivery to humans, are limited. The present study, which sought to explore the relationship between cytotoxic T-cell induction and viral clearing responses, has highlighted the potential of lipopeptide immunogens for this purpose and elucidated some of the essential features for their success as potent inducers of immunity for the control of viral infection.
Acknowledgments
G.D. and D.C.J. contributed equally to this study.
This work was supported by grants from the Cooperative Research Centre for Vaccine Technology and the National Health and Medical Research Council of Australia.
We thank Joanne Pagnon for assistance in the laboratory.
REFERENCES
- 1.Aichele, P., H. Hengartner, R. M. Zinkernagel, and M. Schulz. 1990. Antiviral cytotoxic T cell response induced by in vivo priming with a free synthetic peptide. J. Exp. Med. 171:1815-1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrew, M. E., B. E. Coupar, G. L. Ada, and D. B. Boyle. 1986. Cell-mediated immune responses to influenza virus antigens expressed by vaccinia virus recombinants. Microb. Pathog. 1:443-452. [DOI] [PubMed] [Google Scholar]
- 3.Andrew, M. E., B. E. Coupar, D. B. Boyle, and G. L. Ada. 1987. The roles of influenza virus haemagglutinin and nucleoprotein in protection: analysis using vaccinia virus recombinants. Scand. J. Immunol. 25:21-28. [DOI] [PubMed] [Google Scholar]
- 4.Andrieu, M., E. Loing, J. F. Desoutter, F. Connan, J. Choppin, H. Gras-Masse, D. Hanau, A. Dautry-Varsat, J. G. Guillet, and A. Hosmalin. 2000. Endocytosis of an HIV-derived lipopeptide into human dendritic cells followed by class I-restricted CD8+ T lymphocyte activation. Eur. J. Immunol. 30:3256-3265. [DOI] [PubMed] [Google Scholar]
- 5.BenMohamed, L., H. Gras-Masse, A. Tartar, P. Daubersies, K. Brahimi, M. Bossus, A. Thomas, and P. Druilhe. 1997. Lipopeptide immunization without adjuvant induces potent and long-lasting B, T helper, and cytotoxic T lymphocyte responses against a malaria liver stage antigen in mice and chimpanzees. Eur. J. Immunol. 27:1242-1253. [DOI] [PubMed] [Google Scholar]
- 6.Bodmer, H. C., R. M. Pemberton, J. B. Rothbard, and B. A. Askonas. 1988. Enhanced recognition of a modified peptide antigen by cytotoxic T cells specific for influenza nucleoprotein. Cell 52:253-258. [DOI] [PubMed] [Google Scholar]
- 7.Borges, E., K. H. Wiesmuller, G. Jung, and P. Walden. 1994. Efficacy of synthetic vaccines in the induction of cytotoxic T lymphocytes. Comparison of the costimulating support provided by helper T cells and lipoamino acid. J. Immunol. Methods 173:253-263. [DOI] [PubMed] [Google Scholar]
- 8.Bourgault, I., F. Chirat, A. Tartar, J. P. Levy, J. G. Guillet, and A. Venet. 1994. Simian immunodeficiency virus as a model for vaccination against HIV. Induction in rhesus macaques of GAG- or NEF-specific cytotoxic T lymphocytes by lipopeptides. J. Immunol. 152:2530-2537. [PubMed] [Google Scholar]
- 9.Deprez, B., J. P. Sauzet, C. Boutillon, F. Martinon, A. Tartar, C. Sergheraert, J. G. Guillet, E. Gomard, and H. Gras-Masse. 1996. Comparative efficiency of simple lipopeptide constructs for in vivo induction of virus-specific CTL. Vaccine 14:375-382. [DOI] [PubMed] [Google Scholar]
- 10.Deres, K., H. Schild, K. H. Wiesmuller, G. Jung, and H. G. Rammensee. 1989. In vivo priming of virus-specific cytotoxic T lymphocytes with synthetic lipopeptide vaccine. Nature 342:561-564. [DOI] [PubMed] [Google Scholar]
- 11.Fayolle, C., E. Deriaud, and C. Leclerc. 1991. In vivo induction of cytotoxic T cell response by a free synthetic peptide requires CD4+ T cell help. J. Immunol. 147:4069-4073. [PubMed] [Google Scholar]
- 12.Fazekas De St. Groth, S., and R. G. Webster. 1966. Disquisitions on original antigenic sin. I. Evidence in man. J. Exp. Med. 124:331-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao, X. M., B. Zheng, F. Y. Liew, S. Brett, and J. Tite. 1991. Priming of influenza virus-specific cytotoxic T lymphocytes vivo by short synthetic peptides. J. Immunol. 147:3268-3273. [PubMed] [Google Scholar]
- 14.Harling-McNabb, L., G. Deliyannis, D. C. Jackson, S. Gerondakis, G. Grigoriadis, and L. E. Brown. 1999. Mice lacking the transcription factor subunit Rel can clear an influenza infection and have functional anti-viral cytotoxic T cells but do not develop an optimal antibody response. Int. Immunol. 11:1431-1439. [DOI] [PubMed] [Google Scholar]
- 15.Heeg, K., W. Kuon, and H. Wagner. 1991. Vaccination of class I major histocompatibility complex (MHC)-restricted murine CD8+ cytotoxic T lymphocytes towards soluble antigens: immunostimulating-ovalbumin complexes enter the class I MHC-restricted antigen pathway and allow sensitization against the immunodominant peptide. Eur. J. Immunol. 21:1521-1527. [DOI] [PubMed] [Google Scholar]
- 16.Hioe, C. E., H. Qiu, P. D. Chend, Z. Bian, M. L. Li, J. Li, M. Singh, P. Kuebler, P. McGee, D. O'Hagan, T. Zamb, W. Koff, C. Allsopp, C. Y. Wang, and D. F. Nixon. 1996. Comparison of adjuvant formulations for cytotoxic T cell induction using synthetic peptides. Vaccine 14:412-418. [DOI] [PubMed] [Google Scholar]
- 17.Hruby, D. E. 1990. Vaccinia virus vectors: new strategies for producing recombinant vaccines. Clin. Microbiol. Rev. 3:153-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jackson, D. C., H. E. Drummer, and L. E. Brown. 1994. Conserved determinants for CD4+ T cells within the light chain of the H3 hemagglutinin molecule of influenza virus. Virology 198:613-623. [DOI] [PubMed] [Google Scholar]
- 19.Kast, W. M., L. Roux, J. Curren, H. J. Blom, A. C. Voordouw, R. H. Meloen, D. Kolakofsky, and C. J. Melief. 1991. Protection against lethal Sendai virus infection by in vivo priming of virus-specific cytotoxic T lymphocytes with a free synthetic peptide. Proc. Natl. Acad. Sci. USA 88:2283-2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lawson, C. M., J. R. Bennink, N. P. Restifo, J. W. Yewdell, and B. R. Murphy. 1994. Primary pulmonary cytotoxic T lymphocytes induced by immunization with a vaccinia virus recombinant expressing influenza A virus nucleoprotein peptide do not protect mice against challenge. J. Virol. 68:3505-3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Livingston, B. D., C. Crimi, H. Grey, G. Ishioka, F. V. Chisari, J. Fikes, R. W. Chesnut, and A. Sette. 1997. The hepatitis B virus-specific CTL responses induced in humans by lipopeptide vaccination are comparable to those elicited by acute viral infection. J. Immunol. 159:1383-1392. [PubMed] [Google Scholar]
- 22.Loing, E., M. Andrieu, K. Thiam, D. Schorner, K. H. Wiesmuller, A. Hosmalin, G. Jung, and H. Gras-Masse. 2000. Extension of HLA-A*0201-restricted minimal epitope by N-ε-palmitoyl-lysine increases the life span of functional presentation to cytotoxic T cells. J. Immunol. 164:900-907. [DOI] [PubMed] [Google Scholar]
- 23.Martinon, F., H. Gras-Masse, C. Boutillon, F. Chirat, B. Deprez, J. G. Guillet, E. Gomard, A. Tartar, and J. P. Levy. 1992. Immunization of mice with lipopeptides bypasses the prerequisite for adjuvant. Immune response of BALB/c mice to human immunodeficiency virus envelope glycoprotein. J. Immunol. 149:3416-3422. [PubMed] [Google Scholar]
- 24.Moore, M. W., F. R. Carbone, and M. J. Bevan. 1988. Introduction of soluble protein into the class I pathway of antigen processing and presentation. Cell 54:777-785. [DOI] [PubMed] [Google Scholar]
- 25.Morein, B., M. Villacres-Eriksson, A. Sjolander, and K. L. Bengtsson. 1996. Novel adjuvants and vaccine delivery systems. Vet. Immunol. Immunopathol. 54:373-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murali-Krishna, K., J. D. Altman, M. Suresh, D. J. Sourdive, A. J. Zajac, J. D. Miller, J. Slansky, and R. Ahmed. 1998. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity 8:177-187. [DOI] [PubMed] [Google Scholar]
- 27.Noguchi, Y., T. Noguchi, T. Sato, Y. Yokoo, S. Itoh, M. Yoshida, T. Yoshiki, K. Akiyoshi, J. Sunamoto, E. Nakayama, et al. 1991. Priming for in vitro and in vivo anti-human T lymphotropic virus type 1 cellular immunity by virus-related protein reconstituted into liposome. J. Immunol. 146:3599-3603. [PubMed] [Google Scholar]
- 28.Oseroff, C., A. Sette, P. Wentworth, E. Celis, A. Maewal, C. Dahlberg, J. Fikes, R. T. Kubo, R. W. Chesnut, H. M. Grey, and J. Alexander. 1998. Pools of lipidated HTL-CTL constructs prime for multiple HBV and HCV CTL epitope responses. Vaccine 16:823-833. [DOI] [PubMed] [Google Scholar]
- 29.Oukka, M., J. C. Manuguerra, N. Livaditis, S. Tourdot, N. Riche, I. Vergnon, P. Cordopatis, and K. Kosmatopoulos. 1996. Protection against lethal viral infection by vaccination with nonimmunodominant peptides. J. Immunol. 157:3039-3045. [PubMed] [Google Scholar]
- 30.Reinhardt, R. L., A. Khoruts, R. Merica, T. Zell, and M. K. Jenkins. 2001. Visualizing the generation of memory CD4 T cells in the whole body. Nature 410:101-105. [DOI] [PubMed] [Google Scholar]
- 31.Riberdy, J. M., J. P. Christensen, K. Branum, and P. C. Doherty. 2000. Diminished primary and secondary influenza virus-specific CD8+ T-cell responses in CD4-depleted Ig(−/−) mice. J. Virol. 74:9762-9765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sastry, K. J., B. S. Bender, W. Bell, P. A. Small, Jr., and R. B. Arlinghaus. 1994. Effects of influenza virus-specific cytotoxic T-lymphocyte responses induced by a synthetic nucleoprotein peptide on the survival of mice challenged with a lethal dose of virus. Vaccine 12:1281-1287. [DOI] [PubMed] [Google Scholar]
- 33.Sauzet, J. P., B. Deprez, F. Martinon, J. G. Guillet, H. Gras-Masse, and E. Gomard. 1995. Long-lasting anti-viral cytotoxic T lymphocytes induced in vivo with chimeric-multirestricted lipopeptides. Vaccine 13:1339-1345. [DOI] [PubMed] [Google Scholar]
- 34.Sauzet, J. P., H. Gras-Masse, J. G. Guillet, and E. Gomard. 1996. Influence of strong CD4 epitope on long-term virus-specific cytotoxic T cell responses induced in vivo with peptides. Int. Immunol. 8:457-465. [DOI] [PubMed] [Google Scholar]
- 35.Scalzo, A. A., S. L. Elliott, J. Cox, J. Gardner, D. J. Moss, and A. Suhrbier. 1995. Induction of protective cytotoxic T cells to murine cytomegalovirus by using a nonapeptide and a human-compatible adjuvant (Montanide ISA 720). J. Virol. 69:1306-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schulz, M., R. M. Zinkernagel, and H. Hengartner. 1991. Peptide-induced antiviral protection by cytotoxic T cells. Proc. Natl. Acad. Sci. USA 88:991-993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sedgwick, J. D., and P. G. Holt. 1983. A solid-phase immunoenzymatic technique for the enumeration of specific antibody-secreting cells. J. Immunol. Methods 57:301-309. [DOI] [PubMed] [Google Scholar]
- 38.Sherman, L. A., T. A. Burke, and J. A. Biggs. 1992. Extracellular processing of peptide antigens that bind class I major histocompatibility molecules. J. Exp. Med. 175:1221-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shirai, M., C. D. Pendleton, J. Ahlers, T. Takeshita, M. Newman, and J. A. Berzofsky. 1994. Helper-cytotoxic T lymphocyte (CTL) determinant linkage required for priming of anti-HIV CD8+ CTL in vivo with peptide vaccine constructs. J. Immunol. 152:549-556. [PubMed] [Google Scholar]
- 40.Takahashi, H., T. Takeshita, B. Morein, S. Putney, R. N. Germain, and J. A. Berzofsky. 1990. Induction of CD8+ cytotoxic T cells by immunization with purified HIV-1 envelope protein in ISCOMs. Nature 344:873-875. [DOI] [PubMed] [Google Scholar]
- 41.Tannock, G. A., J. A. Paul, and R. D. Barry. 1984. Relative immunogenicity of the cold-adapted influenza virus A/Ann Arbor/6/60 (A/AA/6/60-ca), recombinants of A/AA/6/60-ca, and parental strains with similar surface antigens. Infect. Immun. 43:457-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taylor, P. M., and B. A. Askonas. 1986. Influenza nucleoprotein-specific cytotoxic T-cell clones are protective in vivo. Immunology 58:417-420. [PMC free article] [PubMed] [Google Scholar]
- 43.Tsunoda, I., A. Sette, R. S. Fujinami, C. Oseroff, J. Ruppert, C. Dahlberg, S. Southwood, T. Arrhenius, L. Q. Kuang, R. T. Kubo, R. W. Chesnut, and G. Y. Ishioka. 1999. Lipopeptide particles as the immunologically active component of CTL inducing vaccines. Vaccine 17:675-685. [DOI] [PubMed] [Google Scholar]
- 44.Ulmer, J. B., J. J. Donnelly, S. E. Parker, G. H. Rhodes, P. L. Felgner, V. J. Dwarki, S. H. Gromkowski, R. R. Deck, C. M. DeWitt, A. Friedman, et al. 1993. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science 259:1745-1749. [DOI] [PubMed] [Google Scholar]
- 45.van-der-Most, R. G., A. Sette, C. Oseroff, J. Alexander, K. Murali-Krishna, L. L. Lau, S. Southwood, J. Sidney, R. W. Chesnut, M. Matloubian, and R. Ahmed. 1996. Analysis of cytotoxic T cell responses to dominant and subdominant epitopes during acute and chronic lymphocytic choriomeningitis virus infection. J. Immunol. 157:5543-5554. [PubMed] [Google Scholar]
- 46.Vitiello, A., G. Ishioka, H. M. Grey, R. Rose, P. Farness, R. LaFond, L. Yuan, F. V. Chisari, J. Furze, R. Bartholomeuz, et al. 1995. Development of a lipopeptide-based therapeutic vaccine to treat chronic HBV infection. I. Induction of a primary cytotoxic T lymphocyte response in humans. J. Clin. Investig. 95:341-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Widmann, C., P. Romero, J. L. Maryanski, G. Corradin, and D. Valmori. 1992. T helper epitopes enhance the cytotoxic response of mice immunized with MHC class I-restricted malaria peptides. J. Immunol. Methods 155:95-99. [DOI] [PubMed] [Google Scholar]
- 48.Wolf, B., S. Hauschildt, B. Uhl, J. Metzger, G. Jung, and W. G. Bessler. 1989. Localization of the cell activator lipopeptide in bone marrow-derived macrophages by electron energy loss spectroscopy (EELS). Immunol. Lett. 20:121-126. [DOI] [PubMed] [Google Scholar]
- 49.Yewdell, J. W., J. R. Bennink, G. L. Smith, and B. Moss. 1985. Influenza A virus nucleoprotein is a major target antigen for cross-reactive anti-influenza A virus cytotoxic T lymphocytes. Proc. Natl. Acad. Sci. USA 82:1785-1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou, F., B. T. Rouse, and L. Huang. 1992. Induction of cytotoxic T lymphocytes in vivo with protein antigen entrapped in membranous vehicles. J. Immunol. 149:1599-1604. [PubMed] [Google Scholar]
- 51.Zhou, X., L. Berg, U. M. Motal, and M. Jondal. 1992. In vivo primary induction of virus-specific CTL by immunization with 9-mer synthetic peptides. J. Immunol. Methods 153:193-200. [DOI] [PubMed] [Google Scholar]