Abstract
Background
This study was conducted to examine, in vitro , the effect of soluble egg antigen (SEA) of S. haematobium on intracellular HCV RNA load in peripheral mononuclear cells (PBMC) as well as on cell proliferation in patients with chronic HCV infection.
Methods
PBMC from 26 patients with chronic HCV infection were cultured for 72 hours in presence and absence of 50 μg SEA/ml medium. Intracellular HCV RNA quantification of plus and minus strands was assessed before and after stimulation. PBMC from five healthy subjects were cultured for 7 days, flow cytometric analysis of DNA content was used to assess the mitogenic effect of SEA on PBMC proliferation compared to phytoheamaglutinine (PHA).
Results
Quantification of the intracellular viral load showed increased copy number/cell of both or either viral strands after induction with SEA in 18 of 26 patients (69.2%) thus indicating stimulation of viral replication. Flow cytometric analysis showed that mean ± S.D. of percent values of cell proliferation was induced from 3.2 ± 1.5% in un-stimulated cells to 16.7 ± 2.5 % and 16.84 ± 1.7 % in cells stimulated with PHA and SEA respectively.
Conclusion
the present study supports earlier reports on SEA proliferative activity on PBMC and provides a strong evidence that the higher morbidity observed in patients co-infected with schistosomiasis and HCV is related, at least in part, to direct stimulation of viral replication by SEA.
Background
Hepatitis C Virus (HCV) is the major agent in non A non B hepatitis with serious complications ranging from chronic inflammatory disease to hepatic cirrhosis and end stage liver failure or hepatocellular carcinoma (HCC). It is estimated that 170 millions world wide are infected with HCV. Egypt has unusually high prevalence of hepatitis C resulting in high morbidity and mortality from liver disease. Approximately 20% of blood donors are seropositive for HCV antibodies [1-5]. Schistosomiasis is another hepatotropic infection with a major burden on Egyptian patient population particularly in rural societies[1,6,7]. Co-infections with Schistosoma mansoni were repeatedly shown to augment pathogenesis induced by HBV and HCV hepatitis [2,8,9]. Subjects co-infected with HCV accelerate advancement of liver disease to the chronicity of HCV infection, cirrhosis and hepatocellular carcinoma and high incidence of viral persistence [10]. However similar studies on Schistosoma haematibium derived SEA has been scarcely reported.
Several reports attempted to reveal the underlying mechanisms of the severity of co-infection. The majority of these have focused on the role of growth factor rich soluble egg antigen (SEA), in contrary to adult worm soluble antigens, on angiogenic processes and granuloma formation mediated by peripheral blood mononuclear cell (PBMC) [11] and endothelial cell proliferation [12] through distinct intracellular signaling pathway[13]. Such mitogenic activity of SEA was shown to be cell specific, dose dependent and involves up regulation of cell cycle engine promoting genes [14].
Although HCV titer was shown to be higher in patients co infected with HCV and Schistosomiasis than patients with HCV infection alone, direct data on the effect of SEA on intracellular HCV replication in PBMC derived from patients with chronic hepatitis C are yet unavailable. Thus the rational of this study is to examine, in vitro , the influence of SEA on intracellular HCV RNA load in PBMC from patients with chronic HCV infection. Careful study of cell proliferation response to SEA allows analysis of relative induction in intracellular viral load versus stimulation of cell proliferation.
Methods
Subjects
Twenty six patients were enrolled in this study. All patients had detectable HCV antibodies by third generation ELISA (Dia Sorin, Torino, Italy). Serum concentrations of HCV RNA varied from 29 × 103 to 3 × 106 . Twenty four patients had genotype 4 of HCV genome, while the remaining two had 1b. None of the patients received treatment for HCV. All the patients had undetectable levels of HBsAg, schistosoma and HIV antibodies. Five subjects served as controls for normal PBMC stimulation studies. None of the 5 control subjects had detectable sero markers for viral or parasitic infections.
Methods
Effect of SEA on cell proliferation
A lyophilized preparation of the soluble fraction of mature live S. haematobium eggs was supplied by Theodore Bilharz Research Institute, Imbaba, Egypt.
Ten ml blood were collected from each normal control subject [approved by the medical ethical committee of the National research center (NRC)] and PBMC were separated from whole blood using Ficoll separating solution. Cells were washed with PBS three times. Mixtures were then centrifuged at 1600 rpm for 10 min to collect the washed cell pellet. After the last wash, cells were re-suspended in 1 ml RPMI-1640, supplemented with 10% FCS, counted and adjusted with RPMI to be 0.75 million cells/ml media. Cells were then plated into a 6 well plate at 2 million cells per well. A preliminary experiment was done to explore the optimum concentration of SEA that induces cell proliferation. Cells were treated with 10, 50, 100 and 150 μg/ml culture of SEA. Results obtained proved that 50 μg/ml culture is optimal (data not shown). To compare the stimulatory effect of SEA with phytoheamaglutinine (PHA), cells were stimulated with PHA at 5 μg/ml culture medium, other two wells were stimulated with SEA at 50 μg/ml medium, and the last two wells were left un-stimulated as control. Cells were cultured in a humidified atmosphere at 37°C, 5% CO2 for 7 days. Media were changed every 48 hours. After 7 days, cells were washed, permeablized with 0.1% triton X-100 solution (v/v) for 6 min at 4°C, then stained with 50 μg/ml propidium iodide (PI) as a DNA-specific fluorochrome for 30 min at 4°C in a dark place. DNA index analysis was performed on FACS Calibur flow cytometer equipped with logarithmic amplifiers.
Effect of SEA on HCV replication in PBMC
Fifteen ml of blood were withdrawn from each patient [approved by the medical ethical committee of (NRC)], five of them were collected on EDTA and were used to extract total cellular RNA from PBMC (to assess the presence of intracellular HCV strands in PBMC), the remaining ten ml blood were collected on heparin to separate PBMC using Ficoll for in vitro experiments. Cells were washed 5 times with PBS, then re-suspended in RPMI 1640 supplemented with 10% FCS at a concentration of 0.75 × 106/ml, plated on a 6 well plate, and cultured at 37°C, 5% CO2 in absence or presence of SEA (50 μg/ml culture). After 72 hr, cells were counted (using haemocytometer), collected, washed seven times with PBS, then subjected to RNA extraction with guanidinium isothiocyanate according to Chomczynski and Sacchi [15].
Reverse transcription – polymerase chain reaction (RT-PCR)
Total cellular RNA was reverse transcribed to cDNA in 25 μl reaction mixture containing 20 U of AMV reverse transcriptase (promega, Madison, WI, USA), 200 μM of each dNTP, 25 pmoles of either antisense primer (1CH: 5' -GGT GCA CGG TCT ACG AGA CCT-3') for plus strand or sense primer (2CH: 5' -AAC TAC TGT CTT CAC GCA GAA -3') for minus strand. The first round PCR was done in a total volume of 50 μl containing 200 μM of each dNTP, 1 × reaction buffer (10 mM Tris-HCl pH 8.8, 1.5 mM MgCl2, 50 mM KCl and 0.1% triton X-100), 2 U Taq polymerase (Finnzyme, Finland), 50 pmoles from each primer (2 CH and P2: 5' -TGC TCA TGG TGC ACG GTC TA -3'). Amplification was performed by 35 cycles of thermal cycling, each consists of denaturation at 94°C for 1 min, annealing at 55°C for 1 min and extension at 72°C for 1 min followed by a final extension step for additional 10 min. The reaction was then cooled to 4°C. Twenty percent of the reaction was taken for a second amplification round (35 cycles) with the internal pair of primers (D1: 5'-CGC AGA AAG CGT CTA GCC AT -3' and D2: 5' -ACT CGG CTA GCA GTC TCG CG -3'). Cycling conditions on the thermal cycler were the same as the first round. Products of PCR were analyzed on 2% agarose gel electrophoresis and photographed.
Quantification of intracellular HCV RNA
Plus-strand RNA was transcribed in vitro (using sp7 primer and transcription in vitro kit, Promega, WI) using as template a cloned fragment containing the entire 5' UTR in PGEM-T plasmid (unpublished data). 5' UTR RNA was quantified spectrophotometrically and standard amounts (2 × 106 – 2 × 107 copies) were reverse transcribed and amplified using the method described above. Amplified products from standard concentrations of 5' UTR RNA and from infected PBMC were resolved on 2% agarose gel stained with ethidium bromide. Polaroid photographed gels were scanned and the intensity of the amplified bands were analyzed using Total Lab software (Phoretix, New Castle, UK). Number of copies of plus-strand RNA in each specimen was calculated on the standard curve. The later was made from serial dilutions of RNA prepared by in vitro transcription and the intensity units of scanned amplified bands using software units.
Results
Detection of HCV in circulating PBMC
Plus HCV RNA strand was detectable in sera and PBMC derived from all the 26 HCV patients. Both plus and minus RNA strands were detectable in PBMC of 21 patients, the remaining 5 patients had only plus strand detectable in their circulating PBMC (Table 1).
Table 1.
Results of RT-PCR amplification of plus and minus RNA strands in circulating PBMC from 26 HCV positive patients.
| Patients | Age | Sex | Genotype | Serum Plus strand | PBMC Plus strand +ve | PBMC Minus strand |
| R.F. | 35 | ♂ | 4 | + | + | + |
| M.H. | 46 | ♂ | 4 | + | + | + |
| S.H. | 45 | ♂ | 4 | + | + | + |
| M.AA. | 40 | ♂ | 4 | + | + | + |
| 809 | 37 | ♂ | 4 | + | + | - |
| K.I. | 30 | ♂ | 4 | + | + | + |
| G.B. | 37 | ♂ | 4 | + | + | + |
| S.A. | 36 | ♀ | 1b | + | + | + |
| F.AA. | 42 | ♀ | 4 | + | + | + |
| no 47 | 45 | ♂ | 4 | + | + | + |
| A.N | 37 | ♂ | 4 | + | + | + |
| M.W. | 40 | ♂ | 4 | + | + | + |
| A.AZ. | 37 | ♂ | 4 | + | + | - |
| Y.AM. | 42 | ♂ | 1b | + | + | + |
| R.M. | 42 | ♂ | 4 | + | + | + |
| no 51 | 53 | ♂ | 4 | + | + | + |
| A.M. | 47 | ♂ | 4 | + | + | + |
| M.A. | 42 | ♂ | 4 | + | + | + |
| AH.AZ. | 45 | ♂ | 4 | + | + | - |
| A 730 | 47 | ♂ | 4 | + | + | + |
| A.A. | 43 | ♂ | 4 | + | + | + |
| M.O. | 45 | ♂ | 4 | + | + | + |
| K.M. | 48 | ♂ | 4 | + | + | + |
| A.S. | 38 | ♂ | 4 | + | + | + |
| S.Y. | 36 | ♂ | 4 | + | + | - |
| S.K. | 43 | ♀ | 4 | + | + | - |
Effect of SEA on PBMC proliferation
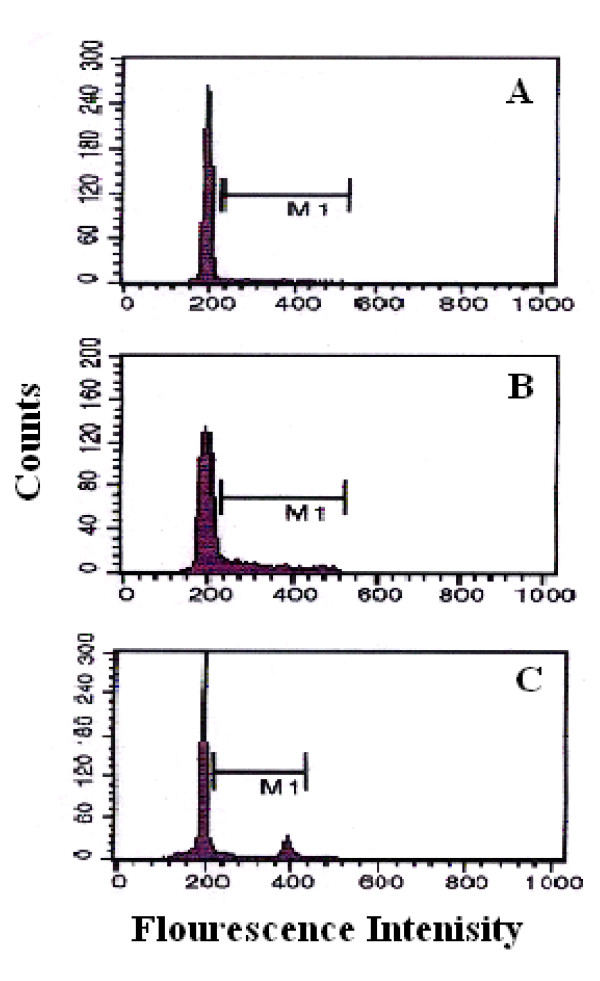
Flow cytometric analysis showed that the mean ± S.D. of percent values of cell proliferation from 5 control un-stimulated cell cultures at day 7 was 3.2 ± 1.5 %. Stimulation of the 5 control cultures with PHA and SEA resulted in a significant (p < 0.01) increase in DNA index to 16.7 ± 2.5 % and 16.84 ± 1.7 % respectively (figure 1).
Figure 1.
Flow cytometric analysis of cell proliferation (S+G2M) of PBMC. A) Normal un-stimulated PBMC, B) Normal PBMC stimulated with 5 μg/ml PHA, C) Normal PBMC stimulated with 50 μg/ml SEA after 7 days of culture.
Effect of SEA on cultured PBMC count from HCV patients
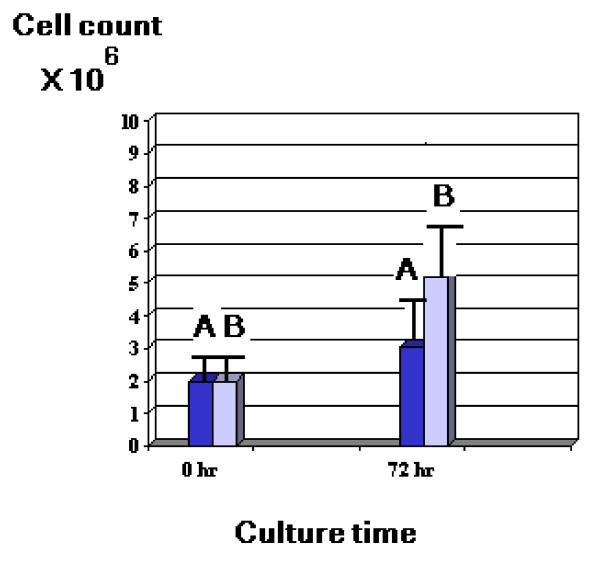
Stimulation of patients' PBMC in culture for 72 hours resulted in increased PBMC count in all cases ranging from 2 – 5 fold increase with a mean value of 4.26 ± 1.5 compared to non stimulated patients' cells that showed mean increase in cell count ranging from 1.3–2.3 fold only with a mean value of 1.75 ± 0.25 (Fig 2).
Figure 2.
Mean ± S.D. value of cell counts of PBMC cultures from 26 HCV patients with (B) and without (A) SEA stimulation.
Effect of SEA on HCV replication in PBMC in vitro
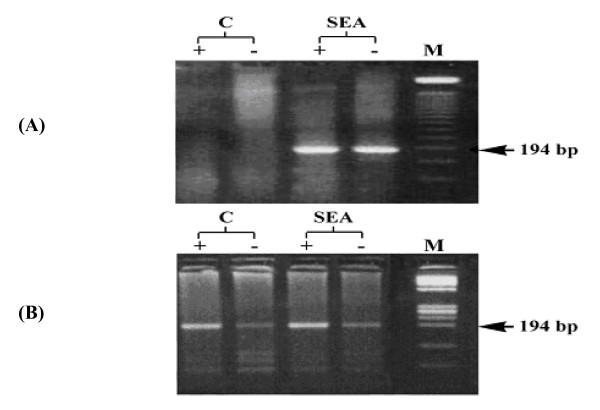
Quantification of the intracellular viral load (table 2) showed increased copy number/cell of both or either viral strands after stimulation with SEA in 18 of 26 patients (69.2%) thus indicating induction of viral replication (Fig 3, table 2). Five out of 21 (23.8%) patients lost preexisting viral strands upon culture. SEA stimulated cells of those patients showed restoration of either or both strands (Table 2).
Table 2.
Effect of SEA on HCV replication in PBMC in vitro
| patients | Before stimulation (copies/cell) | After stimulation (copies/cell) | Induction | ||
| Plus | minus | Plus | minus | ||
| R.F. | 169 | 196 | 496 | 148 | I |
| M.H. | 146 | 93 | UD | 119 | I |
| S.H. | UD | UD | 64 | 64 | I |
| M.AA. | 125 | UD | UD | UD | NI |
| 809 | 73 | 64 | 79 | 64 | I |
| K.I. | UD | UD | UD | 122 | I |
| G.B. | 42 | 51 | 64 | UD | I |
| S.A. | 185 | UD | 234 | UD | I |
| F.AA. | 100 | UD | UD | UD | NI |
| no 47 | 92 | 137 | 126 | UD | I |
| A.N | UD | UD | UD | 86 | I |
| M.W. | 100 | 100 | 100 | 105 | I |
| A.AZ. | UD | 64 | UD | 64 | NI |
| Y.AM. | 64 | 64 | 64 | 86 | I |
| R.M. | UD | 100 | 100 | 100 | NI |
| no 51 | 264 | 264 | 235 | 228 | NI |
| A.M. | UD | UD | UD | 122 | I |
| M.A. | 24 | UD | 38 | 84 | I |
| AH.AZ. | 104 | 100 | 100 | 90 | NI |
| A 730 | 345 | 404 | 100 | 346 | NI |
| A.A. | 73 | 73 | 104 | 100 | I |
| M.O. | 182 | 257 | 185 | 259 | I |
| K.M. | 73 | 73 | 73 | 79 | I |
| A.S. | UD | UD | 64 | UD | I |
| S.Y. | UD | UD | UD | UD | NI |
| S.K. | UD | UD | UD | UD | NI |
UD: undetected, I: induced, NI: non induced
Figure 3.
Results of PCR amplification of intracellular plus (+) and minus (-) RNA strands in PBMC derived from HCV patients before (C) and after stimulation with SEA in inducible (A) and non inducible (B) PBMC cultures. M is the molecular weight marker.
Discussion
In Egypt, viral hepatitis along with schistosomiasis is the major cause of chronic liver disease and liver cirrhosis [16-18]. The present study provides, on molecular bases, ample evidence that SEA treatment in vitro induces HCV replication and therefore may explain the higher disease severity in subjects co-infected with HCV and Schistosomiasis. The notion that intracellular HCV replication was induced in vitro by SEA in 69% of patients represents the first direct evidence on the role of this potent mitogen on HCV replication. It also suggests that individual host factors play distinct roles in determining viral response in vitro and perhaps the overall morbidity in patients co-infected with the two diseases. Several cellular proteins such as Elongation factor 3 (elfa 3), ribosomal proteins S5 and S9 and Eukaryotic translation initiation factor (eIf 3), the polypyrimidine tract binding protein (PTB), heterogenous nuclear ribonucleoprotein C (hnRNPC) and la antigen were shown to regulate translation of HCV polyprotein precursor and hence intracellular replication of viral genome [19-22]. Since PBMC have a major contribution into the circulatory pool of HCV load the results presented herein may explain why HCV titers are higher in patients with co-infection than those infected with HCV alone [23,24]. Furthermore we can not exclude similar role of SEA on hepatocytes harboring HCV genome in co-infected patients. Culturing the PBMC resulted in loss of both plus and minus RNA strands in 23.8% of cases. Interestingly SEA was capable of restoring either one or both strands in those cultures. This strengthens our hypothesis that SEA acts as a direct inducer of viral replication and makes it a valuable stimulatory reagent if future plans are considered to use short term PBMC cultures for antiviral screening. Recently, combinations of mitogens and/or cytokines were found to be able of stimulating synthesis of plus and minus RNA strands of HCV in PBMC and, in certain cases, affinity-purified T and B cells [25]. The exact mechanism whereby SEA stimulates HCV replication is not yet clear. A mechanism involving extracellular signaling of the growth factor components of SEA is perhaps implicated. In agreement with the current data, Kamal et al.,[23] concluded that co-occurrence of the two diseases was characterized by higher HCV RNA titers, histological activity, incidence of cirrhosis and hepatocellular carcinoma (HCC) compared to HCV infection alone. In the present study, the 5 fold increase in DNA index after 7 days and the observed 2.5 fold increase in PBMC count after 72 hour exposure to SEA indicate that SEA mediated cell proliferation involves increased rate of cellular DNA replication. In this regard both SEA and the commercial cellular mitogen PHA exerted similar magnitudes of DNA replication in the studied controls within 7 days of culture. Reports on other cell systems from our laboratory [14] and others [26,27] demonstrated that SEA associated cell proliferation involved up-regulation of cell cycle controlling genes such as peripheral cell nuclear antigen (PCNA) and B-cell translocation gene 1 (BTG1). Previous studies [28] reported that most soluble egg antigen fractions elicited in vitro granuloma reactions by PBMC of almost all Schitosoma haematobium infected patients and in vivo via endothelial cell proliferation [12,29] Conversely, separated soluble adult-worm antigens failed to stimulate PBMC of infected patients to form granulomas [28] suggesting that SEA contains unique factor (s) which are lacking in adult worm antigens. Furthermore, the role of SEA in PBMC proliferation has been well established as an initial step for angiogenesis and granuloma formation in Schistosomiasis patients by up-regulation of vascular endothelial cell growth factor. In HCC, the proliferative activity of vascular endothelial cells is suggested to play an important role in the positive regulation of tumor – associated vascular endothelial cell proliferation [30]. However, in vitro stimulatory effect of SEA on PBMC from chronic HCV patients was scarcely investigated. The significance of PBMC in chronic hepatitis C patients is based on the fact that PBMC represent a large extrahepatic reservoir for HCV replication. We and others have shown that PBMC from chronic HCV patients do not only contain HCV genomes but also function to maintain genomic replication [31-34] and to support viral core and envelope glycoprotein E1 expression in culture [34-36]. The two mitogens tested in the present study appeared not to have the same function on intracellular HCV titers indicating that different mechanisms of mitogen-viral interactions are involved. Induction of viral replication does not always correlate with increased cell proliferation, as SEA induced cell proliferation was notable in all patients even those whom intracellular HCV replication was not induced. Taken together the dual stimulatory function on both PBMC and viral copies per cell, the magnitude of total viral pool response in SEA stimulated culture is obviously larger than the currently presented values, in table 2, as viral copies per cell. The stimulatory function of SEA on replication of DNA viruses was reported as early as 1989 when Ishii et al.,[37] reported that SEA from Schistosoma japonicum induced Epstein Barr virus RNA in lymphoblastoid cells.
Conclusion
The present study suggests that SEA possesses potent in vitro proliferative activity on PBMC and provides the first evidence that SEA directly stimulates HCV replication in vitro . This may explain, at least in part, the higher morbidity observed in patients co-infected with Schistosomiasis and HCV.
Competing interests
The author(s) declare that they have no competing interests.
Authors' contributions
MKE conceived the study, participated in data analysis and revised the manuscript. SSY designed the study, performed all culture steps, carried out molecular detection and quantification of HCV, analyzed the data and drafted the manuscript. MHO performed HCV genotyping, participated in design of the study and revision of the manuscript. AAT performed the flow cytometry experiments. WTE helped in culture work. AMS revised the manuscript carefully. All authors read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Acknowledgments
Acknowledgements
This work was supported by the Egyptian Academy of Scientific Research and technology (ASRT) as project within the National program for genetic engineering and biotechnology.
Contributor Information
Mostafa K El-Awady, Email: mkawady@yahoo.com.
Samar S Youssef, Email: youssefsamar@hotmail.com.
Moataza H Omran, Email: moataza13@yahoo.com.
Ashraf A Tabll, Email: ashraftabll@yahoo.com.
Wael T El Garf, Email: elgarf@yahoo.com.
Ahmed M Salem, Email: ahmedsalem47@hotmail.com.
References
- Kamel MA, Ghaffar YA, Yasef MA, Wright M, Clark LC, Miller F. High HCV prevalence in Egyptian blood donors. Lancet. 1992;340:427. doi: 10.1016/0140-6736(92)91508-6. [DOI] [PubMed] [Google Scholar]
- Darwish MA, Raouf TA, Rushdy P, Constantine N, Rao MR, Edelman R. Risk factors associated with a high seroprevalence of hepatitis C virus infection in Egyptian blood donors. Am J Trop Med Hyg. 1993;49:440–447. doi: 10.4269/ajtmh.1993.49.440. [DOI] [PubMed] [Google Scholar]
- Waked IA, Saleh SM, Moustafa MS, Raouf AA, Thomas DL, Strickland GT. High prevalence of hepatitis C in Egyptian patients with chronic liver disease. Gut. 1995;37:105–107. doi: 10.1136/gut.37.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank C, Mohamed MK, Strickland GT, Lavanchy D, Arthur RR, Magder LS, El Khoby T, Abdel-Wahab Y, Ohn ESA, Anwar W, Sallam I. The role of parenteral antischistosomal therapy in the spread of hepatitis C virus in Egypt. Lancet. 2000;355:887–891. doi: 10.1016/S0140-6736(99)06527-7. [DOI] [PubMed] [Google Scholar]
- WHO Spread of hepatitis C linked to unsafe injection practices in Egypt. Bulletin of the world health organization. 2000;78:562. [Google Scholar]
- Saeed A, Al-Admawi AM, Al-Rasheed A, Fairclough D, Bacchus R. Hepatitis C virus infection in Egyptian volunteer blood donors in Riyadh. Lancet. 1991;338:459–460. doi: 10.1016/0140-6736(91)91094-B. [DOI] [PubMed] [Google Scholar]
- El-Ahmady O, Halim A-B, Mansour O, Salman T. Incidence of hepatitis C virus in Egyptians. J Hepatol. 1994;21:687. doi: 10.1016/S0168-8278(94)80123-1. [DOI] [PubMed] [Google Scholar]
- Badawi AF, Michael MS. Risk factors for hepatocellular carcinoma in Egypt: the role of hepatitis-B viral infection and schistosomiasis. Anticancer Res. 1999;19:4565–9. [PubMed] [Google Scholar]
- Aquino RT, Chieffi PP, Catunda SM, et al. Hepatitis B and C virus markers among patients with hepatosplenic mansonic schistosomiasis. Rev Inst Med Trop Sao Paulo. 2000;42:313–20. doi: 10.1590/s0036-46652000000600003. [DOI] [PubMed] [Google Scholar]
- Kamal SM, Graham CS, He Q, Bianchi L, Tawil AA, Rasenack JW, Khalifa KA, Massoud MM, Koziel MJ. Kinetics of intrahepatic hepatitis C virus (HCV)-specific CD4+ T cell responses in HCV and Schistosoma mansoni coinfection: relation to progression of liver fibrosis. J Infect Dis. 2004;1;189:1140–50. doi: 10.1086/382278. [DOI] [PubMed] [Google Scholar]
- El Ridi R, Ismail S, Gaafar T, El Demellawy M. Differential responsiveness of humans with early-stage schistosomiasis haematobium to Schistosoma haematobium soluble adult-worm and egg antigens. Parasitol Res. 1997;83:471–7. doi: 10.1007/s004360050282. [DOI] [PubMed] [Google Scholar]
- Freedman DO, Ottsen EA. Eggs of schistosoma mansoni stimulate endothelial cell proliferation in vitro . Infectious diseases. 1989;158:556. doi: 10.1093/infdis/158.3.556. [DOI] [PubMed] [Google Scholar]
- Almeida CA, Leite MF, Goes AM. Signal transduction events in peripheral blood mononuclear cells stimulated by Schistosoma mansoni antigens. Hum Immunol. 2001;62:1159–66. doi: 10.1016/S0198-8859(01)00302-0. [DOI] [PubMed] [Google Scholar]
- El Awady MK, Gad YZ, Wen Y, Essawi M, Effat L, Amr KS, Ismail S, Christ GJ. Schistosoma hematobium soluble egg antigen induces proliferation of urothelial and endothelial cells. World J Urol. 2001;19:263. doi: 10.1007/s003450100217. [DOI] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single step method of RNA isolation by acid guanidinium thiocyanate-phenol cloroform extraction. Anal Biochem. 1987;162:156. doi: 10.1016/0003-2697(87)90021-2. [DOI] [PubMed] [Google Scholar]
- Angelico M, Renganathan E, Gandin C, Fathy M, Profili MC, Refai W, De Santis A, Nagi A, Amin G, Capocaccia L, Callea F, Rapicetta M, Badr G, Rocchi G. Chronic liver disease in the Alexandria governorate, Egypt: combination of Schistosomiasis and virus infections. J hepatol. 1997;26:236. doi: 10.1016/S0168-8278(97)80036-0. [DOI] [PubMed] [Google Scholar]
- Halim AB, Garry RF, Dash S, Gribier MA. Effect of schistosomiasis and hepatitis on liver disease. Am J Trop Med Hyg. 1999;60:915. doi: 10.4269/ajtmh.1999.60.915. [DOI] [PubMed] [Google Scholar]
- Zaki A, Bassili A, Amin G, Aref T, Kandil M, Abou Basha LM. Morbidity of schistosomiasis mansoni in rural Alexandria, Egypt. J Egypt Soc Parasitol. 2003;33:695–710. [PubMed] [Google Scholar]
- Buratti E, Tisminetzky S, Zotti M, Baralle FE. Functional analysis of the interaction between HCV 5' UTR and putative subunits of eukaryotic translation initiation factor eIF3. Nucleic Acids Research. 1998;26:3179–3187. doi: 10.1093/nar/26.13.3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushi S, Okada M, Stahli J, Kageyama T, Hoshino FB, Kazuhiko Katayama. Ribosomal Protein S5 Interacts with the Internal Ribosomal Entry Site of Hepatitis C Virus. The J of Biological Chemistry. 2001;276:20824–20826. doi: 10.1074/jbc.C100206200. Issue of June 15. [DOI] [PubMed] [Google Scholar]
- Gontarek RR, Gutshall LL, Herold KM, Tsai J, Sathe GM, Mao J, Prescott G, Del Vecchio AM. hnRNPC and polypyrimidine tract-binding protein specifically interact with the pyrimidine-rich region with the 3' NTR of the HCV genome. Nucleic Acids Research. 1999;27:1457. doi: 10.1093/nar/27.6.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krecic AM, Swanson MS. hnRNP complexes: composition, structure and function. Curr Opin Cell Biol. 1999;363:71. doi: 10.1016/S0955-0674(99)80051-9. [DOI] [PubMed] [Google Scholar]
- Kamal S, Madwar M, Bianchi L, Tawil AE, Fawzy R, Peters T, Rasenack JW. Clinical, virological and histopathological features: long-term follow-up in patients with chronic hepatitis C co-infected with S. mansoni. Liver. 2000;20:281–9. doi: 10.1034/j.1600-0676.2000.020004281.x. [DOI] [PubMed] [Google Scholar]
- Kamal S, Bianchi L, Al Tawil A, Koziel M, El Sayed Khalifa K, Peter T, Rasenack JW. Specific cellular immune response and cytokine patterns in patients coinfected with hepatitis C virus and Schistosoma mansoni. J Infect Dis. 2001;15;184:972–82. doi: 10.1086/323352. [DOI] [PubMed] [Google Scholar]
- Pham TN, Macparland SA, Coffin CS, Lee SS, Bursey FR, Michalak TI. Mitogen-induced upregulation of hepatitis C virus expression in human lymphoid cells. J Gen Virol. 2005;86:657–66. doi: 10.1099/vir.0.80624-0. [DOI] [PubMed] [Google Scholar]
- Kuruda H, Yasunaga Y, Takatera H, Fujioka H, Ohnishi A, Tsujimoyo M. growth factors of bladder cancer determined by proliferating cell nuclear antigen immunostaining. Hinyokika Kiyo. 1994;40:379–82. [PubMed] [Google Scholar]
- Wada T, Shimabukuro T, Kurisu H, Shameam IA, Yashihiro S, Matsuyama H, Naito K, Hashimoto O, Tanaka K. Expression of proliferation cell nuclear antigen in superficial bladder cancer-application to paraffin-embeded tissue sections. Hinyokika Kiyo. 1993;39:313–319. [PubMed] [Google Scholar]
- Gaafar T, Ismail S, Helmy M, Afifi A, Guirguis N, el Ridi R. Identification of Schistosoma haematobium soluble egg antigens that elicit human granuloma formation in vitro . Parasitol Res. 1993;79:103–8. doi: 10.1007/BF00932254. [DOI] [PubMed] [Google Scholar]
- Loeffler DA, Lundy SK, Singh KP, Gerard HC, Hudson AP, Boros DL. Soluble egg antigens from Schistosoma mansoni induce angiogenesis-related processes by up-regulating vascular endothelial growth factor in human endothelial cells. J Infect Dis. 2002;1;185:1650–6. doi: 10.1086/340416. [DOI] [PubMed] [Google Scholar]
- Imura S, Miyake H, Izumi K, Tashiro S, Uehara H. Correlation of vascular endothelial cell proliferation with microvessel density and expression of vascular endothelial growth factor and basic fibroblast growth factor in hepatocellular carcinoma. J Med Invest. 2004;51:202–9. doi: 10.2152/jmi.51.202. [DOI] [PubMed] [Google Scholar]
- Muller HM, Pfaff E, Goeser T, kallinowski B, Salbach C, Theilmann L. Peripheral blood leukocytes serve as possible extrahepatic site for hepatitis C virus replication. J Gen Virol. 1993;74:669. doi: 10.1099/0022-1317-74-4-669. [DOI] [PubMed] [Google Scholar]
- Laskus T, Radkowski M, Piasek A, Nowicki M, Horban A, Cianciara J, Rakcla J. Hepatitis C virus in lymphoid cells of patients coinfected with human immunodeficiency virus type 1: evidence of active replication in monocyte/macrophages and lymphocytes. J Infect Dis. 2000;181:442. doi: 10.1086/315283. [DOI] [PubMed] [Google Scholar]
- El Awady MK, Abdel Rahman MM, Ismail SM, Amr KS, Omran M, Mohamed SA. Prediction of relapse after interferon therapy in hepatitis C virus infected by the use of triple assay. Journal of Gastroenterology and Hepatology. 2002;18:68. doi: 10.1046/j.1440-1746.2003.02919.x. [DOI] [PubMed] [Google Scholar]
- EL-Awady MK, Tabll A, Redwan E, Youssef SS, Omran MH, Thakeb F, El-Demellawy M. Flow Cytometric Detection of Hepatitis C Virus Antigens In Infected Peripheral Blood Leukocytes: Binding and Entry. world. J Gastroenterol. 2005;11:5203–5208. doi: 10.3748/wjg.v11.i33.5203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansonno D, Lacobelli AR, Cornachiulo V, Iodice G, Dammacco F. Detection of HCV proteins by immunofluoresence and HCV RNA genomic sequences by non -isotopic in situ hybridization in bone marrow cells and PBMC of chronically HCV infected patients. Clin Exp Immunol. 1996;103:414–21. doi: 10.1111/j.1365-2249.1996.tb08296.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong GZ, Lai LY, Jiang YF, He Y, Su XS. HCV replication in PBMC and its influence on interferon therapy. World J Gastroenterol. 2003;9:291. doi: 10.3748/wjg.v9.i2.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii A, Matsuoka H, Aji T, Hayatsu H, Wataya Y, Arimoto S, Tokuda H. Evaluation of the mutagenicity and the tumor-promoting activity of parasite extracts: Schistosoma japonicum and Clonorchis sinensis. Mutat Res. 1989;224:229–33. doi: 10.1016/0165-1218(89)90160-2. [DOI] [PubMed] [Google Scholar]