Abstract
Two plasmid vectors encoding the A and B subunits of cholera toxin (CT) and two additional vectors encoding the A and B subunits of the Escherichia coli heat-labile enterotoxin (LT) were evaluated for their ability to serve as genetic adjuvants for particle-mediated DNA vaccines administered to the epidermis of laboratory animals. Both the CT and the LT vectors strongly augmented Th1 cytokine responses (gamma interferon [IFN-γ]) to multiple viral antigens when codelivered with DNA vaccines. In addition, Th2 cytokine responses (interleukin 4 [IL-4]) were also augmented by both sets of vectors, with the effects of the LT vectors on IL-4 responses being more antigen dependent. The activities of both sets of vectors on antibody responses were antigen dependent and ranged from no effect to sharp reductions in the immunoglobulin G1 (IgG1)-to-IgG2a ratios. Overall, the LT vectors exhibited stronger adjuvant effects in terms of T-cell responses than did the CT vectors, and this was correlated with the induction of greater levels of cyclic AMP by the LT vectors following vector transfection into cultured cells. The adjuvant effects observed in vivo were due to the biological effects of the encoded proteins and not due to CpG motifs in the bacterial genes. Interestingly, the individual LT A and B subunit vectors exhibited partial adjuvant activity that was strongly influenced by the presence or absence of signal peptide coding sequences directing the encoded subunit to either intracellular or extracellular locations. Particle-mediated delivery of either the CT or LT adjuvant vectors in rodents and domestic pigs was well tolerated, suggesting that bacterial toxin-based genetic adjuvants may be a safe and effective strategy to enhance the potency of both prophylactic and therapeutic DNA vaccines for the induction of strong cellular immunity.
DNA vaccines are widely recognized for their ability to elicit cellular as well as humoral immune responses to microbial antigens (23). An important characteristic of DNA vaccines is that they can mimic characteristics of live vaccines due to their ability to induce the de novo production of microbial antigens in both transiently transfected nonimmune cells and antigen-presenting cells in the vaccine inoculation site and nearby draining lymph nodes (12, 14, 25). The promise of intramuscularly injected DNA vaccines has yet to be realized in the clinic, since a number of recent human trials investigating this approach have not yielded the strength and breadth of responses obtained previously in animal models (4, 26, 49). This may be in part due to the need for better delivery systems, since a recent human trial employing particle-mediated, intracellular delivery (“gene gun”) of a hepatitis B DNA vaccine to the epidermis resulted in 100% seroconversion to protective titers and the induction of strong cellular responses (38). In addition to improved delivery, any DNA vaccine, with or without a specific delivery system, can potentially benefit from the inclusion of an adjuvant to augment the strength or quality of a given response. To this end, several investigators have demonstrated the ability to modulate the responses to both naked DNA and particle-based DNA vaccines by inclusion of vectors encoding a variety of cytokines and chemokines (23, 24, 36, 40, 46).
Our efforts to identify a safe and potent adjuvant for particle-mediated DNA vaccines have recently focused on cholera toxin (CT) and the related Escherichia coli heat-labile enterotoxin (LT). CT and LT are two of the most powerful adjuvants known, due to their ability to elicit robust mucosal and systemic responses via the mucosal and parenteral routes (37, 50). CT and LT are members of the AB5 class of bacterial toxins and are 80% homologous in their primary structure with essentially identical tertiary structures. These toxins exist as hexamers in which the pentameric B subunit oligomer contains the cellular receptor binding function and is linked to a single A subunit containing an ADP ribosyltransferase activity that is associated with both toxicity and adjuvant effects. Proteolytic cleavage of a trypsin-sensitive site in the A subunit is required for activation of enzymatic activity that is contained in the N-terminal A1 subunit. This activity results in the ADP ribosylation of several G proteins involved in signal transduction. ADP ribosylation of Gs, the regulator of adenylate cyclase, results in the permanent activation of adenylate cyclase and accumulation of cellular cyclic AMP (cAMP) levels.
Although CT and LT are closely related molecules, important differences exist in the adjuvant effects that they exhibit (3, 37, 50). Use of CT as an adjuvant generally results in the induction of antigen-specific Th2 cytokine production, while LT elicits both Th1 and Th2 cytokines (30, 51). This immunological difference may be in part due to a difference in receptor usage, since CT binds to only GM1 ganglioside on the cell surface, while LT has a more general lectin-like activity allowing it to bind to a number of glycoproteins and lipopolysaccharides containing galactose (10, 11). Immunological differences may also be due to differences in the endoplasmic reticulum retention signals located at the C termini of the CT and LT A subunits (9, 27, 28).
The adjuvant effects of CT and LT are likely related to their ability to directly activate dendritic cells (DC) (20), resulting in the induction of antigen-specific cytotoxic T-lymphocyte (CTL) responses in a CD4-independent mechanism (45). The greater Th2 tendency of CT than of LT is correlated with its ability to down-regulate the production of interleukin 12 (IL-12) p70 and both subunits of the IL-12 receptor (5) as well as CD40 ligand (32). Importantly, the high concentration of DC in the skin in the form of Langerhans cells (LC) allows these adjuvants to be effective via the dermal route, achieving the induction of systemic as well as mucosal responses (16, 22). The ability to elicit mucosal responses via skin delivery may be due to the ability of CT to alter the migratory properties of LC (16).
Despite the promising adjuvant potential of CT and LT, parenteral or mucosal administration of these molecules has significant toxic effects that have prevented their incorporation into human vaccines and/or immunotherapeutics. On the other hand, CT and LT proteins can be administered to the skin of laboratory animals (via powder injection) at dosages that exhibit marked adjuvant effects for both systemic and mucosal responses with little measurable toxicity (6, 21, 41, 42). Indeed, dosages of up to 500 μg of LT have been administered to the skin of humans in a transcutaneous immunization clinical trial with no significant toxicity (22). Although genetically detoxified mutants of CT and LT offer the potential for retaining adjuvanticity while reducing toxicity (7, 8, 13, 15, 34, 39, 47), the ability to target native toxins or toxin-encoding genes to the skin offers the greatest potential for realizing the full adjuvant effect of these molecules, since the strongest adjuvant effects are associated with the retention of cAMP-inducing activity (7).
In this report we demonstrate that plasmid vectors encoding native CT or LT can be easily formulated into particle-mediated DNA vaccines encoding a variety of antigens. Delivery of these adjuvanted DNA vaccine formulations to the skin of rodents and pigs is well tolerated and results in substantial adjuvant effects in terms of the antibody subclasses induced and the magnitude of T-cell responses.
MATERIALS AND METHODS
Construction of CT- and LT-encoding vectors.
Genomic DNA from Vibrio cholerae and E. coli strain E078:H11 was used as templates for PCRs to generate DNA fragments containing the coding sequences for the A and B subunits of both CT and LT. For PCR generation of the CT A subunit-encoding fragment, the following two oligodeoxyribonucleotide primers were used: CT A 5′ primer, GGA GCT AGC AAT GAT GAT AAG TTA TAT CGG; and CT A 3′ primer, CCT GGA TCC TCA TAA TTC ATC CTT AAT TCT. The CT A 5′ and 3′ primers contained extra sequences at their 5′ ends, outside the region of homology to the CT A coding sequence, containing recognition sites for NheI and BamHI, respectively, to facilitate insertion into an expression vector. These primers were designed to lead to PCR generation of a fragment of the A subunit coding sequence starting at nucleotide position 164 and ending at nucleotide position 886 (GenBank accession no. D30053). This region encompasses the entire coding sequence for the mature subunit A peptide but does not include the sequence encoding the bacterial signal peptide found at the amino terminus of the pre-subunit A peptide.
For PCR generation of the B subunit-encoding fragment, the following two oligodeoxyribonucleotide primers were used: CT B 5′ primer, GGA GCT AGC ACA CCT CAA AAT ATT ACT GAT; and CT B 3′ primer, CCT GGA TCC TTA ATT TGC CAT ACT AAT TGC. These primers contained extra sequences at their 5′ ends, outside the region of homology to the CT B coding sequence, containing recognition sites for NheI and BamHI, respectively, to facilitate insertion into an expression vector. The CT B primers were designed to lead to PCR generation of a fragment of the B subunit coding sequence starting at nucleotide position 946 and ending at nucleotide position 1257 (GenBank accession no. D30053). This region encompasses the entire coding sequence for the mature subunit B peptide but does not include the sequence encoding the bacterial signal peptide found at the amino terminus of the pre-subunit B peptide.
PCR cloning of the LT A and B subunits was identical to that described above for the CT A and B vectors except for the use of the following LT-specific primers: LT A 5′ primer, GGA GCT AGC AAT GGC GAC AAA TTA TAC CGT; LT A 3′ primer, CCT GGA TCC TCA TAA TTC ATC CCG AAT TCT; LT B 5′ primer, GGA GCT AGC GCT CCC CAG TCT ATT ACA GAA; and the LT B 3′primer, CCT GGA TCC CTA GTT TTC CAT ACT GAT TGC.
All PCRs were performed using Pfu Turbo DNA polymerase from Stratagene (La Jolla, Calif.) along with the supplied PCR buffer. PCR conditions were as follows: 95°C for 2 min; 30 cycles of 95°C for 1 min, 55°C for 2 min 15 s, and 72°C for 1 min; 72°C for 5 min; and 4°C hold.
Following completion of the PCRs, the newly synthesized fragments were digested with NheI and BamHI to generate cohesive ends and the individual fragments were inserted into the NheI- and BamHI-cleaved pWRG7054 expression vector resulting in clones pPJV2002 and pPJV2003 encoding CT A and CT B, respectively, and clones pPJV2004 and pPJV2005 encoding LT A and LT B, respectively. The parental cloning vector pWRG7054 (29) contains the human cytomegalovirus (CMV) immediate early promoter and associated intron A sequence. In addition, the coding sequence for the signal peptide of human tissue plasminogen activator (TPA) is included in pWRG7054 to allow for the secretion from mammalian cells of any protein whose coding sequence is inserted in the correct reading frame at the NheI site.
Versions of all four of the CT and LT A and B subunit vectors lacking the TPA signal peptide coding sequences were also constructed by insertion of the respective NheI-BamHI coding fragments into a similar vector backbone lacking the TPA signal peptide coding sequence.
Antigen-encoding vectors.
The plasmid pM2-FL, encoding the M2 gene of influenza strain A/Sydney/5/97 (H3N2), was constructed by insertion of an M2 coding fragment derived by PCR into the expression vector pWRG7077 (44) containing the human CMV promoter, intron A, and bovine growth hormone polyadenylation site. The M2 PCR fragment was derived by a reverse transcriptase PCR using viral RNA purified from A/Sydney/5/97 (virus was a gift from J. Katz, Centers for Disease Control and Prevention) and the following two PCR primers: 5′ PCR primer, 5′CCCAAGCTTCCACCATGAGCCTTCTAACCGAGGTCGAAACACCTATCAGAAACGAATGGGAGTGC; and 3′ PCR primer, CCCGGATCCTTACTCCAGCTCTATGCTG. The reverse transcriptase PCR was performed using the ProSTAR Ultra-HF RT PCR kit of Stratagene. The extra length of the 5′ primer was necessary to bridge the intron found in the M2 coding sequence in influenza virus RNA. The resulting approximately 300-bp M2 coding fragment was trimmed with HindIII and BamHI (sites specified by the PCR primers) for insertion into pWRG7077. The resultant plasmid was termed pM2-FL and was demonstrated to induce M2 production in transfected cells by an immunohistochemical assay (data not shown).
Plasmid pC-Env/T encoding a truncated version of HIV-1 gp120 was previously described (19). Plasmid pPJV7418 is a dual DNA vaccine vector encoding production of both the hepatitis B surface and core antigens and was derived from the clinical hepatitis B surface antigen vector used in a recent clinical trial (38).
Particle-mediated DNA vaccination.
Six- to eight-week-old female BALB/c mice were vaccinated in the abdominal skin using the PowderJect XR DNA vaccine delivery system (PowderJect Vaccines, Inc., Madison, Wis.) as previously described (35). Each DNA vaccination consisted of two tandem deliveries to the abdominal epidermis of mice following fur removal using clippers. Each delivery consisted of 0.5 mg of 1- to 3-μm-diameter gold particles and 1.0 to 1.5 μg of total DNA. The actual amounts of DNA delivered in each experiment are indicated in Results and/or the figure legends. DNA vaccines were administered at a helium pressure of 400 lb/in2. Primary and booster immunizations were spaced a minimum of 4 weeks apart. For DNA vaccination of domestic pigs, the helium pressure was increased to 500 lb/in2. Each immunization consisted of six tandem deliveries administered to the skin over the groin area for a total dose per immunization of 3.0 mg of gold and 7.5 μg of DNA.
Caco-2 cell transfection assay.
The Caco-2 assay for induction of cAMP was adapted from Bowman and Clements (3). Caco-2 cells (HTB-37; American Type Culture Collection) were grown in minimum essential medium containing nonessential amino acids and 20% fetal bovine serum. For the cAMP induction assay, cells were seeded into six-well plates. When the cells were near confluence, each well was transfected with an equimolar mixture of pPJV2002 and pPJV2003 (CT vectors) or pPJV2004 and pPJV2005 (LT vectors) using Lipofectamine (Stratagene) according to the manufacturer's recommendations. Four hours posttransfection, the transfection medium was replaced with fresh medium containing 1 mM 3-isobutyl-1-methylxanthine (Sigma, St. Louis, Mo.). Transfected cells were harvested at 6, 12, 18, and 24 h posttransfection and assayed for cAMP content using a low pH immunoassay kit (R&D Systems, Minneapolis, Minn.).
Antibody ELISAs.
Serum samples were assayed for M2 and human immunodeficiency virus type 1 (HIV-1) gp120-specific antibody responses using an enzyme-linked immunosorbent assay (ELISA). Ninety-six-well plates were precoated with either 1 μg of an M2 synthetic peptide (HN2-SLLTEVETPIRNEWECR-COOH)/ml or 3 μg of recombinant HIV-1 gp120 (Intracel, Rockville, Md.)/ml in phosphate-buffered saline (PBS). Next day, plates were blocked with 5% nonfat dry milk in PBS for 1 h at room temperature. Plates were then washed three times with wash buffer (10 mM Tris-buffered saline and 0.1% Brij 35). Diluted serum samples were then added to the wells, and the plate contents were incubated for 2 h at room temperature. The plates were washed three times with wash buffer, 100 μl of the secondary antibody was added, and plate contents were incubated for 1 h at room temperature. The secondary antibody consisted of a goat anti-mouse immunoglobulin G (IgG) (heavy plus light chains) biotin-labeled antibody (Southern Biotechnology Associates, Inc., Birmingham, Ala.) that was diluted 1:8,000 in 1% bovine serum albumin (BSA)-PBS-0.1% Tween 20. Plates were then washed three times, and a streptavidin-horseradish peroxidase conjugate (Southern Biotechnology) diluted to 1:8,000 in PBS-0.1% Tween 20 was added, and the plate contents were incubated for 1 h at room temperature. Following three additional washes, 100 μl of tetramethylbenzidine substrate (Bio-Rad, Hercules, Calif.) was added and color development was allowed to proceed for 30 min at room temperature. Color development was stopped by the addition of 1 N H2SO4, and the plates were read at 450 nm. Endpoint dilution titers were determined by identifying the highest dilution of serum that still yielded an absorbance value that was two times the background absorbance value obtained using a nonimmune control sample.
ELISAs specific for the IgG1 and IgG2a antibody subclasses were performed as above except for the substitution of the following two secondary antibodies, respectively: goat anti-mouse IgG1-biotin (catalog no. 1070-08; Southern Biotechnology) at a 1/8,000 dilution and goat anti-mouse IgG2a-biotin (catalog no. 1080-08) at a 1/8,000 dilution.
ELISPOT assays.
To enumerate gamma interferon (IFN-γ)- and Il-4-secreting T cells, enzyme-linked immunospot (ELISPOT) plates (Whatman Polyfiltronics, Clifton, N.J.) were coated with a rat anti-mouse IFN-γ antibody (BD PharMingen, San Diego, Calif.) or with a rat anti-mouse IL-4 antibody (BD PharMingen) using 50 μl per well of 15 μg of coating antibody/ml in carbonate buffer, pH 9.6 (Pierce, Rockford, Ill.), overnight at 4°C. Wells were then washed twice with 100 μl of sterile PBS and then twice with 100 μl of RPMI medium containing 10% fetal bovine serum (R10). Wells were blocked with 200 μl of R10 in a 5% CO2 atmosphere at room temperature. Fresh splenocytes from immune animals were added to individual wells in 100 μl of R10 at concentrations of 1 × 106, 3 × 105, and 1 × 105 per well after which 100 μl of a 20-μg/ml concentration of the M2 peptide (see above) or HBsAg peptide (IPQSLDSWWTSL) was added to each well. The HIV-1 gp120 and HBsAg peptides encode H-2d-restricted CTL epitopes (43, 48). In some HBsAg ELISPOT experiments, an HBsAg peptide mimotope library was used in place of the single CD8+ CTL epitope at a concentration of 10 μM for the whole peptide library. For HBcAg ELISPOTS, recombinant HBcAg (Biodesign, Kennebunk, Maine) was used at a concentration of 10 μg/ml. Wells with fewer than 106 cells from immunized animals were supplemented with cells from naive animals to bring the total to 106 cells per well. For control wells, medium without peptide was added. Plate contents were incubated at 37°C for 24 to 48 h in a 5% CO2 atmosphere. Following incubation, wells were washed twice with PBS, once with water, and twice more with PBS. Detecting antibodies (biotinylated rat anti-mouse IFN-γ [BD PharMingen]) and biotinylated rat anti-mouse IL-4 [BD PharMingen]) were diluted to 1 μg/ml in PBS, and 50 μl of the appropriate antibody was added to each well and incubated for 1 h at room temperature. The plates were then washed five times, and their contents were incubated for 1 h with 50 μl per well of a 1:1,000 dilution of streptavidin-alkaline phosphatase (AP) solution (Mabtech, Nacka, Sweden). The plates were again washed five times, and colorimetric development was accomplished by the addition of alkaline phosphatase membrane substrate (Bio-Rad) until spots formed (2 to 30 min, room temperature). The reaction was stopped by washing with deionized water, and the plates were air dried overnight. Wells were imaged with a video camera linked to a personal computer, and spots were enumerated using ImagePro Plus software (Media Cybernetics, Silver Spring, Md.).
HIV-1 gp120-specific CTL assay.
To generate effector CTLs, splenocytes from immunized mice were cultured with mitomycin C-treated, peptide-pulsed (1 μg of HIV-1 gp120 CTL epitope RGPGRAFVTI-OH/ml) stimulator splenocytes for 7 days in complete R10 media supplemented with 20 U of recombinant rat IL-2 (Collaborative, Bedford, Mass.)/ml. Cytolytic activity was measured on day 7 with a standard 51Cr release assay using 96-well U-bottomed plates containing 3 × 105 targets/well. Target cells used were P815 cells labeled with 100 μCi of sodium chromate (NEN Life Sciences, Boston, Mass.) for 1 h, washed four times to remove excess chromium, and pulsed with CTL epitope peptide at 10 μg/ml. Different effector-to-target ratios were assayed by mixing 3 × 105 51Cr-labeled target cells in 100 μl with 100 μl of different dilutions of effector cells. Duplicate determinations were made for each combination. Plates were centrifuged at 1,000 rpm for 5 min in a Sorvall RT 6000D centrifuge, and their contents were incubated for 5 h at 37°C in humidified air with 5% CO2. After incubation, the plate was centrifuged again, and 25 μl of supernatant was collected per well and transferred to Lumaplates (Packard Instrument Co., Meriden, Conn.). After drying for 2 h or overnight, the plates were counted in a Packard Topcount. Specific cytotoxicity was calculated as the mean percentage of 51Cr release for a particular effector-to-target cell ratio. Percent cytotoxicity was determined from the following formula: 100 × [(experimental release − spontaneous release)/(maximum release − spontaneous release)]. Maximum release was determined by the lysis of targets by detergent (2% Triton X-100; Sigma). Spontaneous release was determined by lysis of 51Cr-labeled target cells incubated in medium without effectors and was consistently <20% of maximal release in all assays.
RESULTS
Construction of adjuvant-encoding vectors.
Genomic DNA from V. cholerae and E. coli strain E078:H11 was used in PCRs to generate fragments encoding the mature forms of the A and B subunits of both CT and LT. Each of the four PCR fragments was separately inserted into the expression vector pWRG7054 (29), resulting in four individual expression vectors encoding the A and B subunits of both toxins. Figure 1 shows a functional map of plasmid pPJV2002, which encodes the A subunit of CT. The human CMV immediate early promoter and its associated intron A were used to drive expression of the CT A coding sequence, which lies downstream of, and in frame with, the human tissue plasminogen activator signal peptide coding sequence. The goal was to develop CT and LT subunit expression vectors that resulted in secretion of their respective subunits following transfection. Cotransfection of A and B subunit plasmids together was designed to result in the secretion of an assembled CT or LT holotoxin. Plasmids pPJV2003, pPJV2004, and pPJV2005 (not shown) encode the remaining CT B, LT A, and LT B subunits, respectively.
FIG. 1.
Functional map of vector pPJV2002. pPJV2002 is a DNA vaccine adjuvant vector encoding the mature form of the CT A subunit fused in frame with the tissue plasminogen activator signal peptide. Expression is driven by the human CMV immediate early promoter, which is functionally linked to the intron A element. Polyadenylation is controlled by the bovine growth hormone polyadenylation sequence. hCMV Pro, human CMV immediate early promoter; TPAsig CDS, tissue plasminogen activator signal peptide coding sequence; CTA CDS, CT A subunit coding sequence; and bGHpA, bovine growth hormone polyadenylation sequence. Vectors pPJV2003, pPJV2004, and pPJV2005 (not shown) encode the CT B subunit, the LT toxin A subunit, and the LT toxin B subunit, respectively.
Biological activity of the CT- and LT-encoding vectors.
Biological activity of the CT-and LT-encoding vectors was demonstrated using the Caco-2 cell assay for the induction of cellular cAMP (3). The CT A and B subunit-encoding vectors (collectively referred to as CT vectors) were mixed at equal molar concentrations and transfected into Caco-2 cells using Lipofectamine (Stratagene). The LT A and B subunit vectors (collectively referred to as LT vectors) were tested in a similar manner. Caco-2 cells treated with 1 μg of purified CT protein were used as a positive control. Four hours posttransfection, the medium was replaced with fresh medium containing 3-isobutyl-1-methylxanthine, a nonspecific inhibitor of cAMP and cGMP phosphodiesterases, after which cAMP levels were quantified at 6, 12, 18, and 24 h posttransfection using a commercial ELISA kit. As shown in Fig. 2, increasing levels of cAMP were detected in CT and LT vector-transfected Caco-2 cells but neither in untransfected cells nor in cells transfected with an empty vector. The highest cAMP levels were observed in the LT vector-transfected cells in which cAMP levels at 24 h were approximately one-third the level observed in positive control cells treated with 1 μg of purified CT protein.
FIG. 2.
Induction of cAMP production in Caco-2 cells following transfection with vectors encoding CT and LT. Four hours following transfection of Caco-2 cells with pPJV2002 + pPJV2003 (CT) or with pPJV2004 + pPJV2005 (LT), growth medium was replaced with fresh medium containing 1 mM 3-isobutyl-1-methylxanthine. Individual wells were harvested at 6, 12, 18, and 24 h posttransfection for quantification of cAMP content. Positive control cells were treated with purified CT protein (1 μg) for 4 h, after which medium was replaced with fresh medium containing 1 mM 3-isobutyl-1-methylxanthine. Two types of negative controls were employed: cells transfected with empty-vector DNA (pWRG7054) or nontransfected cells.
Adjuvant effects of CT and LT vectors on HIV-1 gp120 and M2 DNA vaccines.
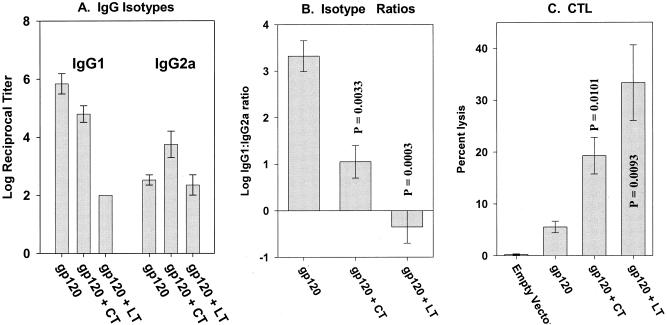
The adjuvant effects of the CT and LT vectors were first evaluated in the context of an HIV-1 gp120 DNA vaccine. Groups of mice were immunized with the gp120 vector plus irrelevant vector or with the gp120 vector supplemented with the CT A + B or LT A + B vectors (1 μg each of the gp120, A subunit, and B subunit vectors). Figure 3 shows the gp120-specific IgG isotype responses and CTL responses following the booster immunization. The CT vectors had little impact on the magnitude of the total antibody response but caused a marked shift in the IgG1-to-IgG2a ratio (Fig. 3A and B). With the LT vectors, a strong reduction in the magnitude of the response (due to IgG1 suppression) was seen resulting in a reversal of the IgG1-to-IgG2a ratio and a slight abundance of gp120-specific IgG2a. The relative magnitude of the CT and LT vector effects on gp120-specific antibody responses was mirrored by the gp120-specific CTL responses (Fig. 3C) in that the strongest augmentation of CTL was seen in the LT vector group. These observations are consistent with the augmentation of Th1-like responses, particularly for the LT vectors.
FIG. 3.
gp120-specific humoral and cellular responses in mice following codelivery of vectors encoding HIV-1 gp120 and CT or LT. All groups (n = 4) received primary and booster immunizations spaced 4 weeks apart. Serum samples and splenocytes were collected 2 weeks following the second immunization. (A) IgG1 and IgG2a responses. (B) IgG1-to-IgG2a ratios. (C) CTL responses. P values shown in panels B and C were calculated using Student's t test. Bars represent standard error.
To examine the applicability of these adjuvant vectors toward additional antigens, an additional study was initiated using a DNA vaccine encoding the M2 protein from influenza type A. The study was designed to determine the importance of the dose of adjuvant vector and incorporated ELISPOT assays for IFN-γ- and IL-4-producing T cells to better quantify the magnitude of the T-cell adjuvant effect.
Plasmid pM2-FL encodes the M2 protein of type A influenza virus, a conserved integrated membrane protein found on the surface of influenza virus particles as well as infected cells (2). M2 exhibits ion pore activity and has been recognized as a potentially important vaccine antigen capable of eliciting cross-protective and heterosubtypic immunity due to its conserved nature (18, 33). In our hands, pM2-FL is strongly immunogenic in mice (M. D. Macklin et al., unpublished data), leading to vigorous antibody titers following two immunizations with 2.0 μg of plasmid DNA per immunization.
The M2 DNA vaccine-plus-adjuvant trial employed 1.0 μg of pM2-FL, either alone or codelivered with decreasing doses of the CT A + B vectors or LT A + B vectors. The largest adjuvant dose consisted of 0.5 μg each of the A and B subunit vectors. All groups of animals received a constant amount of “CMV promoter-containing” DNA by the addition of irrelevant empty-vector DNA where appropriate. All mice received two particle-mediated DNA immunizations (prime and boost) at a 4-week interval after which M2-specific antibody and IFN-γ and IL-4 responses were measured. The cytokine responses were measured by a T-cell ELISPOT assay utilizing a stimulating peptide comprising most of the amino-terminal extracellular domain of M2. Figure 4A and B show the antibody data from this study in which the CT and LT vectors induced a significant increase in total antibody responses compared to the M2 vector alone (Fig. 4A) (P = 0.004 to 0.034). Interestingly, examination of the antibody subclass ratios (Fig. 4B) revealed an additional effect of the adjuvant vectors in that both sets of vectors induced a marked reduction in the IgG1-to-IgG2a ratio, which was statistically significant for all of the LT vector dosages.
FIG. 4.
M2-specific humoral and cellular immune responses in mice following codelivery of pM2-FL with one of four dosage levels of the CT or LT vector. All groups (n = 8) received primary and booster immunizations spaced 4 weeks apart. Blood samples and splenocytes were collected 2 weeks following the second immunization. (A) Total M2-specific IgG responses. (B) M2-specific IgG1-to-IgG2a ratio. (C) IFN-γ ELISPOT responses. (D) IL-4 ELISPOT responses. As noted in the text, * indicates statistical significance using Student's t test when compared to the control group that did not receive adjuvant. Bars represent standard error.
Inasmuch as the humoral response data demonstrated an adjuvant effect with the CT and LT vectors, data from the IL-4 and IFN-γ ELISPOT assays showed a strong augmentation of both Th1 and Th2 cytokine responses (Fig. 4C and D). This was particularly true for the LT vectors in which IFN-γ and IL-4 ELISPOT responses were augmented by 13- to 25-fold (P < 0.0000035 for all groups except the 0.01-μg LT dose for Fig. 4C, where the P value = 0.001). Based on the strength of the IL-4 responses, we believe that it is likely that the observed cytokine responses were due to recognition of an as yet unidentified CD4 epitope near the amino terminus of the M2 protein. Importantly, there was no apparent dose-response relationship in the augmentation of either the IL-4 or IFN-γ responses by either the CT or LT vectors, indicating that only very small quantities of adjuvant vector are required to achieve the observed effects on the cytokine responses.
Effects of signal peptide coding sequence and individual subunit vectors on adjuvant effects of CT and LT vectors.
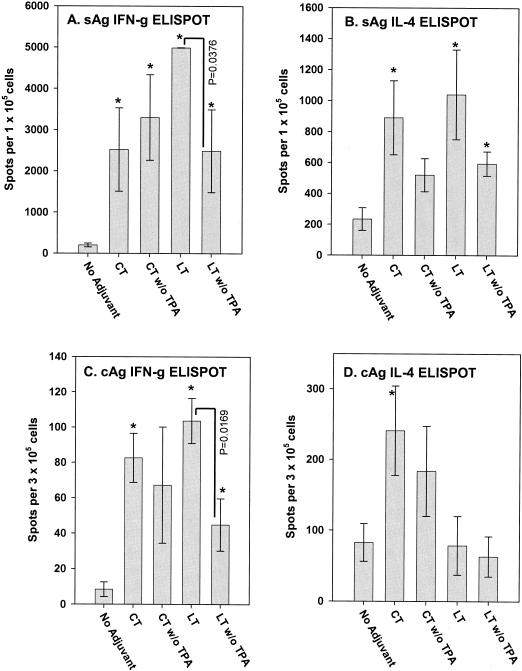
Since the CT and LT vectors were designed to lead to secretion of the A and B subunits of each toxin, it was interesting to determine if the potential for toxin subunit secretion had any bearing on the observed adjuvant activities. We theorized that signal peptide coding sequences would allow for the production and secretion of adjuvant activity by keratinocytes as well as by professional antigen-presenting cells (LC), thereby increasing the potential for activation of both untransfected and transfected LC. Alternatively, with the absence of signal sequences, adjuvant activity would likely be limited to the ADP ribosyltransferase activity produced directly within transfected LC. Figure 5 shows the results of this study, in which various groups of five mice were immunized with a dual DNA vaccine vector encoding both HBsAg and HBcAg, respectively). The control group received the hepatitis vector alone (1 μg), while four remaining groups received the hepatitis vector supplemented with the CT A + B and LT A + B vectors containing and lacking the human TPA signal peptide coding sequence (0.5 μg of each subunit vector). IFN-γ and IL-4 ELISPOT responses to both the surface and core antigens following the booster immunization are shown (Fig. 5). HBsAg-specific IFN-γ responses were measured following in vitro stimulation with either a specific CD8 epitope-containing peptide (43) or a peptide mimotope library with identical results. HBsAg-specific IL-4 responses were measured following stimulation with the peptide mimotope library, while the HBcAg-specific ELISPOT assays employed a recombinant HBcAg for stimulation.
FIG. 5.
HBsAg (sAg) and HBcAg (cAg)-specific IFN-γ and IL-4 ELISPOT responses following immunization with a dual HBsAg/HBcAg vector and CT A + B or LT A + B vectors containing and lacking signal peptide coding sequences. All groups (n = 5) received primary and booster immunizations spaced 4 weeks apart. Blood samples and splenocytes were collected 2 weeks following the second immunization. (A) HBsAg-specific IFN-γ responses. (B) HBsAg-specific IL-4 responses. (C) HBcAg-specific IFN-γ responses. (D) HBcAg-specific IL-4 responses. ∗ indicates statistical significance using Student's t test when compared to the control group that did not receive adjuvant. Bars represent standard error. “w/o TPA” indicates that the vectors did not contain the human TPA signal peptide coding sequence.
The signal sequence-containing CT A + B and LT A + B adjuvant vectors induced significant increases (P ≤ 0.05) in IFN-γ responses to both HBsAg and HBcAg and IL-4 responses to HBsAg (Fig. 5A to C). For IL-4 responses to HBcAg, only the CT vectors exhibited an effect (Fig. 5D). Interestingly, the CT A + B and LT A + B vectors lacking the signal sequences generally exhibited weaker adjuvant activity. This was particularly well demonstrated in the IFN-γ ELISPOT data for both HBsAg and HBcAg (Fig. 5A and C). In these cases there was a statistically significant decrease in adjuvant activity by virtue of the deletion of the signal sequence from the LT A + B vectors. While these data imply that secretion of LT A and B from transfected cells is beneficial, we cannot rule out the possibility that this difference is due simply to differences in expression level or differences in intracellular trafficking of the adjuvant proteins in transfected cells.
The issue of secretion was further examined in a second study involving the HBsAg/HBcAg DNA vaccine, in which the adjuvant activities of individual A and B subunit vectors with and without signal sequences were tested. The controls in this study consisted of the hepatitis HBsAg/HBcAg DNA vaccine alone (1 μg of DNA vaccine + 1 μg of irrelevant vector) and the same vaccine adjuvanted with the signal-containing versions of the CT A + B and LT A + B vectors (1 μg of DNA vaccine + 0.5 μg of CT A or LT A + 0.5 μg of CT B or LT B). Eight test groups in this trial consisted of the HBsAg/HBcAg DNA vaccine supplemented with individual A or B subunit vectors with and without signal sequences (1 μg of DNA vaccine + 1 μg of A or B subunit). Groups of five mice received primary and booster immunizations spaced 4 weeks apart and were sacrificed for ELISPOT assays 2 weeks following the boost. Data are shown in Fig. 6. Importantly, the positive control groups (CT A + B and LT A + B) exhibited adjuvant activities for IFN-γ and IL-4 responses that were indistinguishable from that observed in the previous experiment (compare the first three bars in each graph in Fig. 6 with data in Fig. 5). Data from the individual subunit test groups yielded some interesting patterns. Firstly, no significant adjuvant effects were observed with any of the individual CT A and B subunit vectors with and without signal sequences. Nor were any adjuvant effects observed for the individual LT subunit vectors for the induction of IFN-γ responses to HBcAg. Since the test groups received the same total dose of bacterial-gene-containing DNA as did the positive controls, it can be concluded that the adjuvant effects observed in the CT A + B and LT A + B positive control groups were dependent upon the biological activity of the encoded AB toxins and not due to the adjuvant effects of CpG-containing bacterial DNA (see Discussion for a more thorough examination of this issue).
FIG. 6.
HBsAg (sAg) and HBcAg (cAg)-specific IFN-γ and IL-4 ELISPOT responses following immunization with a dual HBsAg/HBcAg vector and individual CT A or B or LT A or B vectors containing and lacking signal peptide coding sequences. All groups (n = 5) received primary and booster immunizations spaced 4 weeks apart. Blood samples and splenocytes were collected 2 weeks following the second immunization. (A) HBsAg-specific IFN-γ responses. (B) HBsAg-specific IL-4 responses. (C) HBcAg-specific IFN-γ responses. (D) HBcAg-specific IL-4 responses. ∗ indicates statistical significance using Student's t test when compared to the control group that did not receive adjuvant. Bars represent standard error. “w/o TPA” indicates that the vector did not contain the human TPA signal peptide coding sequence.
In contrast to the case with CT subunit vectors, the individual LT subunit vectors exhibited partial adjuvant activity that was influenced by the presence or absence of signal peptide coding sequences. This was particularly well demonstrated for the HBsAg-specific IFN-γ and IL-4 responses, in which a marked adjuvant effect was observed for the LT B vector containing the signal peptide coding sequence (Fig. 6A and B). Interestingly, deletion of the signal peptide coding sequence resulted in loss of the adjuvant effect, as would be expected since the LT B subunit needs to be outside the cell in order to engage its receptors.
As was the case with the LT B subunit vector, the LT A subunit vector alone also exhibited partial adjuvant activity but only in the absence of the signal peptide coding sequence (Fig. 6B and D). This was observed for the IL-4 responses to both HBsAg and HBcAg and is consistent with the need for the intracytoplasmic production of the ADP ribosyltransferase activity in the absence of the cell binding and internalization activity specified by the B subunit. Taken together, the data shown in Fig. 5 and 6 demonstrate that the adjuvant effects imparted by the CT and LT genetic adjuvant vectors are likely due to both the internal and extracellular production of CT AB5 and LT AB5 toxins. Moreover, adjuvant activity is exhibited by the LT B-encoding vector in the absence of ADP ribosyltransferase activity.
In addition to the examination of T-cell responses to HBsAg and HBcAg, antibody responses to both antigens were also examined for all mice from the experiments shown in Fig. 5 and 6. Unlike the results from the gp120 and M2 experiments, no significant effects on antibody responses (magnitude and subclass ratio) to either HBsAg or HBcAg were observed with either the CT A + B or LT A + B vectors or individual subunit vectors (data not shown). The reason for this may be that IgG1-to-IgG2a ratios for HBsAg and HBcAg-specific antibody responses in the absence of adjuvant are more balanced and not wildly skewed toward IgG1 dominance, as they are for the gp120 and influenza DNA vaccines lacking adjuvants (17, 35). While effects on T-cell responses were observed for all antigens tested, the effects that these adjuvants had on antibody responses was clearly antigen dependent.
Preliminary adjuvant vector studies in domestic pigs.
While the adjuvant data reported above were limited to mouse studies, preliminary data from one trial in domestic pigs indicate that similar adjuvant effects can be expected in larger animals. As seen in the mouse M2 experiment (Fig. 4), an M2 DNA vaccine formulation containing equimolar concentrations of the M2, CT A, and CT B vectors elicited a sixfold-higher geometric mean titer to M2 in domestic pigs (P = 0.055, data not shown). While additional experiments were not performed to investigate the nature of the pig responses, the similarity in the degree of enhancement of M2-specific total IgG responses between mice and pigs using a similar antigen-to-adjuvant vector ratio indicates that adjuvant effects will not be limited to rodents. An interesting finding in the pig trial was the absence of any detectable difference in local reactogenicity between the M2 alone and M2 + CT vector formulations (data not shown). Particle-mediated DNA vaccine delivery to the epidermis of pigs, monkeys, and humans traditionally results in a mild, transient erythema that resolves in a few days (38), and this was unchanged in the CT formulation tested in pigs in the above experiment.
Insofar as the CT vector failed to elicit any detectable toxicity or local reactogenicity in pigs when used at a high dosage, we wanted to further examine the CT and LT vectors in a parallel experiment, since the LT vectors remained untested in larger animals and have routinely elicited stronger responses in rodents. Figure 7 shows the results of a simple local reactogenicity comparison in domestic pigs in which the CT and LT vector formulations were compared to a control formulation containing empty-vector DNA. On day 0, a typical mild erythema was observed at all delivery sites with no differences attributable to the DNA formulation. However, while the erythema associated with the empty-vector and CT vector deliveries was completely resolved in a few days, the local reaction associated with the LT vector transformed into a mild induration that persisted beyond day 7 (Fig. 7B) but resolved by day 14 (not shown). The enhanced reaction (induration) due to LT is indicative of a greater level of local infiltration and is consistent with the stronger adjuvant effects observed for the LT A + B vectors. Interestingly, the differences in local reactogenicity between the CT A + B and LT A + B vectors are also seen when CT and LT proteins are administered to the skin using an epidermal powder delivery device (D. Chen, PowderJect Vaccines, personal communication). It will be interesting to determine if the LT vector-associated reactogenicity exhibits a dose-response relationship when the amount of vector delivered is reduced. Since strong adjuvant effects can still be seen at the 10-ng vector dose in mice, it might be possible to identify a reduced adjuvant dose that remains effective in a larger species but is free of any local reactogenicity attributed to LT.
FIG. 7.
Local skin reactogenicity in domestic pigs following epidermal inoculation of CT and LT vectors. CT and LT vectors, as well as a control sample of empty vector, were formulated onto gold particles and delivered into the inguinal epidermis of domestic pigs using the PowderJect XR gene delivery device at a helium pressure of 500 lb/in2. Delivery conditions involved 0.5 mg of gold and 1.0 μg of DNA total per inoculation. Skin sites were observed on days 0, 2, 7, and 14. Days 0 and 7 are shown in panels A and B, respectively. EV, empty vector; CT, CT A and B subunit vectors; and LT, LT toxin A and B subunit vectors.
DISCUSSION
The data presented here demonstrate that the supplementation of DNA vaccines with plasmid vectors encoding the A and B subunits of CT or LT results in a strong adjuvant effect in terms of T-cell responses as quantified by IFN-γ and IL-4 ELISPOT assays. In addition, there was an antigen-dependent effect on antibody responses ranging from no effect to suppression or enhancement of the total response and suppression of the IgG1-to-IgG2a ratio. The adjuvant effects with the LT-encoding vectors were routinely stronger than those observed with the CT vectors, and this phenomenon was correlated with the induction of greater levels of cAMP in cultured cells by the LT A + B than by the CT A + B vectors. While both sets of vectors enhanced both Th1 and Th2 cytokine production, the LT vectors generally elicited more Th1-like responses, as exemplified by the greater suppression of the IgG1-to-IgG2a ratio and the augmentation of HBcAg-specific IFN-γ (but not IL-4) responses. The tendency of the LT vectors to exhibit a stronger effect than the CT vectors is consistent with reported differences in adjuvant effects for the protein versions of these adjuvants (30, 31, 51).
The adjuvant effects of CT and LT, whether in protein or DNA vector form, are likely due in large part to the ADP ribosyltransferase activity exhibited by the A subunit of these closely related toxins. While some adjuvant activity has been attributed to the B subunits of CT and LT alone (via cell surface receptor engagement), the strongest adjuvant effects are routinely observed by wild-type molecules exhibiting the full enzymatic activity imparted by the A subunits (7, 8). The data presented here for the CT-encoding vectors are consistent with this in that adjuvant activity was not observed when the CT A and CT B subunit vectors were used independently, indicating that a functionally assembled AB5 CT toxin molecule is required to elicit an adjuvant effect. Consistent with this was the observation that use of the CT A + B vectors without the signal peptide coding sequences resulted in loss of the statistical significance of the adjuvant effect (three of four experiments [Fig. 5]), implying a role for the release of CT from transfected cells in vivo. This was demonstrated more clearly with the LT A + B vectors (Fig. 5) showing a statistically significant decrease in adjuvant activity, following deletion of the signal peptide coding sequences from both the A and B subunit vectors.
The LT vectors differed from the CT vectors in that the individual LT A and LT B subunit vectors exhibited statistically significant adjuvant activities not seen with the individual CT A and CT B vectors. Moreover, the activities of the LT A and LT B vectors were very much dependent on the presence or absence of signal peptide coding sequences (Fig. 6). Both IFN-γ and IL-4 responses to HBcAg were markedly augmented by the LT B vector alone, and this effect was entirely dependent on the presence of the signal peptide coding sequence in the vector. This is consistent with the need for the B subunit to be in the extracellular environment in order to engage its cell surface receptor. In contrast to this, the IL-4 enhancement activity observed for the LT A subunit vector alone (Fig. 6B and D) was observed only when the signal peptide coding sequence was deleted from the vector, consistent with the intracytoplasmic site of action of the A subunit enzymatic activity. A further peculiarity of the LT vectors was the ability of the individual LT A and LT B vectors to separately augment HBcAg-specific IL-4 responses, while the combined LT A + B vectors exhibited no enhancement of HBcAg-specific IL-4 activity in two separate experiments. This demonstrates that the LT A and B subunits exhibit different roles in vivo and that one may be able to control either the quantity or quality of the adjuvant effect by the choice or ratio of subunit vectors employed.
It is important that the adjuvant effects demonstrated for the CT- and LT-encoding vectors are not due to the presence of immunostimulatory CpG motifs contained within the A and B subunit coding sequences (nor any other regions) of the CT and LT vectors. CpG motifs exert their immunostimulatory potential by a pathway involving receptor-mediated endocytosis of CpG-containing DNA into immune cells (macrophages, DC, and B cells) followed by endosome acidification and likely degradation of the DNA into CpG-containing oligonucleotides (1). Particle-mediated (“gene gun”) DNA vaccines bypass this signaling mechanism because of the direct intranuclear and intracytoplasmic deposition of intact plasmid DNA (23). This is evidenced by the fact that particle-mediated and needle-inoculated DNA vaccines induce qualitatively different responses that are dependent on the method rather than the location of vaccine administration (17, 35). The absence of a CpG effect in the present work can be seen from the data in Fig. 5 in which the CT A and CT B subunit vectors exert no adjuvant effect when administered individually with the antigen vector. Adjuvant effects are only observed when the two subunit vectors are combined, indicating that adjuvanticity is due to biological activity of the encoded CT AB5 product. It should be noted that the CT A and B subunit vectors each contain 22 canonical CpG motifs (that fit the pattern Pu-Pu-C-G-Py-Py), with only one motif present in each of the CT A and CT B subunit coding sequences derived from V. cholerae. The remaining motifs are in the bacterial vector backbone and are commonly found in most of our antigen expression vectors, including the gp120, M2, and hepatitis DNA vaccine vectors used here.
Efforts to develop mutants of the A subunits of CT and LT have met with success in the identification of molecules that exhibit greatly reduced toxicity while retaining a significant degree of adjuvant activity (7, 8, 13, 15, 34, 39, 47). Insofar as the use of mutant toxins is compatible with the technology described here, it should be noted that the ability to use vectors encoding the wild-type toxins without significant toxic effects (see below) allows one to realize the full adjuvant potential that these molecules have to offer.
The ADP ribosylase activity of the CT A and LT A molecules requires proteolytic cleavage of a trypsin-sensitive loop (37) separating the N-terminal A1 and C-terminal A2 fragments. This loop is naturally cleaved when CT is produced in V. cholerae but is uncleaved when toxins are produced in E. coli. The fact that strong cAMP-inducing activity was observed in the in vitro Caco-2 cell transfection assay (especially for the LT vectors) argues that natural proteases in mammalian cells may effectively cleave this loop at some point following synthesis to allow for the observed enzymatic activity and resultant adjuvant effects.
The strong adjuvant potential of CT and LT has been known for many years, but the significant toxicity exhibited by these molecules following parenteral or mucosal administration has prevented the realization of this immunological potential in the clinic. We were thus surprised and encouraged to observe the remarkable absence of apparent toxicity when vectors encoding CT and LT were administered to the epidermis by particle delivery. Recent studies investigating the phenomenon of transcutaneous immunization have demonstrated that large quantities of wild-type CT and LT toxins can be applied directly to the skin of animals and humans, resulting in significant adjuvant effects with no detectable toxicity (21, 22, 41, 42). This lack of toxicity could be explained by the paucity of protein delivery through intact stratum corneum by this method. It was thus surprising to demonstrate strong adjuvant effects and a lack of toxicity following use of an efficient skin delivery system employing CT- and LT-encoding vectors. Administration of the CT vectors to the skin of mice and domestic pigs was completely devoid of any detectable local skin reactogenicity other than the typical transient erythema that is routinely associated with epidermal DNA vaccine administration in domestic pigs, monkeys, and humans (38). Thus, it is conceivable that a DNA-based CT adjuvant for a DNA vaccine could become a routine part of epidermally administered prophylactic DNA vaccines in healthy individuals. In contrast to CT, the LT vectors routinely induced a stronger adjuvant effect as well as a detectable difference in skin reactogenicity characterized by a transient, but mild, induration that persisted beyond the immediate erythema associated with gold particle penetration. This pattern of induration is identical to that observed following LT protein administration to the epidermis (D. Chen, personal communication). Importantly, it should be noted that strong adjuvant effects with LT were observed with as little as 10 ng of vector DNA. Thus, it is possible that dose titration experiments could identify an LT vector dosage that minimizes local reactogenicity while maintaining sufficient adjuvant effects.
A final point to consider is the known ability of CT and LT to serve as mucosal adjuvants. Most studies of the mucosal adjuvant properties of CT and LT deal with mucosal administration of these adjuvants with antigen. However, several recent studies have demonstrated the ability of these molecules to lead to mucosal responses when administered to the skin via transcutaneous immunization (22) or intradermal inoculation (16). In the latter case, the use of CT protein is believed to be responsible for altered migration patterns of activated DC, resulting in their migration to mucosal sites following delivery to the skin (16). Based on these reports, it would follow that DNA vaccine delivery to the epidermis, via powder injection, in the presence of vectors encoding CT or LT would have a similar capacity to induce mucosal immunity. Consistent with this is the recent observation that epidermal powder immunization of antigen plus CT in protein form results in successful mucosal immune response induction (6).
REFERENCES
- 1.Aderem, A., and D. A. Hume. 2000. How do you see CG? Cell 103:993-996. [DOI] [PubMed] [Google Scholar]
- 2.Bauer, C. M., L. H. Pinto, T. A. Cross, and R. A. Lamb. 1999. The influenza virus M2 ion channel protein: probing the structure of the transmembrane domain in intact cells by using engineered disulfide cross-linking. Virology 254:196-209. [DOI] [PubMed] [Google Scholar]
- 3.Bowman, C. C., and J. D. Clements. 2001. Differential biological and adjuvant activities of cholera toxin and Escherichia coli heat-labile enterotoxin hybrids. Infect. Immun. 69:1528-1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boyer, J. D., A. D. Cohen, S. Vogt, K. Schumann, B. Nath, L. Ahn, K. Lacy, M. L. Bagarazzi, T. J. Higgins, Y. Baine, R. B. Ciccarelli, R. S. Ginsberg, R. R. MacGregor, and D. B. Weiner. 2000. Vaccination of seronegative volunteers with a human immunodeficiency virus type 1 env/rev DNA vaccine induces antigen-specific proliferation and lymphocyte production of beta-chemokines. J. Infect. Dis. 181:476-483. [DOI] [PubMed] [Google Scholar]
- 5.Braun, M. C., J. He, C. Y. Wu, and B. L. Kelsall. 1999. Cholera toxin suppresses interleukin (IL)-12 production and IL-12 receptor beta1 and beta2 chain expression. J. Exp. Med. 189:541-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen, D., S. B. Periwal, K. Larrivee, C. Zuleger, C. A. Erickson, R. L. Endres, and L. G. Payne. 2001. Serum and mucosal immune responses to an inactivated influenza virus vaccine induced by epidermal powder immunization. J. Virol. 75:7956-7965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng, E., L. Cardenas-Freytag, and J. D. Clements. 1999. The role of cAMP in mucosal adjuvanticity of Escherichia coli heat-labile enterotoxin (LT). Vaccine 18:38-49. [DOI] [PubMed] [Google Scholar]
- 8.Chong, C., M. Friberg, and J. D. Clements. 1998. LT(R192G), a non-toxic mutant of the heat-labile enterotoxin of Escherichia coli, elicits enhanced humoral and cellular immune responses associated with protection against lethal oral challenge with Salmonella spp. Vaccine 16:732-740. [DOI] [PubMed] [Google Scholar]
- 9.Cieplak, W., Jr., R. J. Messer, M. E. Konkel, and C. C. Grant. 1995. Role of a potential endoplasmic reticulum retention sequence (RDEL) and the Golgi complex in the cytotonic activity of Escherichia coli heat-labile enterotoxin. Mol. Microbiol. 16:789-800. [DOI] [PubMed] [Google Scholar]
- 10.Clements, J. D., and R. A. Finkelstein. 1979. Isolation and characterization of homogeneous heat-labile enterotoxins with high specific activity from Escherichia coli cultures. Infect. Immun. 24:760-769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clements, J. D., R. J. Yancey, and R. A. Finkelstein. 1980. Properties of homogeneous heat-labile enterotoxin from Escherichia coli. Infect. Immun. 29:91-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Condon, C., S. C. Watkins, C. M. Celluzzi, K. Thompson, and L. D. Falo, Jr. 1996. DNA-based immunization by in vivo transfection of dendritic cells. Nat. Med. 2:1122-1128. [DOI] [PubMed] [Google Scholar]
- 13.Del Giudice, G., and R. Rappuoli. 1999. Genetically derived toxoids for use as vaccines and adjuvants. Vaccine 17(Suppl. 2):S44-S52. [DOI] [PubMed] [Google Scholar]
- 14.Donnelly, J. J., J. B. Ulmer, J. W. Shiver, and M. A. Liu. 1997. DNA vaccines. Annu. Rev. Immunol. 15:617-648. [DOI] [PubMed] [Google Scholar]
- 15.Douce, G., M. M. Giuliani, V. Giannelli, M. G. Pizza, R. Rappuoli, and G. Dougan. 1998. Mucosal immunogenicity of genetically detoxified derivatives of heat labile toxin from Escherichia coli. Vaccine 16:1065-1073. [DOI] [PubMed] [Google Scholar]
- 16.Enioutina, E. Y., D. Visic, and R. A. Daynes. 2000. The induction of systemic and mucosal immune responses to antigen-adjuvant compositions administered into the skin: alterations in the migratory properties of dendritic cells appears to be important for stimulating mucosal immunity. Vaccine 18:2753-2767. [DOI] [PubMed] [Google Scholar]
- 17.Feltquate, D. M., S. Heaney, R. G. Webster, and H. L. Robinson. 1997. Different T helper cell types and antibody isotypes generated by saline and gene gun DNA immunization. J. Immunol. 158:2278-2284. [PubMed] [Google Scholar]
- 18.Frace, A. M., A. I. Klimov, T. Rowe, R. A. Black, and J. M. Katz. 1999. Modified M2 proteins produce heterotypic immunity against influenza A virus. Vaccine 17:2237-2244. [DOI] [PubMed] [Google Scholar]
- 19.Fuller, D. H., and J. R. Haynes. 1994. A qualitative progression in HIV type 1 glycoprotein 120-specific cytotoxic cellular and humoral immune responses in mice receiving a DNA-based glycoprotein 120 vaccine. AIDS Res. Hum. Retrovir. 10:1433-1441. [DOI] [PubMed] [Google Scholar]
- 20.Gagliardi, M. C., F. Sallusto, M. Marinaro, A. Langenkamp, A. Lanzavecchia, and M. T. De Magistris. 2000. Cholera toxin induces maturation of human dendritic cells and licences them for Th2 priming. Eur. J. Immunol. 30:2394-2403. [DOI] [PubMed] [Google Scholar]
- 21.Glenn, G. M., T. Scharton-Kersten, R. Vassell, G. R. Matyas, and C. R. Alving. 1999. Transcutaneous immunization with bacterial ADP-ribosylating exotoxins as antigens and adjuvants. Infect. Immun. 67:1100-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glenn, G. M., D. N. Taylor, X. Li, S. Frankel, A. Montemarano, and C. R. Alving. 2000. Transcutaneous immunization: a human vaccine delivery strategy using a patch. Nat. Med. 6:1403-1406. [DOI] [PubMed] [Google Scholar]
- 23.Haynes, J. R. 1999. Genetic vaccines. Infect. Dis. Clin. N. Am. 13:11-26. [DOI] [PubMed] [Google Scholar]
- 24.Kim, J. J., J. S. Yang, K. Dang, K. H. Manson, and D. B. Weiner. 2001. Engineering enhancement of immune responses to DNA-based vaccines in a prostate cancer model in rhesus macaques through the use of cytokine gene adjuvants. Clin. Cancer Res. 7(Suppl. 3):882s-889s. [PubMed] [Google Scholar]
- 25.Larregina, A. T., S. C. Watkins, G. Erdos, L. A. Spencer, W. J. Storkus, D. Beer Stolz, and L. D. Falo, Jr. 2001. Direct transfection and activation of human cutaneous dendritic cells. Gene Ther. 8:608-617. [DOI] [PubMed] [Google Scholar]
- 26.Le, T. P., K. M. Coonan, R. C. Hedstrom, Y. Charoenvit, M. Sedegah, J. E. Epstein, S. Kumar, R. Wang, D. L. Doolan, J. D. Maguire, S. E. Parker, P. Hobart, J. Norman, and S. L. Hoffman. 2000. Safety, tolerability and humoral immune responses after intramuscular administration of a malaria DNA vaccine to healthy adult volunteers. Vaccine 18:1893-1901. [DOI] [PubMed] [Google Scholar]
- 27.Lencer, W. I., C. Constable, S. Moe, M. G. Jobling, H. M. Webb, S. Ruston, J. L. Madara, T. R. Hirst, and R. K. Holmes. 1995. Targeting of cholera toxin and Escherichia coli heat labile toxin in polarized epithelia: role of COOH-terminal KDEL. J. Cell Biol. 131:951-962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lencer, W. I., S. Moe, P. A. Rufo, and J. L. Madara. 1995. Transcytosis of cholera toxin subunits across model human intestinal epithelia. Proc. Natl. Acad. Sci. USA 92:10094-10098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Macklin, M. D., D. McCabe, M. W. McGregor, V. Neumann, T. Meyer, R. Callan, V. S. Hinshaw, and W. F. Swain. 1998. Immunization of pigs with a particle-mediated DNA vaccine to influenza A virus protects against challenge with homologous virus. J. Virol. 72:1491-1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marinaro, M., H. F. Staats, T. Hiroi, R. J. Jackson, M. Coste, P. N. Boyaka, N. Okahashi, M. Yamamoto, H. Kiyono, H. Bluethmann, et al. 1995. Mucosal adjuvant effect of cholera toxin in mice results from induction of T helper 2 (Th2) cells and IL-4. J. Immunol. 155:4621-4629. [PubMed] [Google Scholar]
- 31.Martin, M., D. J. Metzger, S. M. Michalek, T. D. Connell, and M. W. Russell. 2000. Comparative analysis of the mucosal adjuvanticity of the type II heat-labile enterotoxins LT-IIa and LT-IIb. Infect. Immun. 68:281-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martin, M., D. J. Metzger, S. M. Michalek, T. D. Connell, and M. W. Russell. 2001. Distinct cytokine regulation by cholera toxin and type II heat-labile toxins involves differential regulation of CD40 ligand on CD4+ T cells. Infect. Immun. 69:4486-4492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Neirynck, S., T. Deroo, X. Saelens, P. Vanlandschoot, W. M. Jou, and W. Fiers. 1999. A universal influenza A vaccine based on the extracellular domain of the M2 protein. Nat. Med. 5:1157-1163. [DOI] [PubMed] [Google Scholar]
- 34.Partidos, C. D., B. F. Salani, M. Pizza, and R. Rappuoli. 1999. Heat-labile enterotoxin of Escherichia coli and its site-directed mutant LTK63 enhance the proliferative and cytotoxic T-cell responses to intranasally co-immunized synthetic peptides. Immunol. Lett. 67:209-216. [DOI] [PubMed] [Google Scholar]
- 35.Pertmer, T. M., T. R. Roberts, and J. R. Haynes. 1996. Influenza virus nucleoprotein-specific immunoglobulin G subclass and cytokine responses elicited by DNA vaccination are dependent on the route of vector DNA delivery. J. Virol. 70:6119-6125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prayaga, S. K., M. J. Ford, and J. R. Haynes. 1997. Manipulation of HIV-1 gp120-specific immune responses elicited via gene gun-based DNA immunization. Vaccine 15:1349-1352. [DOI] [PubMed] [Google Scholar]
- 37.Rappuoli, R., M. Pizza, G. Douce, and G. Dougan. 1999. Structure and mucosal adjuvanticity of cholera and Escherichia coli heat-labile enterotoxins. Immunol. Today 20:493-500. [DOI] [PubMed] [Google Scholar]
- 38.Roy, M. J., M. S. Wu, L. J. Barr, J. T. Fuller, L. G. Tussey, S. Speller, J. Culp, J. K. Burkholder, W. F. Swain, R. M. Dixon, G. Widera, R. Vessey, A. King, G. Ogg, A. Gallimore, J. R. Haynes, and D. Heydenburg Fuller. 2000. Induction of antigen-specific CD8+ T cells, T helper cells, and protective levels of antibody in humans by particle-mediated administration of a hepatitis B virus DNA vaccine. Vaccine 19:764-778. [DOI] [PubMed] [Google Scholar]
- 39.Ryan, E. T., T. I. Crean, M. John, J. R. Butterton, J. D. Clements, and S. B. Calderwood. 1999. In vivo expression and immunoadjuvancy of a mutant of heat-labile enterotoxin of Escherichia coli in vaccine and vector strains of Vibrio cholerae. Infect. Immun. 67:1694-1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sanchez, G. I., M. Sedegah, W. O. Rogers, T. R. Jones, J. Sacci, A. Witney, D. J. Carucci, N. Kumar, and S. L. Hoffman. 2001. Immunogenicity and protective efficacy of a Plasmodium yoelii Hsp60 DNA vaccine in BALB/c mice. Infect. Immun. 69:3897-3905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scharton-Kersten, T., G. M. Glenn, R. Vassell, J. Yu, D. Walwender, and C. R. Alving. 1999. Principles of transcutaneous immunization using cholera toxin as an adjuvant. Vaccine 17(Suppl. 2):S37-S43. [DOI] [PubMed] [Google Scholar]
- 42.Scharton-Kersten, T., J. Yu, R. Vassell, D. O'Hagan, C. R. Alving, and G. M. Glenn. 2000. Transcutaneous immunization with bacterial ADP-ribosylating exotoxins, subunits, and unrelated adjuvants. Infect. Immun. 68:5306-5313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schirmbeck, R., W. Bohm, K. Melber, and J. Reimann. 1995. Processing of exogenous heat-aggregated (denatured) and particulate (native) hepatitis B surface antigen for class I-restricted epitope presentation. J. Immunol. 155:4676-4684. [PubMed] [Google Scholar]
- 44.Schmaljohn, C., L. Vanderzanden, M. Bray, D. Custer, B. Meyer, D. Li, C. Rossi, D. Fuller, J. Fuller, J. Haynes, and J. Huggins. 1997. Naked DNA vaccines expressing the prM and E genes of Russian spring summer encephalitis virus and Central European encephalitis virus protect mice from homologous and heterologous challenge. J. Virol. 71:9563-9569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Simmons, C. P., P. Mastroeni, R. Fowler, M. Ghaem-maghami, N. Lycke, M. Pizza, R. Rappuoli, and G. Dougan. 1999. MHC class I-restricted cytotoxic lymphocyte responses induced by enterotoxin-based mucosal adjuvants. J. Immunol. 163:6502-6510. [PubMed] [Google Scholar]
- 46.Sin, J. I., J. J. Kim, D. Zhang, and D. B. Weiner. 2001. Modulation of cellular responses by plasmid CD40L: CD40L plasmid vectors enhance antigen-specific helper T cell type 1 CD4+ T-cell-mediated protective immunity against herpes simplex virus type 2 in vivo. Hum. Gene Ther. 12:1091-1102. [DOI] [PubMed] [Google Scholar]
- 47.Stevens, L. A., J. Moss, M. Vaughan, M. Pizza, and R. Rappuoli. 1999. Effects of site-directed mutagenesis of Escherichia coli heat-labile enterotoxin on ADP-ribosyltransferase activity and interaction with ADP-ribosylation factors. Infect. Immun. 67:259-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Takahashi, H., J. Cohen, A. Hosmalin, K. B. Cease, R. Houghten, J. L. Cornette, C. DeLisi, B. Moss, R. N. Germain, and J. A. Berzofsky. 1988. An immunodominant epitope of the human immunodeficiency virus envelope glycoprotein gp160 recognized by class I major histocompatibility complex molecule-restricted murine cytotoxic T lymphocytes. Proc. Natl. Acad. Sci. USA 85:3105-3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang, R., D. L. Doolan, T. P. Le, R. C. Hedstrom, K. M. Coonan, Y. Charoenvit, T. R. Jones, P. Hobart, M. Margalith, J. Ng, W. R. Weiss, M. Sedegah, C. de Taisne, J. A. Norman, and S. L. Hoffman. 1998. Induction of antigen-specific cytotoxic T lymphocytes in humans by a malaria DNA vaccine. Science 282:476-480. [DOI] [PubMed] [Google Scholar]
- 50.Williams, N. A., T. R. Hirst, and T. O. Nashar. 1999. Immune modulation by the cholera-like enterotoxins: from adjuvant to therapeutic. Immunol. Today 20:95-101. [DOI] [PubMed] [Google Scholar]
- 51.Xu-Amano, J., H. Kiyono, R. J. Jackson, H. F. Staats, K. Fujihashi, P. D. Burrows, C. O. Elson, S. Pillai, and J. R. McGhee. 1993. Helper T cell subsets for immunoglobulin A responses: oral immunization with tetanus toxoid and cholera toxin as adjuvant selectively induces Th2 cells in mucosa associated tissues. J. Exp. Med. 178:1309-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]